Adhesion to extracellular ligands through integrins regulates cell shape, migration, growth, and survival. How integrins transmit signals in the outside-to-in direction remains unknown. Whereas in resting integrins the α and β subunit transmembrane domains are associated, ligand binding promotes dissociation and separation of these domains. Here we address whether such separation is required for outside-in signaling. By introduction of an intersubunit disulfide bond, we generated mutant integrin αIIbβ3 with blocked transmembrane separation that binds ligand, mediates adhesion, adopts an extended conformation after ligand binding, and forms antibody-induced macroclusters on the cell surface similarly to wild type. However, the mutant integrin exhibits a profound defect in adhesion-induced outside-in signaling as measured by cell spreading, actin stress-fiber and focal adhesion formation, and focal adhesion kinase activation. This defect was rescued by reduction of the disulfide bond. Our results demonstrate that the separation of transmembrane domains is required for integrin outside-in signal transduction.
Introduction
Integrins constitute a family of heterodimeric cell surface adhesion receptors capable of transmitting bidirectional signals across the plasma membrane. Inside-out signaling occurs when specific intracellular signals impinge on integrin cytoplasmic domains, triggering changes in conformation and ligand-binding affinity in the extracellular domain. In turn, binding of extracellular ligands produces intracellular signals (ie, outside-in signals) such as changes in tyrosine phosphorylation and cytoskeletal organization that critically influence cell shape, migration, growth, and survival.1 The mechanism by which integrins transmit outside-in signals, however, remains unknown.
Integrin α and β subunits each have a large extracellular domain, a single transmembrane (TM) domain, and a short cytoplasmic domain. In the resting state, in which integrins exhibit low affinity for ligand, the extracellular domain exists in a compact bent conformation2,3 that is stabilized by specific α/β interfaces that exist in extracellular, TM,4 and cytoplasmic5,6 domains. Separation of the α and β TM and cytoplasmic domains stimulated by physiologic inside-out activation signals, overexpression of the talin head domain, or mutagenesis of critical cytoplasmic or TM interface residues leads to conformational change in the extracellular domain.5,7,,,,,,–14 Alternatively, stabilization of the cytoplasmic domain5 or TM domain interfaces4 blocks inside-out signal transduction. Such blockade can, however, be readily circumvented by reagents such as activating antibodies and Mn2+, which act directly on the extracellular domain to stabilize the high-affinity conformation,4,5 probably because of the flexibility of the β-subunit extracellular leg.15
It is widely believed that lateral association (ie, “clustering”) of integrin heterodimers, which occurs as a consequence of multivalent ligand binding,16,17 plays a major role in outside-in signaling (see review18 ). However, what role, if any, integrin conformational change (ie, separation of the TM and cytoplasmic domain interfaces) has in outside-in signaling remains uncharacterized.
In this study, we investigate outside-in signaling in the prototypic integrin αIIbβ3 (platelet glycoprotein IIb-IIIa). αIIbβ3 is highly expressed on platelets and plays an essential role in hemostasis, mediating platelet aggregation and spreading on vascular matrices. On resting platelets, αIIbβ3 is inactive. When stimulated by hemostatic agents, such as ADP or thrombin, intracellular signals are generated that initiate αIIbβ3 inside-out signaling, resulting in transition to the high-affinity state and binding of the ligands fibrinogen and von Willebrand factor.18 In turn, binding to multivalent ligands by αIIbβ3 induces outside-in signals that initiate actin reorganization, shape change, and spreading of platelets,18 as well as activation of specific nonreceptor tyrosine kinases, such as focal adhesion kinase (FAK).19,,,,–24
Previously, it has been reported that integrin cross-linking and clustering are required for outside-in signaling.25,,–28 It has also been shown that activating mutations of the TM domains induced FAK activation, but this was proposed to be due to integrin clustering, thought to result from homomeric TM domain association.13 Here, we investigate whether clustering is sufficient for outside-in signaling, or whether integrin conformational change is also required. We use a disulfide bond introduced into the exofacial portion of the αIIb and β3 TM interface to prevent the separation of the two TM helices. Our study demonstrates that the disulfide-bonded mutant exhibits defective cell spreading, actin stress-fiber and focal adhesion formation, and focal adhesion kinase activation, despite intact ligand binding, adhesion, and antibody-induced macroclustering. These results demonstrate that the separation of the integrin α and β subunit TM domains is required for integrin outside-in signaling.
Materials and methods
Plasmid construction and protein expression
Plasmids coding for full-length human αIIb and β3 integrin subunits were subcloned into pEF/V5-HisA and pcDNA3.1/Myc-His (+), respectively.2 Single cysteine substitutions within the TM domains of αIIb and β3 were described previously.4 Constructs were transfected into CHO-K1 cells (American Type Culture Collection, Manassas, VA) using Fugene (Roche Diagnostics, Indianapolis, IN) transfection kit according to the manufacturer's instructions. Stably transfected CHO cells were established as described.29 The expression levels of αIIb, β3, and the hamster αV human β3 chimera were detected by flow cytometry staining with the monoclonal antibodies 10E5 (anti-αIIb mAb, kindly provided by B. S. Coller Rockefeller University, New York, NY),30 AP3 (nonfunctional anti-β3 mAb; American Type Culture Collection), and LM609 (anti-αVβ3 mAb; Chemicon International, Temecula, CA), respectively. To characterize disulfide formation, stably transfected cells were metabolically labeled with [35S] cysteine/methionine and immunoprecipitated by anti-αIIb mAb 10E5 as described.4,5
Soluble ligand binding
The activating anti-aIIb mAb PT25–2 was a generous gift from M. Handa (Keio University Hospital, Tokyo, Japan).31 Soluble binding of FITC-labeled human fibrinogen (Enzyme Research Laboratories, South Bend, IN) and ligand-mimetic PAC-1 IgM (Becton Dickinson, San Jose, CA) was determined as described.29 Cells were also stained in parallel with anti-αIIb mAb 10E5 followed by FITC-conjugated anti–mouse IgG. Binding activity is presented as the percentage of the mean fluorescence intensity (MFI) of PAC-1 or fibrinogen staining relative to the MFI of 10E5 staining.
LIBS epitope expression
Anti–LIBS (ligand-induced–binding site) mAbs LIBS1 and D3 were kindly provided by M. H. Ginsberg (University of California San Diego, La Jolla, CA)32 and L. K. Jennings (University of Tennessee Health Science Center, Memphis, TN).33 LIBS epitope expression was determined as described previously.29 LIBS epitope expression was expressed as the percentage of MFI of anti-LIBS antibody relative to the conformational-independent anti-β3 mAb AP3.
Cell adhesion
Cell adhesion on immobilized human fibrinogen was assessed by the measurement of cellular lactate dehydrogenase (LDH) activity. Cells suspended in 20 mM Hepes-buffered saline pH 7.4 (HBS) supplemented with 5.5 mM glucose and 1% BSA, and either 1 mM Ca2+/1 mM Mg2+ or 1 mM Ca2+/1 mM Mg2+ plus 1 mM DTT, were preincubated with 10 μg/mL mAb LM609 on ice for 30 minutes, and then seeded on flat-bottom 96-well plates (104 cells/well) precoated with different concentrations of fibrinogen and blocked with 1% BSA. After incubation at 37°C for 1 hour, wells were washed 3 times with HBS. The remaining adherent cells were lysed by 1% Triton X-100, and LDH activity was assayed using the Cytotoxicity Detection kit (LDH; Roche Diagnostics, Penzberg, Germany) according to the manufacturer's instructions. Cell adhesion was expressed as a percentage of bound cells relative to total input cells.
