Foxp3 expression was initially thought to be restricted to the CD4+CD25+ regulatory T-cell population. However, recent studies suggest that forkhead box P3 (Foxp3) is expressed in CD4+CD25− T cells in aged mice. In the present study in B-cell non-Hodgkin lymphoma (NHL), we found that a subset of intratumoral but not peripheral blood CD4+CD25− T cells, comprising about 15% of intratumoral CD4+ T cells, express Foxp3 and are capable of suppressing the proliferation of autologous infiltrating CD8+ T cells. In vitro activation with OKT3/anti-CD28 antibody (Ab) or dendritic cells (DCs) induced Foxp3 expression in a subset of these CD4+CD25−Foxp3− T cells. We found that the presence of lymphoma B cells during activation augmented activation-induced Foxp3 expression in CD4+CD25− T cells. We also found that CD70+ lymphoma B cells significantly contributed to the activation-induced Foxp3 expression in intratumoral CD4+CD25− T cells. Furthermore, the blockade of CD27-CD70 interaction by anti-CD70 Ab abrogated lymphoma B-cell–mediated induction of Foxp3 expression in intratumoral CD4+CD25− T cells. Taken together, these studies reveal a novel role for NHL B cells in the development of intratumoral regulatory T cells.
Introduction
It is well established that tumor cells are able to evade detection by the host immune system and to proliferate despite immunotherapeutic strategies to inhibit them. The immune-escape mechanisms of tumors range from impaired antigen presentation and apoptosis resistance to the production of immunosuppressive factors.1,2 Although there is commonly significant infiltration of CD4+ T helper cells and CD8+ cytotoxic T lymphocyte (CTL) cells at the tumor site, tumor cells can use immunosuppressive strategies to induce CD4+ and CD8+ T-cell anergy and create a tolerant tumor microenvironment.2 It has been shown that antigen-presenting cells (APCs) play a crucial role in tolerizing tumor antigen-specific CD4+ and CD8+ T cells.3,4
Significant interest has recently been focused on the premise that tumors may subvert tumor immunity by promoting the expansion, recruitment, and activation of regulatory T (Treg) cells. CD4+ Treg subsets include naturally occurring CD4+CD25+ Treg cells as well as peripherally induced CD4+ Treg cells. Naturally occurring Treg cells arise as a distinct lineage from the thymus and represent approximately 10% of peripheral CD4+ T cells in mice and humans. However, Treg cells can be generated in the periphery under particular conditions of antigenic stimulation.5,–7 Although Treg cells were originally identified for their ability to prevent organ-specific autoimmune disease in mice, Treg cells have also been shown to suppress tumor-specific T-cell immunity8,,,–12 and thereby contribute to the progression of human tumors.9,10,13,14
Expression of transcriptional factor forkhead box P3 (Foxp3) has been demonstrated to be crucial to the development and function of Treg cells.15,–17 Ectopic expression of Foxp3 in CD4+CD25− naive T cells by retroviral gene transfer can convert them to natural Treg-like cells functionally and phenotypically. Transgenic mice lacking Foxp3 lack T cells with regulatory function and have dysregulated T-cell proliferation, resulting in a severe autoimmune disease. These results demonstrate that expression of Foxp3 is important for the function of Treg cells. Although Treg cells express high levels of Foxp3, Foxp3 expression can also be induced in CD4+CD25− T cells by activation with corticosteroids, estrogen, and transforming growth factor-β (TGF-β).5,–7 Although CD4+CD25− T cells expressing Foxp3 have been recently reported in aging mice,18 it is currently unclear whether CD4+CD25−Foxp3+ T cells are present in human tissue and what their significance may be.
Similar to other types of tumors, lymphoma B cells are able to induce immune cell anergy in murine models.19,–21 In mice inoculated with lymphoma B-cell line A20, tumor antigen-specific CD4+ T cells displayed a diminished response to peptide antigen in vitro and were unable to be primed in vivo.19 In vivo disruption of tolerogenic cross-presentation mechanisms by disabling bone-marrow–derived APCs resulted in effective T-cell activation and antitumor immunity in a B-cell lymphoma model.20 Furthermore, recent data in mice showed that, in addition to inducing tumor antigen-specific CD4+ T-cell anergy through antigen-presentation, lymphoma B cells also induced the generation of Treg cells and contributed to the tolerance of immune cells to tumor antigens.21
We have previously shown that intratumoral Treg cells are present in significant numbers in biopsy specimens from patients with non-Hodgkin lymphoma (NHL), and that these cells significantly inhibit the proliferation and cytokine or granule production of intratumoral CD4+ and CD8+ T cells.8,22 Furthermore, we have shown that lymphoma cells produce chemokines capable of attracting Treg cells and thereby recruit Treg cells into the tumor microenvironment. What is unknown in humans, however, is whether malignant B cells influence Foxp3 expression in CD4+ T cells and therefore induce regulatory activity. In the present study, we show that Foxp3 is expressed in a subset of CD4+CD25− T cells in the tumor microenvironment and that CD4+CD25−Foxp3+ T cells display regulatory function. Furthermore, we also show that CD4+CD25−Foxp3- T cells can be induced to express Foxp3 when activated, particularly in the presence of lymphoma B cells that express CD70.
Patients, materials, and methods
Patient samples
Patients providing written informed consent in accordance with the Declaration of Helsinki were eligible for this study if they had a tissue biopsy that on pathologic review showed B-cell lymphoma and adequate tissue to perform the experiments. The biopsy specimens were reviewed and classified using the World Health Organization (WHO) lymphoma classification. The use of human tissue samples for this study was approved by the Institutional Review Board of the Mayo Clinic/Mayo Foundation. All samples used in this study, from either patients or healthy donors, were from fresh tissue samples and were never frozen.
Cell isolation and purification
CD8+ T cells and CD19+ lymphoma B cells were isolated using CD8 and CD19 microbeads (positive selection). CD4+CD25− or CD4+CD25+ T-cell subsets were purified by using a CD4+CD25+ regulatory T-cell isolation kit (Miltenyi Biotec, Auburn, CA) as previously described.22 CD4+CD25−CD127+ T cells and CD70+ or CD70− lymphoma B cells were isolated by flow sorting in the flow cytometry core facility of the Mayo Clinic. Autologous dendritic cells (DCs) were isolated from biopsy specimens by using a monocyte adherence technique. Briefly, a cell suspension was seeded in a 75-cm flask, and the flask was incubated for 2 hours at 37°C and 5% CO2. At the end of the incubation period, the nonadherent cells were removed, and the flask was gently washed with culture medium to remove any residual nonadherent cells. Culture medium containing 800 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) and 1000 U/mL IL-4 was added to the remaining adherent cells in the flask. At day 5, the nonadherent cells were collected, and the pellet was resuspended at a concentration of 1 × 106 cells/mL in culture medium containing 800 U/mL GM-CSF, 1000 U/mL IL-4, 1100 U/mL TNF-α, and 1 μg/mL PGE2 and cultured at 37°C and 5% CO2 for another 48 hours. This resulted in matured DCs for use in the planned experiments.
