Suppressor of cytokine signaling (SOCS) proteins regulate the intensity and duration of cytokine responses. SOCS3 is expressed in peripheral T cells, and recent reports have suggested that overexpression of SOCS3 modulates antigen- and/or costimulation-induced T-cell activation. To study the role of SOCS3 in the regulation of T-cell activation, we used a conditional gene-targeting strategy to generate mice that lack SOCS3 in T/natural killer T cells (Socs3ΔLck/ΔLck mice). SOCS3-deficient CD8 T cells showed greater proliferation than wild-type cells in response to T-cell receptor (TCR) ligation despite normal activation of signaling pathways downstream from TCR or CD28 receptors. Signaling in response to the gp130 cytokines interleukin (IL)–6 and IL-27 was prolonged in Socs3ΔLck/ΔLck T cells, and T cells from gp130Y757F/Y757F mice, in which the SOCS3-binding site on gp130 is ablated, showed a striking similarity to SOCS3-deficient CD8 T cells. Although the proliferative defect of Socs3ΔLck/ΔLck T cells was not rescued in the absence of IL-6, suppression of IL-27 signaling was found to substantially reduce anti-CD3–induced proliferation. We conclude that enhanced responses to TCR ligation by SOCS3-deficient CD8 T cells are not caused by aberrant TCR-signaling pathways but, rather, that increased IL-27 signaling drives unregulated proliferation in the absence of SOCS3.
Introduction
Cytokines are key regulators of many biologic processes including the development and homeostasis of T lymphocytes. Tight control of cytokine responses is critical for proper regulation of T-cell behavior, because alterations in levels of cytokines such as interleukin (IL)–2, IL-4, IL-7, and IL-15 affect T-cell function and, in some instances, cause immunodeficiency or autoimmunity in vivo.1,,–4
Suppressor of cytokine signaling (SOCS) proteins comprise a family of intracellular regulators of cytokine-induced signal transduction.5 SOCS protein expression is inducible by cytokine, and once expressed, SOCS proteins inhibit cytokine signaling by switching off the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway.5 SOCS expression has been observed at many stages of T-cell development and function, and it has been suggested that SOCS1 and SOCS3 are important regulators of T-cell activation and homeostasis.6,,,–10 SOCS1 is highly expressed in thymocytes, where it modulates IL-7 induced signaling and CD8+ T-cell differentiation.8 SOCS3 is expressed in naive T cells and is thought to be important for controlling T-cell activation and maintaining T cells in a quiescent state.7 Studies in vitro have suggested that both SOCS1 and SOCS3 influence T-cell receptor (TCR)–induced signaling via direct interaction with downstream signaling molecules,11,–13 which further highlights the potential importance of SOCS in modulating T-cell function.
The role of SOCS proteins in vivo has been studied using mice that lack individual Socs genes. Socs1−/− mice die as neonates as a result of a severe inflammatory disorder that arises from an inability to appropriately regulate interferon-γ signals in the absence of SOCS1.14,–16 Analyses of Socs1−/−Ifng−/− mice, which are relatively healthy and survive to adulthood, revealed that the immune system is perturbed in young adults.9,17 The ratio of CD4/CD8 T cells is skewed toward CD8+ T cells, with a majority in the periphery having a CD44hi memory-like phenotype.9,17 This expansion of CD8+ memory T cells resembles the phenotypes of IL-7 or IL-15 transgenic (Tg) mice3,18 and is probably caused by increased sensitivity to these and other common γ-chain–dependent cytokines in situations of SOCS1 deficiency.17,19 Indeed, a recent study showed that SOCS1 is critical for regulating IL-15–induced homeostatic proliferation of naive CD8+ T cells in vivo.20 IL-15 induces the activation and proliferation of naive CD8+ T cells that receive survival signals from self-ligands, and these responses are exaggerated in SOCS1-deficient mice.20 Hypersensitivity to IL-15, therefore, is likely to induce the generation and expansion of memory-like CD8+ T cells in these mice.
SOCS3 involvement in T-cell function has been studied in mice that overexpressed SOCS3 in the T-cell compartment. Using SOCS3-Tg mice, SOCS3 was shown to inhibit costimulatory signals mediated through the CD28 receptor, possibly by direct interaction with CD28.21 SOCS3-overexpressing T cells were less responsive than wild-type (wt) cells to anti-CD28–mediated stimulation and produced less IL-2 in response to this signal.21 Ectopic SOCS3 expression in primary murine T cells has also been shown to inhibit IL-2 production, although the site of SOCS3 inhibition in this study was found to be calcineurin, a critical mediator of calcium signaling activated by the TCR, and not CD28.13
Investigation of the role of SOCS3 in vivo by loss-of-function models has been impeded by the embryonic lethality of widespread Socs3 deletion.22,23 Recent studies of mice with conditional deletion of Socs3 in lymphoid cells have indicated roles for SOCS3 in the regulation of CD4+ T-helper (Th) cell differentiation.24,25 Th1 differentiation was found to occur normally in the absence of SOCS3 in both studies. Although no abnormality in Th2 differentiation was observed by Chen et al,24 decreased Th2 immune responses, seen in a Kinjyo et al25 study, were attributed to an increased production of the Th3 cytokines IL-10 and transforming growth factor-β1.25 In contrast, Chen et al24 found SOCS3 to be an important regulator of IL-23 signaling and Th17 generation. The ability of IL-6 to induce Th17 differentiation was augmented in the absence of SOCS3, which suggests that SOCS3 regulates IL-6 signaling.26 The effect of SOCS3 deletion on CD8+ T-cell function, however, has not been examined.
Other studies in which SOCS3 was deleted in the liver or macrophages have also shown SOCS3 to be a critical regulator of IL-6 signals.27,–29 On a molecular level, this has been attributed to the binding of SOCS3 to phosphorylated Tyr757 (Y757) of the gp130 receptor chain, thereby bringing SOCS3 into proximity to and then inhibiting receptor-associated JAKs.30,31
The association of SOCS3 with gp130, the common receptor subunit for the IL-6 family of cytokines, suggests that SOCS3 may regulate activities of other members of this family. Indeed, SOCS3-mediated regulation of leukemia inhibitory factor signaling has been shown to be critical for regulating trophoblast differentiation in SOCS3-deficient embryos.32,33 It is unknown, however, whether SOCS3 regulates signals from other ligands that also trigger heterodimerization of gp130. One of these gp130 ligands is IL-27, a newly described member of the IL-6 family and a potent immunomodulator.34,35
Initially characterized as a proinflammatory cytokine that contributes to Th1 development, IL-27 was shown recently to suppress IL-2 production and the development of Th17 cells.26,36,,–39 IL-27 induces SOCS3 expression,37,38,40 and although it has been proposed that SOCS3 mediates these inhibitory effects of IL-27,36,40 studies with SOCS3-deficient T cells have shown this not to be the case.26,37
To clarify and investigate the role of SOCS3 in T-cell homeostasis and function in vivo, we used the Cre recombinase-LoxP system to generate mice that lack a functional Socs3 gene specifically within the thymus/T/natural killer T (NKT) cell compartment. Socs3ΔLck/ΔLck mice seem to have normal T-cell development in the thymus. After TCR activation in vitro, SOCS3-deficient T cells show enhanced proliferation, although TCR-induced signaling is unaffected. Using T cells from mice that carry a mutant gp130 receptor unable to bind SOCS3, we show that deregulated gp130 signaling leads to T-cell proliferative defects similar to those in SOCS3-deficient T cells, which suggests that gp130 cytokines may be mediating this phenotype. In addition, we find that unregulated IL-6 and IL-27 signaling enhances T-cell proliferation of SOCS3-deficient CD8+ T cells in response to TCR ligation, which indicates that SOCS3 is an important regulator of these gp130 cytokines in T cells.