Cell spreading and microscopy
Cell spreading assays were carried out in Delta T imaging chambers (Bioptechs, Butler, PA). The dishes were coated overnight at 4°C with 100 μg/mL human fibrinogen or 20 μg/mL PAC-1 in HBS, followed by blocking with 1% BSA. The stably transfected cells were detached by trypsin/EDTA, washed once with DMEM containing 0.5 mg/mL soybean trypsin inhibitor (Calbiochem, La Jolla, CA), and then washed twice with DMEM. Cells were incubated in suspension on ice for 30 minutes in DMEM containing 10 μg/mL mAb LM609 before seeding on fibrinogen-coated dishes. Alternately, cells were seeded on PAC-1–coated dishes without prior LM609 treatment. In indicated experiments, 1 mM DTT was added concomitant with cell seeding. After incubation at 37°C for 1 hour, cells were washed 3 times with phosphate-buffered saline at pH 7.4 (PBS) and fixed with 3.7% formaldehyde in PBS at room temperature (RT) for 5 minutes. The fixed cells were treated with 5% nonfat dry milk in PBS at RT for 10 minutes to block nonspecific binding and immunostained with 20 μg/mL Alexa Fluor 488–conjugated anti-αIIb mAb PT25–2 in PBS containing 5% nonfat dry milk at RT for 30 minutes. After PT25–2 staining, cells were washed 3 times with PBS and permeablized with 0.05% Triton X-100 in PBS at RT for 10 minutes, followed by blocking with 5% nonfat dry milk in PBS at RT for 10 minutes, and then stained with Alexa Fluor 546–conjugated phalloidin (Molecular Probes, Eugene, OR) or 20 μg/mL rabbit antivinculin (Sigma, St Louis, MO) in PBS containing 5% nonfat dry milk at RT for 30 minutes. For vinculin staining, cells were finally stained with 20 μg/mL Alexa Fluor 546–labeled goat anti–rabbit IgG (Molecular Probes).
Differential interference contrast (DIC) imaging was conducted on an Axiovert S200 epifluorescence microscope (Carl Zeiss MicroImaging, Heidelberg, Germany), using a 63×/1.4 NA oil objective, coupled to a Hamamatsu Orca CCD camera (Okayama City, Japan). For the quantification of cell spreading, outlines of at least 100 adherent cells (from randomly selected fields in each of 3 separate experiments) were generated and the number of pixels contained within each of these regions was measured using OpenLab software (Improvision, Lexington, MA). Confocal imaging was performed with a Bio-Rad Radiance 2000 laser-scanning confocal system (Hercules, CA) coupled to an Olympus BX50WI microscope (Melville, NY) with a 100×/1.0 NA water-immersion objective.
Antibody-induced integrin clustering
Stably transfected CHO-K1 cells were cultured in DMEM containing 10% FBS on Delta T dishes overnight at 37°C in a CO2 incubator. Adherent cells were washed with PBS and incubated with 10 μg/mL noninhibitory anti-αIIbβ3 mAb D57 (kindly provided by Dr M. H. Ginsberg) in PBS containing 1% BSA for 30 minutes at 37°C, followed by staining with 10 μg/mL goat anti–mouse IgG conjugated to Alexa Fluor 488 for 30 minutes at 37°C. Cells were washed 3 times with PBS and fixed with 3.7% formaldehyde in PBS at RT for 5 minutes before confocal microscopy. Alternatively, cells were fixed with 3.7% formaldehyde in PBS at RT for 5 minutes prior to anti-αIIbβ3 mAb D57 and goat anti–mouse IgG staining.
Immunoprecipitation and Western blotting
Cells were allowed to spread at 37°C for 1 hour on fibrinogen (100 μg/mL)–coated or control (BSA)–coated 10-cm Petri dishes in the presence or absence of 1 mM DTT. After washing with ice-cold PBS, cells were lysed with 1 mL lysis buffer (50 mM Tris-HCl, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF, and 1 μg/mL each aprotinin, leupeptin, and pepstatin) on ice for 30 minutes. The cell lysates were centrifuged at 4°C at 20 000g for 30 minutes and each 1 mL supernatant containing 500 μg total proteins was subjected to immunoprecipitation with 3 μg anti-FAK mAb (BD Bioscience, San Jose, CA) for 1 hour at 4°C followed by incubation with 25 μL protein A agarose (Invitrogen, Frederick, MD) for 1 hour at 4°C. The immunoprecipitated proteins were eluted with 25 μL Tris-glycine sodium dodecyl sulfate (SDS) sample buffer and 10 μL of each sample was subjected to SDS–polyacrylamide gel electrophoresis (PAGE) and Western blotting. The membrane was blotted with rabbit antiphospho-FAK (Tyr397) (Upstate, Lake Placid, NY) and reblotted with anti-FAK mAb (BD Bioscience). To detect protein phosphotyrosine in total cell lysates, supernatants of cell lysates containing 10 μg proteins were subjected to SDS-PAGE and Western blotting. The membranes were blotted with antiphosphotyrosine mAb 4G10 (Upstate) and PY20 (BD Biosciences) and reblotted with anti–β-actin mAb (Sigma). Immunoreactive bands were detected by enhanced chemiluminescence (ECL) Plus Western blotting detection reagents (Amersham Biosciences, Arlington Heights, IL) and scanned on a Storm 860 Imager (GE Healthcare, Piscataway, NJ). The intensities of bands of interest were quantified using ImageQuant software (GE Healthcare). The tyrosine phosphorylation of FAK was expressed as the phospho-FAK (pY397) signal as a percentage of the total FAK signal.
To study the effect of antibody-induced integrin clustering on FAK activation, stably transfected CHO-K1 cells suspended in 0.5 mL HBS supplemented with 5.5 mM glucose and 1% BSA, and 1 mM Ca2+/1 mM Mg2+ were incubated with or without 10 μg/mL anti-αIIbβ3 mAb D57 at 37°C for 30 minutes, followed by incubation with or without 10 μg/mL goat anti–mouse IgG for 60 minutes at 37°C. As a control, cells were incubated with ligand-mimetic PAC-1 IgM in the presence of 1 mM Mn2+ and 10 μg/mL activating mAb PT25–2. After incubation, cells were washed with ice-cold PBS and lysed with lysis buffer. The supernatants (500 μg of proteins) of cell lysates were precleared by incubation with 50 μL protein A agarose at 4°C for 1 hour, and then FAK was immunoprecipitated as described in the previous paragraph. Tyrosine phosphorylation of FAK was detected by antiphosphotyrosine mAb 4G10.