Flow cytometry and Foxp3 intracellular staining
A total of 1 × 106 cells were washed in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) and incubated with CD3, CD4, CD8, CD19, and CD25 specific fluorochrome–conjugated antibodies and analyzed on a FACSCalibur flow cytometry (Becton Dickinson, San Jose, CA). Foxp3 expression was determined by using flow-based intracellular staining following the instructions described by the manufacturer (Biolegend, San Diego, CA). Briefly, cells were stained with CD3-Percp, CD4-PE, and CD25-Allophycocyanin for 30 minutes at 4°C and fixed with 1mL of 1 × Foxp3 Fix/Perm solution for 20 minutes at room temperature (RT). Cells were then spun down, and the supernatant was removed. They were washed once with PBS and 1 mL 1 × Foxp3 Perm buffer. Cells were resuspended in 1 mL 1 × Foxp3 Perm buffer for 15 minutes at RT. Cells were again centrifuged, and the supernatant was discarded. A total of 5μL Alexa-488–conjugated anti-Foxp3 antibody was added and incubated at RT in the dark for 30 minutes. After washing, cells were analyzed by flow cytometry.
CFSE labeling and T-cell proliferation assay
Cells were washed, counted, and resuspended at 1 × 107/mL in PBS. A stock solution of CFSE (carboxyfluorescein succinimidyl ester; 5 mM) was diluted 1:100 with PBS and added to the cells for a final concentration of 5 μM. After 10 minutes at 37°C, cells were washed 3 times with 10 vol PBS containing 10% FBS. CFSE-labeled responding cells were cocultured with or without stimulating cells in the presence or absence of PHA (2.5 μg/mL) at 37°C and 5% CO2. Cells were harvested at day 3, washed, and stained with fluorochrome-conjugated antibodies for detection of surface markers for 30 minutes at 4°C. Cells were analyzed by flow cytometry. The percentage of CFSEdim were measured and calculated as the percentage of proliferated cells.
Immunofluorescence assay
The assay was set up based on an approach described by Hiraoka et al with a few modifications.23 Formalin-fixed paraffin sections (4 μm) were deparaffinized and cleared to 95% ethanol. The sections were treated with 1 mM EDTA (pH 8.0) in a steamer for 30 minutes, cooled down for an additional 5 minutes, and placed in TBS. CD25 (1:500; 4C9; Novocastra Laboratories, Newcastle, United Kingdom), CD4 (1:1000; 4B12; Novocastra Laboratories), and CD20 (1:10 000; L26; DakoCytomation, Carpinteria, CA) were stained with CSA System (DakoCytomation) and in accordance with the manufacturer's instructions with a slight modification. The CSA System modification was to incubate the sections with Texas Red–conjugated AvidinD (1:200; Vector Laboratories, Burlingame, CA) for 30 minutes after the reaction with biotin-conjugated tyramide solution. Prior to staining with second antibody, the sections were incubated in 0.1 M glycine/HCl (pH 2.2) for 2 hours with gentle stirring to detach antibodies. Foxp3 (1:500; ab22510; Abcam, Cambridge, MA) and CD20 were stained with CSAII System (DakoCytomation), also in accordance with manufacturer's instructions. After incubation with FITC-conjugated tyramide, the sections were washed well with TBS and mounted with DAPI-Vectashield (Vector Laboratories). All sections were observed with fluorescence microscopy (Leica DMRxA, Plymouth, MN) with images captured with a SPOT RT camera (63×/1.32-ab oil) and software (Diagnostic Instruments, Burlingame, CA).
Statistical analysis
The Fisher exact test was used to compare differences in nominal variables, while the rank-sum test, paired Student t test, or the Kruskal-Wallis test was used for continuous variables. A P value less than .05 was considered significant.
Results
Foxp3 is expressed in intratumoral CD4+CD25− T cells in B-cell NHL
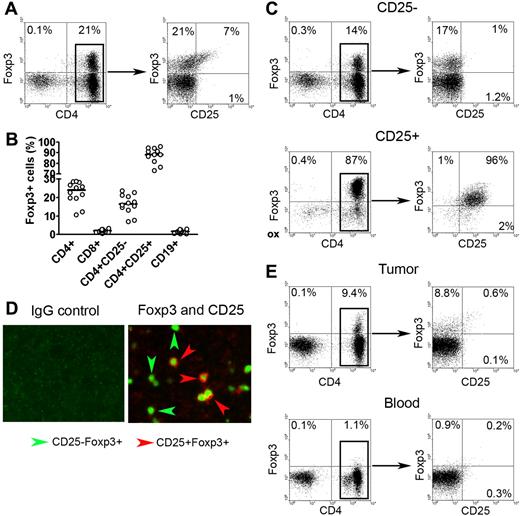
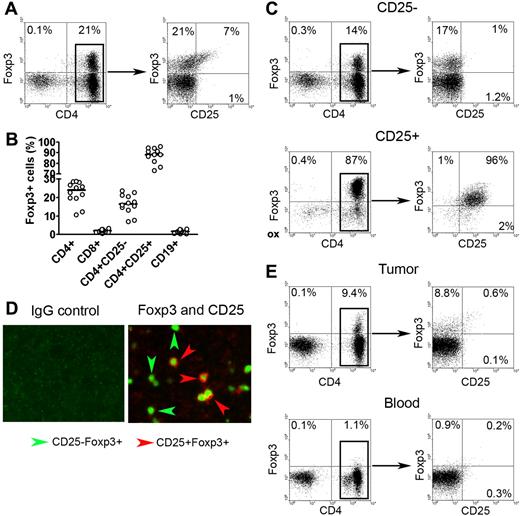
We had previously shown that intratumoral CD4+CD25− T cells had the ability to suppress infiltrating immune effector cells,8 suggesting that an additional subset of these intratumoral cells may have regulatory function. Using Foxp3 as a potential marker of cells with suppressive function, we examined whether Foxp3-expressing CD4+CD25− T cells were present in the biopsy specimens of patients with B-cell NHL. As expected, we found a subset of CD3+CD4+ T cells that expressed Foxp3 (Figure 1A). Further analysis showed that Foxp3+ cells were distributed not only in the CD4+CD25+ population, but also in the CD4+CD25− population. When we performed this same experiment on a panel of NHL specimens (n = 12), we found that Foxp3+ cells accounted for a median of 15% of the total CD4+CD25− T cells (Figure 1B). The vast majority of CD4+CD25+ T cells (92%) expressed Foxp3. Foxp3 expression was not detectable in infiltrating CD8+ T cells or CD19+ lymphoma cells. The frequency of CD4+CD25−Foxp3+ cells was not associated with the age of the patient at the time of the biopsy (data not shown).
