CD40 ligand (CD40L) is an essential effector cytokine for macrophage activation, dendritic cell licensing, and T-cell–dependent antibody responses. Although CD40L is known to be made de novo following antigen recognition, several reports have described surface mobilization of preformed, intracellular CD40L in certain CD4+ effector T cells. Here we show that rapid surface expression of preformed CD40L following antigen recognition is a general property of both effector and memory CD4+ T cells, including in vitro and in vivo activated T-cell–receptor transgenic T cells, memory phenotype CD4+ T cells from pathogen-free naive mice, and polyclonal virus–specific effector and memory T cells. Intracellular CD40L is stored in secretory lysosomes, and colocalizes more strongly with Fas ligand than with CTLA-4, two other molecules that are delivered to the cell surface following antigen recognition. Stimulated surface expression of preformed CD40L is found in memory CD4+ T cells from CD40-deficient mice, indicating that it does not depend on CD40-induced internalization for delivery to the secretory compartment. We suggest that delivery of preformed CD40L to antigen-presenting cells (APCs) could enable antigen-specific activation of APCs in transient interactions that are too brief to permit de novo synthesis of CD40L.
Introduction
The tumor necrosis factor (TNF) family member CD40 ligand (CD40L; CD154) is a transmembrane protein expressed on the surface of activated CD4+ T cells, and triggers CD40-dependent activation of B cells, dendritic cells (DCs), and macrophages.1 CD40L is recognized as an essential cytokine for both humoral and cell-mediated immunity.1,2 Dysregulation of the CD40L-CD40 pathway has been reported in several human diseases, including inflammatory bowel diseases, autoimmune diseases, and graft-versus-host disease. Blockade of CD40L holds promise for treating autoimmune diseases and inducing transplantation tolerance.1,–3
Surface expression of CD40L is tightly regulated.1 After activation with peptide-pulsed antigen-presenting cells (APCs), the de novo synthesis and surface expression of CD40L on naive CD4+ T cells is detectable within 2 hours, peaks at 6 hours, and is significantly reduced by 24 hours after activation.4 The presence of CD40+ cells prevents accurate detection of CD40L through CD40-induced endocytosis, shedding, and blocking by soluble CD40.5,–7 Importantly, the technical difficulties in detecting CD40L by flow cytometry can be overcome by using purified CD4+ T cells,8,9 using a surface mobilization assay in which anti-CD40L monoclonal antibody (mAb) is included in the culture during stimulation,10,–12 adding a blocking mAb to CD40,13,14 or using CD40-deficient animals.9 The studies that have relied on conventional staining methods for detecting surface CD40L may have overlooked the dynamic nature of CD40L expression in the presence of CD40+ cells.
Although CD40L is made de novo following antigen recognition, intracellular sequestration and surface mobilization of preformed CD40L have been reported by several groups. In effector CD4+ T cells from human tonsils, intracellular CD40L comes to the cell surface in 5 to 15 minutes following stimulation with PMA plus ionomycin or immobilized anti-CD3 mAb.15 The early surface expression of CD40L on CD4+CD45RO+ T cells is insensitive to cycloheximide (CHX), while the second phase of CD40L expression beginning at 1 hour after stimulation in both naive and memory T cells is sensitive to CHX treatment.15 Other groups have reported rapid and inducible surface expression of preformed CD40L in specialized subsets of effector CD4+ T cells, including CD4+CXCR5+ follicular B-helper T cells,16 P-selectinhi and P-selectin− L-selectin− effector CD4+ T cells,17 synovial fluid CD4+ T cells from patients with rheumatoid arthritis,18 and memory CD4+ T cells from lupus-prone, young, clinically healthy mice.19 Recently, Lesley et al reported the discovery of a different type of preformed CD40L that is constitutively sorted to the cell surface in naive CD4+ T cells and natural regulatory T cells.9 However, a systematic analysis of the existence and the surface mobilization of intracellular, preformed CD40L among naive, effector, and memory CD4+ T-cell populations in normal immune responses has not been conducted.
The secretion of effector molecules from T cells occurs via the constitutive secretory pathway, in which newly synthesized proteins are released by exocytosis of small vesicles directly after Golgi processing, or the regulated secretory pathway, in which effector proteins are stored in intracellular vesicles until cells are stimulated.20 For the latter pathway, secretory vesicles can be divided into at least 2 categories: specialized secretory vesicles that lack lysosomal markers (eg, RANTES storage vesicles and CXCR3/1-storing granules)21,22 and “secretory lysosomes” (SLs), a heterogeneous group of organelles that share lysosomal markers and includes the lytic granules of cytolytic T lymphocytes.23 Naive T lymphocytes do not possess SLs, but acquire them upon activation.23 Fas ligand (FasL; CD178), another member of the TNF family, is stored in SLs in both CD4+ and CD8+ activated T cells and is released to the cell surface upon T-cell receptor (TCR) signaling.24 CTLA-4 (CD152) is also reported to be stored in SLs and is mobilized toward the contact site between T cells and APCs upon antigen recognition.25,–27 Experiments to determine if intracellular CD40L is stored in SLs have not been reported.
Here we show that stimulated surface mobilization of intracellular, preformed CD40L is a general property of effector and memory T helper 1 (Th1) cells, whether generated in vitro or in vivo. We show that preformed CD40L is stored in SLs and colocalizes more strongly with FasL than with CTLA-4. Naive and effector/memory CD4+ T cells from unmanipulated animals contain intracellular CD40L, although only effector/memory CD4+ T cells exhibit rapid surface mobilization of intracellular CD40L. We also show that preformed CD40L in lymphocytic choriomeningitis virus (LCMV)–specific effector and memory Th1 cells can be recruited to the cell surface in response to antigen presented by APCs. These results suggest that effector and memory CD4+ T cells have a rapid and antigen-specific way to execute their effector functions by mobilizing preformed CD40L from intracellular compartments before they express newly synthesized proteins.
Materials and methods
Animals
Heterozygous AD10 TCR transgenic mice (Vβ3+, Vα11+), reactive against moth cytochrome c (MCC) fragment 88-103 on I-Ek, were maintained on a B10.BR (H-2k) background, and have been described previously.28 C57BL/6J mice expressing a transgenic I-Ek/MCC peptide complex under a major histocompatibility complex (MHC) class II promoter have been described previously.29 AND TCR-transgenic T cells are efficiently selected in the thymus on I-Ab in C57BL/6 mice but recognize MCC only in the context of I-Ek.29 CD40L-deficient and RAG-1–deficient C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). CD40L-deficient AD10 transgenic animals were bred in-house. CD40-deficient mice were kindly provided by H. Kikutani (Osaka, Japan). Mice were housed under specific pathogen–free conditions at the Oregon Health and Science University animal facility. These studies were approved by the Institutional Animal Care and Use Committee at the Oregon Health and Science University.