Results
Expression and disulfide bond formation of the wild-type and mutant αIIbβ3 receptors
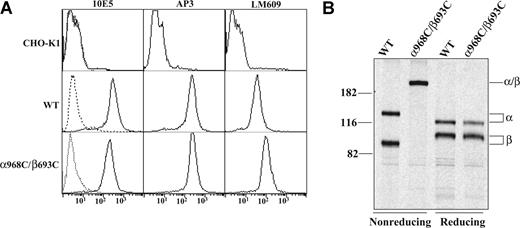
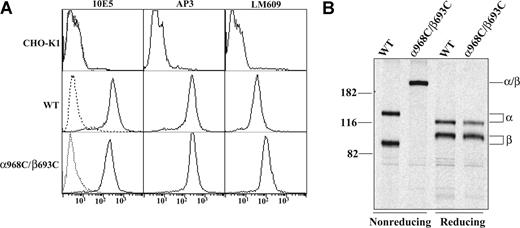
Disulfide bond scanning studies have revealed in the resting state a specific interface between the alpha-helices of the αIIb and β3 TM domains.4 For several pairs of cysteines introduced at the exofacial region of the interface, disulfides form constitutively.4 We focused on one of these (αIIbW968C/β3I693C, denoted α968C/β693C) that forms disulfides with approximately 100% efficiency to study its effect on integrin outside-in signaling. CHO-K1 cells have been widely used to study αIIbβ3 outside-in signaling.21,22,34 We generated stable CHO-K1 cell lines expressing either wild-type (WT) or disulfide mutant (α968C/β693C) αIIbβ3. Stable WT and α968C/β693C CHO clones were selected that expressed similar levels of receptors, as assessed by staining with anti-αIIb monoclonal antibody (mAb) 10E5 and anti-β3 mAb AP3 (Figure 1A). Disulfide bond formation between α968C and β693C was confirmed to be approximately 100% by nonreducing SDS-PAGE of 35S-labeled, 10E5-immunoprecipitated receptors in the α968C/β693C stable CHO-K1 cell line (Figure 1B).
Expression and disulfide bond formation of αIIbβ3 integrins on CHO-K1 cells. (A) Immunofluorescence flow cytometry of CHO-K1 transfectants. CHO-K1 cells stably transfected with the wild-type (WT) or cysteine mutant (α968C/β693C) αIIbβ3 integrin were immunostained with 10E5 (anti-αIIb), AP3 (anti-β3), and LM609 (anti-αVβ3 complex) and detected by flow cytometry (–). Mock-transfected CHO-K1 cells were used as a control. Cells immunostained with X63 control IgG are shown (----). (B) Immunoprecipitation. Lysates from (35)S-labeled CHO-K1 stable transfectants were immunoprecipitated with mAb 10E5 (anti-αIIb). The precipitates of WT or α968C/β693C mutant were treated with nonreducing or reducing loading buffer and subjected to 7.5% SDS-PAGE. Bands of αIIb (α), β3 (β), and αIIb/β3 (α/β) heterodimer are indicated. Positions of protein molecular size markers (in kDa) are shown on the left.
Expression and disulfide bond formation of αIIbβ3 integrins on CHO-K1 cells. (A) Immunofluorescence flow cytometry of CHO-K1 transfectants. CHO-K1 cells stably transfected with the wild-type (WT) or cysteine mutant (α968C/β693C) αIIbβ3 integrin were immunostained with 10E5 (anti-αIIb), AP3 (anti-β3), and LM609 (anti-αVβ3 complex) and detected by flow cytometry (–). Mock-transfected CHO-K1 cells were used as a control. Cells immunostained with X63 control IgG are shown (----). (B) Immunoprecipitation. Lysates from (35)S-labeled CHO-K1 stable transfectants were immunoprecipitated with mAb 10E5 (anti-αIIb). The precipitates of WT or α968C/β693C mutant were treated with nonreducing or reducing loading buffer and subjected to 7.5% SDS-PAGE. Bands of αIIb (α), β3 (β), and αIIb/β3 (α/β) heterodimer are indicated. Positions of protein molecular size markers (in kDa) are shown on the left.
It is known in CHO cells that transfected human β3 integrin can associate with the endogenous hamster αV to form chimeric αVβ3. The chimeric αVβ3 is also a receptor for fibrinogen35 but does not bind the αIIbβ3 ligand-mimetic mAb PAC-1. We confirmed, using the anti-αVβ3 complex-specific mAb LM609, that chimeric αVβ3 was expressed along with αIIbβ3 in both WT and α968C/β693C CHO-K1 cell lines (Figure 1A). In this context, αVβ3 was found to make a minor contribution to the overall adhesion to fibrinogen, which could be completely blocked by the αVβ3 function-blocking antibody LM609 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Thus, to exclude possible contribution of αVβ3 outside-in signaling, we have included LM609 in all fibrinogen-binding experiments.
TM disulfide-bonded αIIbβ3 mediates externally stimulated soluble ligand binding and cell adhesion similarly to that of wild type
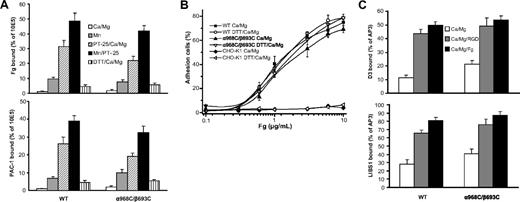
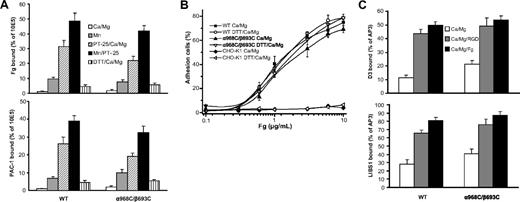
Whereas neither WT- nor α968C/β693C-expressing cells bound significant soluble fibrinogen (50 μg/mL) or αIIbβ3 ligand-mimetic mAb PAC-1 (10 μg/mL) in the presence of 1 mM Ca2+, both cell types efficiently bound these ligands in the presence of activating cation (Mn2+) or activating antibody (anti-αIIb mAb PT25–2)31 (Figure 2A). Ligand binding by both cell lines was augmented even further by combined addition of Mn2+ and PT25–2.29 Treatment with reducing agent (1 mM DTT) slightly increased ligand binding in Ca2+ for both WT and α968C/β693C cells (Figure 2A). Most importantly, under all conditions tested, ligand binding of the mutant α968C/β693C was similar to that of the WT. This suggests that the TM disulfide had little effect on ligand binding stimulated from outside the cells by Mn2+ or activating mAbs. Similar results were obtained in 293T cells transiently transfected with the WT and α968C/β693C mutant αIIbβ3 (data not shown and see Luo et al4 ).
Ligand binding and LIBS exposure. (A) Soluble fibrinogen (Fg) or PAC-1 binding of CHO-K1 stable transfectants in the presence of either 1 mM Ca2+/1 mM Mg2+, 1 mM Mn2+, 10 μg/mL PT25–2 plus 1 mM Ca2+/1 mM Mg2+, 10 μg/mL PT25–2 plus 1 mM Mn2+, or 1 mM DTT plus 1 mM Ca2+/1 mM Mg2+ as indicated. Binding activity was determined by flow cytometry and expressed as described in “Soluble ligand binding.” (B) Adhesion of CHO-K1 transfectants (preincubated with 10 μg/mL mAb LM609) in the presence of 1 mM Ca2+/1 mM Mg2+ or 1 mM Ca2+/1 mM Mg2+ plus 1 mM DTT to surfaces coated with fibrinogen at indicated concentrations. The amount of bound cells was determined by measuring the lactate dehydrogenase (LDH) activity as described in “Cell adhesion.” Data are representative of 3 independent experiments, each in triplicate. (C) LIBS exposure. Cells were stained with 2 anti-LIBS antibodies, D3 and LIBS1, in the presence or absence of 50 μM RGD peptides (GRGDSP) or 3 mg/mL human fibrinogen in 1 mM Ca2+/1 mM Mg2+ condition. LIBS epitope expression was expressed as the percentage of mean fluorescence intensity of anti-LIBS antibody relative to the conformational-independent anti-β3 mAb AP3. Error bars are standard deviation (SD).