Foxp3 expression in CD4+CD25− T cells in biopsy specimens of B-cell NHL. (A) Representative dot plots showing Foxp3 expression in CD4+CD25+ and CD4+CD25− T cells in biopsy specimens of patients with NHL. Intracellular Foxp3 expression was determined by using fluorochrome-conjugated Foxp3 antibody. (B) Percentage of Foxp3 expression in different subsets of cells in NHL specimens (n = 12). The horizontal bars indicate median expression levels. (C) Representative dot plots showing Foxp3 expression in positive (CD25+) and negative (CD25−) fractions of CD25-depleted sample. (D) Representative immunofluorescence images showing Foxp3 expression in CD25+ and CD25− cells in biopsy specimens of patients with NHL. Tissues were stained with antibodies to human CD25 and Foxp3. Left panels shows isotype control for each antibody; right, double immunofluorescence staining of CD25 (red; surface) and Foxp3 (green; intracellular). Original magnification, × 200. See “Immunofluorescence assay” for image acquisition details. (E) Representative dot plots showing Foxp3 expression in CD4+CD25− T cells (CD25-depleted) from tumor tissue (top panel) and peripheral blood mononuclear cells (bottom panel) from patients with NHL. Percentages in panels A,C, and E represent number of cells in quadrant as a percentage of all cells.
Foxp3 expression in CD4+CD25− T cells in biopsy specimens of B-cell NHL. (A) Representative dot plots showing Foxp3 expression in CD4+CD25+ and CD4+CD25− T cells in biopsy specimens of patients with NHL. Intracellular Foxp3 expression was determined by using fluorochrome-conjugated Foxp3 antibody. (B) Percentage of Foxp3 expression in different subsets of cells in NHL specimens (n = 12). The horizontal bars indicate median expression levels. (C) Representative dot plots showing Foxp3 expression in positive (CD25+) and negative (CD25−) fractions of CD25-depleted sample. (D) Representative immunofluorescence images showing Foxp3 expression in CD25+ and CD25− cells in biopsy specimens of patients with NHL. Tissues were stained with antibodies to human CD25 and Foxp3. Left panels shows isotype control for each antibody; right, double immunofluorescence staining of CD25 (red; surface) and Foxp3 (green; intracellular). Original magnification, × 200. See “Immunofluorescence assay” for image acquisition details. (E) Representative dot plots showing Foxp3 expression in CD4+CD25− T cells (CD25-depleted) from tumor tissue (top panel) and peripheral blood mononuclear cells (bottom panel) from patients with NHL. Percentages in panels A,C, and E represent number of cells in quadrant as a percentage of all cells.
To confirm these results, we used a CD25 depletion approach to determine if Foxp3 was expressed in CD4+CD25− T cells. As shown in Figure 1C, CD25 depletion efficiently removed almost all CD25+ cells from the samples as indicated by lack of CD25 staining on cells from the CD25− fraction. Contrary to expression of CD25, Foxp3 expression remained in a subset of CD4+ cells (17%) after CD25 depletion. As expected, CD4+CD25+ cells exhibited strong staining for Foxp3 (96%) (Figure 1C). Similar results were seen in a minimum of 6 experiments.
In addition to flow cytometry, we also applied an immunofluorescence technique to formalin-fixed paraffin sections from B-cell NHL samples to further confirm Foxp3 expression in CD4+CD25− T cells. Dual staining by immunofluorescence enabled simultaneous staining for Foxp3 and CD25. As shown in Figure 1D, cells with positive CD25 staining were normally accompanied by positive Foxp3 staining, indicated by cells with red surface staining (CD25) and green intracellular staining (Foxp3). Although many cells exhibited dual staining, we have found that many cells were stained green without red cell-surface staining, indicating Foxp3 expression in CD25− cells. In contrast, the CD8 or CD19 cell populations were negative for CD25 and Foxp3 (data not shown). Taken together, these results demonstrate that, in addition to CD4+CD25+ T cells, Foxp3 is also expressed in a subpopulation of intratumoral CD4+CD25− T cells.
Next, we wanted to determine if CD4+CD25−Foxp3+ T cells were specific to tumor sites, or whether they were also present in peripheral blood. To test this, we collected peripheral blood and tumor tissue from the same patients with B-cell NHL (n = 3). Using the CD25 depletion approach shown in Figure 1E, we found that Foxp3+ cells could be detected in CD25− cells isolated from tumor tissue (9.4%) (Figure 1E top left panel). Costaining with anti-CD4 and anti-CD25 confirmed that the Foxp3+ cells were CD4+ and CD25− (Figure 1E top right panel). Contrary to the tumor tissue, very few Foxp3+ cells could be detected in the CD25− fraction of peripheral blood mononuclear cells (PBMCs) isolated from the same patient (1.1%) (Figure 1E bottom panel). These findings suggest that the lymphoma microenvironment may be involved in the generation of intratumoral CD4+CD25−Foxp3+ T cells in B-cell NHL.
Next, we examined the phenotype of the intratumoral CD4+CD25−Foxp3+ cells (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The phenotype of CD4+CD25−Foxp3+ T cells was similar to that of CD4+CD25+Foxp3+ T cells but distinct from that of CD4+CD25−Foxp3− T cells. Both CD4+CD25+Foxp3+ T and CD4+CD25−Foxp3+ cells express CD27, CD45RO, and CD62L and lack expression of CD70, PD-1, and CD127, indicating a memory phenotype. In contrast, most CD4+CD25−Foxp3− T cells lack CD62L expression, and some CD4+CD25−Foxp3− T cells express PD-1, suggesting Foxp3 expression can be used to distinguish different T-cell populations.