Antibodies and reagents
PerCP–anti-CD4, biotin–anti-Vβ3, biotin–anti-CD62L, and labeled isotype controls were purchased from BD Biosciences (San Jose, CA). The following reagents were purchased from Biolegend (San Diego, CA): purified anti-FcγRII/III antibody, FITC–anti–TNF-α, PE–anti–CTLA-4 (UC10–4B9), PE–anti-CD40L (MR1), PE–anti-FasL (MFL3), allophycocyanin–anti–interferon-γ (IFN-γ), Alexa Fluor 647–anti-CD107a (1D4B), and PE/Cy7–anti-CD8α. FITC–anti-CD44 and allophycocyanin-streptavidin were purchased from eBiosciences (San Diego, CA). Polyclonal goat anti-CD40L Ab was purchased from R&D Systems (Minneapolis, MN). The following purified Abs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA): anti–β2-microglobulin (S19.8), anti–cathepsin D (H-75), anti–CTLA-4 (H-126), anti-FasL (C-178), and anti–Lamp-2 (H-207). Anti-EEA1 Ab was purchased from Abcam (Cambridge, MA). Antiserum for giantin was purchased from Covance Research Products (Baltimore, MD). Alexa Fluor 488 donkey anti–goat IgG was purchased from Molecular Probes (Eugene, OR). Texas Red donkey anti–rabbit IgG, Texas Red donkey anti–mouse IgG, and Cy5-streptavidin were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
T-cell priming
A mixture of 106 AND T cells in an AND RAG-1 knock-out spleen cell suspension, 40 × 106 splenocytes from I-Ek/MCC antigen-transgenic mice, and 12.5 μg LPS (L6761; Sigma-Aldrich, St Louis, MO) in HBSS was injected intravenously into RAG-1 knock-out mice. In vivo–primed AND T cells were recovered from recipient spleens 14 days later. In vitro–primed effector CD4+ T cells were generated as described previously.28
Flow cytometric analysis of CD40L expression
A total of 3 different methods (the conventional method, the surface mobilization assay, and intracellular staining) were used to detect CD40L, FasL, CTLA-4, and CD107a by flow cytometry. For the conventional method, cells were stained with PE-labeled anti-CD40L mAb at 4°C for 15 minutes in staining buffer (PBS plus 2% FBS plus 0.1% NaN3). For the surface mobilization assay, cells were blocked with anti-FcγRII/III antibody and 10 μg/mL PE-labeled anti–CTLA-4, anti-CD40L, anti-FasL, or Alexa Fluor 647–labeled anti-CD107a antibody, or the respective isotype control antibody, was introduced into the cell culture immediately before stimulation with an equal number of peptide-pulsed APCs (CH12 B lymphoma cells) or 50 ng/mL PMA (Sigma-Aldrich) plus 1 μg/mL ionomycin (Sigma-Aldrich), and then incubated for 30 minutes at 37°C. After 3 washes, T cells were surface stained for 15 minutes at 4°C in staining buffer to identify subsets of CD4+ T cells. To block new protein synthesis in indicated experiments, T cells were pretreated with 10 μg/mL of CHX (Calbiochem, San Diego, CA) for 1 hour at 37°C, and the surface mobilization assay was conducted in the presence of CHX. Supernatants were collected, and the concentration of IFN-γ in the culture media was measured with an enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences). For intracellular CD40L staining, cells were stained for surface antigens, fixed for 5 minutes at 4°C, and stained with 2 μg/mL of PE–anti-CD40L mAb for 1 hour on ice using the Cytofix/Cytoperm kit (BD Biosciences). Stained cells were analyzed on a FACSCalibur or an LSRII (BD Biosciences). Data obtained by FACSCalibur and LSRII are presented in logarithmic and “logicle” displays, respectively.30 Data were analyzed with FlowJo software (Tree Star, Ashland, OR).
Fixed cell microscopy, data processing, and analysis of colocalization
In vitro– or in vivo–primed TCR transgenic T cells were fixed and permeabilized as described previously.28 For in vivo–primed T cells, CD4+ T cells were enriched by negative selection using the SpinSep kit following the manufacturer's instructions (Stem Cell Technologies, Vancouver, BC). In the case of giantin staining, cells were fixed and permeabilized with ice cold 90% methanol at −20°C for 5 minutes. Permeabilized T cells were blocked overnight at 4°C with PBS plus 5% normal donkey serum (NDS; Jackson Immunoresearch Laboratories), then stained with primary Abs at 10 μg/mL in PBS plus 2% NDS for 1.5 hours at room temperature in a humidified chamber. Following 3 PBS washes of 3 minutes each, cells were incubated with 5 μg/mL secondary Abs in PBS plus 1% NDS for 45 minutes at room temperature. After 3 more PBS washes, Fluoromount G (SouthernBiotech, Birmingham, AL) was added to the wells. Stained cells were examined on a DeltaVision system (Applied Precision, Issaquah, WA) based on a Nikon TE200 inverted fluorescence microscope (Nikon, Melville, NY) using a 60×/1.40 NA oil-immersion objective lens. Images were acquired with a CH350L camera (Applied Precision) using softWoRx 2.5 software (Applied Precision). TCR transgenic T cells were identified by Vβ3 staining. A stack of 30 to 50 fluorescent images spaced 0.2 μm apart in the z-axis was obtained and deconvolved with softWoRx Suite 1.2 software (Applied Precision), and was processed with Photoshop 7.0 software (Adobe Systems, San Deigo, CA) as described previously.28 To evaluate the degree of colocalization, the Pearson correlation coefficient (r) between CD40L and compartment markers or FasL and CTLA-4 was calculated from the difference in signal intensity for each voxel in the image stack using Volocity software (Improvision, Coventry, United Kingdom). A Pearson correlation coefficient greater than 0.5 defines strong colocalization. Significant differences between groups as a whole for Figures 2B and 3E were confirmed with a 1-way ANOVA test. Continuous variables were compared across groups with the Wilcoxon rank-sum test. A P value less than .05 was considered significant.