Ligand binding and LIBS exposure. (A) Soluble fibrinogen (Fg) or PAC-1 binding of CHO-K1 stable transfectants in the presence of either 1 mM Ca2+/1 mM Mg2+, 1 mM Mn2+, 10 μg/mL PT25–2 plus 1 mM Ca2+/1 mM Mg2+, 10 μg/mL PT25–2 plus 1 mM Mn2+, or 1 mM DTT plus 1 mM Ca2+/1 mM Mg2+ as indicated. Binding activity was determined by flow cytometry and expressed as described in “Soluble ligand binding.” (B) Adhesion of CHO-K1 transfectants (preincubated with 10 μg/mL mAb LM609) in the presence of 1 mM Ca2+/1 mM Mg2+ or 1 mM Ca2+/1 mM Mg2+ plus 1 mM DTT to surfaces coated with fibrinogen at indicated concentrations. The amount of bound cells was determined by measuring the lactate dehydrogenase (LDH) activity as described in “Cell adhesion.” Data are representative of 3 independent experiments, each in triplicate. (C) LIBS exposure. Cells were stained with 2 anti-LIBS antibodies, D3 and LIBS1, in the presence or absence of 50 μM RGD peptides (GRGDSP) or 3 mg/mL human fibrinogen in 1 mM Ca2+/1 mM Mg2+ condition. LIBS epitope expression was expressed as the percentage of mean fluorescence intensity of anti-LIBS antibody relative to the conformational-independent anti-β3 mAb AP3. Error bars are standard deviation (SD).
The ligand binding of the WT and disulfide mutant was further assessed by cell adhesion assays on immobilized fibrinogen. As we previously observed29 and in contrast to soluble ligand binding, at coating concentrations higher than 1 μg/mL both WT and α968C/β693C cells mediated efficient adhesion to immobilized fibrinogen even in the absence of activation (ie, Mn2+ or PT25–2) (Figure 2B). As a negative control, parent CHO-K1 cells showed no adhesion even at the highest coating concentration of fibrinogen (10 μg/mL) (Figure 2B). Treatment of WT and α968C/β693C cells with 1 mM DTT had little effect on cell adhesion for both WT and mutant integrins (Figure 2B), as was the case for soluble ligand-binding assays (Figure 2A).
TM disulfide-bonded αIIbβ3 adopts an extended conformation after binding to ligand
Previously, we demonstrated that disulfide-bonded receptor α968C/β693C adopted an extended conformation upon binding to an RGD peptide, as determined by flow cytometry staining with anti-LIBS antibodies.4 Here, we further investigated integrin conformation by determining LIBS expression in the presence of human fibrinogen (3 mg/mL). As expected, binding of RGD significantly increased the staining of 2 anti-LIBS antibodies, D3 and LIBS1, in both WT and α968C/β693C CHO-K1 cells. The presence of fibrinogen also substantially increased the staining (Figure 2C). The data demonstrate that the disulfide-bonded receptors could adopt an extended conformation upon binding physiological ligand even though the TM helices were associated. This is consistent with previous observations that, due to flexibility of the lower β leg, the linkage between TM domain separation and integrin extension is not rigid.15
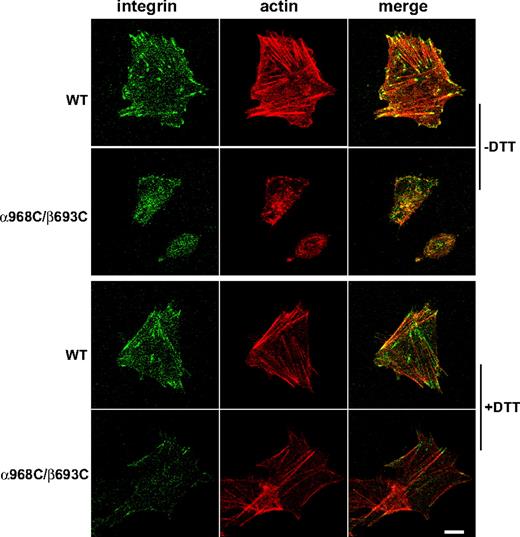
Integrin TM separation is critical for cell spreading
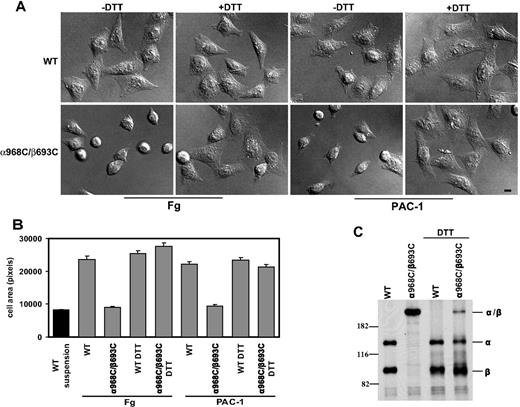
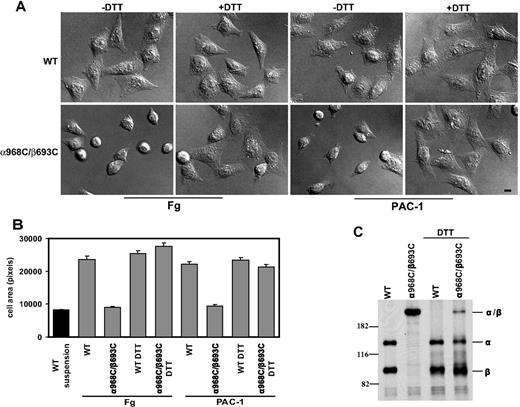
To assess efficiency of spreading, CHO-K1 transfectants were allowed to adhere to immobilized fibrinogen or ligand-mimetic antibody PAC-1 at 37°C for 1 hour, followed by fixation and microscopic analysis. WT αIIbβ3-expressing cells were found to undergo substantial spreading on both substrates (Figure 3A). However, despite strong binding and adhesion to these substrates (Figure 2), cells expressing mutant α968C/β693C exhibited little or no spreading and instead remained rounded (Figure 3A). Quantification of cell area revealed that, compared with controls (cells fixed in suspension prior to settling on the substrates), WT αIIbβ3 transfectants increased their surface area by greater than 200% on average, whereas mutant αIIbβ3 transfectants failed to spread (Figure 3B). Similar results were obtained when the cell spreading assay was carried out in the presence of Mn2+ and activating antibody (data not shown).
Cell spreading assay. (A) Differential interference contrast (DIC) images of CHO-K1 stable transfectants after adhering at 37°C to immobilized human fibrinogen (in the presence of 10 μg/mL mAb LM609) or ligand-mimic mAb PAC-1 in the presence or absence of 1 mM DTT. The images are representatives from 1 of 3 independent experiments. Scale bar represents 10 μm. (B) Quantification of the areas of adhering/spreading cells as described in “Cell spreading and microscopy.” Error bars are SD. (C) Reduction of the disulfide bond by DTT. The (35)S-labeled transfectants were allowed to adhere on immobilized fibrinogen at 37°C for 1 hour in the presence or absence of 1 mM DTT, followed by immunoprecipitation with 10E5 (anti- αIIb), and loaded on 7.5% SDS-PAGE under nonreducing condition. The bands corresponding to αIIb (α), β3 (β), or αIIbβ3 (α/β) heterodimer are indicated. Positions of protein molecular size markers (in kDa) are shown on the left.