Materials and methods
Mice
Mice containing a loxP-flanked (floxed) Socs3 allele on a C57Bl/6 background have been described previously.27 Cre recombinase was expressed under the control of a Tg Lck promoter.41 Mice expressing Cre were bred with mice heterozygous for the Socs3-null allele and then were mated with Socs3fl/fl mice to produce progeny containing one floxed (fl) or Cre-excised allele (Δ) and either a wt (+) or null (−) Socs3 allele. Mice containing 2 Cre-excised alleles (Socs3ΔLck/ΔLck) were then generated. Mice homozygous for the gp130(Y757F) knock-in mutation (gp130Y757F/Y757F mice) on a C57BL/6 background have been described previously.42 Socs3ΔLck/ΔLck mice were generated on an IL-6–deficient background by mating with Il-6−/− mice.43 Mice were used for experiments at 8 to 14 weeks of age. All mouse studies were approved by the St Vincent's Health Animal Ethics Committee.
To test the specificity of Socs3 excision, cells from Socs3+/ΔLck mice were stained with antibodies specific for granulocytes (Gr1), B cells (B220), erythrocytes (Ter119), macrophages (Mac-1), T cells (TCR-β), and NK cells (NK1.1) (BD Pharmingen, San Diego, CA), and subsets were separated using a MoFlo (DakoCytomation, Carpinteria, CA) fluorescence-activated cell sorter (FACS). Polymerase chain reaction (PCR) genotyping was carried out on genomic DNA from each cell subset using primers amplifying the wt, floxed, and excised Socs3 alleles.27
T-cell purification
Single-cell suspensions were prepared from pooled lymph node (LN) cells from C57BL/6 wt or Socs3ΔLck/ΔLck mice. Total T cells or CD8+ T cells were purified using either T-cell or CD8+ subset enrichment columns, respectively (R&D Systems, Minneapolis, MN), which enrich T-cell populations by negative selection of B cell, myeloid, dendritic, and other non–T-cell lineages. Naive CD8+ T cells were purified by labeling LN T cells with antibodies specific for CD8 and CD44 and sorting CD8+ CD44lo cells using a FACS Aria (BD Pharmingen).
Gene-expression analysis
Quantitative reverse transcription PCR.
T cells were either untreated or incubated with 200 ng/mL recombinant murine IL-2 (R&D Systems) for 1 hour at 37°C. Socs3 and porphobilinogen deaminase (Pbgd) messenger RNA expression levels were quantitated by real-time PCR analysis using the primers 5′-TGAGCGTCAAGACCCAGTCG-3′ (forward) and 5′-CACAGTCGAAGCGGGGAACT-3′ (reverse) (Socs3) and 5′-CCTGGTTGTTCACTCCCTGA-3′ (forward) and 5′-CAACAGCATCACAAGGGTTTT-3′ (reverse) (Pbgd) for 45 cycles at 94°C for 15 seconds, 65°C (Socs3) or 60°C (Pbgd) for 20 seconds, and 72°C for 15 seconds. Expression of Socs3 was quantified by comparison with the expression of the single-copy housekeeping gene, Pbgd. Il-2 expression, normalized against 18S, was measured in purified T cells stimulated with 5 μg/mL anti-CD3 for 24 hours using the primers 5′-TCAATTGGAAGATGCTGAGA-3′ (forward) and 5′-GAAGGCTATCCATCTCCTCA-3′ (reverse) (Il-2) and 5′-GTAACCCGTTGAACCCCATT-3′ (forward) and 5′-CCATCCAATCGGTAGTAGCG-3′ (reverse) (18S) for 40 cycles at 94°C for 20 seconds, 62°C for 30 seconds, and 72°C for 30 seconds after an initial denaturation step at 95°C for 10 minutes.
Reverse transcription PCR.
Il-6 and Il-27p28 expression in purified T cells was determined by reverse transcription (RT)–PCR using primer kits (R&D Systems). Hprt (housekeeping gene) expression was measured by PCR amplification using the primers 5′-AAGCTTGCTGGTGAAAAGGA-3′ (forward) and 5′-CAAGGGCATATCCAACAACA-3′ (reverse) for 35 cycles at 94°C for 45 seconds, 55°C for 45 seconds, and 72°C for 45 seconds.
Carboxyfluorescein diacetate succinimidyl ester proliferation assays
Purified T cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR), as described previously9 and plated into 96-well plates at 5 × 105 cells per mL in T-cell medium (RPMI 1640 containing 10% [vol/vol] heat-inactivated fetal calf serum [Sigma-Aldrich, St Louis, MO], 1 mM l-glutamine [Gibco, Grand Island, NY] and 50 μM 2-mercaptoethanol [Sigma-Aldrich]). Plates were coated with purified anti-CD3 (clone KT3-1-1) or anti-CD28 (clone 37.51, a kind gift from Prof James Allison, University of California, Berkeley, CA44 ) antibodies by incubation overnight at 4°C before addition of cells. Cells were harvested after 72 hours of culture, stained, and analyzed by flow cytometry. For certain experiments, cells were stimulated for 3 days with 2.5 μg/mL anti-CD3 in the presence of 5 μg/mL murine WSX-1(IL-27R)-murineFc (Amgen Inc, Thousand Oaks, CA) to inhibit IL-27 signaling or 20 μg/mL neutralizing anti-mIL-2 antibody (clone JES6-1A12). The division index (the average number of divisions that a cell in the starting population has undergone) was calculated using FlowJo software (Tree Star, Ashland, OR).
Immunoblotting
Purified LN T cells (1.5 × 106 per sample) were stimulated for the indicated times with hamster antimouse CD3 (1 μg/mL; clone 2C11) and antihamster IgG (1 μg/mL; BD Pharmingen) or cytokine (20 ng/mL IL-2, 10 ng/mL IL-6, or 50 ng/mL IL-27; R&D Systems) in T-cell medium at 37°C. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of total cell lysates and immunoblots using antibodies specific for phospho–extracellular signal-regulated kinase (ERK), phospho–Jun N-terminal kinase (JNK), or phospho-STAT1 (all from Cell Signaling Technologies, Beverly, MA), nuclear factor of activated T cells (NFATp), ERK or STAT1 (Santa Cruz Biotechnology, Santa Cruz, CA), phospho-STAT5a/b (Upstate Biotechnology, Lake Placid, NY), or STAT5a/b (Zymed Laboratories, South San Francisco, CA) were performed essentially as described previously.45
Intracellular FACS analysis
Measurement of IL-2 production.