Regulatory function of CD4+CD25−Foxp3+ T cells
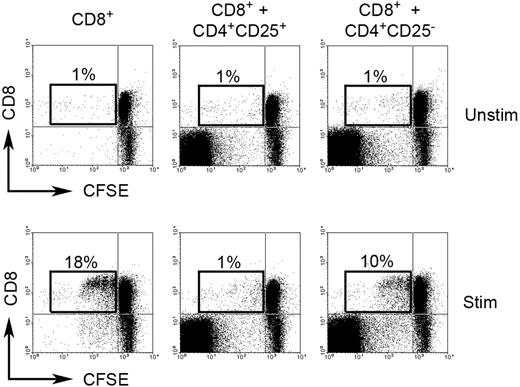
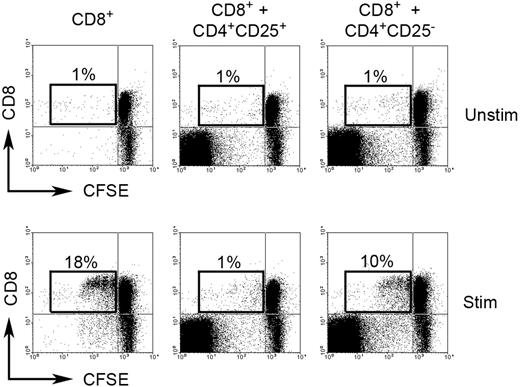
Because of Foxp3 expression, we wanted to establish if CD4+CD25−Foxp3+ cells have regulatory function. To address this question, we determined the effect of intratumoral CD4+CD25− T cells on proliferation of CD8+ T cells. Infiltrating CFSE-labeled CD8+ T cells (responding cells) were cultured either alone or with intratumoral CD4+CD25− T cells (stimulating cells) in the presence or absence of PHA for 3 days at a ratio of 1:4 (stimulating cells to responding cells). As a control, CD8+ T cells were also cultured in the same conditions with intratumoral Treg (CD4+CD25+) cells. To exclude effect of competition for space/nutrients on cell proliferation in the coculture wells, we added equal numbers of extra CD8+ T cells to CD4+CD25− or Treg cells to each well to ensure that each well contained an identical number of cells. Our results suggest that intratumoral CD4+CD25− T cells strongly suppressed the proliferation of PHA-activated infiltrating CD8+ T cells. Coculture of CD8+ T cells with CD4+CD25− T cells decreased CFSEdim cells (proliferated cells) from 18% to 10% when compared with CD8+ T cells cultured alone (Figure 2). As expected, intratumoral Treg cells displayed complete inhibition of proliferation of PHA-activated infiltrating CD8+ T cells, reducing CFSEdim cells from 18% to 1% when compared with CD8+ T cells cultured alone (Figure 2). Because Foxp3 staining requires permeabilization of the cells, we were unable to sort on Foxp3+ cells for these experiments. However, these results do suggest that intratumoral CD4+CD25− T cells have regulatory function.
Regulatory function of CD4+CD25− T cells in biopsy specimens of B-cell NHL. CFSE-labeled CD8+ T cells were cocultured either alone or with intratumoral CD4+CD25− T cells or intratumoral Treg cells (CD4+CD25+) in the presence (Stim) or absence (Unstim) of PHA for 3 days. Proliferation of CD8+ T cells is measured based on CFSEdim cells. The figure shown is representative of 3 independent experiments (3 samples) with similar results. Percentages indicate number of proliferated CD8+ T cells (CFSEdim) of just the CD8+ cell population.
Regulatory function of CD4+CD25− T cells in biopsy specimens of B-cell NHL. CFSE-labeled CD8+ T cells were cocultured either alone or with intratumoral CD4+CD25− T cells or intratumoral Treg cells (CD4+CD25+) in the presence (Stim) or absence (Unstim) of PHA for 3 days. Proliferation of CD8+ T cells is measured based on CFSEdim cells. The figure shown is representative of 3 independent experiments (3 samples) with similar results. Percentages indicate number of proliferated CD8+ T cells (CFSEdim) of just the CD8+ cell population.
Regulation of Foxp3 expression in intratumoral CD4+CD25− T cells
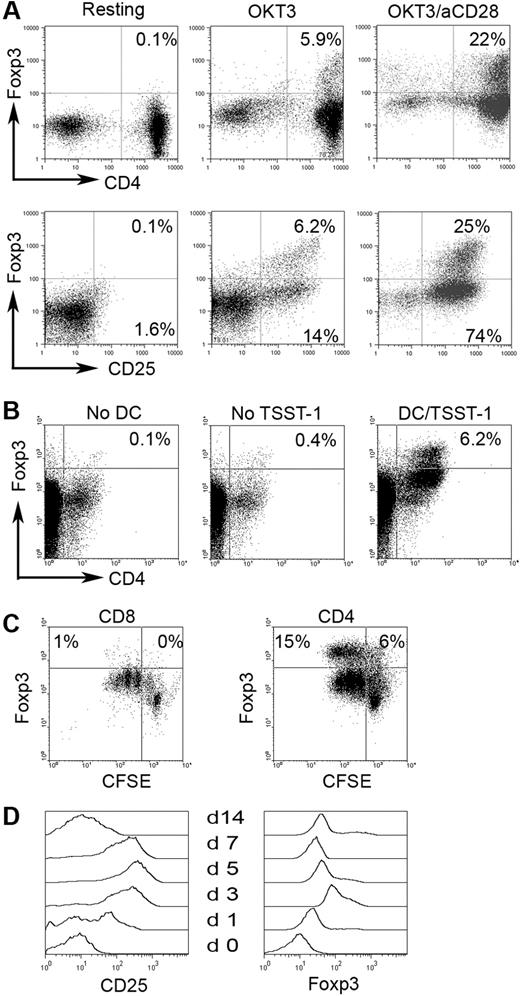
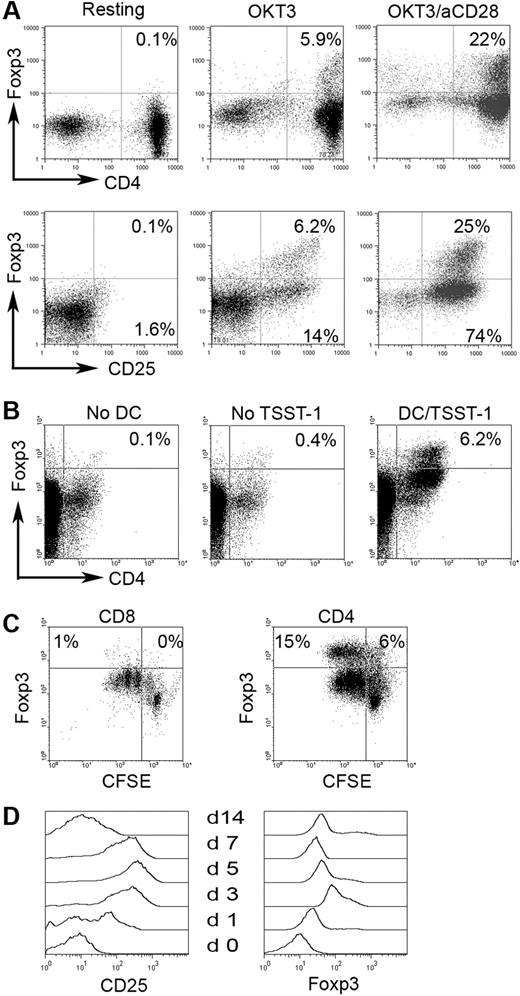
The origin of CD4+CD25−Foxp3+ cells is unclear—it is possible that at one time these cells were CD25+ and lost expression, or they may arise directly from the CD4+CD25− population. We therefore wanted to determine if Foxp3 expression could be up-regulated in CD4+CD25−Foxp3− cells through in vitro activation. As previously mentioned, we could not sort cells based on Foxp3 expression for technical reasons. We therefore used CD127 (IL-7Rα), which has been shown to be inversely correlated with CD25 and Foxp3 expression in CD4+CD25+ T cells,24 to isolate CD4+CD25−Foxp3− cells. Before sorting, we confirmed that CD127 expression was inversely associated with Foxp3 and CD25 expression (Figure S2A). Using a fluorescence-activated cell sorter (FACS)–based cell-sorting approach, we were able to obtain a pure population of CD4+CD25−Foxp3− T cells (Figure S2B). We then cultured the CD4+CD25−CD127+ T cells with OKT3 and anti-CD28 (Figure 3A) or cocultured them with matured DCs pulsed with superantigen toxic shock syndrome toxin-1 (TSST-1) for 3 days (Figure 3B). As shown in Figure 3A (top panel), activation with OKT3 alone induced a moderate amount of Foxp3 expression (5.9%) while activation using a combination of OKT3 and anti-CD28 resulted in substantial Foxp3 expression (22%), suggesting the necessity of costimulation in activation-induced Foxp3 expression. As expected, stimulation with OKT3 alone or in combination with anti-CD28 resulted in expression of CD25 (Figure 3A bottom panel). The induction of Foxp3 expression appeared to be predominantly in a subset of CD4+CD25− T cells, because Foxp3 was only expressed in a small portion of CD25+ (activated) T cells (Figure 3A bottom panel).