Cell spreading assay. (A) Differential interference contrast (DIC) images of CHO-K1 stable transfectants after adhering at 37°C to immobilized human fibrinogen (in the presence of 10 μg/mL mAb LM609) or ligand-mimic mAb PAC-1 in the presence or absence of 1 mM DTT. The images are representatives from 1 of 3 independent experiments. Scale bar represents 10 μm. (B) Quantification of the areas of adhering/spreading cells as described in “Cell spreading and microscopy.” Error bars are SD. (C) Reduction of the disulfide bond by DTT. The (35)S-labeled transfectants were allowed to adhere on immobilized fibrinogen at 37°C for 1 hour in the presence or absence of 1 mM DTT, followed by immunoprecipitation with 10E5 (anti- αIIb), and loaded on 7.5% SDS-PAGE under nonreducing condition. The bands corresponding to αIIb (α), β3 (β), or αIIbβ3 (α/β) heterodimer are indicated. Positions of protein molecular size markers (in kDa) are shown on the left.
To demonstrate that the defect in spreading was due to the TM disulfide linkage, we treated the cells with 1 mM DTT. This resulted in reduction of the majority of engineered disulfides in the mutant receptor, as seen by SDS-PAGE (Figure 3C). Such reversal of the TM linkage led to a rescue of mutant αIIbβ3 CHO spreading on fibrinogen and PAC-1 (Figure 3A,B). The effect of DTT treatment was unlikely to be due to direct effect on ligand binding because DTT could only slightly increase ligand binding for both the WT and the mutant receptors (Figure 2) and DTT had little effect on cell spreading for the WT (Figure 3A,B). These data show that failure of mutant receptors to support spreading on immobilized ligands was due specifically to the inability to undergo separation of the α and β subunit TM helices.
The above results were supported by experiments using transient transfection of either 293T or CHO-K1 cells with the WT or mutant receptors. In this setting, cells expressing WT or the single cysteine mutant receptors (α968C/β3 and αIIb/β693C) spread efficiently on immobilized fibrinogen. By contrast, 293T or CHO-K1 cells transiently expressing the disulfide mutant α968C/β693C failed to spread (Figure S2 and data not shown). Moreover, 293T cells expressing 2 other mutants with disulfides introduced to the C-termini of the αIIbβ3 extracellular domain to prevent integrin TM separation, αIIbL959C/β3P688C and αIIbA958C/β3E686C, were also defective in spreading (data not shown). These results demonstrate that the observed spreading defects were not cell-type specific or due to specific effects of cysteine point mutations.
Integrin TM separation is important for focal adhesion and actin stress-fiber formation
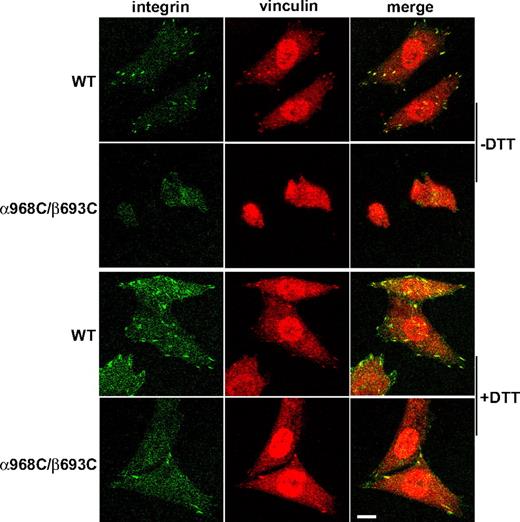
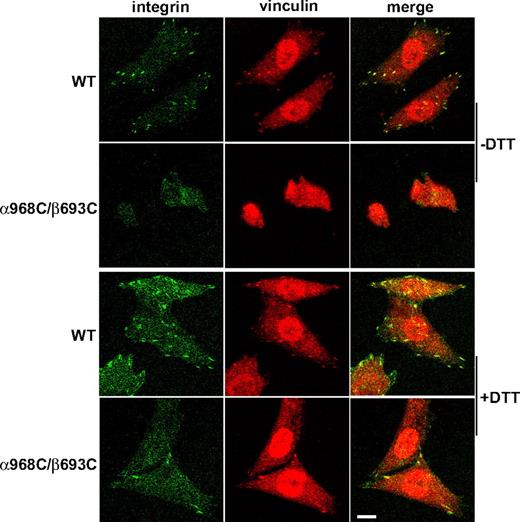
Ligand binding, and the resulting outside-in signaling by integrins, is strongly coupled to cytoskeleton rearrangements including formation of focal adhesion and actin stress fibers.36,–38 Focal adhesions are initiated with the recruitment of integrins into elongated, micron-scale macroclusters associated with the substrate.35 To assess focal adhesion formation, CHO-K1 transfectants were allowed to adhere to fibrinogen-coated substrates followed by fixation and staining with fluorescent αIIbβ3 and vinculin antibodies. Under these conditions WT integrins could be readily detected in clustered patterns that colocalized with vinculin typical of focal adhesions, whereas α968C/β693C receptors showed no detectable focal adhesion formation (Figure 4). Consistent with the above results, DTT treatment resulted in spreading and focal adhesion formation in the mutant cells (Figure 4). However 1 mM DTT did not completely restore the mutant cells to the WT levels, which was probably due to incomplete reduction of the disulfide bond as shown in Figure 3C. Although higher concentration of DTT (> 5 mM) could completely reduce the engineered disulfide, such conditions produced secondary effects, including significantly increased integrin affinity for ligands (data not shown), that precluded meaningful interpretation in this setting.
Recruitment of αIIbβ3 integrin at focal adhesion sites. Cells were allowed to adhere on immobilized fibrinogen (in the presence of 10 μg/mL mAb LM609) at 37°C for 1 hour in the presence or absence of 1 mM DTT, followed by fixation and staining with antivinculin and anti-αIIb mAb PT25–2 conjugated with Alexa Fluor 488 as described in “Cell spreading and microscopy.” The stained cells were subjected to confocal microscopy. Scale bar represents 10 μm.
Recruitment of αIIbβ3 integrin at focal adhesion sites. Cells were allowed to adhere on immobilized fibrinogen (in the presence of 10 μg/mL mAb LM609) at 37°C for 1 hour in the presence or absence of 1 mM DTT, followed by fixation and staining with antivinculin and anti-αIIb mAb PT25–2 conjugated with Alexa Fluor 488 as described in “Cell spreading and microscopy.” The stained cells were subjected to confocal microscopy. Scale bar represents 10 μm.
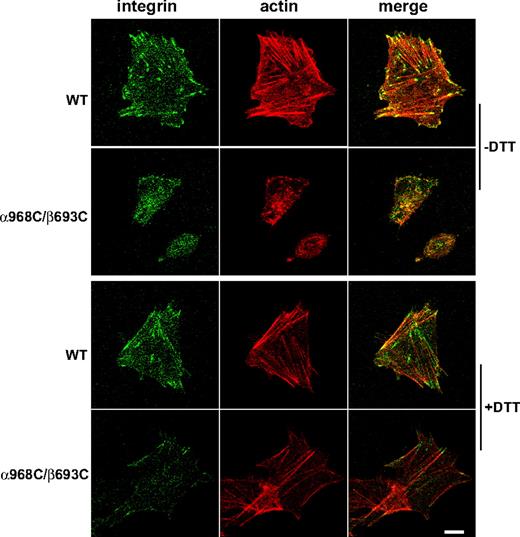
To assess actin stress-fiber formation, fibrinogen-adherent cells were prepared as above and stained with fluorescent phalloidin to visualize F-actin. Focal contacts were concomitantly visualized by staining integrins with fluorescent antibody. In cells expressing WT αIIbβ3, robust actin stress fibers were observed that colocalized with integrin-enriched focal contacts at their termini (Figure 5). Consistent with the assessment of focal adhesions, cells expressing TM disulfide-bonded αIIbβ3 failed to exhibit significant stress-fiber formation (Figure 5). However, after DTT treatment, the actin stress fibers, as well as cell spreading and colocalization of integrin and actin, in mutant CHO-K1 cells were detected (Figure 5). These results further demonstrate that ligand-dependent integrincytoskeleton signaling was blocked by the disulfide bond formed in the TM domain.