Purified T cells (2.5 × 105 per well) were stimulated in 96-well plates either uncoated or coated with anti-CD3 (10 μg/mL) with or without anti-CD28 (5 μg/mL) for 20 hours at 37°C. Brefeldin A (10 μg/mL; Sigma-Aldrich) was added to the cultures for the final 15 hours. Cells were harvested, fixed, and permeabilized using the Cytofix/Cytoperm kit (BD Pharmingen) and stained using antibodies specific for CD8 and IL-2 (BD Pharmingen) before analysis by flow cytometry.
Cytokine-induced STAT activation.
Unfractionated splenocytes were stimulated at 37°C in T-cell medium that contained recombinant mouse IL-6 (10 ng/mL; a kind gift from Dr Richard Simpson, Ludwig Institute for Cancer Research, Melbourne, Australia) or recombinant human IL-27 (50 ng/mL; R&D Systems) with or without murine WSX-1(IL-27R)-murineFc (5 μg/mL; Amgen Inc) fixed in 1% buffered formalin followed by permeabilization in ice-cold 90% methanol. Cells then were stained with antibodies specific for CD8 and phospho-STAT1 or phospho-STAT3 (PhosFlow; BD Pharmingen) and analyzed by flow cytometry.
IL-2 enzyme-linked immunosorbent assay
Purified T cells were plated at 5 × 105 cells per mL in T-cell medium into 96-well plates precoated with 10 μg/mL anti-CD3. Supernatants were harvested after a 24-hour culture, and IL-2 was measured using the Quantikine enzyme-linked immunosorbent assay kit (R&D Systems) according to manufacturer instructions.
Statistical analyses
Data from in vitro assays were analyzed using a Student 2-tailed t test for independent events.
Results
Generation of mice that lack SOCS3 in T cells
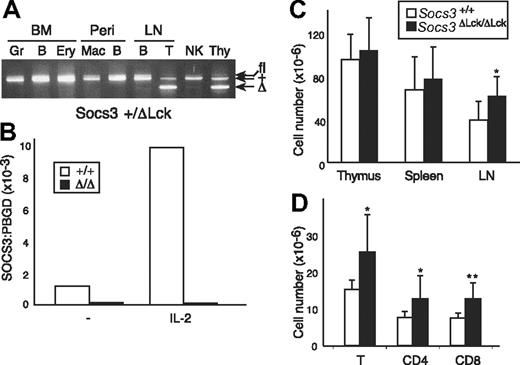
To overcome embryonic lethality associated with genome-wide loss of SOCS3 expression and assess the contribution of SOCS3 in the T-cell compartment, we crossed mice that were homozygous for the conditional allele of Socs3 (Socs3fl/fl) with mice that carried a Tg Cre recombinase under the control of an Lck promoter. Cre expression in Lck-Cre mice is activated early in thymopoiesis, and excision of Socs3fl in the resulting Socs3ΔLck /ΔLck mice is expected to occur specifically within T and NKT cells, which share a common thymocyte precursor.8,41 The conditional allele was able to functionally compensate for the loss of wt Socs3 during embryonic development, because Socs3fl/fl and Socs3ΔLck /ΔLck mice were viable and healthy. Socs3ΔLck/ΔLck mice were born in the expected Mendelian ratio and showed no signs of illness or obvious pathologic abnormalities to at least 9 months of age (data not shown). To test for the efficiency and specificity of the Cre-mediated excision reaction, cell suspensions from the major lymphoid organs were sorted by fluorescence-activated cell sorting into individual cell subsets, and PCR genotyping of the Socs3 locus was performed (Figure 1A and data not shown). As expected, excision of the Socs3 allele occurred only in thymocytes and peripheral T cells, whereas granulocytes, macrophages, B cells, NK cells, and erythroid cells from the same mice all contained an intact conditional allele. Real-time PCR confirmed the absence of Socs3 gene expression in LN T cells from Socs3ΔLck/ΔLck mice, and IL-2 was unable to induce Socs3 expression in these cells (Figure 1B). The expression level of SOCS1, the most closely related SOCS family member, was unchanged in SOCS3-deficient T cells compared with wt T cells (data not shown).
Verification of Socs3fl deletion in T cells. (A) Cells expressing lineage-specific markers were purified from Socs3+/ΔLck bone marrow (BM), peritoneal cavity (Peri), LN, or spleen. Genomic DNA was genotyped at the Socs3 locus by PCR. The bands corresponding to the different Socs3 alleles are indicated. Gr indicates granulocytes; B, B cells; Ery, erythroid cells; Mac, macrophages; T, T cells; Thy, thymocytes. (B) SOCS3 expression in purified T cells from wt (open bars) or Socs3ΔLck/ΔLck (filled bars) mice, either unstimulated or after IL-2 stimulation for 1 hour, was analyzed by real-time, quantitative RT PCR. Expression of SOCS3 is presented as arbitrary units standardized against expression of the control housekeeping gene, Pbgd. (C) Thymocytes, splenocytes, and LN cells were harvested and counted from 8- to 12-week-old wt or Socs3ΔLck/ΔLck mice (n = 5-8). (D) Cells were pooled from inguinal, submandibular, brachial, axillary, and mesenteric LN cells from wt or Socs3ΔLck/ΔLck mice and counted, and the lymphocyte subsets were analyzed by flow cytometry. Values shown in C and D are means (± SD) of values from 6 to 9 mice. *P < .05; **P < .01.
Verification of Socs3fl deletion in T cells. (A) Cells expressing lineage-specific markers were purified from Socs3+/ΔLck bone marrow (BM), peritoneal cavity (Peri), LN, or spleen. Genomic DNA was genotyped at the Socs3 locus by PCR. The bands corresponding to the different Socs3 alleles are indicated. Gr indicates granulocytes; B, B cells; Ery, erythroid cells; Mac, macrophages; T, T cells; Thy, thymocytes. (B) SOCS3 expression in purified T cells from wt (open bars) or Socs3ΔLck/ΔLck (filled bars) mice, either unstimulated or after IL-2 stimulation for 1 hour, was analyzed by real-time, quantitative RT PCR. Expression of SOCS3 is presented as arbitrary units standardized against expression of the control housekeeping gene, Pbgd. (C) Thymocytes, splenocytes, and LN cells were harvested and counted from 8- to 12-week-old wt or Socs3ΔLck/ΔLck mice (n = 5-8). (D) Cells were pooled from inguinal, submandibular, brachial, axillary, and mesenteric LN cells from wt or Socs3ΔLck/ΔLck mice and counted, and the lymphocyte subsets were analyzed by flow cytometry. Values shown in C and D are means (± SD) of values from 6 to 9 mice. *P < .05; **P < .01.