Foxp3 induction in CD4+CD25− T cells in biopsy specimens of B-cell NHL. Foxp3 expression was examined in CD4+CD25− T cells stimulated with OKT3 or OKT3/anti-CD28 Ab (A) (n = 10) or with in vitro–generated DCs pulsed with or without super-Ag TSST-1 (B) (n = 3). (C) Foxp3 expression in CD4+CD25− or CD8+ T cells upon activation. CFSE was used to monitor activation and proliferation (n = 6). Percentages indicate number of proliferated CD8+ T cells (CFSEdim) of just the CD8+ cell population. (D) CD25 and Foxp3 expression determined at specific day after activation in intratumoral CD4+CD25− T cells in B-cell NHL (n = 4).
Foxp3 induction in CD4+CD25− T cells in biopsy specimens of B-cell NHL. Foxp3 expression was examined in CD4+CD25− T cells stimulated with OKT3 or OKT3/anti-CD28 Ab (A) (n = 10) or with in vitro–generated DCs pulsed with or without super-Ag TSST-1 (B) (n = 3). (C) Foxp3 expression in CD4+CD25− or CD8+ T cells upon activation. CFSE was used to monitor activation and proliferation (n = 6). Percentages indicate number of proliferated CD8+ T cells (CFSEdim) of just the CD8+ cell population. (D) CD25 and Foxp3 expression determined at specific day after activation in intratumoral CD4+CD25− T cells in B-cell NHL (n = 4).
Foxp3 expression could also be induced in CD4+CD25− T cells activated by autologous DCs pulsed with TSST-1 (Figure 3B). DCs induced the expression of Foxp3 in intratumoral CD4+CD25− T cells (6.2%) compared with the no DC (0.1%) and no TSST-1 (0.4%) controls. Taken together, these results indicate that a subset of intratumoral CD4+CD25− T cells are capable of up-regulating Foxp3 expression.
We next wanted to determine if up-regulation of Foxp3 expression specifically occurred in CD4+CD25− T cells or whether it was a common activation event that occurred in other types of T cells during activation. To address this, we measured the Foxp3 expression in infiltrating CD8+ T cells. In contrast to CD4+CD25− T cells, infiltrating CD8+ T cells did not express Foxp3 upon activation with OKT3/anti-CD28 or DCs, although significant activation and proliferation could be seen in both CD8+ and CD4+CD25− T cells as demonstrated by substantial CFSE dilution (Figure 3C). These results suggest that Foxp3 induction in CD4+CD25− T cells is cell-type specific and not just a common activation event.
Because Foxp3 expression could be found independent of CD25 (Figure 1), we wanted to determine the expression patterns of both CD25 and Foxp3 upon activation over time. Therefore, we studied Foxp3 and CD25 expression in CD4+CD25− T cells after activation by OKT3/anti-CD28 stimulation for 0 to 14 days (n = 4). The expression level of CD25 and Foxp3 reached a peak at day 5 and day 3, respectively. After this, both of CD25 and Foxp3 expression declined gradually over time. However, Foxp3 expression was maintained at detectable levels, while CD25 expression decreased to negligible levels by day 14 (Figure 3D). This result suggests that CD4+CD25−Foxp3+ T cells may be the long-term result of previous activation via the T-cell receptor.
Malignant lymphoma B cells induce Foxp3 expression in CD4+CD25− T cells
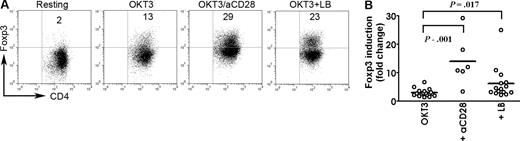
The presence of CD4+CD25−Foxp3+ T cells in tumor tissue but not in peripheral blood suggested that malignant lymphoma B cells might be involved in the induction of Foxp3 in intratumoral CD4+CD25− T cells in B-cell NHL. To test this, we isolated CD4+CD25−CD127+ T cells (Foxp3−) and cultured them in OKT3-coated plates (OKT3) or with the addition of anti-CD28 (OKT3/aCD28) or CD19+ lymphoma cells (OKT3 + LB [lymphoma B cells]) for 3 days. Unstimulated cells (resting) were used as the baseline for calculating Foxp3 induction. As shown in Figure 4A, activation with OKT3 alone induced a small number of CD4+CD25− T cells to express Foxp3 (13%), while costimulation with anti-CD28 strongly enhanced Foxp3 induction (29%). The culture of CD19+ lymphoma B cells with OKT3 also resulted in an increase in Foxp3 expression (23%) to a level comparable with OKT3/anti-CD28 activation. In the absence of OKT3, lymphoma B cells are not able to induce Foxp3 expression in CD4+CD25− T cells (data not shown). Data acquired from multiple NHL samples indicated that lymphoma B cells significantly induced Foxp3 expression in CD4+CD25− T cells, with mean fold induction (over resting cells) of 5.64 (n = 15; P = .017 compared with OKT3 alone), while OKT3 alone increased Foxp3 by 2.59-fold (n = 15) and OKT3 plus anti-CD28 by 14.01-fold (n = 6) (Figure 4B). In addition, using a CD19- and CD25-depletion approach, we also found that Foxp3 expression can be induced more efficiently in activated CD4+CD25− T cells from a CD25-depleted population (CD19+ lymphoma B cells present; 41%) than in CD4+CD25− T cells from CD25 and CD19 double-depleted population (CD19+ lymphoma B cells absent, 13%; data not shown). These results suggest that malignant lymphoma B cells are involved in the generation of CD4+CD25−Foxp3+ T cells in tumor sites of B-cell NHL.
