Homing of hematopoietic stem cells (HSCs) into the bone marrow (BM) is a prerequisite for establishment of hematopoiesis during development and following transplantation. However, the molecular interactions that control homing of HSCs, in particular, of fetal HSCs, are not well understood. Herein, we studied the role of the α6 and α4 integrin receptors for homing and engraftment of fetal liver (FL) HSCs and hematopoietic progenitor cells (HPCs) to adult BM by using integrin α6 gene–deleted mice and function-blocking antibodies. Both integrins were ubiquitously expressed in FL Lin−Sca-1+Kit+ (LSK) cells. Deletion of integrin α6 receptor or inhibition by a function-blocking antibody inhibited FL LSK cell adhesion to its extracellular ligands, laminins-411 and -511 in vitro, and significantly reduced homing of HPCs to BM. In contrast, the anti-integrin α6 antibody did not inhibit BM homing of HSCs. In agreement with this, integrin α6 gene–deleted FL HSCs did not display any homing or engraftment defect compared with wild-type littermates. In contrast, inhibition of integrin α4 receptor by a function-blocking antibody virtually abrogated homing of both FL HSCs and HPCs to BM, indicating distinct functions for integrin α6 and α4 receptors during homing of fetal HSCs and HPCs.
Introduction
During transplantation, intravenously injected hematopoietic stem and progenitor cells (HSPCs) selectively transmigrate through the sinusoidal walls into the bone marrow (BM) niches to engraft and reconstitute hematopoiesis. This process, called homing, is a rapid multistep process involving multiple adhesion receptor and ligand pairs, leading first to reversible adhesion of circulating HSPCs to endothelial cells of BM sinusoids and subsequently transmigration through the sinusoidal walls into the specific hematopoietic niches.1 The adhesive interactions mediated by distinct receptors on hematopoietic cells regulate the localization, migration, and other HSPC functions within their native microenvironments, and are likely to define their homing potential in a transplantation setting.2
The rate of reconstitution following transplantation varies depending on the source of hematopoietic stem cells (HSCs), suggesting developmentally regulated and cell type–specific differences in expression and function of homing receptors. Cord blood grafts are associated with a particularly delayed hematopoietic reconstitution.3 In contrast, experimental studies have shown a more rapid and robust reconstitution of both human and mouse fetal liver (FL) HSCs than adult BM HSCs.4,5 Increasing HSC homing and engraftment efficiency might offer a means to enhance reconstitution, which may be critically important if donor cell numbers are limiting, as frequently observed in cord blood units.3 Therefore, knowledge of the specific molecular mechanisms involved in homing and engraftment of different types of HSPCs is of clinical significance for the development of clinical transplantation methods.
Mouse models for mammalian hematopoiesis are widely used to study developmentally regulated HSC functions, including mobilization, homing, and engraftment.6 During mammalian ontogeny, HSCs develop sequentially in distinct hematopoietic tissues, beginning in the yolk sac and subsequently in the aorta-gonad-mesonephros region in the embryo proper. At embryonic day 10 (E10) in mice, definitive hematopoiesis arises in the liver, which is the main site of hematopoiesis throughout subsequent fetal development.7 In later gestation, hematopoiesis expands into spleen (E15) and thereafter into BM (E17). This shift in hematopoiesis is accomplished by migration of liver HSPCs into spleen and BM, and consequently, is associated with mobilization of large numbers of HSPCs into the bloodstream.6 Despite the central role of migration and homing in stem-cell biology and in stem-cell therapy, the molecular pathways underlying FL HSC homing into BM remain to be elucidated.
Members of the integrin family of adhesion receptors are major mediators of cell migration during development and in the adult organism, including the hematopoietic tissue.8 All integrins are heterodimeric transmembrane molecules consisting of α and β subunits. They mediate binding to extracellular matrix (ECM) and cell surface ligands by their extracellular domains and provide a link between cytoskeletal proteins and the cytoplasmic domain, thereby acting as bidirectional signaling molecules.9 In vivo studies using gene-deleted mice and function-blocking antibodies have established the fundamental role for β1 integrins during HSC migration both into FL and fetal and adult BM.10,–12 So far, 12 distinct β1 integrins, containing different α chains (α1-11, αv), have been characterized.8 Of these, the function of integrin α4 receptor during embryonic hematopoiesis has been studied by using gene-ablated mice and function-blocking antibodies.13,,–16 Although these data indicate an important role for the integrin α4 chain during fetal hematopoiesis, its role in homing of FL HSPCs has not been defined.
The integrin α6 chain assembles with integrin β1 or β4 chains to form major receptors for ECM laminins.17,18 Integrin α6 is ubiquitously expressed in both human and adult mouse primitive hematopoietic cells.19,–21 In adult BM, the major ligands of α6 integrins, laminin isoforms laminin-411 and laminin-511, previously called laminin-8 and laminin-10, respectively,22 are present in subendothelial basement membranes of sinusoids.23,–25 We have previously shown that the integrin α6 receptor promotes migration of human hematopoietic progenitor cells (HPCs) in vitro21 and functions as a homing receptor for adult mouse BM HSPCs in vivo.19 Herein, we have studied the role of the integrin α6 receptor for homing, engraftment, and mobilization of FL HSPCs in vivo by using function-blocking antibodies and gene-deleted mice.26 Our results show that the α6 integrin receptor is functional during homing of FL HPCs, but not during homing and engraftment of multilineage repopulating HSCs in vivo. In contrast, we show the critical role of integrin α4 receptor for homing of both FL HPCs and multilineage repopulating HSCs to adult BM, indicating distinct functions for integrin α6 and α4 receptors during fetal hematopoiesis.
Materials and methods
Animals and genotyping
Integrin α6+/− (C57BL/6 CD45.2) and wild-type congenic (CD45.1, CD45.2) mice were maintained under specific pathogen-free conditions. All adult mice used in the study were 8 to 14 weeks old. All animal protocols were approved by the local ethics committee at Lund University. The integrin α6+/− mouse strain26 has been backcrossed to a C57BL/6 background for more than 10 generations. Integrin α6−/− or wild-type CD45.2 embryos were obtained by interbreeding integrin α6+/− mice. Genotyping was performed by polymerase chain reaction (PCR). The wild-type integrin α6allele was detected by using a forward primer (5′-GTGATAACTCCAGCTTGTGTGTCAAG-3′) and a reverse primer (5′-CCTCTGCAGCGGGAGTGCTTC-3′),27 producing a 500-bp DNA fragment. The forward primer 5′-ATCGTCATGCTCCTGTTTCTCC-3′ and the reverse primer 5′-TCAGAGCAGCCGATTGTC-3′, which is located in the neomycin cassette, produced a 1000-bp band in the mutant integrin α6-allele. The PCR parameters were as follows: 1.5 minutes denaturation at 94°C; 40 cycles of amplification at 94°C for 1 minute; 61°C for 0.5 minutes; and 72°C for 1 minute, followed by 72°C for 10 minutes.