Lymphoid cellularity and composition of thymus, spleen, and LN from age- and sex-matched Socs3+/+ and Socs3ΔLck/ΔLck mice were compared to determine whether T-cell development and homeostasis were altered in the absence of SOCS3. No significant differences were found in cellularity in the thymus or spleen (Figure 1C). LNs from Socs3ΔLck/ΔLck mice were slightly enlarged, which reflected an increase in the number of both CD4+ and CD8+ T cells (Figure 1C,D). In contrast to SOCS1-deficient T cells,9 there were no differences in the proportions of CD4+ and CD8+ T cells, and SOCS3-deficient T cells did not appear to be abnormally activated, because expression of the activation markers CD25, CD69, and CD44 was indistinguishable from that of wt mice (data not shown).
Increased responses to TCR engagement by Socs3ΔLck/ΔLck T cells
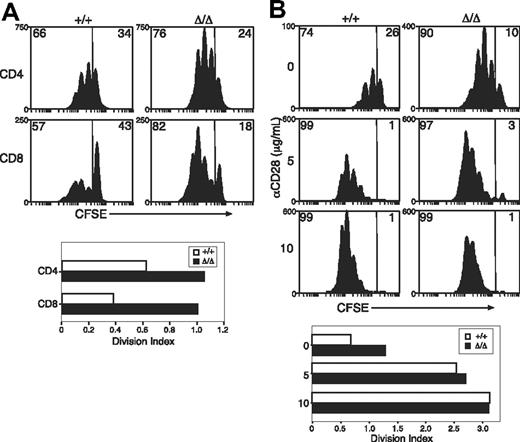
Previous reports have suggested that SOCS3 regulates TCR- or CD28-mediated activation of T cells.13,21 To investigate T-cell responses in the absence of SOCS3, purified T cells from LNs of Socs3+/+ or Socs3ΔLck/ΔLck mice were labeled with the cytoplasmic dye CFSE, and proliferation was measured in response to anti-CD3. As shown in Figure 2A, anti-CD3–induced proliferation was greatly enhanced in SOCS3-deficient CD8+ T cells. Minor increases were seen in the proliferation of SOCS3-deficient CD4+ T cells (Figure 2A). The enhanced proliferation seemed to result from an increase in the number of cells that were mobilized to enter division combined with an increase in the average number of divisions completed by dividing cells. SOCS3-deficient and wt CD8+ T cells showed a similar dose-dependent increase in proliferation in response to anti-CD3 plus anti-CD28 (Figure 2B), which suggests that there was no increase in sensitivity to CD28-mediated costimulation.
Increased anti-CD3–induced proliferation of SOCS3-deficient CD8+ T cells. (A) CFSE-labeled T cells were purified from wt (+/+) or Socs3ΔLck/ΔLck (Δ/Δ) LN cells and stimulated with 1.25 μg/mL anti-CD3 for 3 days. (B) Purified CD8+ T cells were labeled with CFSE and stimulated with 5 μg/mL anti-CD3+ at the indicated concentrations of anti-CD28 for 3 days. The division index shows the average number of cell divisions in each culture. Data are representative of 2 to 3 independent experiments.
Increased anti-CD3–induced proliferation of SOCS3-deficient CD8+ T cells. (A) CFSE-labeled T cells were purified from wt (+/+) or Socs3ΔLck/ΔLck (Δ/Δ) LN cells and stimulated with 1.25 μg/mL anti-CD3 for 3 days. (B) Purified CD8+ T cells were labeled with CFSE and stimulated with 5 μg/mL anti-CD3+ at the indicated concentrations of anti-CD28 for 3 days. The division index shows the average number of cell divisions in each culture. Data are representative of 2 to 3 independent experiments.
TCR- and CD28-signaling pathways are intact in Socs3ΔLck/ΔLck T cells
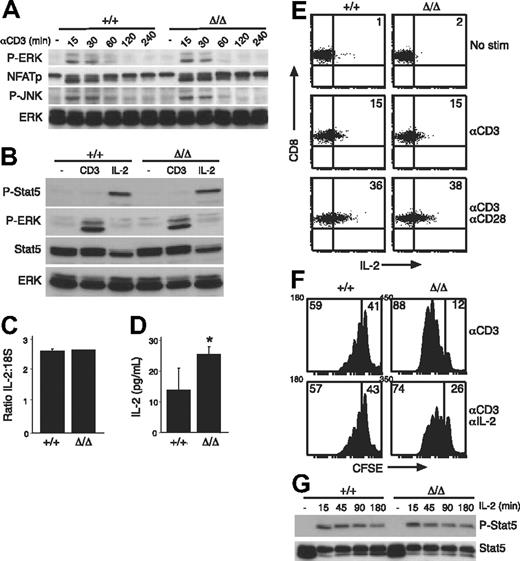
Previous studies have implicated SOCS3 in the regulation of TCR- or CD28-signaling pathways by inhibition of calcineurin or CD28, respectively.13,21 Our results suggest that there was no perturbation in CD28 signaling in the absence of SOCS3 (Figure 2B). Rather, Socs3ΔLck/ΔLck T cells responded abnormally to TCR engagement alone (Figure 2A), which could result from unregulated signaling through the TCR in the absence of SOCS3. Therefore, we examined the activation of critical mediators involved in TCR signaling. For each parameter we examined, there was no difference in either the intensity or the duration of the response between wt and SOCS3-deficient T cells (Figure 3A,B and data not shown). Anti-CD3–induced dephosphorylation of nuclear factor of activated T cells, and phosphorylation of ERK and Jun N-terminal kinase were all similar in wt and Socs3ΔLck/ΔLck T cells (Figure 3A). Furthermore, there was no difference in the up-regulation of the activation markers CD25, CD69, and CD44 over a 20-hour time course in CD4+ or CD8+ cells in response to either anti-CD3 or anti-CD3 plus anti-CD28 stimulation (data not shown). We also found no evidence of STAT5 activation in response to anti-CD3 stimulation (Figure 3B), which discounts the possibility that SOCS3 may be regulating JAK/STAT activation induced directly by anti-CD3 stimulation.46
TCR-signaling pathways are intact in SOCS3-deficient T cells. (A) Total LN T cells from wt (+/+) or Socs3ΔLck/ΔLck (Δ/Δ) mice were stimulated with 10 μg/mL anti-CD3 for the indicated times and immunoblotted with antibodies specific for the indicated proteins. An antibody specific for ERK was used as a loading control. (B) LN T cells were either unstimulated or stimulated with 10 ng/mL IL-2 or anti-CD3 and immunoblotted with the indicated antibodies. Antibodies specific for ERK and STAT5 were used as loading controls. LN T cells were stimulated for 24 hours with anti-CD3, and the expression of IL-2 was measured by quantitative RT-PCR (C) and enzyme-linked immunosorbent assay (D). (E) CD8+ T cells were stimulated for 20 hours with anti-CD3 with or without anti-CD28 and analyzed by an intracellular FACS for IL-2 production. Numbers in quadrants refer to the percentage of cells that fall within that quadrant. (F) Purified LN CD8+ T cells were CFSE labeled and cultured for 3 days with 5 μg/mL anti-CD3 either alone or in the presence of 20 μg/mL anti–IL-2 antibody. Numbers in quadrants refer to the percentage of cells that fall within that quadrant. (G) LN T cells were stimulated with IL-2 (20 ng/mL) for the indicated times, and STAT5 phosphorylation was measured by immunoblotting. Data are representative of 2 to 3 independent experiments. P-ERK indicates phospho-ERK; NFATp, nuclear factor of activated T cells; P-JNK, phospho-Jun N-terminal kinase; P-Stat5, phospho-STAT5. Values shown in C and D are means (± SD) of triplicate measurements. *P < .05.