Surface mobilization assay with intracellular cytokine staining for LCMV-specific T cells
C57BL/6J mice were infected intraperitoneally with 2 × 105 plaque-forming units of LCMV-Armstrong (53b) strain and were used at the indicated time points after infection. A surface mobilization assay was combined with intracellular cytokine staining to detect rapid surface expression of CD40L on either IFN-γ+ or TNF-α+, viral glycoprotein (GP61–80)–specific, effector or memory T cells. In this assay, uninfected splenocytes were pulsed with 10 μM of GP61–80 for at least 2 hours at 37°C and were used as APCs to stimulate either uninfected or LCMV-infected splenocytes in the presence of PE-labeled anti-CD40L mAb for 30 minutes. All splenocytes were preblocked with anti-FcγRII/III antibody. Cells were washed 3 times with fresh media to remove anti-CD40L mAb. After washing, cells were incubated another 5.5 hours in the presence of 2 μg/mL Brefeldin A (Sigma-Aldrich) to block further surface expression of CD40L and cytokine secretion, and then fixed, permeabilized, and stained for intracellular IFN-γ and TNF-α using the Cytofix/Cytoperm kit.
Results
Intracellular CD40L quickly comes to the cell surface upon antigenic stimulation of effector CD4+ T cells
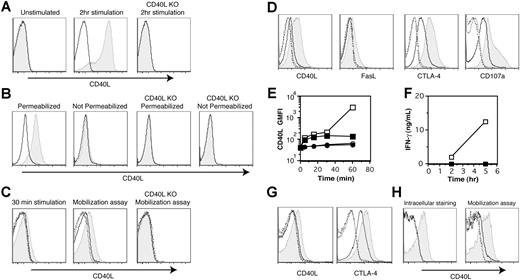
Several studies have shown that under some circumstances, effector CD4+ T cells store preformed CD40L that can be rapidly expressed on the plasma membrane upon stimulation with either PMA plus ionomycin or TCR cross-linking by anti-CD3 mAb.15,,,–19 We started our study by verifying these finding using in vitro–generated effector CD4+ T cells from TCR transgenic animals. We analyzed surface and intracellular CD40L expression with 3 different methods: conventional surface staining, intracellular staining, and the surface mobilization assay. First, surface CD40L staining was conducted by conventional staining of cells at 4°C. Surface CD40L expression was not detectable in effector CD4+ T cells, although acutely activated effector CD4+ T cells expressed substantial amounts of CD40L on the cell surface upon stimulation with PMA plus ionomycin for 2 hours (Figure 1A). Next, we measured intracellular CD40L using optimized fixation and permeabilization conditions for effector CD4+ T cells without acute stimulation as described in “Materials and methods.” T cells stained under permeabilizing conditions exhibited a significant shift for CD40L staining, although there was negligible staining without permeabilization (Figure 1B), indicating that most CD40L in in vitro–generated effector CD4+ T cells is intracellular. Finally, we assessed the surface expression of CD40L upon stimulation with the surface mobilization assay. In this assay, a relatively high concentration of PE-labeled anti-CD40L mAb (MR1; 10 μg/mL) is included in a 30-minute culture to enhance the detection of CD40L by capturing CD40L that would otherwise be downmodulated by several mechanisms (endocytosis, shedding, etc) following CD40 engagement.10,11 Indeed, the surface mobilization assay revealed a significant amount of CD40L expression after a 30-minute stimulation with PMA plus ionomycin (Figure 1C middle panel), while conventional staining at 4°C after 30 minutes of stimulation showed partial detection of CD40L (Figure 1C left panel). Even without stimulation, the surface mobilization assay detected low levels of CD40L expression during a 30-minute incubation (Figure 1C middle panel). This was mistaken for nonspecific “background” staining in a previous report,12 but here the specificity of the staining was confirmed with effector CD4+ T cells from CD40L-deficient TCR transgenic animals (Figure 1C right panel). The same assay was applied to the detection of FasL, CTLA-4, and CD107a (Lamp-1) (Figure 1D), since these are molecules known to be expressed on the cell surface by regulated secretion.24,25,31 FasL expression analyzed by the mobilization assay showed a pattern similar to that of CD40L, although the magnitude of mobilization was less than that of CD40L. The mobilization assay detected the constitutive surface expression of CTLA-4, probably reflecting active cycling of CTLA-4 as previously reported,25 with further enhancement of the mobilization upon PMA plus ionomycin stimulation. The pattern of mobilization for CD107a resembled that of CTLA-4. However, the conventional method of staining at 4°C failed to detect surface expression of these effector molecules without stimulation, and showed a significantly reduced signal upon stimulation for 30 minutes (Figure 1C; data not shown). These results show the usefulness of the mobilization assay to analyze stimulated surface expression of effector molecules in CD4+ T cells.
Antigen recognition induces the mobilization of preformed CD40L in effector CD4+ T cells. (A) In vitro–generated effector CD4+ T cells from CD40L-sufficient and -deficient (CD40L KO) TCR transgenic mice were stimulated or left unstimulated with PMA plus ionomycin for 2 hours. Levels of CD40L (shaded) and isotype control (line) staining on gated effector CD4+ T cells after surface staining at 4°C are shown. (B) In vitro–generated effector CD4+ T cells from CD40L-sufficient and CD40L KO TCR transgenic mice were fixed, permeabilized or left unpermeabilized, and stained for intracellular CD40L at 4°C without acute stimulation. Levels of CD40L (shaded) and isotype control (line) staining on gated effector CD4+ T cells are shown. (C) The surface mobilization assay was conducted as described in “Materials and methods, Surface mobilization assay” by addition of anti-CD40L mAb at 37°C for 30 minutes (middle and right panels), and was compared with the surface staining at 4°C following 30 minutes of stimulation (left panel). CD40L KO effector CD4+ T cells were used as a specificity control for the mobilization assay. Shaded histogram indicates levels of CD40L with stimulation. –––– indicates levels of CD40L without stimulation. –––– indicates levels of isotype control with stimulation. -------- indicates levels of isotype control without stimulation. (D) The surface mobilization assay was conducted for CD40L, FasL, CTLA-4, and CD107a. Histograms are assigned as in panel C. (E) The kinetics of surface mobilization of CD40L were assessed by the mobilization assay. ■ indicates CHX-pretreated and PMA plus ionomycin-stimulated; □, CHX-untreated and PMA plus ionomycin-stimulated; ●, CHX-pretreated and unstimulated; and ○, CHX-untreated and unstimulated. Raw geometric mean fluorescent intensity of CD40L staining are shown. (F) IFN-γ levels in the supernatants from CHX-pretreated (■) or -untreated (□) effector CD4+ T cells cultured in the presence of PMA plus ionomycin for the indicated periods of time were measured by ELISA. (G) The mobilization of CD40L and CTLA-4 on in vitro effector CD4+ T cells was induced with antigen-pulsed CH12 B cells for 30 minutes. Histograms are assigned as in panels C and D. (H) Day-14, in vivo–primed TCR transgenic effector CD4+ T cells were analyzed for intracellular CD40L without stimulation and surface mobilization of CD40L upon PMA plus ionomycin stimulation as described in panels B and C. The levels of CD40L staining gated on CD4+, Vα11+, and Vβ3+ T cells are shown. See the descriptions in panels B and C for the histogram assignment. Experiments were repeated at least 5 times with similar results.