Effect of lymphoma B cells on activation-induced Foxp3 expression in CD4+CD25− T-cell biopsy specimens of B-cell NHL. (A) Purified CD4+CD25−CD127+ T cells were cultured in OKT3-coated plates (OKT3) or with the addition of anti-CD28 Ab (OKT3/aCD28) or CD19+ lymphoma cells (OKT3 + LB) for 3 days. Unstimulated cells (Resting) were used as the baseline for calculating Foxp3 induction. Foxp3 expression was examined by intracellular staining. Numbers indicate CD4+Foxp3+ cells as a percentage of all cells. (B) Summarization of Foxp3 induction in CD4+CD25− T cells upon activation with OKT3 alone (OKT3; n = 15) or plus anti-CD28 (+ aCD28; n = 6) or in the presence of lymphoma B cells (+ LB; n = 15). Foxp3 induction is indicated by fold change to group without stimulation (Resting). The horizontal bars indicate median.
Effect of lymphoma B cells on activation-induced Foxp3 expression in CD4+CD25− T-cell biopsy specimens of B-cell NHL. (A) Purified CD4+CD25−CD127+ T cells were cultured in OKT3-coated plates (OKT3) or with the addition of anti-CD28 Ab (OKT3/aCD28) or CD19+ lymphoma cells (OKT3 + LB) for 3 days. Unstimulated cells (Resting) were used as the baseline for calculating Foxp3 induction. Foxp3 expression was examined by intracellular staining. Numbers indicate CD4+Foxp3+ cells as a percentage of all cells. (B) Summarization of Foxp3 induction in CD4+CD25− T cells upon activation with OKT3 alone (OKT3; n = 15) or plus anti-CD28 (+ aCD28; n = 6) or in the presence of lymphoma B cells (+ LB; n = 15). Foxp3 induction is indicated by fold change to group without stimulation (Resting). The horizontal bars indicate median.
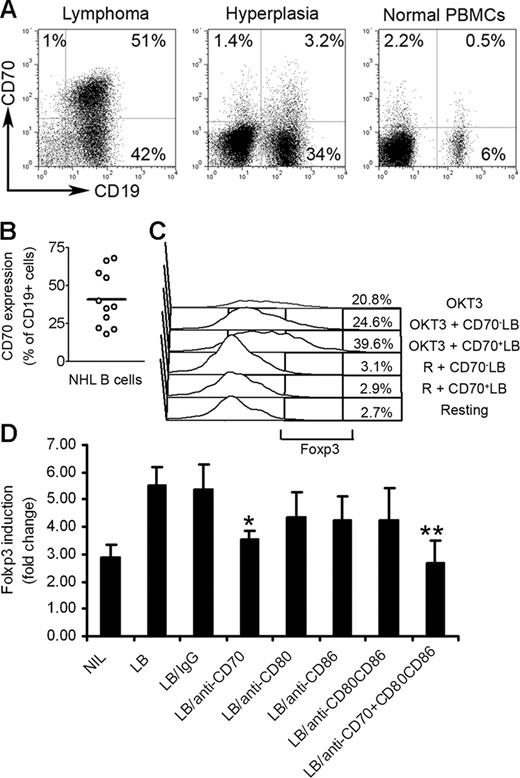
CD70 and CD27 contribute to lymphoma B-cell–mediated Foxp3 expression
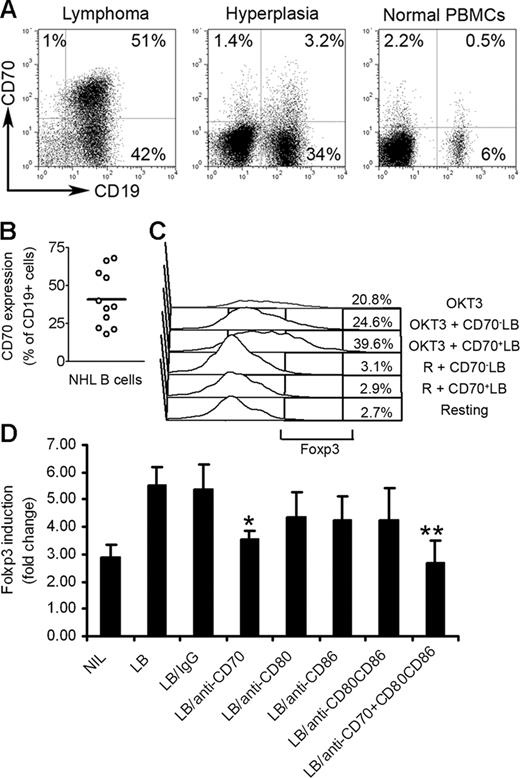
We next wanted to better understand the contribution of NHL B cells to up-regulation of Foxp3 expression. Lymphoma B cells have been previously reported to express low levels of costimulatory molecules CD80 and CD86. However, unlike their normal counterparts, they express high levels of CD70, the ligand for the tumor necrosis factor receptor superfamily member CD27.25 Because of this unique expression profile, we investigated the role of CD70 on the induction of Foxp3 expression in CD4+CD25− T cells. We first determined the expression of CD70 on normal, hyperplastic, and lymphoma B cells. As shown in Figure 5A, malignant CD19+ B cells in biopsy specimens from patients with B-cell NHL expressed abundant CD70 on approximately 48% of total CD19+ lymphoma B cells (Figure 5B). Interestingly, CD19+ cells in biopsy specimens from patients with follicular hyperplasia (nonmalignant) and PBMCs from healthy individuals express negligible amounts of CD70 (3.2% and 0.5%, respectively; Figure 5A). We also found that the CD70 receptor, CD27, was expressed on CD4+CD25− T cells (Figure S1).