TCR-signaling pathways are intact in SOCS3-deficient T cells. (A) Total LN T cells from wt (+/+) or Socs3ΔLck/ΔLck (Δ/Δ) mice were stimulated with 10 μg/mL anti-CD3 for the indicated times and immunoblotted with antibodies specific for the indicated proteins. An antibody specific for ERK was used as a loading control. (B) LN T cells were either unstimulated or stimulated with 10 ng/mL IL-2 or anti-CD3 and immunoblotted with the indicated antibodies. Antibodies specific for ERK and STAT5 were used as loading controls. LN T cells were stimulated for 24 hours with anti-CD3, and the expression of IL-2 was measured by quantitative RT-PCR (C) and enzyme-linked immunosorbent assay (D). (E) CD8+ T cells were stimulated for 20 hours with anti-CD3 with or without anti-CD28 and analyzed by an intracellular FACS for IL-2 production. Numbers in quadrants refer to the percentage of cells that fall within that quadrant. (F) Purified LN CD8+ T cells were CFSE labeled and cultured for 3 days with 5 μg/mL anti-CD3 either alone or in the presence of 20 μg/mL anti–IL-2 antibody. Numbers in quadrants refer to the percentage of cells that fall within that quadrant. (G) LN T cells were stimulated with IL-2 (20 ng/mL) for the indicated times, and STAT5 phosphorylation was measured by immunoblotting. Data are representative of 2 to 3 independent experiments. P-ERK indicates phospho-ERK; NFATp, nuclear factor of activated T cells; P-JNK, phospho-Jun N-terminal kinase; P-Stat5, phospho-STAT5. Values shown in C and D are means (± SD) of triplicate measurements. *P < .05.
Next, we asked whether the hyperproliferative response of Socs3ΔLck/ΔLck T cells to anti-CD3 stimulation was a result of increased production or sensitivity to IL-2, because SOCS3 has been shown to inhibit IL-2–induced signaling in overexpression studies.47 Quantitative RT-PCR analysis confirmed that there was no difference in IL-2 gene transcription in response to anti-CD3 stimulation in the absence of SOCS3 (Figure 3C), but there seemed to be more IL-2 in cultures that contained SOCS3-deficient cells, presumably because of the increased number of cells in these cultures (Figure 3D). When IL-2 production was assessed on a per-cell basis, there was no difference in either the number of IL-2–producing CD8+ T cells or the amount of IL-2 produced by each cell in response to anti-CD3 (Figure 3E). Proliferation of SOCS3-deficient CD8 T cells was still enhanced compared with wt in the presence of a neutralizing anti–IL-2 antibody (Figure 3F), which further suggests that there is an IL-2–independent mechanism by which SOCS3 regulates CD8 T-cell responses. To exclude the possibility that the CD3-induced hyperproliferation in Socs3ΔLck/ΔLck T cells was a result of increased IL-2 sensitivity, IL-2–induced STAT5 activation was examined and showed no difference between wt and SOCS3-deficient T cells (Figure 3G), which is in agreement with recent data from another SOCS3-deficient model.24
Given that the SOCS3-deficient T-cell hyperproliferation cannot be explained by heightened signaling through the TCR, we sought to examine more conventional targets of SOCS proteins. Obvious candidates would be cytokines such as γc-cytokines, which are known to influence T-cell proliferation and survival.48,49 However, analysis of STAT activation and/or cell survival in response to cytokines such as IL-2, IL-4, and IL-7 showed no deviations between wt and SOCS3-deficient T cells (Figure 3G and data not shown), which is consistent with the results of recent studies.24
T cells from gp130Y757F/Y757F mice phenocopy Socs3ΔLck/ΔLck T cells
Previous studies have shown that SOCS3 binds to phosphorylated Y757 of the gp130 receptor and inhibits gp130-dependent signal transduction.27,,,,–32 Mice engineered with the Y757F knock-in mutation, to simultaneously ablate SOCS3 and SHP2 binding to gp130, are essentially devoid of SOCS3 activity with respect to gp130 cytokines, particularly those that use gp130 homodimers.50 Accordingly, although SOCS3 is produced in cells from these mice,42 it is unable to down-regulate signals from gp130 cytokines because the SOCS3 docking site on gp130 is absent.30,31 Thus, with respect to gp130 cytokines, gp130Y757F/Y757F T cells are functionally SOCS3 deficient.
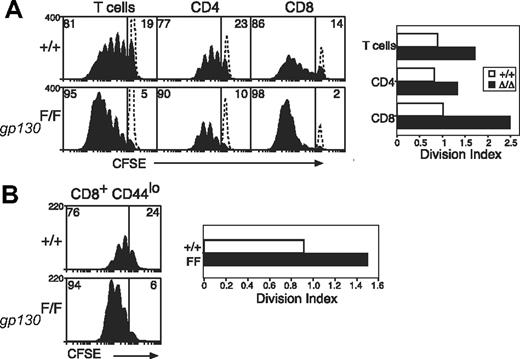
It has been shown that IL-6 induces hyperactivation of STAT1 and STAT3 in gp130Y757F/Y757F cells,26,42 and, as would be expected, T cells from gp130Y757F/Y757F mice also displayed enhanced STAT activation in response to IL-27 (data not shown). To test whether altered gp130 cytokine sensitivity can augment TCR responses, we examined the response of gp130Y757F/Y757F T cells to TCR stimulation and found that these cells behaved in a similar manner to that of SOCS3-deficient T cells. Thus, although TCR-signaling pathways are unaltered in these cells, T cells from gp130Y757F/Y757F mice were hyperproliferative in response to anti-CD3, and similar to Socs3ΔLck/ΔLck mice, this effect was most prominent in CD8+ T cells (Figure 4A).