Antigen recognition induces the mobilization of preformed CD40L in effector CD4+ T cells. (A) In vitro–generated effector CD4+ T cells from CD40L-sufficient and -deficient (CD40L KO) TCR transgenic mice were stimulated or left unstimulated with PMA plus ionomycin for 2 hours. Levels of CD40L (shaded) and isotype control (line) staining on gated effector CD4+ T cells after surface staining at 4°C are shown. (B) In vitro–generated effector CD4+ T cells from CD40L-sufficient and CD40L KO TCR transgenic mice were fixed, permeabilized or left unpermeabilized, and stained for intracellular CD40L at 4°C without acute stimulation. Levels of CD40L (shaded) and isotype control (line) staining on gated effector CD4+ T cells are shown. (C) The surface mobilization assay was conducted as described in “Materials and methods, Surface mobilization assay” by addition of anti-CD40L mAb at 37°C for 30 minutes (middle and right panels), and was compared with the surface staining at 4°C following 30 minutes of stimulation (left panel). CD40L KO effector CD4+ T cells were used as a specificity control for the mobilization assay. Shaded histogram indicates levels of CD40L with stimulation. –––– indicates levels of CD40L without stimulation. –––– indicates levels of isotype control with stimulation. -------- indicates levels of isotype control without stimulation. (D) The surface mobilization assay was conducted for CD40L, FasL, CTLA-4, and CD107a. Histograms are assigned as in panel C. (E) The kinetics of surface mobilization of CD40L were assessed by the mobilization assay. ■ indicates CHX-pretreated and PMA plus ionomycin-stimulated; □, CHX-untreated and PMA plus ionomycin-stimulated; ●, CHX-pretreated and unstimulated; and ○, CHX-untreated and unstimulated. Raw geometric mean fluorescent intensity of CD40L staining are shown. (F) IFN-γ levels in the supernatants from CHX-pretreated (■) or -untreated (□) effector CD4+ T cells cultured in the presence of PMA plus ionomycin for the indicated periods of time were measured by ELISA. (G) The mobilization of CD40L and CTLA-4 on in vitro effector CD4+ T cells was induced with antigen-pulsed CH12 B cells for 30 minutes. Histograms are assigned as in panels C and D. (H) Day-14, in vivo–primed TCR transgenic effector CD4+ T cells were analyzed for intracellular CD40L without stimulation and surface mobilization of CD40L upon PMA plus ionomycin stimulation as described in panels B and C. The levels of CD40L staining gated on CD4+, Vα11+, and Vβ3+ T cells are shown. See the descriptions in panels B and C for the histogram assignment. Experiments were repeated at least 5 times with similar results.
To address whether the rapid surface expression of CD40L is dependent on de novo protein synthesis, we examined the effect of CHX on rapid CD40L expression. Kinetic experiments with T cells pretreated with CHX showed that early CD40L expression from 5 to 30 minutes after stimulation is CHX resistant, while CD40L expression at 1 hour is partially sensitive (Figure 1E). Complete inhibition of IFN-γ production from T cells by CHX verified that protein synthesis was blocked (Figure 1F). These results indicate that the mobilization of CD40L within 30 minutes after stimulation relies on intracellular, preformed CD40L rather than de novo synthesized CD40L. To extend these finding to more physiologic conditions, we used antigen-pulsed APCs to stimulate the T cells, and found that antigenic stimulation for 30 minutes also induced the surface expression of preformed CD40L as well as CTLA-4 (Figure 1G).
Next, we tested the existence of preformed CD40L in effector CD4+ T cells generated in vivo by transferring naive AND TCR transgenic CD4+ T cells with antigen transgenic splenocytes29 as APCs and LPS as an adjuvant into Rag-1–deficient mice. CD4+ T cells recovered from the spleens of recipient mice on day 14 were Th1 effector cells. They were uniformly CD44high and CD62Llow, and about 50% were intracellular IFN-γ+ following stimulation with PMA plus ionomycin for 5 hours (data not shown). Of the total recovered spleen cells, 15.3% plus or minus 3.2% were CD4+ T cells, and among those, 36.3% plus or minus 3.1% were Vα11+ Vβ3+ TCR transgenic T cells (data not shown). Preformed CD40L on effector CD4+ T cells generated in vivo can be detected on day 5, and is maintained at relatively high levels even 8 weeks after in vivo priming (data not shown). The level of preformed CD40L assessed by intracellular staining and the surface mobilization assay was higher in effector CD4+ T cells generated in vivo than in those generated in vitro (Figure 1H). These results indicate that preformed CD40L is a shared property of effector CD4+ T cells generated in vitro or in vivo.
CD40L is found in secretory lysosomes and colocalizes more strongly with FasL than with CTLA-4
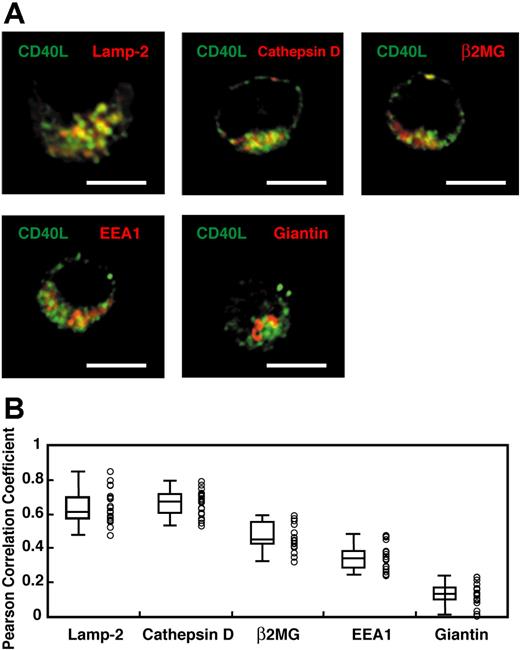
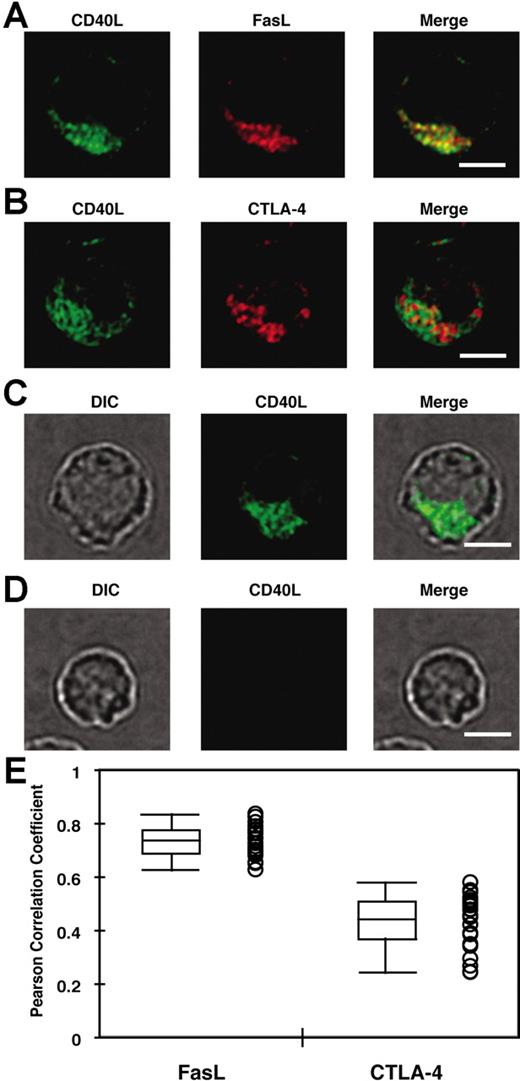
Yellin et al5 showed that ammonium chloride or chloroquine treatment of CD4+ T cells increased the amount of intracellular CD40L, suggesting that intracellular CD40L might be localized in an acidic, lysosomal compartment. Also, FasL, another TNF family member, is stored in SLs.24 Therefore, we hypothesized that CD40L might exist in a lysosomal compartment in activated CD4+ T cells. Day-14 in vivo–generated effector CD4+ T cells were enriched by negative selection and then fixed, permeabilized, and stained with anti-Vβ3 mAb and polyclonal anti-CD40L Ab together with antibodies to several cellular compartment markers. Transgenic T cells were identified based on Vβ3 staining by fluorescent microscopy (data not shown), and were subsequently analyzed for colocalization of CD40L with compartment markers by fluorescent microscopy as described in “Materials and methods, Fixed cell microscopy, data processing, and analysis of colocalization.” As shown in Figure 2, CD40L staining showed a vesicular distribution and colocalized with the lysosome markers Lamp-2 (r = 0.607 ± 0.021) and cathepsin D (r = 0.636 ± 0.016). CD40L also weakly colocalized with a marker for endosomes (EEA1; r = 0.317 ± 0.017) and a marker for the constitutive secretory pathway (β2-microglobulin; r = 0.466 ± 0.018). CD40L did not colocalize with a Golgi marker, giantin (r = 0.095 ± 0.015). Statistical analysis confirmed that CD40L staining colocalized with the lysosomal markers more than any other markers (Figure 2B). Finally, the colocalization of CD40L with FasL and CTLA-4 was compared in in vitro–generated effector CD4+ T cells. As shown in Figure 3A,B,E, CD40L colocalized more strongly with FasL (r = 0.753 ± 0.010; n = 25) than with CTLA-4 (r = 0.481 ± 0.018). The specificity of the polyclonal anti-CD40L Ab was confirmed by lack of staining of effector CD4+ T cells from CD40L-deficient TCR transgenic mice (Figure 3C,D). Taken together, these results indicate that CD40L exists in lysosmal compartments with FasL and is less strongly associated with CTLA-4.
Preformed CD40L exists in lysosomal compartments. (A) In vivo–primed TCR transgenic CD4+ effector T cells were enriched by negative selection and identified based on Vβ3 staining by fluorescent microscopy (data not shown) as described in “Flow cytometric analysis of CD40L expression.” These cells were subsequently analyzed for colocalization of CD40L with compartment markers. Merged pictures between CD40L (green) and the compartment markers, Lamp-2, cathepsin D, β2-microglobulin (β2MG), EEA-1, and giantin (red) are shown. Representative pictures of 1 Z-section from a stack are shown. Scale bar equals 4 μm. (B) The Pearson correlation coefficient between CD40L and each compartment marker was obtained as described in “Fixed cell microscopy, data processing, and analysis of colocalization.” A total of 20 cells and 30 to 50 Z sections for each cell from 2 experiments were analyzed. Each circle represents data from a single cell. Median (bar in the box), interquartile ranges (boxes), and maximums and minimums (whiskers) are also shown. The Pearson correlation coefficient for CD40L–Lamp-2 and CD40L–cathepsin D are significantly higher than those for CD40L-β2MG, CD40L–EEA-1, and CD40L-giantin (P < .005; Wilcoxon rank-sum test). There is no statistical difference between CD40L–Lamp-2 and CD40L–cathepsin D (P = .20; Wilcoxon rank-sum test).
Preformed CD40L exists in lysosomal compartments. (A) In vivo–primed TCR transgenic CD4+ effector T cells were enriched by negative selection and identified based on Vβ3 staining by fluorescent microscopy (data not shown) as described in “Flow cytometric analysis of CD40L expression.” These cells were subsequently analyzed for colocalization of CD40L with compartment markers. Merged pictures between CD40L (green) and the compartment markers, Lamp-2, cathepsin D, β2-microglobulin (β2MG), EEA-1, and giantin (red) are shown. Representative pictures of 1 Z-section from a stack are shown. Scale bar equals 4 μm. (B) The Pearson correlation coefficient between CD40L and each compartment marker was obtained as described in “Fixed cell microscopy, data processing, and analysis of colocalization.” A total of 20 cells and 30 to 50 Z sections for each cell from 2 experiments were analyzed. Each circle represents data from a single cell. Median (bar in the box), interquartile ranges (boxes), and maximums and minimums (whiskers) are also shown. The Pearson correlation coefficient for CD40L–Lamp-2 and CD40L–cathepsin D are significantly higher than those for CD40L-β2MG, CD40L–EEA-1, and CD40L-giantin (P < .005; Wilcoxon rank-sum test). There is no statistical difference between CD40L–Lamp-2 and CD40L–cathepsin D (P = .20; Wilcoxon rank-sum test).
CD40L colocalizes more strongly with FasL than with CTLA-4. (A, B) In vitro–primed effector CD4+ T cells were used to analyze the degree of colocalization between CD40L and FasL (A) or CTLA-4 (B) as described in “Flow cytometric analysis of CD40L expression.” (C,D) The specificity of the polyclonal anti-CD40L Ab was tested with in vitro–primed effector CD4+ T cells from CD40L-sufficient (C) or -deficient (D) mice. DIC indicates differential interference contrast image. Scale bar equals 4 μm. (E) The Pearson correlation coefficient between CD40L and FasL or CTLA-4 was obtained as described in “Materials and methods, Fixed cell microscopy, data processing, and analysis of colocalization.” A total of 25 cells and 30 to 50 Z sections for each cell from 2 experiments were analyzed. Each circle represents data from a single cell. Median (bar in the box), interquartile ranges (boxes), and maximum and minimum (whiskers) are also shown. The Pearson correlation coefficient for CD40L-FasL is significantly higher than that for CD40L–CTLA-4 (P < .001; Wilcoxon rank-sum test).
CD40L colocalizes more strongly with FasL than with CTLA-4. (A, B) In vitro–primed effector CD4+ T cells were used to analyze the degree of colocalization between CD40L and FasL (A) or CTLA-4 (B) as described in “Flow cytometric analysis of CD40L expression.” (C,D) The specificity of the polyclonal anti-CD40L Ab was tested with in vitro–primed effector CD4+ T cells from CD40L-sufficient (C) or -deficient (D) mice. DIC indicates differential interference contrast image. Scale bar equals 4 μm. (E) The Pearson correlation coefficient between CD40L and FasL or CTLA-4 was obtained as described in “Materials and methods, Fixed cell microscopy, data processing, and analysis of colocalization.” A total of 25 cells and 30 to 50 Z sections for each cell from 2 experiments were analyzed. Each circle represents data from a single cell. Median (bar in the box), interquartile ranges (boxes), and maximum and minimum (whiskers) are also shown. The Pearson correlation coefficient for CD40L-FasL is significantly higher than that for CD40L–CTLA-4 (P < .001; Wilcoxon rank-sum test).
Effector/memory but not naive CD4+ T cells in unmanipulated animals exhibit mobilization of preformed CD40L upon stimulation
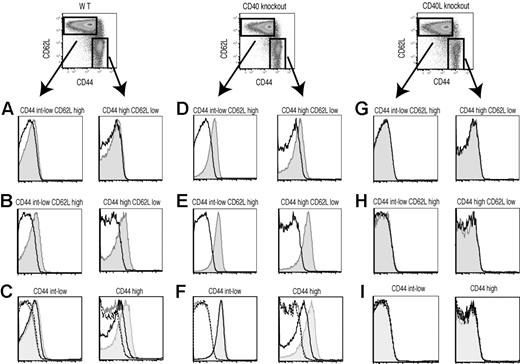
Previous reports showed preformed CD40L in effector or memory CD4+ T cells under pathologic conditions.18,19 Therefore, we asked whether the existence of an intracellular pool of CD40L is a general property of CD4+ T cells. To address this question, spleen cells from unmanipulated C57BL/6, CD40-deficient, or CD40L-deficient animals were examined by conventional surface and intracellular staining and the surface mobilization assay for expression of CD40L by naive and effector/memory CD4+ T cells. Naive and effector/memory CD4+ T cells were distinguished based on CD44 and CD62L staining (naive, CD44int-low and CD62Lhigh; effector/memory, CD44high and CD62Llow). Conventional surface staining did not detect CD40L expression on either naive or effector/memory CD4+ T cells directly ex vivo (Figure 4A). As expected, effector/memory CD4+ T cells had intracellular CD40L, and surprisingly, naive CD4+ T cells also possessed intracellular CD40L (Figure 4B). In accordance with results from Lesley et al,9 both naive and effector/memory CD4+ T cells from CD40-deficient mice exhibited clear surface CD40L expression (Figure 4D), probably due to the lack of CD40-dependent downmodulation of surface CD40L on CD4+ T cells. Intracellular CD40L staining was also enhanced in CD40-deficient mice (Figure 4E). Data for CD44high, CD62Lhigh, “central memory” CD4+ T cells were very similar to CD44high CD62Llow effector/memory CD4+ T cells (data not shown). As shown in Figure 4C, effector/memory CD4+ T cells, but not naive CD4+ T cells, released intracellular CD40L in response to stimulation with PMA plus ionomycin during the first 30 minutes of incubation. CHX pretreatment did not reduce the mobilization of preformed CD40L (data not shown). Interestingly, the surface mobilization assay revealed that CD40L was sorted to the correct intracellular compartment independently of CD40 (Figure 4F). Staining of CD40L-deficient splenocytes confirmed the specificity of all 3 different staining methods for CD40L detection (Figure 4G-I). Although we32 and others33 have reported that activated CD8+ T cells synthesize and express CD40L, none of the staining methods detect CD40L expression in resting CD8+ T cells (Beadling and Slifka;32 data not shown). These data indicate that preformed CD40L is a shared property of naive and effector/memory CD4+ T cells, but only effector/memory CD4+ T cells can respond to external stimulation by deploying preformed CD40L.
Effector/memory but not naive CD4+ T cells from unmanipulated animals mobilize preformed CD40L. CD40L expression in naive and effector/memory CD4+ T cells from unmanipulated normal (A-C), CD40-deficient (D-F), and CD40L-deficient (G-I) animals was analyzed by conventional surface staining (A,D,G), intracellular staining (B,E,H), and the surface mobilization assay (C,F,I). (A,B,D, E,G,H) The levels of CD40L (shaded) and isotype control (line) staining for the indicated population of CD4+ T cells are shown. (C,F,I) Shaded indicates CD40L with stimulation. –– indicates CD40L without stimulation. –– indicates isotype control with stimulation. ------ indicates isotype control without stimulation. Data shown are representative of 4 independent experiments.
Effector/memory but not naive CD4+ T cells from unmanipulated animals mobilize preformed CD40L. CD40L expression in naive and effector/memory CD4+ T cells from unmanipulated normal (A-C), CD40-deficient (D-F), and CD40L-deficient (G-I) animals was analyzed by conventional surface staining (A,D,G), intracellular staining (B,E,H), and the surface mobilization assay (C,F,I). (A,B,D, E,G,H) The levels of CD40L (shaded) and isotype control (line) staining for the indicated population of CD4+ T cells are shown. (C,F,I) Shaded indicates CD40L with stimulation. –– indicates CD40L without stimulation. –– indicates isotype control with stimulation. ------ indicates isotype control without stimulation. Data shown are representative of 4 independent experiments.
Virus-specific effector and memory CD4+ T cells rapidly express surface CD40L upon antigenic stimulation
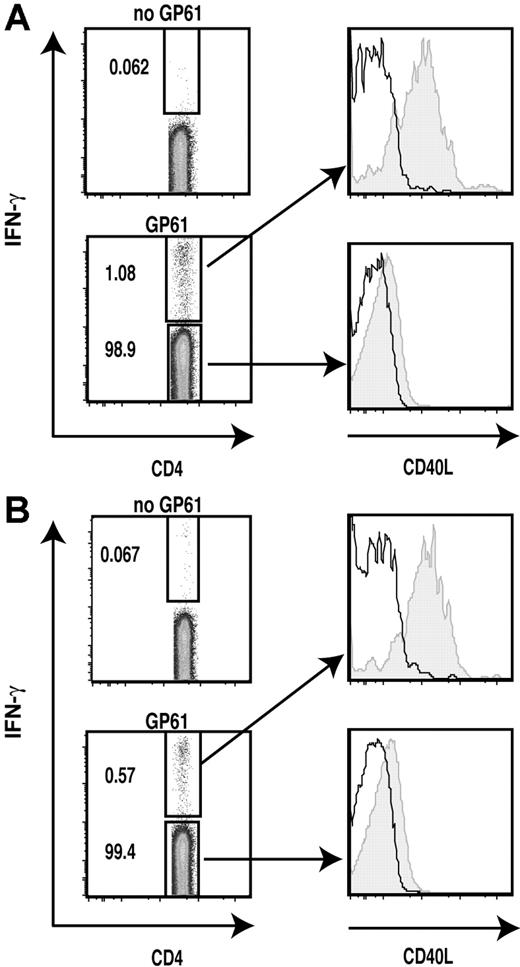
Previous studies reported that some naive T cells masquerade as memory T cells and advised caution in considering CD44high T cells from uninfected animals to be genuine memory T cells based on the expression of CD44 alone.34,35 To ask if the acquisition of preformed CD40L is a general property of genuine effector and memory CD4+ T cells during the normal immune response, we analyzed virus-specific effector and memory CD4+ T cells in the well-characterized LCMV infection model (Armstrong strain) in which the virus is cleared within 8 to 10 days and memory CD4+ cells persist in the absence of virus.36,37 We chose to examine T cells from mice 9 days and 3 to 6 months after infection to analyze effector and memory CD4+ T cells, respectively.37 We tested rapid surface expression of CD40L on virus-specific intracellular IFN-γ+ or TNF-α+ CD4+ T cells as described in “Materials and methods.” IFN-γ+ virus-specific effector cells exhibited high levels of mobilization of preformed CD40L in response to antigenic stimulation (Figure 5A). The intracellular IFN-γ− populations showed only marginal CD40L surface expression. Surprisingly, virus-specific memory CD4+ T cells examined up to 6 months after infection still displayed rapid surface CD40L expression at high levels comparable with those of effector CD4+ T cells (Figure 5B). Analysis of TNF-α+ CD4+ T cells provided very similar results to those of IFN-γ+ CD4+ T cells (data not shown). These results indicate that preformed CD40L is a shared property of effector and memory CD4+ T cells, and that preformed CD40L is not merely being degraded in lysosomes in recently activated effector T cells, but is actively maintained in CD4+ memory T cells in the absence of antigen, possibly for prompt execution of effector functions upon antigen recognition.
LCMV-specific effector and memory Th1 cells rapidly mobilize preformed CD40L upon antigenic stimulation. The surface mobilization assay was combined with intracellular cytokine staining of LCMV-infected splenocytes to detect mobilization of preformed CD40L on the cell surface of LCMV-specific effector (A) (day 9 after infection) and memory (B) (6 months after infection) Th1 cells 30 minutes after stimulation with LCMV GP61 peptide-pulsed APCs as described in “Materials and methods, Flow cytometric analysis of CD40L expression.” The levels of CD40L (shaded) and isotype control (line) for IFN-γ+ and IFN-γ− populations are shown. Numbers in plots indicate the percentage of that population among CD4+ cells in the sample. Data shown are representative of 3 independent experiments.
LCMV-specific effector and memory Th1 cells rapidly mobilize preformed CD40L upon antigenic stimulation. The surface mobilization assay was combined with intracellular cytokine staining of LCMV-infected splenocytes to detect mobilization of preformed CD40L on the cell surface of LCMV-specific effector (A) (day 9 after infection) and memory (B) (6 months after infection) Th1 cells 30 minutes after stimulation with LCMV GP61 peptide-pulsed APCs as described in “Materials and methods, Flow cytometric analysis of CD40L expression.” The levels of CD40L (shaded) and isotype control (line) for IFN-γ+ and IFN-γ− populations are shown. Numbers in plots indicate the percentage of that population among CD4+ cells in the sample. Data shown are representative of 3 independent experiments.
Discussion
CD40L is generally believed to be made de novo following antigen recognition, and to be transported to the surface by the constitutive secretory pathway. In this paper, we show that effector and memory CD4+ T cells also store preformed CD40L in SLs and mobilize it rapidly to the cell surface following antigen recognition. We show that inducible rapid surface expression of preformed CD40L is not limited to recently activated15,–17 or pathogenic18,19 T-cell subsets, but is a general property of effector and memory Th1 cells. Although further work will need to be done to establish the functional significance of rapid, stimulated surface expression of preformed CD40L, these findings suggest a previously overlooked mechanism for effector and memory CD4+ T cells to activate APCs bearing specific antigen during brief interactions that are too short to allow de novo cytokine synthesis.
How does CD40L get to lysosomal compartments to be stored as preformed CD40L? Cyster and colleagues recently reported that CD40L is made constitutively by all CD4+ T cells, including resting naive T cells, but accumulates on the cell surface only in CD40-deficient animals,9 probably owing to efficient CD40-induced internalization and destruction of CD40L.5,18 Our data from normal and CD40-deficient mice suggests the existence of 2 distinct pools of intracellular CD40L in resting CD4+ T cells: one is sorted to the cell surface by constitutive secretion and is used by all CD4+ T cells,9 and the other appears on the cell surface in response to external stimulation and is used by effector/memory but not naive CD4+ T cells. Entry of CD40L into the secretory compartment in effector/memory cells does not depend on CD40-induced internalization, because we observed increased intracellular CD40L and surface mobilization of intracellular, preformed CD40L in effector/memory CD4+ T cells, even when the T cells were obtained from CD40-deficient animals (Figure 4). CD40L has a very short 22–amino acid cytoplasmic domain without any recognizable sorting motifs.1 It has been suggested that the polar residues within the transmembrane domain of CD40L determine its lysosomal distribution, although this has not been verified experimentally.38 It is also possible that external ligands for CD40L other than CD4039 could direct CD40L to SLs.
Levels of preformed, mobilizable CD40L were higher in virus-specific effector CD4+ T cells and in memory CD4+ T cells examined up to 6 months after infection than in effectory/memory phenotype cells from unmanipulated mice, indicating the existence of a TCR-independent mechanism to regulate the levels of preformed CD40L (Figure 5). Previously, we demonstrated that cytokine (IL-12 plus IL-18)–induced CD40L expression on LCMV-specific effector CD8+ T cells is delayed and sustained compared with TCR triggering over at least 12 hours,32 and others have shown that IL-12 prolongs the expression of CD40L in CD4+ T cells for more than 24 hours following TCR triggering.4 It is possible that IL-12 and IL-18 maintain the production of preformed CD40L during the acute phase of inflammation. In addition, IL-7 is a known survival factor for memory CD4+ T cells,40 and induces CD40L expression on CD4+ T cells.41 Therefore, we speculate that IL-7 might not only provide the survival signal but also contribute to maintaining intracellular CD40L levels in memory CD4+ T cells.
We found that CD40L colocalizes with FasL more strongly than with CTLA-4 (Figure 3), although both FasL and CTLA-4 are reported to exist in SLs.24,,–27 Incubation of unstimulated T cells with antibodies at 37°C for 30 minutes labeled CTLA-4 and CD107a much more effectively than CD40L and FasL (Figure 1D), which may be attributed to the cycling of CTLA-4 and CD107a between the endosomal compartment and the cell surface.25,42 The lack of participation of CD40L and FasL in the endosomal recycling compartment may explain the weaker colocalization between CD40L and CTLA-4 that we describe here. Alternatively, this difference may reflect heterogeneity among SLs. Although localization in different compartments may allow surface expression of FasL and CD40L to be regulated independently from CTLA-4, it is possible that effector molecules localized in lysosomes and endosomes eventually use a common exit strategy to get to the plasma membrane.43
We speculate that directional secretion of preformed and newly synthesized CD40L may be regulated independently. Pioneering work by Kupfer suggested that polarization of the Golgi apparatus toward the APC mediates polarized secretion of cytokines.44 Recently, Davis's group extended these findings by showing that some newly synthesized cytokines (IFN-γ and IL-2) are secreted toward the APCs, while others (CCL-3 and TNF-α) are secreted in all directions, perhaps owing to differential association of secretory vesicles with Rab GTPases.45 Interestingly, they observed TNF-α staining at the immunologic synapse 30 minutes after T-cell activation, although it was released in all directions 2 hours after stimulation, suggesting that the spatial distribution of preformed and newly synthesized TNF-αs are different.45 Our preliminary data with live-cell imaging of T cells transfected with fluorescent protein-tagged CD40L suggests that preformed CD40L is polarized toward the APCs upon antigen recognition, as previously reported for FasL24 and CTLA-427 (Y.K. and D.C.P., unpublished observation June 2005). It remains to be determined whether newly synthesized CD40L is secreted toward the APCs or in all directions as described for TNF-α.45,–47
Very little is known about the functions of preformed CD40L. Two studies addressed this issue using activated, fixed effector CD4+ T cells in conditions under which T cells expressed surface CD40L from preformed stores but not by de novo synthesis. They showed that these fixed cells could activate DCs to produce IL-12p40 or enhance survival, but could not induce proliferation of B cells.18,19 These studies suggest that the amount of surface CD40L provided from preformed stores can be sufficient to activate APCs. We and others have shown that mobilization of CD40L is already detectable at 5 minutes after stimulation, reaches a maximum at 15 minutes, and remains at the same level until 30 minutes after stimulation. From 30 minutes after stimulation, further increases in CD40L expression become dependent on de novo synthesis (Figure 1E).15 Intravital 2 photon microscopy of T cell/B cell interactions showed that cognate T cell/B cell interactions range from 8 to 40 minutes in lymph nodes.48,49 In addition, several groups have reported that transient interactions may dominate in vivo, both during the initial encounters between naive T cells and antigen-bearing DCs, and again after T-cell division begins on the second day after antigenic stimulation.50,–52 Therefore, it is possible that most interactions between CD4+ T cells and B cells or DCs in vivo may be too short to allow for de novo synthesis of CD40L but long enough to permit delivery of CD40L from preformed stores. Preformed CD40L might convert transient interactions into longer interactions by inducing cytoskeletal reorganization within DCs.53 Preformed CD40L could be particularly important in ensuring that rare interactions between memory CD4+ T cells and antigen-bearing APCs are productive during initiation of a secondary immune response.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Aurelie Snyder and the Oregon Health and Science University Molecular Microbiology and Immunology Microscopy Core Facility for expert technical assistance. We thank Hans-Peter Raué, Ian J. Amanna, and Carol Beadling for their assistance with LCMV infection of mice. We thank Cortny A. Williams for her assistance with the in vivo priming of effector CD4+ T cells. We also thank Susan E. Murray for critical review of the manuscript.
This work was supported by National Institutes of Health (NIH) grants AI29544 (D.C.P.), AI070934 (D.C.P.), AI054458 (M.K.S.), and AI051346 (M.K.S.), and Oregon National Primate Research Center grant RR000163 (M.K.S.).
National Institutes of Health
Authorship
Contribution: Y.K., T.J.T., M.K.S., and D.C.P. designed research; Y.K. performed research; Y.K. and D.C.P. analyzed data; Y.K. and D.C.P. wrote the paper; and all authors checked the final version of manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David C. Parker, Department of Molecular Microbiology and Immunology, Oregon Health and Science University, 3181 SW Sam Jackson Park Rd, L220, Portland, OR 97239; e-mail:parkerd@ohsu.edu.