Actin stress-fiber formation in the stably transfected CHO-K1 cells adhering on immobilized fibrinogen. Cells were seeded on fibrinogen-coated surfaces (in the presence of 10 μg/mL mAb LM609) and incubated at 37°C for 1 hour in the presence or absence of 1 mM DTT. After fixation and permeabilization, the adhering cells were stained with phalloidin conjugated with Alexa Fluor 546 and anti-αIIb mAb PT25–2 conjugated with Alexa Fluor 488. The stained cells were subjected to confocal microscopy. Scale bar represents 10 μm.
Actin stress-fiber formation in the stably transfected CHO-K1 cells adhering on immobilized fibrinogen. Cells were seeded on fibrinogen-coated surfaces (in the presence of 10 μg/mL mAb LM609) and incubated at 37°C for 1 hour in the presence or absence of 1 mM DTT. After fixation and permeabilization, the adhering cells were stained with phalloidin conjugated with Alexa Fluor 546 and anti-αIIb mAb PT25–2 conjugated with Alexa Fluor 488. The stained cells were subjected to confocal microscopy. Scale bar represents 10 μm.
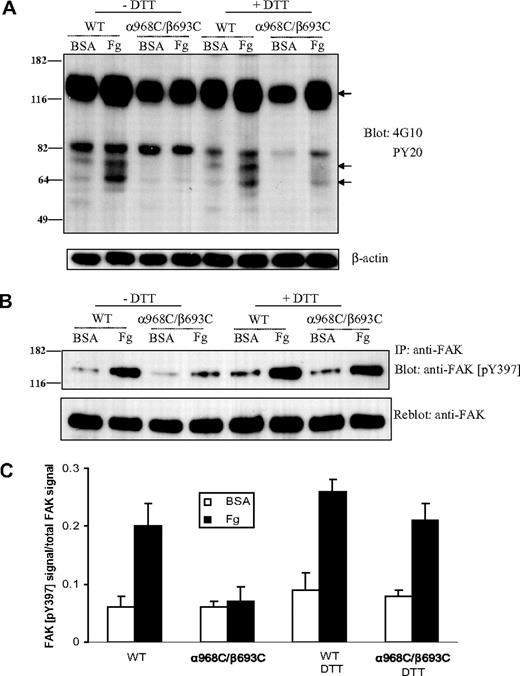
Integrin TM separation is critical for adhesion-promoted protein tyrosine phosphorylation
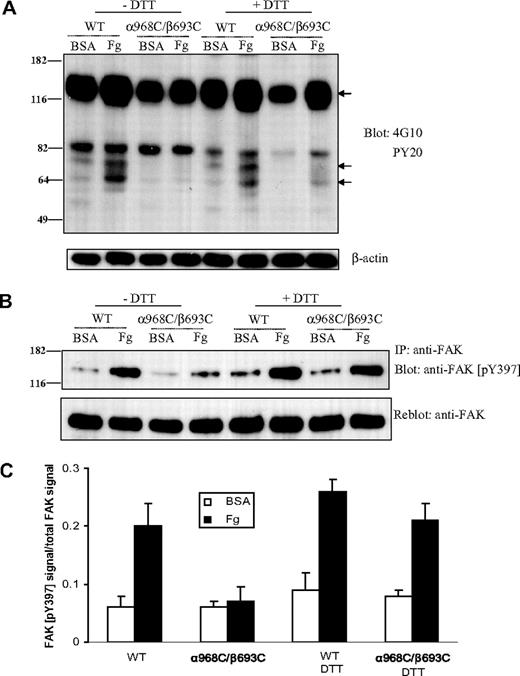
Activation of intracellular tyrosine phosphorylation is a central component of integrin outside-in signaling.39 Thus, as another measure of integrin outside-in signal transduction, we measured the protein tyrosine phosphorylation in whole cell lysates from CHO-K1 cells expressing the WT or mutant integrins before and after adhering to immobilized ligands. In cells transfected with WT αIIbβ3, adhesion to immobilized fibrinogen (at 37°C for 1 hour) induced significant tyrosine phosphorylation of at least 3 proteins, with molecular weights of approximately 66, 75, and 125 kDa (Figure 6A). By contrast, there was no detectable increase of protein tyrosine phosphorylation in α968C/β693C-transfected cells after adhering to fibrinogen. However, when α968C/β693C-transfected cells were treated with DTT, adhesion-dependent increase in protein tyrosine phosphorylation of these 3 proteins was detected (Figure 6A).
Protein tyrosine phosphorylation after cell adhesion on immobilized ligand. (A) Protein tyrosine phosphorylation in CHO-K1 cells stably transfected with WT or mutant (α968C/β693C) αIIb/β3 after adhering to immobilized fibrinogen or BSA (in the presence of 10 μg/mL mAb LM609) in the presence or absence of 1 mM DTT for 1 hour. Total cell lysates were blotted with antiphosphotyrosine mAb 4G10 and PY20 and reblotted with anti–β-actin (lower panel). The arrows indicate the position of 3 proteins that exhibited significantly increased phosphotyrosine signal following adhesion on fibrinogen. Positions of protein molecular size marker (in kDa) are shown on the left. (B) Tyrosine phosphorylation of focal adhesion kinase (FAK) in the transfected CHO-K1 cells. Cells were adhered on fibrinogen or BSA-coated culture dish for 1 hour followed by lysis. Lysates of adhering cells were immunoprecipitated with anti-FAK mAb and blotted with anti-FAK (pY397) (rabbit antiserum) (upper panel). The same membrane was then stripped and reblotted with anti-FAK rabbit polyclonal IgG (lower panel). (C) Quantification of tyrosine phosphorylation of FAK. The intensities of blotted phospho-FAK (Tyr397) and total FAK were quantified as described in “Immunoprecipitation and Western blotting.” The extent of FAK tyrosine phosphorylation was expressed as a percentage of phospho-FAK (pY397) signals relative to total FAK signals. Results are average of 3 independent experiments. Error bars are SD.
Protein tyrosine phosphorylation after cell adhesion on immobilized ligand. (A) Protein tyrosine phosphorylation in CHO-K1 cells stably transfected with WT or mutant (α968C/β693C) αIIb/β3 after adhering to immobilized fibrinogen or BSA (in the presence of 10 μg/mL mAb LM609) in the presence or absence of 1 mM DTT for 1 hour. Total cell lysates were blotted with antiphosphotyrosine mAb 4G10 and PY20 and reblotted with anti–β-actin (lower panel). The arrows indicate the position of 3 proteins that exhibited significantly increased phosphotyrosine signal following adhesion on fibrinogen. Positions of protein molecular size marker (in kDa) are shown on the left. (B) Tyrosine phosphorylation of focal adhesion kinase (FAK) in the transfected CHO-K1 cells. Cells were adhered on fibrinogen or BSA-coated culture dish for 1 hour followed by lysis. Lysates of adhering cells were immunoprecipitated with anti-FAK mAb and blotted with anti-FAK (pY397) (rabbit antiserum) (upper panel). The same membrane was then stripped and reblotted with anti-FAK rabbit polyclonal IgG (lower panel). (C) Quantification of tyrosine phosphorylation of FAK. The intensities of blotted phospho-FAK (Tyr397) and total FAK were quantified as described in “Immunoprecipitation and Western blotting.” The extent of FAK tyrosine phosphorylation was expressed as a percentage of phospho-FAK (pY397) signals relative to total FAK signals. Results are average of 3 independent experiments. Error bars are SD.