Hematopoietic growth factors
Human granulocyte colony-stimulating factor (G-CSF) and murine granulocyte-macrophage colony-stimulating factor (GM-CSF) were provided by Amgen (Thousand Oaks, CA). Human fms-like tyrosine kinase-3 ligand was a kind gift of Immunex (Seattle, WA). Mouse interleukin-3 was from PeproTech (Rocky Hill, NJ).
Proteins and function-blocking antibodies
Recombinant laminin-411 and laminin-511 were produced as described.28,29 Human placental laminin-511/521 (laminin-10/11) was from Chemicon (Chandlers Ford, United Kingdom). The purified preservative-free antibody GoH3 against integrin α6 (CD49f) was from Immunotech (Marseille, France), and PS/2 against integrin α4 (CD49d) was from Chemicon. HA2/5 against integrin β1 (CD29) and the rat IgG2a and hamster IgM antibodies were from PharMingen (San Diego, CA).
Isolation and analysis of FL HSPCs
Single cell suspensions from E14.5 FLs were prepared with 25-gauge needles and 70-μm filters. Cells not expressing lineage markers (Lin− cells) were selected by using antibodies against B220 (RA3–6B2), CD5 (53-7.3), CD8a (53-6.7), Gr-1 (RB6–8C5), and Ter-119 (Ly76) (PharMingen) and sheep anti–rat IgG (Fc)–conjugated immunomagnetic beads (Dynal, Oslo, Norway), and thereafter stained with goat antirat-tricolor (Caltag, Burlingame, CA), rat antimouse Sca-1-FITC (E13-161.7), c-Kit-APC (2B8), Flt3-PE (AZF10.1), or isotype-matched control antibodies (PharMingen). 7-amino actinomycin (Sigma-Aldrich, St Louis, MO) was used to exclude dead cells. Lin−Sca-1+Kit+ (LSK) cells were sorted on a FACSVantage or FACSDiva (Becton Dickinson, San Jose, CA). Reanalysis reproducibly showed a purity of more than 95%.
Integrin expression on FL LSK and Lin−Sca-1−c-Kit+ cells was analyzed by using PE-conjugated antibodies against integrin α6 (GoH3), α5 (5H10-27), or α4 (R1-2) (PharMingen). Analyses were performed using by FACSCalibur using FlowJo (TreeStar, San Carlos, CA) or CELLQuest (Becton Dickinson) programs.
Cell adhesion assay
Flat-bottom 96-well plates (MaxiSorp; Nunc, Roskilde, Denmark) were coated with ECM proteins in PBS (Gibco, Carlsbad, CA) at 10 to 30 μg/mL. The cell adhesion and antibody inhibition assays were performed as described.19
Assays for HPC homing
E14.5 FL cells were incubated with anti-integrin or control antibodies (2 μg/106 cells) in IMDM, 5% fetal calf serum (FCS) on ice for 30 to 40 minutes. The cells (5 × 106/mouse) were injected into tail vein of adult recipient mice, which were irradiated 4 to 8 hours before transplantation with a single 900-cGy dose using a cesium Cs 137 source (Instrument AB Scanditronix, Uppsala, Sweden). Three hours after injection, blood, BM, and spleen were harvested for granulocyte-macrophage colony-forming units (CFU-GM) assay, as described.19 The myeloid colonies (CFU-GMs) containing more than 50 cells were scored using an inverted Olympus IX 70 microscope (Tokyo, Japan). The mean value of each duplicate was calculated.
The number of recovered CFU-GMs was corrected to represent the whole BM, estimating that one femur represents 5.9% of the total BM.30 The number of injected CFU-GMs was assayed in a fraction of the donor FL cells. Host-derived residual CFU-GMs (0-2 in BM and spleen, 0 in blood) were assessed in irradiated mice but not receiving a transplant (1-2/experiment) and were subtracted from CFU-GMs recovered after transplantation. The percentage of recovered CFU-GMs of injected cells was calculated.
To analyze homing of integrin α6−/− FL HPCs, FLs were dissected in separate dishes containing PBS, 5% FCS. The cells from each liver were transplanted to 1 to 2 mice (3.5 to 5 × 106 cells/mouse), and CFU-GM homing was analyzed.
Antibody perturbation of FL HSC homing
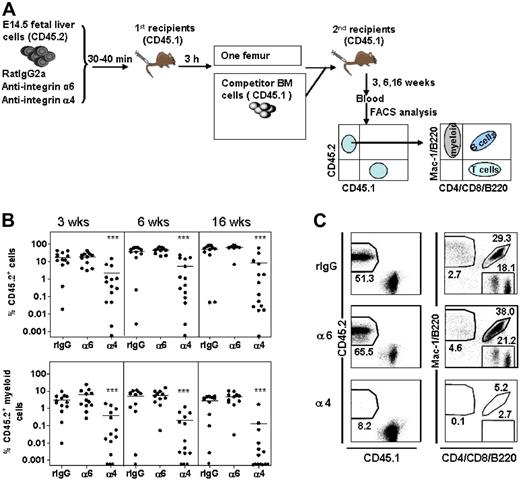
Wild-type CD45.2 E14.5 FL cells (donor) were incubated with anti-integrin or control antibodies (2μg/106 cells) for 30 to 40 minutes on ice.19 The anti-integrin α6 antibody was used in a concentration previously shown to inhibit homing of adult HSCs to BM.19 The cells (10 × 106 cells/mouse) were injected intravenously into lethally irradiated CD45.1 mice. Because of a possible transient effect of function-blocking antibodies, homing was assayed at 3 hours. The BM cells were harvested and washed with IMDM, 5% FCS to remove nonbound antibodies. The cells from one femur of each primary recipient were injected together with 2 × 105 BM cells from adult CD45.1 mice (competitor) into lethally irradiated CD45.1 secondary recipient mice. Blood was harvested at 3, 6, and 16 weeks after transplantation and analyzed for reconstitution of donor HSCs (CD45.2) and competitor/recipient cells (CD45.1). The reconstitutions at 3 and 6 weeks are achieved by both short-term and long-term HSCs, and the reconstitution at 16 weeks is derived from LT-HSCs only.31,–33 Lineage reconstitution was examined using fluorochrome-conjugated antibodies against CD4 and CD8 (T cells), B220 (B cells), and Mac-1 (CD11b) (myeloid cells) along with antibodies against CD45.1 (A20) and CD45.2 (104) (PharMingen) as described.19 Since myeloid cells, including myeloid progenitors, in contrast to lymphocytes, are short lived and must be continuously generated from the HSCs, sustained myeloid reconstitution is the best indicator of HSC reconstitution.34,35
Competitive repopulation assay of integrin α6−/− FL and fetal bone marrow (FBM) HSCs
Integrin α6+/+ or α6−/− E14.5 FL cells (1 × 106/mouse) were injected intravenously together with 1.5 × 106 wild-type adult BM competitors (CD45.1) into lethally irradiated CD45.1 recipients. Seventeen weeks after transplantation, a half femur equivalent BM cells were transplanted into lethally irradiated secondary recipient mice. Reconstitution was analyzed at 3 to 40 weeks after primary transplantation and 12 weeks after secondary transplantation.
E18.5 FBM cells from integrin α6−/− fetuses or WT littermates were obtained by homogenizing femurs and tibias through a 70-μm filter. Competitive repopulating units (CRUs) were measured by transplanting limiting doses of cells (1/15 and 1/7.5 leg equivalents corresponding to 2.0-2.2 × 104 and 4.0-4.4 × 104 cells, respectively) into lethally irradiated recipients (7 mice/dose) together with 2 × 105 BM competitor cells. Reconstitution was analyzed at 8 and 16 weeks after transplantation. Reconstituted mice were defined as having a minimum of 0.1% CD45.2+ total cells and 0.02% CD45.2+ cells of each of the myeloid, B, and T lineages with an analysis of a minimum 50 000 blood cells.36 The specificity of the fluorescence-activated cell sorting (FACS) staining was controlled by analyzing the blood of CD45.1 mice that did not undergo transplantation, and in all the assays the background CD45.2 staining was less than 0.01% and CD45.2+Mac-1+ staining was less than 0.002% of total nucleated blood cells. CRUs were calculated based on the frequencies of positive mice at each dose,37 using Limit Dilution Analysis software (StemCell Technologies, Vancouver, BC).
Mobilization assays
The mice reconstituted with integrin α6−/− or α6+/+ FL cells (CD45.2) together with CD45.1 competitor BM cells 5 to 8 months after transplantation were injected with G-CSF (100 μg/kg subcutaneously, twice daily) for 3 days.38 The following day, blood and femora were collected. Lin−Sca-1+ cells expressing CD45.1 or CD45.2 markers in blood and BM were analyzed as described above.
Reverse-transcription (RT)–PCR analysis of integrin α6 splice variants
Total RNA from adult BM and E14.5 FL LSK and Lin−Sca-1−c-Kit+ cells was isolated using Trizol (Life Technologies, Bethesda, MD). First-strand synthesis was performed by using oligo dT primer and Superscript II RT (Invitrogen, Frederick, MD). The primers for the integrin α6A and α6B variants were as follows: forward, 5′-GTGAACGTGAGGTGTGTGAAC-3′ and reverse, 5′-CGCATGGTATCGGGGAATGC-3′39 ; and for glyceraldehyde-3-phosphate dehydrogenase (GAPDH): forward, 5′-TTGTCAGCAATGCATCCTGC-3′ and reverse, 5′-CCGTTCAGCTCTGGGATGAC-3′.40 The cycling conditions were 94°C for 2 minutes, followed by 35 cycles of 94°C for 15 seconds, 50°C for 30 seconds, and 72°C for 2 minutes.
Statistical analysis
Statistical significance was determined using the unpaired t test for parametric or Mann-Whitney t test for nonparametric comparisons.
Results
Expression integrin α4, α6, and α5 on FL HSCs and HPCs
We analyzed the expression of integrin α4 and α6 chains on LSK cells, which are highly enriched for HSCs and contain all short-term and long-term reconstituting activity in mouse FL.5,41 All LSK cells from E14.5 FL expressed integrin α4 and α6 receptors (Figure 1).
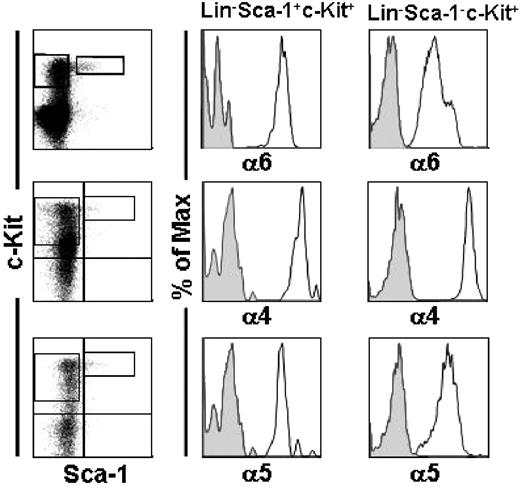
Integrin expression in mouse fetal liver HSCs. E14.5 FL cells were stained with lineage antibodies (Lin) and gated for Lin− and viable cells. Lin− viable cells were gated for Sca-1−c-Kit+ clonogenic progenitor cells and the more primitive Sca-1+c-Kit+ (LSK) cells. The left panels show gates for Sca-1+c-Kit+ and Sca-1−c-Kit+ on Lin− cells. A ubiquitous expression of integrin α6, α4, and α5 chains was seen in LSK cells (right panels) and Lin−Sca-1−c-Kit+ progenitors (middle panels). Staining with the anti-integrin antibodies is shown as open histograms and staining with isotype control antibodies, as gray histograms. Shown is 1 representative analysis of 2.
Integrin expression in mouse fetal liver HSCs. E14.5 FL cells were stained with lineage antibodies (Lin) and gated for Lin− and viable cells. Lin− viable cells were gated for Sca-1−c-Kit+ clonogenic progenitor cells and the more primitive Sca-1+c-Kit+ (LSK) cells. The left panels show gates for Sca-1+c-Kit+ and Sca-1−c-Kit+ on Lin− cells. A ubiquitous expression of integrin α6, α4, and α5 chains was seen in LSK cells (right panels) and Lin−Sca-1−c-Kit+ progenitors (middle panels). Staining with the anti-integrin antibodies is shown as open histograms and staining with isotype control antibodies, as gray histograms. Shown is 1 representative analysis of 2.
Furthermore, 100% and 95% of committed myeloid progenitors, which have the phenotype Lin−Sca-1−c-Kit+,6 expressed the integrin α4 and α6 chains, respectively. Expression of integrin α5 was similar on FL cells. In agreement with this, a ubiquitous expression of these integrins has been reported in less well-defined fetal HSPCs.16,42,43
Integrin α6β1 mediates FL LSK adhesion to laminins
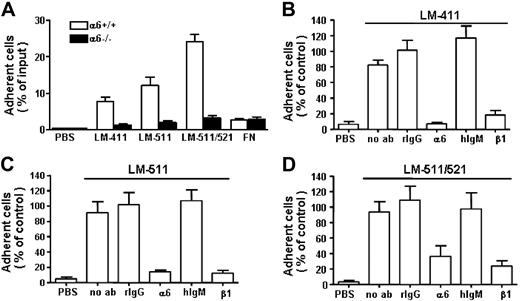
In in vitro adhesion assay (Figure 2), 10% to 15% of the isolated FL LSK cells adhered to recombinant laminin-411 and -511 isoforms and 25% adhered to placental laminin-511/521. Pretreatment of the cells with a protein kinase C activator, TPA, did not increase cell adhesion to the laminins (not shown). In addition to integrin α6β1, other laminin receptors, including integrin α2β1, α-dystroglycan, p67 receptor, and heparan sulfate proteoglycans are also expressed on HSPCs.20,44,–46 We therefore analyzed the contribution of integrin α6β1 receptor for FL LSK adhesion to laminin-411 and -511 by using integrin α6−/− FL LSK cells and function-blocking antibodies against α6 and β1 chains. Deletion of α6 integrin receptor effectively reduced LSK cell adhesion to all laminin isoforms in vitro (Figure 2A). In agreement with this, adhesion of wild-type LSK cells to laminins was inhibited by both antibodies (Figure 2B-D), indicating that integrin α6β1 is a major lamininreceptor in FL LSK cells. However, some cells still adhered to laminins after inhibition of integrin α6 function, which might indicate expression of other laminin receptors in a fraction of FL LSK cells.
Adhesion of mouse fetal liver LSK cells to laminins and inhibition of cell adhesion by antibodies against integrin α6 and β1 chains. (A) The adhesion of integrin α6−/− FL LSK cells (α6−/−) and wild-type littermates (α6+/+) to laminin-411 (LM-411) (30 μg/mL), laminin-511 (LM-511) (10 μg/mL), and laminin-511/521 (LM-511/521) (10 μg/mL). PBS indicates adhesion to wells coated with PBS instead of proteins; FN, adhesion to wells coated with fibronectin. Data represent mean (± SEM) from 2 experiments performed in triplicate. Asterisks indicate differences compared with wild-type cells. (B-D) Adhesion of LSK cells to wells coated with LM-411 (B), LM-511 (C), and LM-511/521 (D) in the presence of antibodies GoH3 against integrin α6 chain (α6), Ha2/5 against integrin β1 chain (β1), or control rat or hamster monoclonal antibodies (rIgG and hIgM, respectively). PBS indicates cell adhesion to wells coated with PBS instead of proteins. The results are shown as percentage adherent cells of mean adhesion obtained after treatment with rIgG control antibody. Data are mean (± SEM) from 2 to 3 experiments performed in triplicate.
Adhesion of mouse fetal liver LSK cells to laminins and inhibition of cell adhesion by antibodies against integrin α6 and β1 chains. (A) The adhesion of integrin α6−/− FL LSK cells (α6−/−) and wild-type littermates (α6+/+) to laminin-411 (LM-411) (30 μg/mL), laminin-511 (LM-511) (10 μg/mL), and laminin-511/521 (LM-511/521) (10 μg/mL). PBS indicates adhesion to wells coated with PBS instead of proteins; FN, adhesion to wells coated with fibronectin. Data represent mean (± SEM) from 2 experiments performed in triplicate. Asterisks indicate differences compared with wild-type cells. (B-D) Adhesion of LSK cells to wells coated with LM-411 (B), LM-511 (C), and LM-511/521 (D) in the presence of antibodies GoH3 against integrin α6 chain (α6), Ha2/5 against integrin β1 chain (β1), or control rat or hamster monoclonal antibodies (rIgG and hIgM, respectively). PBS indicates cell adhesion to wells coated with PBS instead of proteins. The results are shown as percentage adherent cells of mean adhesion obtained after treatment with rIgG control antibody. Data are mean (± SEM) from 2 to 3 experiments performed in triplicate.
Normal development of FL HSPCs in integrin α6−/− mice
FL hematopoiesis was studied in E14.5 integrin α6 gene–deleted embryos and wild-type littermates (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The numbers of LSK cells, the more primitive Flt3− fractions,47 and CFU-GMs were similar in gene-deleted and in wild-type embryos, indicating normal development of FL HSPCs in the absence of α6 integrin.
Homing of integrin α6−/− FL HPCs to adult BM is impaired
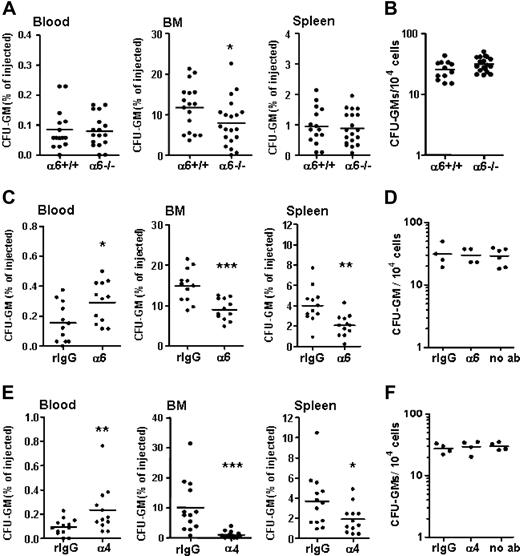
To analyze the role of integrin α6 receptor for FL HPC homing, FL cells from integrin α6−/− embryos or wild-type littermates were injected into lethally irradiated mice. About 12% of the injected wild-type FL CFU-GMs were recovered in BM (Figure 3A). Deletion of integrin α6 receptor resulted in a partially but significantly reduced homing of FL HPCs to BM (67% of wild type). In contrast, CFU-GM homing to spleen was not affected by the absence of integrin α6 receptor. The numbers of α6−/− and α6+/+ CFU-GMs recovered in blood were similar. Since only a fraction of the transplanted CFU-GMs were recovered in spleen and in blood, it is likely that the injected FL CFU-GMs transiently home to other organs, such as lung and liver, as previously described.48 The colony growth of integrin α6−/− FL cells was similar to that of wild-type cells (Figure 3B).
The role of integrin α6 and α4 receptors for homing of fetal liver progenitors to BM and spleen. FL cells were injected into lethally irradiated recipient mice. Three hours after injection, cells from blood, BM, and spleen were collected for CFU-GM assay. Data are pooled from 3 independent experiments. (A) Percentages of injected FL HPCs (GFU-GM) from integrin α6−/− FL (α6−/−) embryos and wild-type littermates (α6+/+) recovered in blood, BM, and spleen. (B) Frequency of CFU-GMs in integrin α6−/− and wild-type (α6+/+) FLs. (C) Percentages of FL CFU-GMs recovered in blood, BM, and spleen after treatment with a function-blocking antibody against integrin α6 receptor (α6) or isotype control antibody (rIgG). (D) Frequency of CFU-GMs in FL cells cultured after treatment with the antibody against integrin α6 receptor (α6) or isotype control antibody (rIgG), or without pretreatment with antibodies (no ab). (E) Percentages of FL CFU-GMs recovered in blood, BM, and spleen after treatment with a function-blocking antibody against integrin α4 receptor (α4) or isotype control antibody (rIgG). (F) Frequency of CFU-GMs in FL cells cultured after treatment with the antibody against integrin α4 receptor (α4) or isotype control antibody (rIgG), or without pretreatment with antibodies (no ab). The results in panels C and E are pooled from 2 separate experiments. *P < .05, **P < .01, ***P < .001, compared with results obtained from wild-type FL cells (A,B) or cells treated with the isotype control antibodies (C-F). Each dot represents mean of 2 duplicate measurements from one recipient mouse. The horizontal bars show mean values.
The role of integrin α6 and α4 receptors for homing of fetal liver progenitors to BM and spleen. FL cells were injected into lethally irradiated recipient mice. Three hours after injection, cells from blood, BM, and spleen were collected for CFU-GM assay. Data are pooled from 3 independent experiments. (A) Percentages of injected FL HPCs (GFU-GM) from integrin α6−/− FL (α6−/−) embryos and wild-type littermates (α6+/+) recovered in blood, BM, and spleen. (B) Frequency of CFU-GMs in integrin α6−/− and wild-type (α6+/+) FLs. (C) Percentages of FL CFU-GMs recovered in blood, BM, and spleen after treatment with a function-blocking antibody against integrin α6 receptor (α6) or isotype control antibody (rIgG). (D) Frequency of CFU-GMs in FL cells cultured after treatment with the antibody against integrin α6 receptor (α6) or isotype control antibody (rIgG), or without pretreatment with antibodies (no ab). (E) Percentages of FL CFU-GMs recovered in blood, BM, and spleen after treatment with a function-blocking antibody against integrin α4 receptor (α4) or isotype control antibody (rIgG). (F) Frequency of CFU-GMs in FL cells cultured after treatment with the antibody against integrin α4 receptor (α4) or isotype control antibody (rIgG), or without pretreatment with antibodies (no ab). The results in panels C and E are pooled from 2 separate experiments. *P < .05, **P < .01, ***P < .001, compared with results obtained from wild-type FL cells (A,B) or cells treated with the isotype control antibodies (C-F). Each dot represents mean of 2 duplicate measurements from one recipient mouse. The horizontal bars show mean values.
Integrin α6 antibody inhibits FL HPC homing to BM and spleen
Since the integrin α6 gene deletion resulted in only partial reduction of HPC homing, it is possible that the loss of function may have been compensated by other cell adhesion molecules. To circumvent this possibility of compensation, we also used a function-blocking antibody to evaluate the effect of a more immediate loss of integrin α6 function for progenitor cell homing. The anti-integrin α6 antibody GoH3 used has previously been shown to inhibit transendothelial migration of neutrophils in vivo49,50 and homing of adult mouse HPCs and HSCs to BM vivo.19 FL cells were preincubated with the anti-integrin or control antibodies and injected intravenously into lethally irradiated recipient mice. Three hours after transplantation, 14.9% and 4.0% of the CFU-GMs pretreated with the control antibody were recovered in BM and spleen, respectively (Figure 3C). In full agreement with the reduced homing efficiency of integrin α6 gene–deleted HPCs, the function-blocking antibody against integrin α6 receptor significantly inhibited homing of CFU-GMs into BM (60% of control). Notably, treatment with the anti-integrin α6 antibody also resulted in reduced homing of HPCs to spleen and significant retention of HPCs in blood, demonstrating that HPC migration to extramedullary sites is more effectively inhibited by the antibody than gene deletion.
The inhibition of FL CFU-GM homing to BM by the anti-integrin α6 antibody was similar to that previously observed with adult BM cells.19 However, homing of FL but not adult BM CFU-GM to spleen was reduced by the anti-integrin α6 antibody treatment, suggesting developmentally regulated differences in receptor engagement in homing of embryonic and adult BM HPCs to spleen. Importantly, antibody treatment did not influence colony formation of FL HPCs (Figure 3D).
Integrin α4 antibody abrogates FL HPC homing to BM
The partial inhibition of FL HPC homing to BM after treatment with anti-integrin α6 antibody suggests functional collaboration of additional adhesion molecules. Previous16,42,43 and our present results indicate that integrin α4 is ubiquitously expressed on FL HSPCs. Thus, we analyzed the role of integrin α4 receptor for homing of FL HPCs into adult BM, using a function-blocking antibody PS/2, previously shown to be functionally active in vitro and in vivo.19,51,52 Treatment of FL cells with the antibody almost completely inhibited CFU-GM homing into BM (< 10% of control). The anti-integrin α4 antibody also inhibited HPC homing to spleen, in contrast to the reported increase in uptake of adult HPCs to spleen after blocking of integrin α4 receptor or its counterreceptor VCAM-1.51 Correspondingly, significantly more CFU-GMs were retained in blood after treatment with the anti-integrin α4 antibody, compared with treatment with the control antibody (Figure 3E). Pretreatment of cells with anti-integrin α4 antibody did not affect colony growth (Figure 3F).
Integrin α6-deficient FL HSCs engraft normally in adult BM
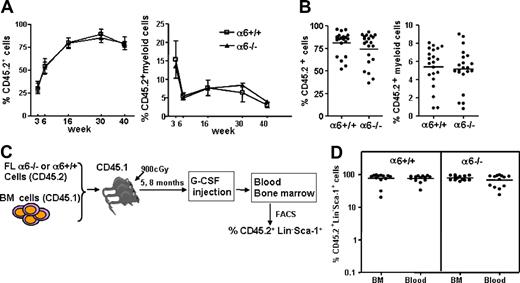
To study the contribution of integrin α6 receptor to HSC homing and engraftment in BM, FL cells from integrin α6−/− embryos or wild-type littermates were transplanted together with adult BM competitor cells into lethally irradiated recipients. Engraftment of short-term and long-term integrin α6−/− FL HSCs was similar to that of wild-type cells. Reconstitution of total and myeloid cells (Figure 4A), as well as lymphoid cells (not shown), from both genotypes was identical at 3 to 40 weeks after transplantation. The homing efficiency of most primitive HSCs was studied by transplanting BM cells from the primary recipients into lethally irradiated secondary recipients. At 12 weeks after serial transplantation, reconstitution was still similar in both groups (Figure 4B), suggesting that FL cells lacking integrin α6 home and engraft efficiently in adult BM.
Reconstitution and mobilization of integrin α6−/− fetal liver HSCs. (A) FL cells (106) from integrin α6−/− (α6−/−) embryos or wild-type littermates (α6+/+) expressing CD45.2 isotype together with 1.5 × 106 CD45.1 competitor adult BM cells were injected into lethally irradiated CD45.1 mice. Blood was harvested at 3, 6, 16, 30, and 40 weeks after transplantation. Shown is reconstitution of CD45.2+ cells and CD45.2 myeloid cells (Mac-1) of all nucleated cells (mean ± SD; n = 13-14/group at 3, 6, and 16 weeks and n = 3-4/group at 30 and 40 weeks). (B) After 17 weeks, BM cells were transplanted into secondary recipients and reconstitution was analyzed 12 weeks after transplantation. Data are pooled from 2 independent experiments. The horizontal bars show mean values for each measurement. (C) Schematic representation of HSPC mobilization. Mice reconstituted with integrin α6−/− (α6−/−) or wild-type (α6+/+) FL cells (CD45.2), together with CD45.2 competitor cells, 5 and 8 months after transplantation, were injected subcutaneously with G-CSF for 3 days. On the fourth day, the femora and blood were collected and the cells in blood and BM were analyzed by flow cytometry. (D) Shown is the proportion of CD45.2+Lin−Sca-1+ cells of all Lin−Sca-1+ cells in BM and blood. The data are pooled since there was no difference of mobilization response in the 2 experiments.
Reconstitution and mobilization of integrin α6−/− fetal liver HSCs. (A) FL cells (106) from integrin α6−/− (α6−/−) embryos or wild-type littermates (α6+/+) expressing CD45.2 isotype together with 1.5 × 106 CD45.1 competitor adult BM cells were injected into lethally irradiated CD45.1 mice. Blood was harvested at 3, 6, 16, 30, and 40 weeks after transplantation. Shown is reconstitution of CD45.2+ cells and CD45.2 myeloid cells (Mac-1) of all nucleated cells (mean ± SD; n = 13-14/group at 3, 6, and 16 weeks and n = 3-4/group at 30 and 40 weeks). (B) After 17 weeks, BM cells were transplanted into secondary recipients and reconstitution was analyzed 12 weeks after transplantation. Data are pooled from 2 independent experiments. The horizontal bars show mean values for each measurement. (C) Schematic representation of HSPC mobilization. Mice reconstituted with integrin α6−/− (α6−/−) or wild-type (α6+/+) FL cells (CD45.2), together with CD45.2 competitor cells, 5 and 8 months after transplantation, were injected subcutaneously with G-CSF for 3 days. On the fourth day, the femora and blood were collected and the cells in blood and BM were analyzed by flow cytometry. (D) Shown is the proportion of CD45.2+Lin−Sca-1+ cells of all Lin−Sca-1+ cells in BM and blood. The data are pooled since there was no difference of mobilization response in the 2 experiments.
Normal mobilization of integrin α6−/− HSPCs by G-CSF
To test whether integrin α6 receptor is involved in HSPC mobilization, HSPCs were mobilized by G-CSF in mice stably reconstituted with integrin α6−/− or wild-type FL cells together with competitor cells at 5 and 8 months after transplantation. The proportions of FL-derived Lin−Sca-1+ cells in blood and BM were analyzed by flow cytometry (Figure 4C). The proportion of integrin α6−/− Lin−Sca-1+ cells in blood and BM was similar to that derived from wild-type littermates (Figure 4D), showing that absence of α6 integrin does not influence HSPC mobilization from BM.
Integrin α4, but not α6, receptor is required for homing of FL HSCs to BM
Since FL HSCs have a greater proliferative capacity than adult HSCs,4 a possible homing defect in integrin α6 gene–deleted HSCs might be masked by the high proliferation rate during engraftment. In addition, the efficient engraftment of integrin α6−/− FL HSCs might be due to functional compensation by other molecules. To specifically address the impact of α6 integrin receptor on HSC homing, we studied the effect of inhibition of integrin function by antibody on short-term homing of HSCs to BM as described.30 The HSCs were assayed by the competitive repopulation assay (Figure 5A). Homing of FL HSCs, in contrast to adult HSCs,19 was not affected by inhibition of integrin α6 receptor function (Figure 5B). In contrast, treatment with the anti-integrin α4 antibody nearly abrogated homing of both short-term and long-term HSCs, indicating a dominant role for integrin α4-receptor in recruiting FL HSCs into BM.
Perturbation of fetal liver HSC homing by antibodies against integrin α6 and α4. (A) Schematic representation of the experimental setup: FL cells from CD45.2 mice were incubated with rat IgG2a antibody (rIgG) or antibodies against integrin α4 (α4) or integrin α6 (α6) and injected into lethally irradiated CD45.1 mice. BM cells were harvested 3 hours after injection and cells from one femur each were injected together with CD45.2 competitor BM cells into lethally irradiated CD45.1 secondary recipient mice. Blood was harvested at 3, 6, and 16 weeks after transplantation and reconstitution was analyzed by flow cytometry. (B) Peripheral blood reconstitution of CD45.2 cells and CD45.2 myeloid cells at 3, 6, and 16 weeks. ***P < .001 compared with reconstitution from cells incubated with rat IgG. Data are pooled from 2 independent experiments. The horizontal bars show mean values. (C) Representative FACS dot plots showing blood reconstitution 16 weeks after secondary transplantation. The left panels show reconstitution of CD45.2 FL cells. The right panels show blood reconstitution of myeloid (Mac-1) cells derived from CD45.2 cells. The numbers indicate the mean percentage of cells in the corresponding fraction (n = 11-14).
Perturbation of fetal liver HSC homing by antibodies against integrin α6 and α4. (A) Schematic representation of the experimental setup: FL cells from CD45.2 mice were incubated with rat IgG2a antibody (rIgG) or antibodies against integrin α4 (α4) or integrin α6 (α6) and injected into lethally irradiated CD45.1 mice. BM cells were harvested 3 hours after injection and cells from one femur each were injected together with CD45.2 competitor BM cells into lethally irradiated CD45.1 secondary recipient mice. Blood was harvested at 3, 6, and 16 weeks after transplantation and reconstitution was analyzed by flow cytometry. (B) Peripheral blood reconstitution of CD45.2 cells and CD45.2 myeloid cells at 3, 6, and 16 weeks. ***P < .001 compared with reconstitution from cells incubated with rat IgG. Data are pooled from 2 independent experiments. The horizontal bars show mean values. (C) Representative FACS dot plots showing blood reconstitution 16 weeks after secondary transplantation. The left panels show reconstitution of CD45.2 FL cells. The right panels show blood reconstitution of myeloid (Mac-1) cells derived from CD45.2 cells. The numbers indicate the mean percentage of cells in the corresponding fraction (n = 11-14).
Normal HSC colonization to FBM in integrin α6−/− mice
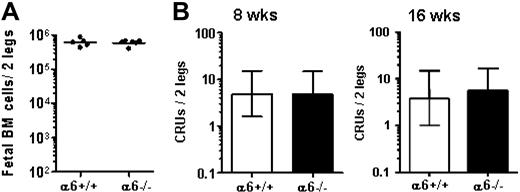
The role of integrin α6 receptor during physiological migration of HSCs from FL into FBM was studied in E18.5 integrin α6 gene–deleted embryos and wild-type littermates. Fetal BM cellularity (Figure 6A) was not affected by integrin α6 gene deletion. Furthermore, the CRU numbers in α6−/− FBM were similar to that in WT littermates (Figure 6B), suggesting normal HSC homing and engraftment in the absence of integrin α6.
Normal FBM hematopoiesis in absence of integrin α6. E18.5 FBM from integrin α6 gene–deleted mice (α6−/−) and wild-type littermates (α6+/+) was analyzed for total cell number (A) total cell number (horizontal bars show mean values), and (B) competitive repopulating units (CRUs). CRUs were assayed by transplanting 2 to 4.4 × 104 FBM cells together with 2 × 105 adult BM cells into lethally irradiated adult mice. Three of 13 and 3 of 12 mice that received integrin α6−/− FBM cells, and 3 of 14 and 2 of 12 mice that received wild-type FBM cells, were reconstituted with FBM cells at 8 weeks and 16 weeks, respectively. The CRU numbers/2 legs (femur and tibia) are shown as mean (± 95% confidence interval).
Normal FBM hematopoiesis in absence of integrin α6. E18.5 FBM from integrin α6 gene–deleted mice (α6−/−) and wild-type littermates (α6+/+) was analyzed for total cell number (A) total cell number (horizontal bars show mean values), and (B) competitive repopulating units (CRUs). CRUs were assayed by transplanting 2 to 4.4 × 104 FBM cells together with 2 × 105 adult BM cells into lethally irradiated adult mice. Three of 13 and 3 of 12 mice that received integrin α6−/− FBM cells, and 3 of 14 and 2 of 12 mice that received wild-type FBM cells, were reconstituted with FBM cells at 8 weeks and 16 weeks, respectively. The CRU numbers/2 legs (femur and tibia) are shown as mean (± 95% confidence interval).
Expression of integrin α6 mRNA splice variants in BM and FL HSPCs
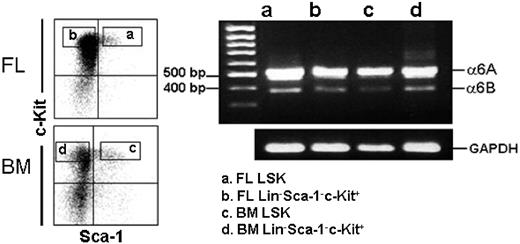
The integrin α6 polypeptide chain is expressed in 2 functionally different variants, α6A and α6B, with different cytoplasmic domains generated by alternative mRNA splicing.53 Of these, the larger α6A polypeptide is more active in promoting migration than the smaller isoform.54 We studied the expression of the 2 integrin α6 mRNA splice variants on FL and adult BM LSK cells and the more mature Lin−Sca-1−c-Kit+ cells. Both the 510-bp and 378-bp cDNA fragments corresponding to α6A and α6B mRNAs, with a higher expression of the larger splice variant, were detected in LSK and Lin−Sca-1−c-Kit+ fractions from both FL and adult BM (Figure 7), indicating that the differences in integrin α6 receptor function in FL and adult BM HSCs are not due to differential expression of the functionally different splice variants.
Expression of integrin α6A and α6B mRNA splice variants in adult mouse BM and FL HSPCs. Adult mouse BM and E14.5 FL LSK and Lin−Sca-1−c-Kit+ cells were gated for sorting (left panels). The vertical and horizontal bars were set on the basis of isotype-matched negative control profiles (99.3% of cells negative). Reanalysis of each cell population showed more than 99% purity. RT-PCR analysis for integrin α6A and α6B mRNA splice variants of FL and BM LSK and Lin−Sca-1−c-Kit+ cells is shown in the right panel. MW indicates positions of 500-bp and 400-bp molecular weight markers on the gel. The bands corresponding to 510-bp and 378-bp integrin α6A and α6B splice variants are seen in all 4 cell populations. PCR for GAPDH shows the relative amounts of mRNA in the corresponding samples. Shown is 1 representative of 2 experiments.
Expression of integrin α6A and α6B mRNA splice variants in adult mouse BM and FL HSPCs. Adult mouse BM and E14.5 FL LSK and Lin−Sca-1−c-Kit+ cells were gated for sorting (left panels). The vertical and horizontal bars were set on the basis of isotype-matched negative control profiles (99.3% of cells negative). Reanalysis of each cell population showed more than 99% purity. RT-PCR analysis for integrin α6A and α6B mRNA splice variants of FL and BM LSK and Lin−Sca-1−c-Kit+ cells is shown in the right panel. MW indicates positions of 500-bp and 400-bp molecular weight markers on the gel. The bands corresponding to 510-bp and 378-bp integrin α6A and α6B splice variants are seen in all 4 cell populations. PCR for GAPDH shows the relative amounts of mRNA in the corresponding samples. Shown is 1 representative of 2 experiments.
Discussion
Clinical and experimental studies have revealed several phenotypic and functional differences in fetal and adult HSCs.6 However, the molecular differences underlying differential homing and engraftment capacities of primitive and definitive HSC populations are still largely unknown. In the present study, we have specifically addressed the role of integrin α6 and α4 receptors for homing and engraftment of FL HSC and HPC populations. In addition, we have studied the consequences of integrin α6 gene deletion for FL hematopoiesis and reconstitution of FL HSCs in BM. Embryonic deletion of integrin α6 chain leads to severe skin blistering, cerebral malformations, and neonatal death. However, specific aspects of hematopoiesis in integrin α6–deficient mice have not been previously studied.26
We here show that 10% to 15% of wild-type FL HPCs, injected into recipient mice after treatment with control antibody or without antibody treatment, were recovered in BM within 3 hours. This fraction of homed FL HPCs is similar as previously observed in adult BM cells.19,30,51 In contrast to our results, one study43 reported a severely reduced homing capacity of FL HPCs compared with adult BM HPCs, with 1% to 1.5% of transplanted FL and 6% of adult BM HPCs recovered in BM 3 hours after intravenous injection. The reason for the difference and for the overall lower frequencies of homed HPCs in this study might be a more extensive manipulation of cells before the colony assay. Furthermore, in another study, approximately 10% of transplanted HSCs from both mouse FL and adult BM were recovered in BM of lethally irradiated recipients 24 hours after injection,55 suggesting constant homing efficiency of HSCs and HPCs during ontogeny.
We show here that homozygous deletion of integrin α6 receptor resulted in reduced homing of FL CFU-GMs into BM. In agreement with this, the function-blocking antibody against integrin α6 chain significantly inhibited homing of FL CFU-GMs into BM, with a slightly more pronounced effect than obtained by the gene deletion. However, HPC homing in both experimental assays was only partially inhibited, and approximately 60% of HPCs were still recovered in the BM, compared with wild-type FL cells or cells treated with control antibody. A similar degree of inhibition by a function-blocking anti-integrin α6 antibody was previously shown for adult BM HPCs,19 indicating a role for integrin α6 receptor for HPC homing throughout ontogeny.
In contrast to FL HPCs, homing of long-term or short-term repopulating HSCs was not inhibited by the function-blocking antibody against α6 integrin. In line with this, homing and engraftment of integrin α6 gene–deleted FL HSCs, assayed 3 to 40 weeks after transplantation, did not differ from that of wild-type HSCs. Reconstitution analysis 12 weeks after serial transplantation did not show any engraftment defect of α6−/− FL HSCs. Furthermore, the HSC frequencies were similar in BM of integrin α6 gene–deleted fetuses and in wild-type littermates, indicating that fetal HSC development is normal in the absence of α6 integrin. Taken together, the present and previous results19 show ubiquitous expression but distinct developmentally regulated functions of the integrin α6 receptor in fetal and adult HSPCs.
The observed differences in integrin α6 receptor function in HSCs suggest differences in integrin activational status, influencing the binding capacity to particular ligands.56 Activation of integrin receptors can be modulated both by ligand-dependent extracellular signals and intracellular signaling influenced by a variety of cytokines and chemokines.57,58 Such modulation might be an important regulatory mechanism influencing transendothelial or stromal migration and homing of HSPCs.58,,,–62 Our results show a relatively low frequency of LSK cells adherent to laminins, which might indicate an inactive conformation of the α6 integrin receptor in most cells. Protein kinase C pathway activator TPA, which mimics the effect of physiological agonists,63 did not increase adhesion, indicating a protein kinase C–independent mechanism. Other possible mechanisms for integrin activation, determined by the specific cellular environments, including posttranslational modifications, interactions with growth factor receptors,58 or association with other membrane molecules such as tetraspanins,44 should be addressed in further studies.
A role of integrin α6 receptor for mobilization of HSPCs was studied by using mice reconstituted with integrin α6−/− or wild-type FL HSCs, together with adult BM competitor cells. In line with the reported high proliferation and engraftment capacity of FL cells,5,64 the majority of Lin−Sca-1+ cells in BM 5 to 8 months after transplantation was derived from FL cells. Mobilization of Lin−Sca-1+ cells was not influenced by deletion of integrin α6 receptor, in agreement with our previous findings, which did not show any mobilizing effect of intravenously injected anti-integrin α6 antibodies in adult mouse HSPCs.19 Recently, a p67 nonintegrin laminin receptor was reported to be functionally active in homing and G-CSF–induced mobilization of human HPCs.45,65 In addition, several other laminin receptors including integrin α2β1, α-dystroglycan, Lutheran glycoprotein, and heparan sulfate proteoglycans,20,44,–46,66,67 have been reported in adult hematopoietic cells. The possible expression and role of these receptors during fetal HSPC mobilization and homing should be addressed in further studies.
Our present findings show a ubiquitous expression of integrin α4 receptor in FL LSK cells and more mature Lin−Sca-1−c-Kit+ progenitors. We therefore analyzed its contribution for FL HSPC homing. Remarkably, homing of FL HPCs to BM was completely inhibited by a function-blocking antibody against integrin α4 chain. Furthermore, homing of short-term and long-term repopulating HSCs was abrogated by inhibition of integrin α4 function. Previous studies where integrin α4−/− embryos and chimeric mice were used have established an important role for α4 integrin for maintenance and development of multilineage progenitors in fetal liver, BM, and spleen, as well as for postnatal multilineage hematopoietic development.13,14,68 In vivo homing of fetal α4−/− HSPCs was not specifically addressed in these studies, although a functional defect in transmigration through adherent stromal cell layer in cultures, restricted to erythroid and B-lineage cells, was noted in vitro. In a recent study,69 reconstitution, albeit in a relatively low level, from E12.5 integrin α4−/− FL cells was reported after transplantation. However, the significance of this is unclear since a homing defect might have been compensated by increased proliferative potential of integrin gene–deleted HPCs, as recently reported in adult mice.70,71 In contrast, studies on inducible integrin α4 gene deletion during adult hematopoiesis have shown decreased self-renewal capacity of HSCs and selective impairment in homing of α4-deficient HPCs to BM.70,72 Our recent findings19 have shown that inhibition of integrin α4 receptor by a function-blocking antibody dramatically reduces homing of both short-term and long-term HSCs from adult BM. Taken together, the present and previous findings suggest a role for integrin α4 during homing of both fetal and adult HSPCs into myeloablated recipients after transplantation, whereas its role during shift of hematopoiesis from liver to BM during fetal development is currently unknown.
In conclusion, our results from in vivo transplantation assays show that α6-integrin receptor functions as a homing receptor for FL HPCs during their transmigration into adult BM, whereas homing and engraftment of FL HSCs is independent of α6-integrin receptor function. Furthermore, our findings indicate an essential role for integrin α4-receptor during homing of both FL HPCs and multilineage reconstituting HSCs into BM. Altogether, these and previous findings show intrinsic differences in function of cell adhesion receptors in HSCs and HPCs from developing hematopoietic tissues compared with those from adult sources,6,19 which may be one of the mechanisms underlying the differences in reconstitution in the transplantation setting.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from ALF (Government Public Health Grant), Kungliga fysiografiska sällskapet in Lund, the Swedish Cancer Society, University Hospital of Lund Foundation, and Georg Danielsson's Foundation.
We thank Yutaka Sasaki, Jens Nygren, Lilian Wittman, Zhi Ma, and Anna Fossum for technical help and scientific discussions.
Authorship
Contribution: K.T. and A.D. provided laminin-411 and laminin-511; E.G.-L. provided integrin α6−/− mice; H.Q. and M.E. designed and performed research, analyzed data, and wrote the paper; A.N. performed research and analyzed data; and S.E.W.J. designed research and analyzed data.
Conflict-of-interest disclosure: K.T. and A.D. have declared a financial interest in a company (BioStratum) whose products (recombinant laminin-411 and -511) were studied in the present work. All other authors declare no competing financial interests.
Correspondence: Marja Ekblom, Hematopoietic Stem Cell Laboratory, BMC B12, Lund University, 221 84 Lund, Sweden; e-mail:marja.ekblom@med.lu.se.