Increased anti-CD3–induced proliferation of gp130F757/F757 T cells. (A) Purified, CFSE-labeled wt (+/+) or gp130F757/F757 (gp130F/F) LN T cells were stimulated for 3 days with anti-CD3 (10 μg/mL) and analyzed by flow cytometry. The profile of unstimulated, CFSE-labeled cells cultured for 3 days is indicated by the dotted lines. (B) Naive CD44lo CD8+ LN T cells were purified by fluorescence-activated cell sorting and cultured as described for panel A. The division index shows the average number of cell divisions in each culture. Data are representative of 3 to 4 independent experiments.
Increased anti-CD3–induced proliferation of gp130F757/F757 T cells. (A) Purified, CFSE-labeled wt (+/+) or gp130F757/F757 (gp130F/F) LN T cells were stimulated for 3 days with anti-CD3 (10 μg/mL) and analyzed by flow cytometry. The profile of unstimulated, CFSE-labeled cells cultured for 3 days is indicated by the dotted lines. (B) Naive CD44lo CD8+ LN T cells were purified by fluorescence-activated cell sorting and cultured as described for panel A. The division index shows the average number of cell divisions in each culture. Data are representative of 3 to 4 independent experiments.
It was shown previously that T cells expressing a mutant gp130 receptor unable to bind SOCS3 acquire an activated phenotype.51 Similarly, gp130Y757F/Y757F T cells were often more activated than T cells from wt controls and expressed higher levels of the activation marker CD44 (data not shown). To assess whether increased initial T-cell activation contributed to hyperactivation in response to TCR ligation, we sorted naive CD44lo CD8+ T cells and measured the proliferation of this subset individually. As shown in Figure 4B, naive CD8+ T cells from gp130Y757F/Y757F mice were still hyperproliferative in response to TCR stimulation, which confirms the importance of gp130 cytokines in these responses.
Socs3ΔLck/ΔLck T cells are hypersensitive to gp130 cytokines
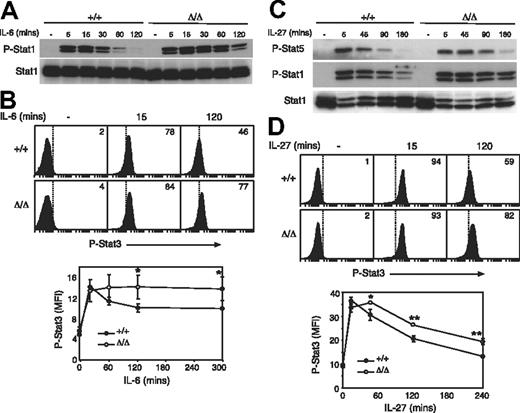
Several cytokines signal through the cytokine receptor subunit gp130. Considering that only IL-6 and IL-27 have appreciable effects on T cells, we chose to study responses to these cytokines in greater detail. Although better characterized for its roles in B-cell maturation and acute-phase protein production in the liver, IL-6 has also been shown to provide costimulatory survival signals to T cells and to be important in initiating T-cell proliferation, IL-2 secretion and responsiveness, and T-cell survival via STAT3.52,,–55 We examined IL-6–induced STAT activation in Socs3ΔLck/ΔLck T cells and found that, similar to other cell lineages, SOCS3-deficient T cells showed enhanced responses to IL-6. There was no difference in the intensity of IL-6–induced STAT1 or STAT3 tyrosine phosphorylation in Socs3ΔLck/ΔLck T cells relative to wt, but the duration of signaling was substantially prolonged in Socs3ΔLck/ΔLck T cells (Figure 5A,B).
SOCS3-deficient T cells are hyperresponsive to gp130 cytokines. Purified LN T cells were stimulated with IL-6 (A) or IL-27 (C) for the indicated times, and STAT activation was measured by immunoblotting with antibodies specific for phosphorylated STAT (P-Stat) proteins, as indicated. Antibodies specific for total STAT were used as loading controls. Activation of STAT3 in response to IL-6 (B) and IL-27 (D) was assayed by intracellular flow cytometry in CD8+ T cells. The mean fluorescence intensity (MFI) of phospho-STAT3 staining is shown below. Each data point represents mean (± SD) of values from 3 mice of each genotype. ■ indicates wt cells; □, Socs3ΔLck/ΔLck cells. Data are representative of 2 to 3 independent experiments. *P < .05; **P < .001.
SOCS3-deficient T cells are hyperresponsive to gp130 cytokines. Purified LN T cells were stimulated with IL-6 (A) or IL-27 (C) for the indicated times, and STAT activation was measured by immunoblotting with antibodies specific for phosphorylated STAT (P-Stat) proteins, as indicated. Antibodies specific for total STAT were used as loading controls. Activation of STAT3 in response to IL-6 (B) and IL-27 (D) was assayed by intracellular flow cytometry in CD8+ T cells. The mean fluorescence intensity (MFI) of phospho-STAT3 staining is shown below. Each data point represents mean (± SD) of values from 3 mice of each genotype. ■ indicates wt cells; □, Socs3ΔLck/ΔLck cells. Data are representative of 2 to 3 independent experiments. *P < .05; **P < .001.
IL-27 is a newly described cytokine that has profound effects on T-cell function.34 IL-27 signals through a heterodimeric receptor complex composed of WSX-1 and gp13056 and has been shown to be a potent activator of STAT1, STAT3, and STAT5.57 After examination of IL-27–induced STAT activation in Socs3ΔLck/ΔLck T cells, we found that, similar to IL-6, the magnitude of the response to IL-27 was unchanged in SOCS3-deficient T cells, but IL-27–induced STAT1, STAT3, and STAT5 tyrosine phosphorylation was prolonged relative to wt cells, which suggests that SOCS3 is important for regulating IL-27–induced signals (Figure 5C,D).
IL-6 and IL-27 are produced by activated T cells and provide mitogenic signals
Given that TCR-signaling pathways are unaffected by the absence of SOCS3 and SOCS3-deficient T cells have enhanced responses to gp130 cytokines, the increased proliferation of Socs3ΔLck/ΔLck T cells in vitro may be driven by gp130 cytokines that are produced during culture. Although IL-27 is considered to be produced primarily by antigen-presenting cells,58 we found that both IL-6 and IL-27p28 are produced by T cells within 24 hours of stimulation with anti-CD3 in either the presence or absence of costimulation (Figure 6A and data not shown). In addition, although IL-6 and IL-27 were poor T-cell mitogens on their own, both gp130 cytokines synergized with TCR signals and strongly promoted the proliferation of wt CD8+ T cells when combined with anti-CD3 (data not shown).