Effect of CD27-CD70 interaction on Foxp3 induction in CD4+CD25− T-cell biopsy specimens of B-cell NHL. (A) Representative dot plots showing CD70 expression on CD19+ B cells in biopsy specimens from patients with lymphoma or biopsy specimens from patients with follicular hyperplasia or PBMCs from healthy individuals. Percentages indicate number of CD19+CD70+ cells as a percentage of all unsorted mononuclear cells. (B) Summarization of frequency of CD19+CD70+ cells in biopsy specimens of patients with NHL (n = 11). The horizontal bar represents the median Foxp3 expression level. (C) CD70+ (CD70+ LB) and CD70− (CD70− LB) lymphoma B cells were isolated by flow sorting and cocultured with intratumoral CD4+CD25− T cells in plates coated with (OKT3) or without (R) OKT3 for 3 days. Foxp3 expression was determined by intracellular staining. (D) Summarization of Foxp3 induction in CD4+CD25− T cells cocultured with lymphoma B cells (LB) treated with anti-CD70 Ab alone (LB/anti-CD70), or combination with anti-CD80 and CD86 Abs (LB/anti-CD70 + CD80CD86) in plates precoated with OKT3 for 3 days. Foxp3 expression was determined by intracellular staining. Foxp3 induction is indicated by fold change to group without stimulation (mean ± SE). *P < .05 compared with group with LB alone; **P < .01 compared with group with LB alone.
Effect of CD27-CD70 interaction on Foxp3 induction in CD4+CD25− T-cell biopsy specimens of B-cell NHL. (A) Representative dot plots showing CD70 expression on CD19+ B cells in biopsy specimens from patients with lymphoma or biopsy specimens from patients with follicular hyperplasia or PBMCs from healthy individuals. Percentages indicate number of CD19+CD70+ cells as a percentage of all unsorted mononuclear cells. (B) Summarization of frequency of CD19+CD70+ cells in biopsy specimens of patients with NHL (n = 11). The horizontal bar represents the median Foxp3 expression level. (C) CD70+ (CD70+ LB) and CD70− (CD70− LB) lymphoma B cells were isolated by flow sorting and cocultured with intratumoral CD4+CD25− T cells in plates coated with (OKT3) or without (R) OKT3 for 3 days. Foxp3 expression was determined by intracellular staining. (D) Summarization of Foxp3 induction in CD4+CD25− T cells cocultured with lymphoma B cells (LB) treated with anti-CD70 Ab alone (LB/anti-CD70), or combination with anti-CD80 and CD86 Abs (LB/anti-CD70 + CD80CD86) in plates precoated with OKT3 for 3 days. Foxp3 expression was determined by intracellular staining. Foxp3 induction is indicated by fold change to group without stimulation (mean ± SE). *P < .05 compared with group with LB alone; **P < .01 compared with group with LB alone.
We then isolated CD70+ or CD70− lymphoma B cells by flow sorting and cocultured them with CD4+CD25− T cells in the presence or absence of OKT3 for 3 days. We found that compared with CD70− lymphoma B cells, CD70+ lymphoma B cells more efficiently costimulated the activation and proliferation of infiltrating CD4+CD25− T cells (Figure S3). More importantly, CD70+ lymphoma B cells strongly enhanced Foxp3 expression in CD4+CD25− T cells (Figure 5C). CD70+ lymphoma B cells increased Foxp3 expression in CD4+CD25− T cells from 20.8% to 38.6%, while CD70− lymphoma B cells had little effect, only increasing Foxp3 from 20.8% to 24.6%. These results suggest that CD70+ lymphoma B cells contribute to activation-induced Foxp3 expression in CD4+CD25− T cells.
We next sought to further define the role of CD70 in regulation of Foxp3 expression. Using a blocking antibody approach, we inhibited the specific interaction between CD27 and CD70 and determined the effects on Foxp3 expression. In addition, we examined the effect of blockade of CD80 or CD86 on lymphoma-mediated Foxp3 expression in CD4+CD25− T cells, as CD80 and CD86 have been reported to be involved in the generation of CD4+CD25+ Treg cells from CD4+CD25− T cells.26,27 As shown in Figure 5D, treatment of lymphoma B cells with a blocking antibody specific to CD70 partially reversed the lymphoma B-cell–mediated Foxp3 expression in CD4+CD25− T cells (n = 10, P = .018 compared with the group with lymphoma cells alone). Importantly, the addition of anti-CD70 antibody combined with anti-CD80 and anti-CD86 Abs completely abrogated the ability of NHL B cells to up-regulate Foxp3 expression in CD4+CD25− T cells (n = 6; P < .001 compared with the group with lymphoma cells alone). We saw a moderate reversal of Foxp3 expression in CD4+CD25− T cells when treated with anti-CD80 Ab; however, this was not a statistically significant difference. Isotype controls for the blocking antibodies used in the study were also tested and found to have no effect on Foxp3 expression (Figure 5D). Taken together, these results suggest that interaction between CD70 and CD27 alone, or in combination with CD80 and CD86, contributes to lymphoma B-cell–mediated Foxp3 expression in intratumoral CD4+CD25− T cells.
Discussion
The key observations of this study are (1) CD4+CD25− T cells that express Foxp3 and have regulatory activity are present in biopsy specimens of patients with B-cell NHL; (2) lymphoma B cells, especially CD70+ lymphoma B cells, mediate activation-induced Foxp3 expression in CD4+CD25− T cells; and (3) the interaction between CD27 and CD70 contributes to the induction of Foxp3 expression. Initially, murine studies showed that Foxp3 is a lineage-specific marker of CD4+CD25+ Treg cells15,–17 and, consistent with this finding, that Foxp3 expression was not up-regulated in CD4+CD25−Foxp3− T cells following T-cell receptor (TCR) stimulation.16 Early work in human systems supported this conclusion, with Foxp3 being found only in CD4+CD25+ T cells with in vitro suppressive activity.28,29 However, later studies suggested that TCR stimulation of CD4+CD25−Foxp3− T cells led to the induction of Foxp3 expression7,30,31 More recently, Nishioka et al reported that a population of CD4+CD25−Foxp3+ T cells are present in aged mice, suggesting chronic stimulation of T cells over time changed the differentiation of T cells.18
In the present study, we have identified a subset of CD4+CD25− T cells that express Foxp3 present in the tumor site in B-cell NHL. Because Foxp3 expression confers regulatory function on T cells, we hypothesized that CD4+CD25−Foxp3+ cells may also have regulatory activity. Similar to Treg cells, we found that intratumoral CD4+CD25− T cells inhibit proliferation of tumor infiltrating CD8+ T cells. The inhibitory capabilities of CD4+CD25− T cells were not as high as that seen with CD4+CD25+ cells and this finding is likely due to the fact that only a subset of CD4+CD25− cells express Foxp3. Taken together with the finding that Foxp3 expression can be independent of CD25 expression, the actual number of T cells with regulatory function in areas of B-cell lymphoma may in fact be much higher than what we may have expected when using CD25 to identify Treg cells.
The mechanisms by which both CD4+CD25−Foxp3+ and CD4+CD25−Foxp3+ T cells suppress CD8+ T cells is poorly understood; however, it has been shown that both TCR activation and cell contact is required.32,33 Previous work from our lab also suggests that interactions between PD1 on CD4+ T cells and B7H1 on Treg cells may also be involved in Treg mediated-suppression of proliferation.22 Given the similar phenotypes between CD4+CD25−Foxp3+ and CD4+CD25+Foxp3+ T cells, we would hypothesize that both subtypes use similar mechanisms. However, further investigation is needed to definitively elucidate the exact mechanisms by which CD4+CD25−Foxp3+ exert their inhibitory function.
In addition to finding that CD4+CD25−Foxp3+ cells have regulatory function, our data also demonstrates that Foxp3 expression can be induced in CD4+CD25−Foxp3− T cells, confirming observations from other studies.7,34 The inducible properties of Foxp3 raise the question whether Foxp3 expression is a common activation marker. Our data indicated that induction of Foxp3 is cell-type specific and was not found in CD8+ T cells or CD19+ B cells. Activation-induced Foxp3 expression in intratumoral CD4+CD25− T cells was found to be associated with CD25 up-regulation, which was expected. However, CD25 expression was transient, while Foxp3 expression remained stable up to 14 days. The results suggest that CD4+CD25−Foxp3+ T cells may have at one time expressed CD25 after their initial activation. These data are consistent with data showing that continued culturing of human CD4+ T cells in the absence of continued stimulation led to maintenance of Foxp3 expression and acquisition of Treg functionality.7,35 Interestingly, Wang et al35 showed that long-term maintenance (14 days) of Foxp3 expression in CD4+CD25− T cells after activation correlated with suppressive activity. Their findings are similar to those reported in our study. However, not all cells retained Foxp3 expression, and it is therefore difficult to draw a general conclusion that all intratumoral CD4+CD25− T cells will maintain their Foxp3 expression upon activation based on the fact that not all peripheral blood T cells will maintain Foxp3 expression after activation.
A number of studies have supported the hypothesis that tumors evade immunologic rejection by the creation of an immunosuppressive microenvironment.19,36,,,–40 Specifically in B-cell lymphoma, it has been shown that lymphoma B cells induce antigen-specific T-cell anergy and significantly promote the development of a more generalized state of immunosuppression in mice.19 More recently, Zhou et al described a subset of tumor-induced CD25− Treg cells (TMTreg) in mice that arise after the mice are inoculated with lymphoma B cells.21 These TMTreg cells have increased expression of Foxp3 and IL-10, develop independently of preexisting natural Treg cells, and maintain suppressive properties long-term in the absence of antigen stimulation. Similar to our observations in patients with B-cell NHL, CD25 expression did not distinguish these tumor-induced regulatory cells. In conjunction with natural Treg cells, TMTreg cells induced tumor-specific CD4+ T-cell tolerance.21,41
In previous work, we and others have highlighted the role of tumor-secreted chemokines in the attraction of Treg cells into the tumor microenvironment9,22,42 ; however, once there, it is not clear how malignant cells further support Treg cells. CD70, a member of the TNF superfamily interacting with CD27, has been shown to play a role in cell differentiation and T helper 1 (Th1)/Th2 switch.43,44 In addition, CD70-expressing cells (nonhematopoietic origin) in the intestinal mucosa can function as APCs to control the proliferation and differentiation of T cells in the intestinal mucosa.45 Because CD70 has been shown by us as well as others25 to be expressed by malignant B cells, we hypothesized that CD70-expressing lymphoma B cells may uniquely support Treg cells. Our data clearly showed that CD70+ lymphoma B cells contribute to the lymphoma-mediated Foxp3 induction in intratumoral CD4+CD25− T cells. In addition, we also have found that CD70+ lymphoma B cells were able to serve as APCs and induce Foxp3 expression in CD4+CD25− T cells (Figure S3). These results suggest that the interaction between CD70−CD27 is involved in T-cell differentiation to Treg cells. Although CD80/CD86 have been reported to be involved in the generation of CD4+CD25+ Treg cells from CD4+CD25− T cells,26,27 we have not found a consistent and statistically significant reversion of Foxp3 induction by anti-CD86 Ab treatment, although we do see a moderate reversal of Foxp3 expression by anti-CD80 treatment. Weak expression of CD80 and CD86 on lymphoma B cells may account for this result.
The costimulatory and antigen-presenting functions of these CD70+ lymphoma B cells in mediating activation-induced Foxp3 expression in intratumoral CD4+CD25− T cells suggests that CD70 could be a potential therapeutic target for monoclonal antibody therapy in patients with B-cell NHL. In fact, recent publications have shown that CD70 is an attractive target for antibody-based therapeutics and that anti-CD70 antibodies may have antitumor activity, particularly in renal cell carcinoma.46,47
In summary, this study provides evidence for the first time in humans that CD4+CD25− T cells expressing Foxp3 are present in tumor sites of patients with B-cell NHL and that lymphoma B cells, especially CD70+ lymphoma B cells, are involved in activation-induced Foxp3 expression in intratumoral CD4+CD25− T cells. These results suggest an important mechanism by which lymphoma cells may escape immunosurveillance by inducing CD4+CD25− T cells to become suppressive and thereby generating a profoundly immune-suppressive tumor microenvironment. Our data suggest that while targeting CD4+CD25+ Treg cells may augment tumor-specific immune responses, residual CD4+CD25−Foxp3+ cells capable of mediating immune suppression would still remain and would continue to inhibit the host's antitumor response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants CA92104 and CA97274 from the National Institutes of Health and by a translational research grant from the Leukemia and Lymphoma Society.
National Institutes of Health
Authorship
Contribution: Z.-Z.Y. designed the research, performed experiments, analyzed data, and wrote the paper; A.J.N. designed the research, analyzed data, and wrote the paper; S.C.Z. performed experiments; T.E.W. analyzed data and wrote the paper; and S.M.A. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen M. Ansell, Division of Hematology and Internal Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail:ansell.stephen@mayo.edu.