Among the many tyrosine kinases associated with integrin signaling, focal adhesion kinase (FAK, a 125-kDa protein) plays, perhaps, the most prominent role. Tyrosine phosphorylation of FAK itself (at residue Tyr397) is induced by integrin signaling and is a reliable indicator of FAK activation status.40 Thus, FAK was immunoprecipitated from CHO cell transfectants and Western blotting was performed using a well-known anti-FAK antibody that recognizes the phosphorylated form of Tyr397 specifically. As shown in Figure 6B,C, FAK became strongly phosphorylated at Tyr397 in the WT αIIbβ3 cells upon adhesion to fibrinogen. However, there was little adhesion-dependent increase of Tyr397 phosphorylation in the α968C/β693C cells (Figure 6B,C). After reducing the disulfide bond in the mutant receptors with DTT, fibrinogen-dependent FAK phosphorylation at Tyr397 was detected (Figure 6B,C). These data demonstrate a critical role for TM domain separation for integrin induction of intracellular tyrosine phosphorylation and, specifically, activation of FAK.
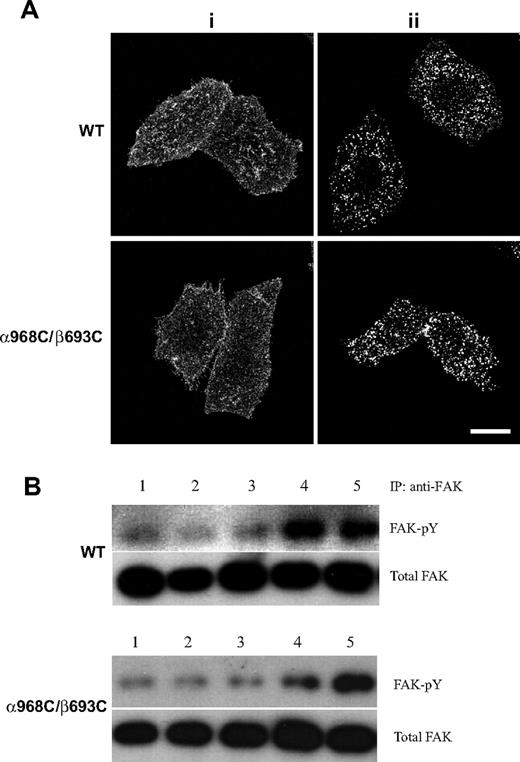
Integrin macroclustering is not perturbed by TM disulfide linkage
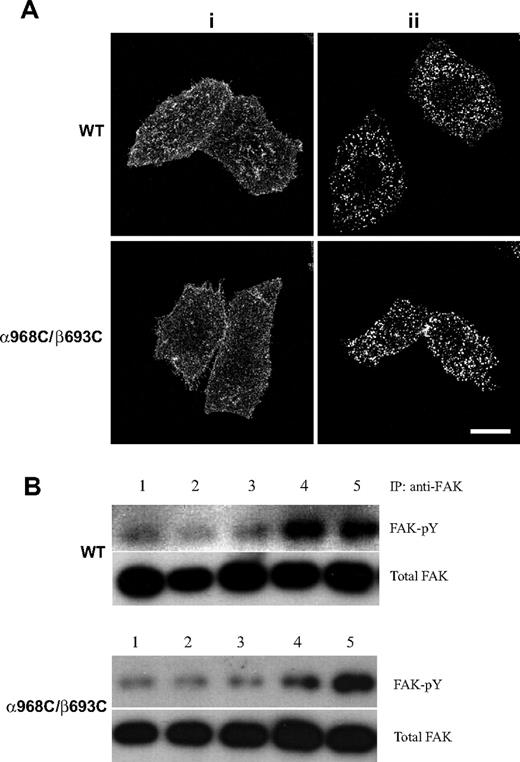
Activation of the full range of integrin-mediated signaling events has been shown to require both receptor aggregation (clustering) and occupancy (ligand binding).17,25,,–28 We demonstrated above that wild-type and TM disulfide mutant integrins adhere to fibrinogen substrates and undergo stimulated (via Mn2+ or activating mAb) binding to soluble ligand similarly (Figure 2A,B). Here we assessed the overall capacity of wild-type and TM disulfide mutant integrins to undergo cell surface redistribution and macroclustering. Antibody-mediated receptor cross-linking has been broadly used as a surrogate for multivalent ligand-induced integrin clustering.17,25 To determine whether the α968C/β693C receptors retained the ability to form macroclusters, we cross-linked cell surface WT and α968C/β693C αIIbβ3 with primary and secondary antibodies at 37°C for 30 minutes followed by fixation (Figure 7Aii). As a control, cells were fixed before adding secondary antibody (Figure 7Ai). Confocal microscopy revealed that upon cross-linking, but not under control condition, WT and mutant receptors both readily formed macroclusters to a similar extent (Figure 7A). These results suggested that the mutant α968C/β693C receptors were not defective in their overall capacity to undergo cell-surface redistribution and to macrocluster upon interaction with multivalent ligand/substrate.
Antibody-induced integrin clustering and its effect on FAK activation. (A) Confocal images of integrin clustering induced by antibody. The stably transfected cells grown on Delta T dishes were stained with nonfunctional anti-αIIbβ3 mAb, followed by staining with goat anti–mouse IgG conjugated with Alexa Fluor 488 after (i) or before (ii) fixation. Scale bar represents 10 μm. (B) WT or α968C/β693C mutant CHO-K1 cell transfectants in suspension were incubated with (lanes 2, 3) or without (lane 1) noninhibitory anti-αIIbβ3 mAb in the absence (lane 2) or presence (lane 3) of goat anti–mouse IgG at 37°C for 1 hour. As a control, cells were incubated with ligand-mimetic antibody PAC-1 (lane 4) in the presence of 1 mM Mn2+ and 10 μg/mL activating antibody PT25–2 or adhered on Fg-coated dish (lane 5) in the absence (for WT) or presence (for α968C/β693C) of 1 mM DTT. FAK was immunoprecipitated from the cell lysate and blotted with antiphosphotyrosine mAb 4G10 (FAK-pY) and reblotted with anti-FAK (total FAK).
Antibody-induced integrin clustering and its effect on FAK activation. (A) Confocal images of integrin clustering induced by antibody. The stably transfected cells grown on Delta T dishes were stained with nonfunctional anti-αIIbβ3 mAb, followed by staining with goat anti–mouse IgG conjugated with Alexa Fluor 488 after (i) or before (ii) fixation. Scale bar represents 10 μm. (B) WT or α968C/β693C mutant CHO-K1 cell transfectants in suspension were incubated with (lanes 2, 3) or without (lane 1) noninhibitory anti-αIIbβ3 mAb in the absence (lane 2) or presence (lane 3) of goat anti–mouse IgG at 37°C for 1 hour. As a control, cells were incubated with ligand-mimetic antibody PAC-1 (lane 4) in the presence of 1 mM Mn2+ and 10 μg/mL activating antibody PT25–2 or adhered on Fg-coated dish (lane 5) in the absence (for WT) or presence (for α968C/β693C) of 1 mM DTT. FAK was immunoprecipitated from the cell lysate and blotted with antiphosphotyrosine mAb 4G10 (FAK-pY) and reblotted with anti-FAK (total FAK).
To study the signaling events mediated by antibody-induced macroclustering, FAK tyrosine phosphorylation was measured using cells in suspension on which αIIbβ3 integrins were cross-linked with primary and secondary antibodies. As shown in Figure 7B, cross-linking with a nonfunctional antibody (ie, non–conformation dependent/altering, non–ligand mimetic) did not induce detectable FAK tyrosine phosphorylation either in WT or mutant cells (lanes 2,3). By contrast, binding of ligand-mimetic PAC-1 (mouse IgM) induced obvious FAK tyrosine phosphorylation in WT cells, but not in mutant cells (Figure 7B lane 4). PAC-1–induced FAK phosphorylation was, however, recovered in the mutant integrin when treated with DTT (data not shown). These results are consistent with the above assay using adherent cells (ie, Figure 6B,C). As a positive control, WT cells or mutant cells treated with DTT, which adhered to immobilized fibrinogen, induced FAK phosphorylation (Figure 7B lane 5). These results indicate that both integrin clustering and TM domain separation are important for outside-in signaling.
Discussion
Cell membrane receptors transmit signals across the plasma membrane by mechanisms that are still poorly understood. Activation of the full range of integrin-mediated signaling events has been shown to require both receptor aggregation (clustering) and ligand occupancy.26,27 However, a requirement for conformational change in integrin outside-in signaling has not previously been demonstrated. Moreover, the specific nature of any such conformational change required for this process has not been identified. In this study, we demonstrate that outside-in signaling is highly dependent on the ability of integrins to undergo conformational rearrangement and that signal transmission through the membrane requires separation of the TM, and presumably, cytoplasmic domains.
We show that preventing TM domain separation by introducing a disulfide bond between the α and β subunit TM domains, or between the ectodomains adjacent to the TM domains, strongly inhibits ligand-dependent outside-in signaling. Binding to soluble ligands required the high-affinity integrin conformation and was not mediated by wild-type and mutant αIIbβ3 in the absence of stimulation. However, in the presence of extracellular activation stimuli (ie, Mn2+ and/or PT25–2 activating mAb) both the disulfide-linked and wild-type receptors bound soluble ligands efficiently and to a similar extent. Contrasting to soluble ligand binding, adhesion to multivalent substrates bearing immobilized fibrinogen did not require activation. But importantly here again both wild-type and mutant receptors behaved similarly and showed a comparable dependence on the fibrinogen coating concentration. Moreover, both wild-type and disulfide-bonded receptors adopted an extended conformation upon ligand binding and were similarly susceptible to antibody-induced cell surface redistribution (ie, macroclustering). Nonetheless, as assessed by cell spreading, focal adhesion and actin stress-fiber formation, and FAK tyrosine phosphorylation, the TM disulfide bond greatly inhibited outside-in signal transduction. Importantly, single cysteine substitutions or reduction of the disulfide in the double cysteine mutant rescued signaling, demonstrating that the observed defects were not attributable to cysteine point mutations, but rather to the restraint imposed by the disulfide bond. Thus, our results demonstrate that outside-in signaling requires conformational communication between extracellular and cytoplasmic regions and specifically requires TM domain separation.
Precisely how integrin TM domain separation is involved in the outside-in signaling process remains to be determined. Based on previous FRET studies,10 we assume that ligand-induced TM separation is likely coupled to cytoplasmic domain separation, implying that α-β cytoplasmic domain interactions somehow constrain or inhibit integrin signaling capacity. Interestingly, and in support of this idea, chimeric integrin β (β1, β3, or β5) subunit cytoplasmic domains (coupled to an unrelated TM and extracellular domain) expressed in the absence of α subunit cytoplasmic domains were competent for signal transduction and, upon antibody- and bead-mediated clustering, induced FAK phosphorylation to levels comparable with those elicited by adhesion of the endogenous integrins to ligand-coated substrates.41 It has been known that many cytoplasmic proteins, including nonreceptor protein tyrosine kinases, can bind integrin cytoplasmic tails.42 Thus, one possibility is that ligand binding-induced separation of TM and cytoplasmic domains is required for kinases or other intracellular proteins to bind integrin cytoplasmic tails. Several studies suggested that some proteins (eg, talin7,14,43 ) bind to the integrin β-subunit tail at the same location as does the α-subunit tail. In this context, dissociation of α- and β-subunit tails would seem to be required for the binding of such proteins. On the other hand, some studies have shown that certain tyrosine kinases, such as Src, associate constitutively with integrin αIIbβ3 in platelets, and platelet adhesion to fibrinogen causes a rapid increase in Src activation.44 Thus, another possibility is that the association of the integrin TM and cytoplasmic domains somehow constrains the activity of integrin tail-bound kinases, whereas dissociation of the TM and cytoplasmic domains releases these constraints.
An important role for integrin clustering has been well established for outside-in signaling.25,,–28 Given that some outside-in signaling (although not typically the full range of signaling events) can be induced in certain settings by integrin cross-linking strategies (eg, with nonfunctional antibodies), which themselves do not proactively stabilize the active integrin conformation, it has often been inferred that conformation does not play a direct or critical role in such signaling. Importantly, however, in no such studies has conformation been specifically controlled. It is now well understood that integrins exist in a dynamic equilibrium between various inactive and active conformational states; even in the absence of specific stimuli (eg, inside-out signals or extracellular ligand), at any given instant some fraction of cell surface integrins may spontaneously transition to intermediate or open conformations.15 Although under our experimental conditions no activation of FAK phosphorylation was observed upon cross-linking with a nonfunctional integrin antibody, our results suggest that in settings where signaling has resulted from such treatments, that the resulting increase in density of the spontaneously active conformers (with dissociated α and β cytoplasmic domains) is responsible for activation. When integrin occupancy by soluble monomeric ligand (which stabilizes the active integrin conformers and promotes transmembrane domain separation) has been combined with antibody cross-linking, total outside-in signal transduction is greatly elevated.26,27 This result is consistent with an increase in the local density of active integrin conformers and our finding that transmembrane domain separation is required for integrin outside-in signaling. While dissociation of the α and β cytoplasmic domains may be important for the association and/or activity of specific kinases, clustering may serve to facilitate interactions among integrin-bound kinases to promote transphosphorylation/transactivation events in a fashion loosely analogous to receptor tyrosine kinase activation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH) (HL48675 to T.A.S.), the Arthritis Foundation (C.V.C.), and the American Heart Association (0535403T to B.-H.L.).
National Institutes of Health
Authorship
Contribution: J.Z. and C.V.C. designed and performed research, analyzed data, and wrote the paper; M.K. performed research; M.S. analyzed data; T.A.S. analyzed data and wrote the paper; and B.-H.L. conceived, designed, and performed research, analyzed data, and wrote the paper. J.Z. and C.V.C. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bing-Hao Luo, CBR Institute for Biomedical Research and Department of Pathology, Harvard Medical School, 200 Longwood Ave, Boston, MA 02115; e-mail:luo@lsu.edu.