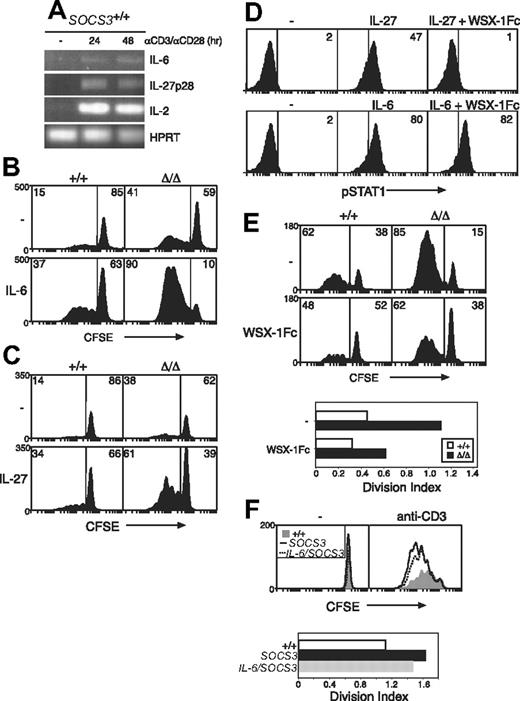
Hypersensitivity to IL-27, and not IL-6, drives increased CD8+ T-cell proliferation in the absence of SOCS3. (A) Purified wt LN T cells were stimulated for the indicated times with anti-CD3 (10 μg/mL) plus anti-CD28 (5 μg/mL), and the expression of IL-6, IL-27p28, and IL-2 was measured by RT-PCR. CFSE-labeled wt (+/+) or Socs3ΔLck/ΔLck (Δ/Δ) LN CD8+ T cells were stimulated for 3 days with anti-CD3 (0.6 μg/mL) with or without IL-6 (10 ng/mL) (B) or IL-27 (100 ng/mL) (C) and analyzed by flow cytometry. (D) The capacity of the WSX-1Fc reagent to inhibit IL-27 signaling was assessed by intracellular flow cytometry. STAT1 activation was measured in splenocytes from wt mice stimulated for 15 minutes with IL-6 (10 ng/mL) or IL-27 (5 ng/mL), with or without 5 μg/mL WSX-1Fc chimeric receptor. (E) CFSE-labeled wt or Socs3ΔLck/ΔLck LN CD8+ T cells were stimulated for 3 days with anti-CD3 with or without 5 μg/mL WSX-1Fc chimeric receptor. (F) Purified, CFSE-labeled wt (+/+, shaded), Socs3ΔLck/ΔLck (SOCS3, –), or IL-6–deficient Socs3ΔLck/ΔLck (IL-6/SOCS3, ---) CD8+ LN T cells were stimulated for 3 days with anti-CD3 (10 μg/mL). The division index shows the average number of cell divisions in each culture. Data are representative of 2 to 3 independent experiments.
Hypersensitivity to IL-27, and not IL-6, drives increased CD8+ T-cell proliferation in the absence of SOCS3. (A) Purified wt LN T cells were stimulated for the indicated times with anti-CD3 (10 μg/mL) plus anti-CD28 (5 μg/mL), and the expression of IL-6, IL-27p28, and IL-2 was measured by RT-PCR. CFSE-labeled wt (+/+) or Socs3ΔLck/ΔLck (Δ/Δ) LN CD8+ T cells were stimulated for 3 days with anti-CD3 (0.6 μg/mL) with or without IL-6 (10 ng/mL) (B) or IL-27 (100 ng/mL) (C) and analyzed by flow cytometry. (D) The capacity of the WSX-1Fc reagent to inhibit IL-27 signaling was assessed by intracellular flow cytometry. STAT1 activation was measured in splenocytes from wt mice stimulated for 15 minutes with IL-6 (10 ng/mL) or IL-27 (5 ng/mL), with or without 5 μg/mL WSX-1Fc chimeric receptor. (E) CFSE-labeled wt or Socs3ΔLck/ΔLck LN CD8+ T cells were stimulated for 3 days with anti-CD3 with or without 5 μg/mL WSX-1Fc chimeric receptor. (F) Purified, CFSE-labeled wt (+/+, shaded), Socs3ΔLck/ΔLck (SOCS3, –), or IL-6–deficient Socs3ΔLck/ΔLck (IL-6/SOCS3, ---) CD8+ LN T cells were stimulated for 3 days with anti-CD3 (10 μg/mL). The division index shows the average number of cell divisions in each culture. Data are representative of 2 to 3 independent experiments.
Next, we compared the ability of IL-27 to induce proliferation in wt and Socs3ΔLck/ΔLck T cells. Neither IL-6 nor IL-27 alone induced the proliferation of either wt or Socs3ΔLck/ΔLck T cells (data not shown). To examine IL-6– and IL-27–induced proliferation in the presence of anti-CD3, it was necessary to titrate the concentration of anti-CD3 to a level at which basal proliferation was similar between wt and Socs3ΔLck/ΔLck T cells. In response to 0.6 μg/mL anti-CD3, wt and Socs3ΔLck/ΔLck CD8+ T cells proliferated to a similar extent (Figure 6B-C). Both IL-6 and IL-27 slightly increased this basal proliferation in wt cells. The response of Socs3ΔLck/ΔLck CD8+ T cells to IL-6 (Figure 6B) or IL-27 (Figure 6C) was substantially greater, however, which suggests that increased IL-6 and IL-27 signaling in the absence of SOCS3 promotes CD8+ T-cell proliferation.
To directly assess the involvement of IL-27, we measured anti-CD3–induced proliferation in wt and SOCS3-deficient CD8+ T cells in the presence of a WSX-1Fc chimeric receptor, which will bind IL-27 but is unable to activate intracellular signaling pathways. We first showed that this reagent specifically inhibits STAT1 activation in response to IL-27 but not IL-6 (Figure 6D). Anti-CD3–induced proliferation of CD8+ T cells from both wt and Socs3ΔLck/ΔLck mice was reduced substantially in the presence of the WSX-1Fc chimeric receptor, which suggests that IL-27 produced in these cultures enhances T-cell proliferation induced by TCR ligation (Figure 6E). IL-6, in contrast, was not found to have a major effect on these processes, because the proliferation defect of Socs3ΔLck/ΔLck T cells was not rescued in Socs3ΔLck/ΔLck mice generated on an IL-6–deficient background (Figure 6F).
Discussion
The importance of SOCS proteins in the regulation of T-cell function has become increasingly evident within recent years. SOCS1 involvement in T-cell homeostasis in vivo was highlighted by the high frequency of abnormally activated T cells and increased homeostatic proliferation characteristic of SOCS1-deficient mice.8,9,17 SOCS1 and SOCS3 have similar functions in vitro and are more similar to each other than to other SOCS family members, which suggests that they may have overlapping functions in vivo. A key difference between SOCS1 and SOCS3, however, is their expression in T cells. SOCS1 expression is low in naive cells but is up-regulated rapidly in response to antigen, whereas SOCS3 expression is high in resting T cells but is down-regulated in newly activated T cells.7
To investigate the role of SOCS3 in T-cell function and homeostasis in vivo, we generated Socs3ΔLck/ΔLck mice in which the Socs3 gene was deleted specifically within T and NKT cells.
We found that SOCS3-deficient T cells were hyperproliferative in response to a TCR stimulus. This effect was seen in both CD4+ and CD8+ T cells but was most pronounced in SOCS3-deficient CD8+ T cells. Hyperproliferation in the absence of SOCS3 is consistent with previous studies that showed that overexpression of SOCS3 in T cells, in vivo or in vitro, results in reduced proliferation in response to TCR and antigenic stimulation.13,21 Our results, however, differ from the previous studies, which concluded that perturbed signaling downstream of the TCR13 or CD28 receptor21 accounts for this defect. First, SOCS3-deficient T cells respond normally to TCR stimulation in combination with costimulation (anti-CD3 plus anti-CD28), which suggests that costimulatory pathways are unaffected by the absence of SOCS3. Second, although responses to TCR ligation are enhanced, signaling pathways downstream of the TCR (including calcium-signaling pathways) are activated to a normal intensity and duration in the absence of SOCS3. Third, exaggerated TCR signaling would be expected to result in increased IL-2 production and activation-marker expression, neither of which are characteristic of Socs3ΔLck/ΔLck T cells.
Our results suggest that these T-cell defects are most probably caused by enhanced cytokine signaling in the absence of SOCS3. Previously, we and others showed that SOCS1 regulates CD8+ T-cell development and function by modulating γc cytokines.8,17,19,20 Socs3ΔLck/ΔLck T cells, however, showed normal signaling in response to IL-2, IL-4, and IL-7. Previous studies have shown that SOCS3 regulates gp130 cytokines.27,–29,32 We showed that CD8+ T cells from gp130Y757F/Y757F mice, which lack the SOCS3-binding site on gp130, also proliferate strongly in response to TCR ligation despite the TCR-signaling pathways being unaffected in these cells. This result suggested that hypersensitivity to a gp130 cytokine drives the hyperproliferative phenotype of SOCS3-deficient T cells. SOCS3-deficient T cells had prolonged STAT activation in response to both IL-6 and IL-27, the main gp130 cytokines that act on T cells. Finally, we showed that inhibition of IL-27 signaling limits anti-CD3–induced proliferation in CD8+ T cells, whereas the absence of IL-6 had no effect. Collectively, these data suggest that IL-27 synergizes with TCR-signaling pathways to provide costimulatory signals, which supports the hypothesis that the T-cell phenotype in Socs3ΔLck/ΔLck mice is caused primarily by hypersensitivity to IL-27 signaling.
In contrast to other gp130 family members, IL-27 has profound effects on T-cell behavior. IL-27 is a proinflammatory cytokine that promotes Th1 inflammatory responses.58 Notably, CD4+ T cells from IL-27 receptor (WSX-1) Tg mice are hyperproliferative in response to TCR ligation, and IL-27, in combination with anti-CD3, has been shown to augment the proliferation of naive CD4+ T cells.57,59 Paradoxically, IL-27 also has anti-inflammatory properties, and WSX-1−/− mice develop a lethal CD4+ inflammatory disease after parasite infection because of a failure to down-regulate the T-cell response.60 IL-27 inhibits IL-2 production in CD4+ T cells and the differentiation of Th17 inflammatory cells, both of which are likely to contribute to its anti-inflammatory actions.26,36,,,–40
There is considerable conflict in the literature regarding these anti-inflammatory properties of IL-27. First, although several studies have shown that IL-27 suppresses IL-2 production,38,–40 no increase in IL-2 production was seen in cells from Il27ra−/− mice with autoimmune encephalomyelitis.36 Although our data show that SOCS3-deficient T cells are hypersensitive to IL-27, we found no differences in IL-2 production by SOCS3-deficient CD8+ T cells. Second, there are also conflicting data regarding the involvement of STAT1 in IL-27–inhibitory actions.38,40
Our results suggest that IL-27 has a positive effect on CD8+ T-cell activation. In contrast to other studies, our experiments analyzed the consequences of TCR engagement in the absence of CD28 costimulation, which may account for the proinflammatory IL-27 responses observed in our study. Indeed, it was shown recently that the activation state of T cells influences the response to IL-27, with IL-27 potently suppressing cytokine production in fully activated, but not early activated, CD4+ T cells.37 In addition, the activation of STAT pathways by IL-27 differed in early and fully activated CD4 T cells in this study. IL-27 preferentially induced STAT3 phosphorylation in fully activated cells, whereas IL-27 induced the phosphorylation of both STAT1 and STAT3 in early activated cells.37 We have shown that IL-27–induced activation of STAT1, STAT3, and STAT5 is prolonged in SOCS3-deficient T cells, which suggests that these cells more closely represent early activated T cells.
Given that both IL-6 and IL-27 signal through gp130 and both of them are regulated by SOCS3, it is surprising that the hyperproliferative phenotype of SOCS3-deficient T cells is caused primarily by enhanced responses to IL-27. Differences in the actions of IL-6 and IL-27, however, have been suggested in other recent studies. For example, unlike IL-27, IL-6 does not suppress IL-2 production by CD4+ T cells.38 We found qualitative differences in the STAT pathways activated by these cytokines, with STAT5 activated by IL-27 but not IL-6, and differences in the kinetics of STAT activation by IL-6 and IL-27 (Figure 5). The differences in biologic responses probably originate from signaling via different cell surface receptors, with IL-27 binding to a receptor composed of gp130 and the ligand-specific subunit WSX-1, whereas IL-6 signals via gp130 homodimers together with the ligand-binding IL-6Rα subunit. Collectively, these data highlight the differences in IL-6 and IL-27 function and regulation.
SOCS3 has now been shown to regulate the signaling of several gp130 cytokines, those that signal through both homodimeric gp130 receptors (IL-627,–29 ) and heterodimeric receptors (leukemia inhibitory factor,32,33 IL-27). It remains to be determined whether SOCS3 also regulates other members of the gp130 family.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Janelle Lochland, Ailsa Frew, Lei Shong Lau, and Hayley Croom for first-class technical assistance and Melanie Rowe, Kylie Gilbert, and Kelly Trueman for expert animal husbandry.
This work was supported by Australian National Health and Medical Research Council program grant 257500, US National Institutes of Health (NIH) grant CA22556, and AMRAD Operations. R.S. was supported by a QEII Fellowship from the Australian Research Council and a Sylvia and Charles Viertel Senior Medical Research Fellowship. C.B. was supported by the Danish Medical Research Council.
National Institutes of Health
Authorship
Contribution: C.B. and R.S. designed and performed research, analyzed data, and wrote the article; G.M.T., B.J.J., J.F., and R.C. performed research; B.J.J., M.E., W.S.A., and C.J.M.S. contributed vital new reagents; and N.A.N., D.J.H., and W.S.A. designed the research and analyzed data.
Conflict-of-interest disclosure: The authors disclose that this work was supported in part by AMRAD Operations, for which N.A.N. and D.J.H. consult and/or have a financial interest. C.J.M.S. is an employee of Amgen. All other authors declare no competing financial interests.
Correspondence: Robyn Starr, St Vincent's Institute, 41 Victoria Parade, Fitzroy, Victoria 3065, Australia; e-mail address:rstarr@svi.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal