CD37 is a lineage-specific B-cell antigen that to date has been neglected as an attractive therapeutic target. To exploit this, novel CD37-specific small modular immunopharmaceuticals (CD37-SMIP) that include variable regions linked to modified human IgG1 hinge, CH2, and CH3 domains were designed. The lead CD37-SMIP molecule induces potent apoptosis in the presence of a cross-linker, and antibody-dependent cellular cytotoxicity against B-cell leukemia/lymphoma cell lines and primary chronic lymphocytic leukemia (CLL) cells superior to therapeutic antibodies used in these diseases. The CD37-SMIP–dependent ADCC function in vitro was mediated by natural killer (NK) cells but not naive or activated monocytes. Significant in vivo therapeutic efficacy was demonstrated in a SCID mouse xenograft leukemia/lymphoma model. Depletion of NK cells in this mouse model resulted in diminished efficacy further supported the in vivo importance of NK cells in SMIP therapy. These findings provide strong justification for CD37 as a therapeutic target and introduce small modular immunopharmaceuticals as a novel class of targeted therapies for B-cell malignancies.
Introduction
Immunotherapy using monoclonal antibodies (MAbs) is emerging as a safe and selective method for the treatment of cancer.1 The role of monoclonal antibodies in B-cell malignancies has expanded since the introduction of rituximab (Rituxan) targeted against the CD20 antigen on the B-cell surface in 1997. Numerous studies have confirmed the efficacy of rituximab as a single agent and in combination therapy in low-grade non-Hodgkin lymphoma (NHL),2,,,–6 mantle-cell lymphoma,7,,,–11 diffuse large-cell lymphoma,12,13 and Burkitt leukemia/lymphoma.14 However, only a subset of patients respond to therapy and the majority of those eventually relapse after rituximab treatment. Therefore, identification of new therapeutic targets on B cells that are potentially more effective than CD20 represents a novel strategy for therapy of B-cell malignancies.
The CD37 antigen is one potential target that has not been adequately evaluated. CD37 is a heavily glycosylated 40- to 52-kDa glycoprotein and a member of the tetraspan transmembrane family of proteins.15,16 CD37 is expressed strongly on the surface of B cells and transformed mature B-cell leukemia and lymphoma cells17,,–20,22,23,25,26 but is either absent or minimally expressed on normal T cells.21 The CD37 antigen is expressed on monocytes and granulocytes at very low density and is absent on natural killer (NK) cells, platelets, and erythrocytes.15,22 During B-cell development, CD37 is expressed in cells progressing from pre-B to peripheral mature B-cell stages and is absent on terminal differentiation to plasma cells.23 Although the precise function of CD37 remains unknown, it has been found to form complexes with CD53, CD81, CD82, and class II glycoprotein on B-cell surface that may represent an ion channel or a transporter.24 CD37 has modest internalization and shedding in transformed B cells expressing the antigen.25,26 It is highly expressed in endosomes and exosomes in B lymphocytes, reflecting possible involvement in intracellular trafficking and antigen presentation.15 Targeted inactivation of CD37 in mice revealed no changes in the development of lymphoid organs but a reduced IgG1 level in the sera and an alteration of response to T-cell–dependent antigens, indicating a possible role of CD37 in T cell–B cell interaction.27
Given the relative B-cell selectivity, CD37 thus represents a valuable therapeutic target for malignancies derived from peripheral mature B cells, such as B-cell chronic lymphocytic leukemia (CLL), hairy-cell leukemia (HCL), and B-cell NHL.25,26 In particular, CLL may be a good target of CD37-based immunotherapy, because the expression of CD37 is relatively high, even compared with CD20, in this type of leukemia.17 Efforts to target CD37 clinically have been limited. One reported preclinical trial performed in the late 1980s examined the efficacy of 131I-labeled MB-1, a murine CD37 MAb in a mouse model.28 This was later examined as part of a clinical trial in patients with NHL,29,,,–33 in which both CD37 and CD20 antibodies were evaluated. Despite clinical responses observed in this study, CD20 was chosen as the target antigen by many for therapeutic antibody therapy, and no subsequent efforts have been made to target CD37.
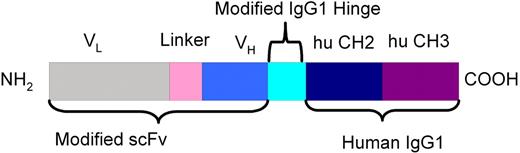
A CD37-small modular immunopharmaceutical (SMIP) was developed by Trubion Pharmaceuticals, using variable regions (VL and VH) from G28-1 hybridoma and engineered constant regions encoding human IgG1 domains (hinge, CH2, and CH3) (Figure 1). Initial expressions were performed by transfection of COS-7 monkey kidney cells and screened for specific binding to human B cell lines. The selected recombinant expression plasmid was used to transfect Chinese hamster ovary (CHO) cells and further selected under methotrexate pressure. The final stably expressing cell line was used in production of the fusion protein by purification from CHO culture supernatant by chromatography. To enhance the production of sufficient high-quality material, acceptable pharmacokinetics, and therapeutic efficacy, several technical considerations were made. These modifications provided a production efficiency that will allow sufficient production of CD37-SMIP for clinical investigation and were further screened for their ability to recruit effector cells to mediate cellular cytotoxicity. In addition, CD37-SMIP was engineered to have a molecular weight above that filtered by the glomerulus to avoid rapid elimination. This size feature of the CD37 SMIP offers the potential advantage of an extended half-life in vivo compatible with other biologic therapies such as monoclonal antibodies. Herein, we validate that CD37 is an exciting therapeutic target and provide strong in vitro and in vivo evidence to support clinical development of this novel CD37-SMIP in CLL, B-NHL, and related B-cell malignancies.
Patients, materials, and methods
Reagents and antibodies
CD37-SMIP (G28-1 scFv-Ig) and fluorescein isothiocyanate (FITC)–labeled CD37-SMIP were provided by Trubion Pharmaceuticals (Seattle, WA). Phycoerythrin (PE)–labeled mouse anti–human CD37 antibody (clone BL14), PE-labeled isotype control mouse IgG1, FITC-labeled annexin V, and propidium iodide (PI) were purchased from BD Pharmingen (San Diego, CA). N-benzyloxycarbonyl-Val-Ala-Asp-[Ome]-fluoromethylketone (Z-VAD-fmk) was purchased from EMD Biosciences (San Diego, CA). Alemtuzumab was produced by Ilex Pharmaceuticals (San Antonio, TX) and purchased commercially. Rituximab and trastuzumab were produced by Genentech (South San Francisco, CA) and purchased commercially. Goat anti–human IgG antibody (Fcγ fragment-specific) was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Anti-Caspase-3 (MAb AR-14) was gift from John Reed (Burnham Institute, La Jolla, CA). Anti-poly(ADP-ribose) polymerase (PARP; MAb C-2-10) was from Calbiochem (San Diego, CA). Anti-phosphotyrosine antibody clone 4G10 was from Upstate Biotechnology (Lake Placid, NY). Anti-GAPDH antibody was from Chemicon International Inc. (Temecula, CA). Herbimycin was purchased from Sigma (St. Louis, MO).
Patient sample processing and cell culture
All the patients enrolled in this study had immunophenotypically defined B-CLL as outlined by National Institute criteria.34 Blood was obtained from patients with informed consent in accordance with the Declaration of Helsinki and under a protocol approved by the institutional review board of Ohio State University (Columbus, OH). All of the patients with B-CLL had been without prior therapy for a minimum of 2 months. B-CLL cells were isolated immediately after donation using Ficoll density gradient centrifugation (Ficoll-Paque Plus; GE Healthcare, Chalfont St Giles, Buckinghamshire, United Kingdom). Isolated mononuclear cells were incubated in RPMI 1640 media supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT), 2 mM l-glutamine (Invitrogen, Carlsbad, CA), and penicillin (100 U/mL)/streptomycin (100 μg/mL; Sigma-Aldrich) at 37°C in an atmosphere of 5% CO2. Freshly isolated B-CLL cells were used for all the experiments described herein except for the surface staining. Samples used were more than 90% B cells as determined by CD19 surface staining and fluorescence-activated cell sorting (FACS) analysis. For those samples with less than 90% B cells, negative selection was applied to deplete non–B cells using B cell Isolation Kit II (Miltenyi Biotec, Auburn, CA) or by “Rosette-Sep” kit from Stem Cell Technologies (Vancouver, BC, Canada) according to the manufacturer's suggested protocol. Normal T-lymphocytes were separated from human peripheral blood mononuclear cells (PBMC) using negative selection using Pan T Cell Isolation Kit II (Miltenyi Biotec Inc).
In vitro treatment of cells with antibodies
Cells were suspended in media at a density of 1 × 106 cells/mL immediately after isolation. CD37-SMIP treatment used a 5 μg/mL concentration except for the dose-response studies. All other antibodies (trastuzumab, rituximab, and alemtuzumab), were used at 10 μg/mL. The cross-linker, goat anti–human IgG (Fc specific) was added to the cell suspension 5 minutes after adding the primary antibodies, at a concentration 5 times that of the primary antibodies (ie, 25 μg/mL for 5 μg/mL). For all CD37-SMIP treatment, a group of samples with the same concentration of trastuzumab treatment was applied as isotype control. In addition, a group of samples with no treatment was collected as media control, and a group treated with fludarabine (6.6 μmol/L) was also set up as a positive control. For examination of caspase inhibition, 150 μmol/L Z-VAD-fmk was added 10 minutes before the addition of the indicated reagents.
Assessment of apoptosis by flow cytometry
The apoptosis of cells was measured using annexin V-FITC/PI staining followed by FACS analysis according to the manufacturer's protocol (BD Pharmingen). Unstained cell samples and cells stained with annexin V-FITC or PI only were also processed for compensation. Results were presented as percentage cytotoxicity, which was defined as (% annexin V+ and/or PI+ cells of treatment group) − (% annexin V+ and/or PI+ cells of media control). FACS analysis was performed using a Beckman Coulter EPICS XL cytometer (Beckman Coulter, Fullerton, CA). Ten thousand events were collected for each sample and data were acquired in list mode. System II software package (Beckman Coulter) was used to analyze the data.
Cell-surface immunostaining and flow cytometry analysis
For CD37 surface expression analysis, B-CLL cells were isolated as described above, and used fresh or preserved in cryovials in 10% dimethyl sulfoxide (DMSO), 40% FBS, and 50% RPMI 1640 media in −180°C for less than 4 months. The expression level of CD37 was not altered as a result of the cryopreservation process. Cryopreserved samples were quickly thawed and washed twice in ice-cold PBS before use. Cells were incubated with PE-labeled anti-CD37, CD37-SMIP, or mouse IgG1 isotype control antibodies at 4°C for 30 minutes. The cells were then spun down at 300g for 10 minutes and rinsed twice with ice-cold PBS and analyzed by FACS. For the binding study, cells were preincubated with CD37-SMIP or trastuzumab (5 μg/mL) for 5 minutes followed by PE-labeled anti-CD37 mouse IgG. The incubation and wash procedure was identical to the surface-staining protocol. For cell lines, cells were split the night before, and fresh cells were used for immunostaining as described for B-CLL cells.
Western blotting
Immunoblot assays were performed with the multiple antigen detection (MAD) immunoblotting method as described previously.35 Whole-cell lysates were prepared and stored in −80°C. Protein concentration in the lysates was quantified by the bicinchoninic acid (BCA) method (Pierce, Rockford, IL). Protein samples (50 μg) were separated by SDS-PAGE (10% for PARP and phosphotyrosine and 12% for Caspase-3 blots), and transferred to 0.2 μmol/L nitrocellulose membranes (Whatman Schleicher & Schuell, Keene, NH). After incubation with indicated primary antibodies, horseradish peroxidase (HRP)-conjugated goat anti–rabbit IgG or goat anti–mouse IgG (Bio-Rad Laboratories, Richmond, CA) were used as secondary antibodies. The immune complexes were detected using a chemiluminescent substrate kit as described by the manufacturers (SuperSignal; Pierce, Rockford, IL).
Antibody-dependent cellular cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC) assay
ADCC activity was determined by standard 4-hour 51Cr-release assay. 51Cr-labeled target cells (5 × 104 B -CLL cells, Raji cells, or 697 cells) were placed in 96-well plates and various concentrations of antibodies were added to wells. Effector cells (PBMC, NK cells, or monocytes from healthy donors) were then added to the plates at indicated effector-to-target (E:T) ratios. After 4-hour incubation, supernatants were removed and counted in a gamma counter. The percentage of specific cell lysis was determined by: % lysis = 100 × (ER − SR)/(MR − SR), where ER, SR, and MR represent experimental, spontaneous, and maximum release, respectively.
For CDC assay, B-CLL cells at 106/mL were suspended in RPMI 1640 media, media with 30% autologous plasma from the patient blood samples, and media with 30% heat-inactivated (56°C, 30 minutes) plasma. Cells were then treated with antibodies for 10 minutes. After incubation at 37°C for 1 hour, cells were pelleted and resuspended with 100 μL 1× binding buffer (BD Pharmingen), then stained with 5 μL PI solution (BD Pharmingen). The extent of CDC was measured by FACS analysis of PI+ cells in duplicate samples.
Development of disseminated leukemia xenograft model
Female 4- to 6-week-old C.B.-17 SCID mice (Taconic Farms, Germantown, NY) were housed in pathogen-free, isolated cages. To ensure the consistency of engraftment, Raji cells were frozen in cryovials (1 × 107 cells each) and stored in a vapor-phase liquid nitrogen envivronment (−180°C). Before in vivo inoculation, cryopreserved cells were thawed, cultured for 10 days, and examined for the viability, CD37, CD20, and CD19 positivity and sensitivity to CD37 or CD20 antibody treatment. Only cells with greater than 90% viability and greater than 50% CD37 positivity were used for injection. Cells were resuspended in PBS at room temperature at a density of 107 cells/mL, in 200 μL (2 × 106 cells) and inoculated intravenously through tail vein using a mouse tail illuminator (Braintree Scientific, Braintree, MA). Untreated SCID mice developed symptomatic central nervous system, liver, and pulmonary metastasis that resulted in death from massive tumor burden 17 to 20 days after inoculation. Tissues obtained from killed tumor-bearing SCID mice were subjected to histopathologic examination to confirm the presence of disease. Gross pathologic, histologic, and histopathologic examination and immunohistochemical analysis of the tissues from treated and untreated mice were performed at the Ohio State University Comprehensive Cancer Center mouse phenotyping core facility. The residual human Raji cells were evaluated in formalin-fixed paraffin-embedded tissue sections of 4 to 5 μm by immunohistochemistry using monoclonal mouse anti–human CD45 (BD Pharmingen) followed by biotinylated goat anti-mouse secondary antibody Counterstaining was done with Richard-Allan Hematoxylin 2. Slides were then dehydrated and coverslipped. All washes were carried out in Dako Wash Buffer (Tris-buffered saline/Tween 20) (Dako Denmark A/S, Glostrup, Denmark). Bone marrow cell suspension was prepared by flushing fresh marrow-containing femurs with PBS and stained with PE-labeled antibodies to check the existence of human leukemia cells.
In vivo therapeutic efficacy evaluation in xenograft model
Antibody treatment was started 3 days after inoculation of Raji cells. CD37-SMIP or rituximab dissolved in 1 mg/mL saline were injected via tail vein, and maintained an every-other-day intravenous schedule for 2 weeks (5 mg/kg injection, 7 injections each mouse). Placebo (saline) and isotype control (trastuzumab) were administered on the same schedule and at the same dose. Animals were monitored daily for signs of illness and killed immediately if hind limb paralysis, respiratory distress, or 30% loss in body weight was noted. Body weight was measured once every week. Survival time (paralysis time) was used as primary endpoint for evaluation. For NK-cell depletion, anti-asialo GM1 antibody (30-μg injection in 200 μL of saline) was injected to mouse through tail vein at −6, −1, 4, 9, and 14 d after Raji cell inoculation. At day 10, 50 μL peripheral blood was obtained by retro-orbital bleeding was analyzed for NK cells by flow cytometry to confirm the depletion effect. In a separate group, 4 control SCID mice were treated with or without the same dose of anti-asialo GM1 antibody, and the splenocytes were prepared 2 days after the injection for cytotoxicity experiment using YAC-1 cells as target cells.
Statistical analysis of data
Analysis was performed by statisticians in the Center for Biostatistics, the Ohio State University, using SAS software (SAS Institute, Cary, NC). Comparisons were made using a 2-sided α = .05 level of significance. Mixed effects models were used to account for the dependencies in the cell- donor experiments, and analysis of variance (ANOVA) was used for the cell-line experiments. Synergy hypotheses were tested using interaction contrasts. Kaplan-Meier survival functions and log-rank tests were used to compare animal survival among different treatment groups.
Results
CD37-SMIP induced apoptosis of CD19+ B cells from CLL patients
Analysis of B cells from patients with CLL and T cells from healthy donors revealed a large number of cells expressing CD37 in B cells compared with T cells (96 ± 3% in CLL B cells and 3 ± 2% in T cells). The specific binding of genetically engineered CD37-SMIP to CD37 molecule was confirmed by flow cytometric analysis of B cells from patients with CLL. Furthermore, CD37-SMIP but not control antibodies such as trastuzumab (Herceptin, anti-HER2), rituximab (Rituxan, anti-CD20) or alemtuzumab (Campath, anti-CD52) blocked the binding of CD37-FITC to CD19+ B cells from patients with CLL (data not shown).
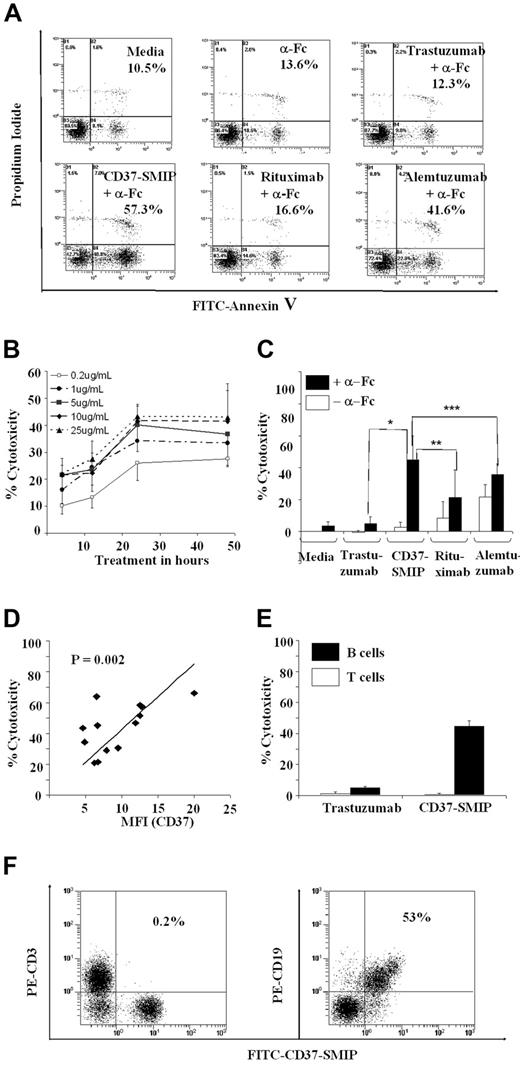
Induction of apoptosis in B cells from patients with CLL has been reported with therapeutic antibodies such as alemtuzumab29 and rituximab37 that are currently used to treat patients with CLL. To determine the effect of CD37-SMIP on B-cell survival, CD19+ cells from patients with CLL were treated with CD37-SMIP in the presence or absence of a secondary cross-linking, Fc-specific, goat anti–human IgG antibody (α-Fc[b]). Although no apoptosis was observed with CD37-SMIP in the absence of α-Fc, addition of α-Fc promoted maximal apoptosis (Figure 2A). The CD37-SMIP mediated apoptosis in a dose-dependent manner, with a maximal effect seen at 5 μg/mL, the CD37 saturating dose of CD37-SMIP in B-CLL cells (Figure 2B). We have thus applied 5 μg/mL for CD37-SMIP in the rest of our in vitro studies, and 10 μg/mL for other antibodies. This in part reflects the fact that CD37-SMIP has a lower molecular weight than other antibodies and thus is able to achieve sufficient molar concentration by using lower mass concentration. The cumulative results of these experiments with CD37-SMIP showed an increase in cytotoxicity in the presence of α-Fc compared with rituximab (P < .0001) or alemtuzumab (P < .0001) (Figure 2C) that represent the 2 therapeutic antibodies used to treat CLL currently. In addition, apoptosis with α-Fc was significantly higher for CD37 compared with rituximab (24.4% increase; 95% confidence interval [CI], 17%, 32%; P < .0001) or alemtuzumab (9% increase; 95% CI, 2%, 16%; P = .0130) (Figure 2C). The apoptosis induced by CD37-SMIP in B-CLL cells correlates with the expression of the target CD37 antigen (r = 0.78; P = .002; n = 13) (Figure 2D). Consistent with this, CD37-SMIP induced apoptosis in CD37+ B cells but not T cells that lack significant expression of CD37 (Figure 2E,F).
CD37-SMIP–induced cell death in B-CLL cells. (A) CD37-SMIP–induced cell death in CLL B cells. CLL B cells were treated with media, goat anti–human IgG (α-Fc), trastuzumab with α-Fc, CD37-SMIP with α-Fc, rituximab with α-Fc, or alemtuzumab with α-Fc for 24 hours. The cells were stained with FITC-annexin V and propidium iodide. The numbers shown in each panel represent percentage Annexin V+ and/or PI+ cells. Shown is a representative result from 14 patient samples analyzed. (B) Dose- and time-dependent induction of cytotoxicity by CD37-SMIP. B-CLL cells were treated with indicated concentrations of CD37-SMIP with cross-linker α-FC. The direct cell death at indicated time points was assessed by FITC-Annexin V/PI staining. Percentage cytotoxicity shown represents Annexin V+ and/or PI+ cells normalized with the media control. Error bars represent standard deviation (SD) among 4 B-CLL patient cell samples. (C) Summary of CD37-SMIP–induced direct cytotoxicity. B-CLL cells isolated from 14 patients were subjected to indicated treatment for 24 hours with or without cross-linker (α-Fc). Cell death was examined with FITC-Annexin V and PI. Percentage cytotoxicity shown represents Annexin V+ and/or PI+ cells normalized with the media control. Error bars represent SD among 14 B-CLL patient samples. Statistical analysis of difference between antibody treatments is shown by P values: *, P < .001; **, P < .001; **, P = .04. (D) Correlation of CD37 expression level and direct cytotoxicity by CD37-SMIP. MFI (CD37): mean fluorescence intensity of CD37 staining of different B-CLL cell samples. Percentage cytotoxicity shown represents Annexin V+ and/or PI+ cells normalized with the media control. (E) Flow cytometric analysis showing selective binding of CD37-SMIP to CD19+ but not CD3+ human PBMC. (F) CD37-SMIP–induced cytotoxicity in B but not T cells. Results shown are summary of FITC-Annexin V+ and/or PI+ cells normalized with media control in normal T cells (open bar; n = 5) or B-CLL cells (filled bar; n = 15) 24 hours after treatment with CD37-SMIP or trastuzumab. Error bars represent SD among samples.
CD37-SMIP–induced cell death in B-CLL cells. (A) CD37-SMIP–induced cell death in CLL B cells. CLL B cells were treated with media, goat anti–human IgG (α-Fc), trastuzumab with α-Fc, CD37-SMIP with α-Fc, rituximab with α-Fc, or alemtuzumab with α-Fc for 24 hours. The cells were stained with FITC-annexin V and propidium iodide. The numbers shown in each panel represent percentage Annexin V+ and/or PI+ cells. Shown is a representative result from 14 patient samples analyzed. (B) Dose- and time-dependent induction of cytotoxicity by CD37-SMIP. B-CLL cells were treated with indicated concentrations of CD37-SMIP with cross-linker α-FC. The direct cell death at indicated time points was assessed by FITC-Annexin V/PI staining. Percentage cytotoxicity shown represents Annexin V+ and/or PI+ cells normalized with the media control. Error bars represent standard deviation (SD) among 4 B-CLL patient cell samples. (C) Summary of CD37-SMIP–induced direct cytotoxicity. B-CLL cells isolated from 14 patients were subjected to indicated treatment for 24 hours with or without cross-linker (α-Fc). Cell death was examined with FITC-Annexin V and PI. Percentage cytotoxicity shown represents Annexin V+ and/or PI+ cells normalized with the media control. Error bars represent SD among 14 B-CLL patient samples. Statistical analysis of difference between antibody treatments is shown by P values: *, P < .001; **, P < .001; **, P = .04. (D) Correlation of CD37 expression level and direct cytotoxicity by CD37-SMIP. MFI (CD37): mean fluorescence intensity of CD37 staining of different B-CLL cell samples. Percentage cytotoxicity shown represents Annexin V+ and/or PI+ cells normalized with the media control. (E) Flow cytometric analysis showing selective binding of CD37-SMIP to CD19+ but not CD3+ human PBMC. (F) CD37-SMIP–induced cytotoxicity in B but not T cells. Results shown are summary of FITC-Annexin V+ and/or PI+ cells normalized with media control in normal T cells (open bar; n = 5) or B-CLL cells (filled bar; n = 15) 24 hours after treatment with CD37-SMIP or trastuzumab. Error bars represent SD among samples.
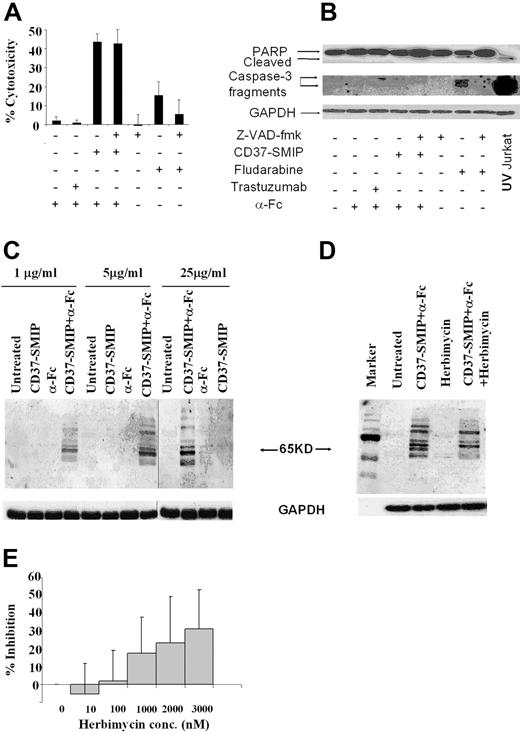
CD37-SMIP–induced CLL cell apoptosis was independent of caspase activation
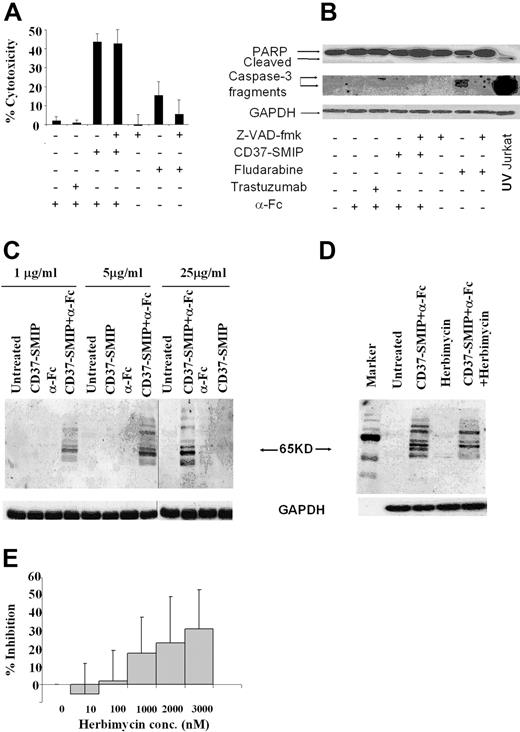
Antibody-induced cellular cytotoxicity is mediated through various mechanisms, including caspase activation dependent35,36 and/or independent pathways.37 In this context, we examined the mechanism by which CD37-SMIP induced apoptosis in CLL B cells. Fludarabine is widely used as a therapeutic agent in CLL and mediates death through a caspase-dependent pathway.39,40 Treatment of CLL B cells with fludarabine promoted modest apoptosis that was inhibited by the pan caspase inhibitor Z-VAD-fmk (Figure 3A). The caspase dependence of fludarabine was further supported by demonstrable processing of the inactive pro-form of caspase 3 and cleavage of downstream target poly(ADP-ribose) polymerase (PARP) after fludarabine treatment (Figure 3B). In contrast to fludarabine, CD37-SMIP promoted rapid apoptosis at 24 hours that was not prevented by Z-VAD-fmk treatment (Figure 3A). Furthermore, caspase 3 and downstream PARP were not processed after treatment with CD37-SMIP (Figure 3B). These data collectively suggest that CD37-SMIP induces apoptosis through a novel pathway different from fludarabine in primary CLL B cells. To determine protein tyrosine phosphorylation as a potential activation event responsible for CD37-SMIP–induced cell death, CD19+ B cells from patients with CLL were stimulated with CD37-SMIP with or without α-Fc. Western blot analysis of cellular lysates with anti-phosphotyrosine antibody revealed several tyrosine phosphorylated proteins including a predominant ∼65 kDa protein in response to the cross-linking of CD37-SMIP within 10 minutes (Figure 3C). Further, herbimycin, an inhibitor of tyrosine kinase, prevented phosphorylation of several of these proteins including the predominant ∼65 kDa protein (Figure 3D). It is noteworthy that increasing concentrations of herbimycin inhibited CD37-SMIP-induced apoptosis of CD19+ primary CLL cells (Figure 3E), supporting a potential role for tyrosine phosphorylation events in CD37-SMIP–induced apoptosis.
CD37-SMIP induces caspase-independent cell death in B-CLL cells. Freshly isolated B cells from 5 patients with CLL were treated with cross-linker alone (α-Fc), trastuzumab + α-Fc, CD37-SMIP + α-Fc, or fludarabine in the presence or absence of the pan-caspase inhibitor Z-VAD-fmk (150 μmol/L). (A) Z-VAD-fmk failed to inhibit CD37-SMIP–induced cell death. Percentage cytotoxicity shown represents Annexin V+ and/or PI+ cells normalized with the media control. Error bars represent SD among 5 B-CLL patient cell samples. (B) CD37-SMIP failed to induce activation of caspases in CLL cells. Cell lysates from primary CLL B cells treated with indicated conditions for 24 hours were assessed by Western blotting to detect PARP and caspase-3 cleavage. UV-irradiated Jurkat cell lysate was used as a positive control for both PARP and caspase 3 cleavage. (C) CD37-SMIP–induced tyrosine phosphorylation of cellular proteins. CLL cells were treated with indicated concentrations of CD37-SMIP with or without cross-linker in PBS for 10 minutes, and phosphotyrosine proteins were detected by Western blot analysis using anti-phosphotyrosine antibody 4G10. (D) Effect of tyrosine kinase inhibitor herbimycin on CD37-SMIP–induced tyrosine phosphorylation. Primary CLL cells were pretreated with media or herbimycin (3 μmol/L) before stimulation with CD37 SMIP + α-Fc. The tyrosine-phosphorylated proteins were detected by Western blot using anti-phosphotyrosine antibody 4G10. (E) Herbimycin inhibited CD37-SMIP–induced cell death in primary CLL cells. CLL B cells were treated with indicated concentrations of herbimycin followed by CD37-SMIP (5 μg/mL) + α-Fc. The cellular cytotoxicity was measured by FITC-Annexin V/PI staining at 48 hours after treatment. Data shown represents percentage inhibition of CD37-SMIP–induced cytotoxicity in the presence of indicated concentrations of herbimycin. Results are summary of 11 CLL patient samples. Error bars are the SD among these samples.
CD37-SMIP induces caspase-independent cell death in B-CLL cells. Freshly isolated B cells from 5 patients with CLL were treated with cross-linker alone (α-Fc), trastuzumab + α-Fc, CD37-SMIP + α-Fc, or fludarabine in the presence or absence of the pan-caspase inhibitor Z-VAD-fmk (150 μmol/L). (A) Z-VAD-fmk failed to inhibit CD37-SMIP–induced cell death. Percentage cytotoxicity shown represents Annexin V+ and/or PI+ cells normalized with the media control. Error bars represent SD among 5 B-CLL patient cell samples. (B) CD37-SMIP failed to induce activation of caspases in CLL cells. Cell lysates from primary CLL B cells treated with indicated conditions for 24 hours were assessed by Western blotting to detect PARP and caspase-3 cleavage. UV-irradiated Jurkat cell lysate was used as a positive control for both PARP and caspase 3 cleavage. (C) CD37-SMIP–induced tyrosine phosphorylation of cellular proteins. CLL cells were treated with indicated concentrations of CD37-SMIP with or without cross-linker in PBS for 10 minutes, and phosphotyrosine proteins were detected by Western blot analysis using anti-phosphotyrosine antibody 4G10. (D) Effect of tyrosine kinase inhibitor herbimycin on CD37-SMIP–induced tyrosine phosphorylation. Primary CLL cells were pretreated with media or herbimycin (3 μmol/L) before stimulation with CD37 SMIP + α-Fc. The tyrosine-phosphorylated proteins were detected by Western blot using anti-phosphotyrosine antibody 4G10. (E) Herbimycin inhibited CD37-SMIP–induced cell death in primary CLL cells. CLL B cells were treated with indicated concentrations of herbimycin followed by CD37-SMIP (5 μg/mL) + α-Fc. The cellular cytotoxicity was measured by FITC-Annexin V/PI staining at 48 hours after treatment. Data shown represents percentage inhibition of CD37-SMIP–induced cytotoxicity in the presence of indicated concentrations of herbimycin. Results are summary of 11 CLL patient samples. Error bars are the SD among these samples.
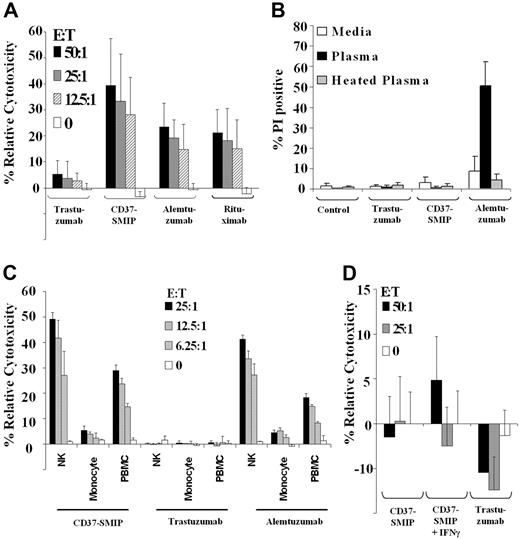
CD37-SMIP efficiently mediates ADCC but not CDC
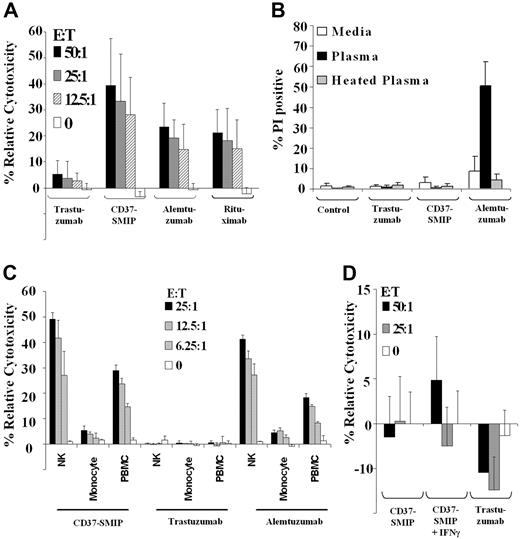
We next investigated whether the modified Fc region on CD37-SMIP is efficient in mediating ADCC and CDC against primary CD19+ CLL cells. Peripheral blood mononuclear cells (PBMCs) from normal healthy volunteers mediated ADCC in the presence of CD37-SMIP (39.3% ± 18%, E:T = 50:1) but not in the presence of the negative control trastuzumab (5.3% ± 5%, E:T = 50:1; Figure 4A). Furthermore, for all effector/target ratios tested, the ADCC with CD37-SMIP was found to be significantly higher than the ADCC with either alemtuzumab (14.5% higher; 95% CI, 9.0%, 19.9%; P < .0001) or rituximab (15% higher; 95% CI, 9.7%, 20.6%; P < .0001) that are directed against CD52 and CD20 molecules, respectively (Figure 4A). In contrast to their ability to mediate ADCC function, CD37-SMIP or rituximab failed to mediate CDC against CLL cells, whereas alemtuzumab effectively mediated CDC in vitro (Figure 4B).
CD37-SMIP induced ADCC but not CDC in primary CLL B cells. (A) CD37-SMIP induced ADCC against CLL B cells. Ability of trastuzumab, CD37-SMIP, alemtuzumab, or rituximab to mediate ADCC was evaluated using fresh human PBMC as effector cells and CD19+ primary CLL B cells as target cells at the indicated effector/target (E:T) ratios. Data shown here are summary of 6 patient samples and error bars represent SD among patients. (B) CD37 SMIP does not mediate CDC. Primary CLL B cells were treated with media, trastuzumab, CD37-SMIP, or alemtuzumab in the presence of media, human plasma or heat-inactivated human plasma for 1 hour. The CDC function was evaluated by propidium iodide staining. Summary of the results from 4 CLL patient samples are shown. Error bars show SD among patients. (C) In vitro evaluation of CD37-SMIP–induced ADCC function by NK cells, monocytes, or PBMC effector cells. Effect of purified NK cells, monocytes, or PBMC effector cells to mediate CD37-SMIP-, trastuzumab-, or alemtuzumab-dependent ADCC function against CD19+ primary CLL B cells as target cells was evaluated at the indicated effector/target (E:T) ratios by standard chromium release assay as described under “Materials and methods.” Data shown here are the summary of results from 6 patient samples, and error bars represent SD among patients. (D) Naive or IFN-γ activated monocytes failed to mediate CD37-SMIP–dependent ADCC function against CLL B cell targets. The effects of CD37-SMIP–induced ADCC using naive or IFN-γ–activated monocytes were measured as described above. Data shown here are summary of 3 patient samples (± SD).
CD37-SMIP induced ADCC but not CDC in primary CLL B cells. (A) CD37-SMIP induced ADCC against CLL B cells. Ability of trastuzumab, CD37-SMIP, alemtuzumab, or rituximab to mediate ADCC was evaluated using fresh human PBMC as effector cells and CD19+ primary CLL B cells as target cells at the indicated effector/target (E:T) ratios. Data shown here are summary of 6 patient samples and error bars represent SD among patients. (B) CD37 SMIP does not mediate CDC. Primary CLL B cells were treated with media, trastuzumab, CD37-SMIP, or alemtuzumab in the presence of media, human plasma or heat-inactivated human plasma for 1 hour. The CDC function was evaluated by propidium iodide staining. Summary of the results from 4 CLL patient samples are shown. Error bars show SD among patients. (C) In vitro evaluation of CD37-SMIP–induced ADCC function by NK cells, monocytes, or PBMC effector cells. Effect of purified NK cells, monocytes, or PBMC effector cells to mediate CD37-SMIP-, trastuzumab-, or alemtuzumab-dependent ADCC function against CD19+ primary CLL B cells as target cells was evaluated at the indicated effector/target (E:T) ratios by standard chromium release assay as described under “Materials and methods.” Data shown here are the summary of results from 6 patient samples, and error bars represent SD among patients. (D) Naive or IFN-γ activated monocytes failed to mediate CD37-SMIP–dependent ADCC function against CLL B cell targets. The effects of CD37-SMIP–induced ADCC using naive or IFN-γ–activated monocytes were measured as described above. Data shown here are summary of 3 patient samples (± SD).
The efficient ADCC function prompted us to evaluate the potential effector cell populations that may be involved in CD37-SMIP–mediated ADCC. We compared the ability of PBMC, NK, or monocyte effector cells to mediate ADCC in vitro. Figure 4C shows that NK cells enriched from PBMC, mediated an increase in CD37-SMIP dependent ADCC compared with normal mononuclear effector cells. In contrast, enriched monocytes failed to cause CD37-SMIP dependent ADCC (NK cells, 49.2% ± 2.6%; PBMC, 29.0% ± 2.1%; monocytes, 5.4% ± 1.8%). Activation of monocytes by interferon-γ (IFNγ) also failed to result in CD37-SMIP–mediated ADCC in CLL cells (Figure 4D). The importance of NK cells in CD37-SMIP–mediated ADCC was not unique to this target, as demonstrated by similar findings of NK-cell importance over monocytes in alemtuzumab-mediated ADCC (Figure 4C). These results indicate that NK cells, but not monocytes, are the major effector cell population in mediating the CLL cell death induced by CD37-SMIP.
CD37-SMIP mediated apoptosis and ADCC against CD37+ B- cell lymphoma cell lines
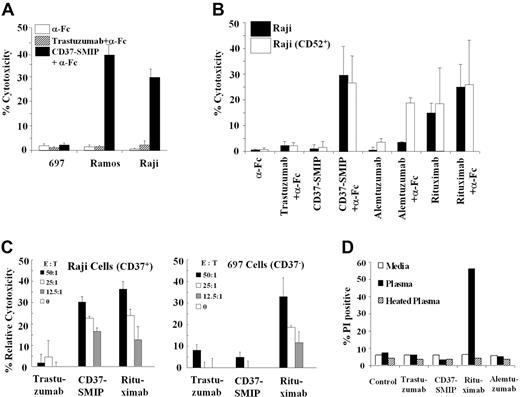
To confirm the relevance of our initial preclinical findings with CD37-SMIP described above, we sought to validate the efficacy of this agent in vivo. Initial screening of a panel of lymphoblastic B- cell lines, representing a variety of lymphoma/leukemia types revealed significant levels of expression of CD37 in Raji (62%, mean fluorescence intensity [MFI] = 70), Daudi (100%, MFI = 189), Ramos (100% MFI = 122), and RS11846 (99%, MFI = 70) lymphoblastic cell lines, whereas MEC-1 B-CLL cells (23%, MFI = 12) had medium expression. Both 697, an acute lymphoblastic lymphoma cell line (0.1% MFI = 0.93), and WAC B-CLL cell line (0.3%, MFI = 1) were negative for this antigen. We used the Raji and Ramos cells as 2 representative CD37+ cell lines and 697 cells as a CD37− cell line for our initial in vitro studies. CD37-SMIP promoted apoptosis in the CD37+ Ramos or Raji cell with the cross-linking antibody, whereas no death was noted in the CD37− 697 cell line (Figure 5A). In Raji cells, the level of cell death induced by CD37-SMIP exposure was similar to that achieved by rituximab (Figure 5B). Raji cells lack significant CD52 expression and were not sensitive to alemtuzumab treatment, unless presented as a CD52 high expression clone (R.L., unpublished data, October 2006). We also examined the healthy donor-derived PBMC for ADCC after treatment of target cells with CD37-SMIP. A strong interaction effect between cell lines (Raji vs 697) and treatment was suggested (P < .0001), indicating no effect of CD37-SMIP in 697 (CD37−) cells but a strong effect in Raji (CD37+) cells (Figure 5C). Similar to primary B-CLL cells, no CD37-SMIP-mediated CDC was observed in Raji cells (Figure 5D).
CD37-SMIP induced direct cytotoxicity and ADCC in human B-cell lines. (A) CD37-SMIP induced direct cytotoxicity in CD37+ (Raji and Ramos), but not in CD37−(697) human B-cell lines. Cytotoxicity was measured using FITC-Annexin V/PI staining at 24 hours after treatment as described in the legend to Figure 1A. Representative results from 3 independent experiments are shown. Error bars are SD among triplicate samples within the same experiment. (B) CD37-SMIP induced direct cytotoxicity in Raji B-cell lines comparable with that seen with alemtuzumab or rituximab. In vitro direct cytotoxic effects of CD37-SMIP, alemtuzumab, or rituximab in the presence or absence of cross-linker (α-Fc) was evaluated by FITC-Annexin V/PI staining. Percentage cytotoxicity was measured using FITC-annexin V/PI staining at 24 hours after treatment as described above. Raji parent clone (CD52−) and variant CD52+ clones were used in these studies. (C) CD37-SMIP induced ADCC in CD37+ Raji cells but not in CD37− 697 B-cell line. Effect of PBMC from healthy donors to mediate trastuzumab-, CD37-SMIP-, alemtuzumab-, or rituximab-dependent ADCC function against CD37+ (Raji cells) or CD37− (697 cells) target cells was evaluated at the indicated effector: target (E:T) ratios by standard chromium release assay as described in legend to Figure 3A. (D) Rituximab but not CD37-SMIP mediated CDC function against Raji B-cell line. Raji cells were treated with media, trastuzumab, CD37-SMIP, rituximab, or alemtuzumab in the presence of media, human plasma, or heat-inactivated human plasma for 1 hour. The CDC function was evaluated by propidium iodide staining and presented as percentage of PI positive cells in response to the various treatments. Results shown are representative of three independent experiments.
CD37-SMIP induced direct cytotoxicity and ADCC in human B-cell lines. (A) CD37-SMIP induced direct cytotoxicity in CD37+ (Raji and Ramos), but not in CD37−(697) human B-cell lines. Cytotoxicity was measured using FITC-Annexin V/PI staining at 24 hours after treatment as described in the legend to Figure 1A. Representative results from 3 independent experiments are shown. Error bars are SD among triplicate samples within the same experiment. (B) CD37-SMIP induced direct cytotoxicity in Raji B-cell lines comparable with that seen with alemtuzumab or rituximab. In vitro direct cytotoxic effects of CD37-SMIP, alemtuzumab, or rituximab in the presence or absence of cross-linker (α-Fc) was evaluated by FITC-Annexin V/PI staining. Percentage cytotoxicity was measured using FITC-annexin V/PI staining at 24 hours after treatment as described above. Raji parent clone (CD52−) and variant CD52+ clones were used in these studies. (C) CD37-SMIP induced ADCC in CD37+ Raji cells but not in CD37− 697 B-cell line. Effect of PBMC from healthy donors to mediate trastuzumab-, CD37-SMIP-, alemtuzumab-, or rituximab-dependent ADCC function against CD37+ (Raji cells) or CD37− (697 cells) target cells was evaluated at the indicated effector: target (E:T) ratios by standard chromium release assay as described in legend to Figure 3A. (D) Rituximab but not CD37-SMIP mediated CDC function against Raji B-cell line. Raji cells were treated with media, trastuzumab, CD37-SMIP, rituximab, or alemtuzumab in the presence of media, human plasma, or heat-inactivated human plasma for 1 hour. The CDC function was evaluated by propidium iodide staining and presented as percentage of PI positive cells in response to the various treatments. Results shown are representative of three independent experiments.
CD37-SMIP had potent in vivo anti-lymphoma activity
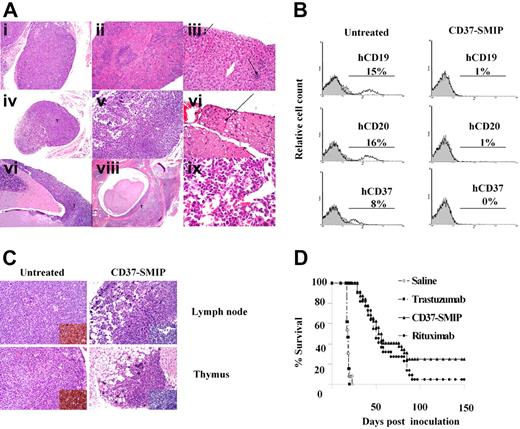
Given that primary B-CLL cells fail to successfully engraft in immunodeficient mice with recapitulation of the disease or a measurable end point, we chose to test in vivo therapeutic efficacy of CD37-SMIP in an aggressive Raji cell-inoculated disseminated leukemia/lymphoma xenograft mouse model. Sixteen days after inoculation, the SCID mice revealed neoplastic cell infiltrates throughout the body, as revealed by tissue sections (Figure 6A). Extensive replacement by tumor cells was observed in the lymph nodes, thymus, bone marrow, liver, spleen, soft tissue, and middle ear. In central nerve system (CNS), marked multifocal neoplastic cell infiltration was observed in meninges, and mild multifocal mineralization was also observed in brain. This was accompanied by neuropathy, degenerative spinal nerves and marked paresis of the hind legs at 17 to 20 days that preceded death by 1 to 2 days. In vitro, Raji cells in culture are also CD19 and CD20 positive (data now shown). Tumor cells derived from the bone marrow of engrafted mice maintained expression of hCD37 and hCD20 (Figure 6B), making this a valid model for in vivo investigation of CD37-SMIP and the relevant control antibody rituximab.
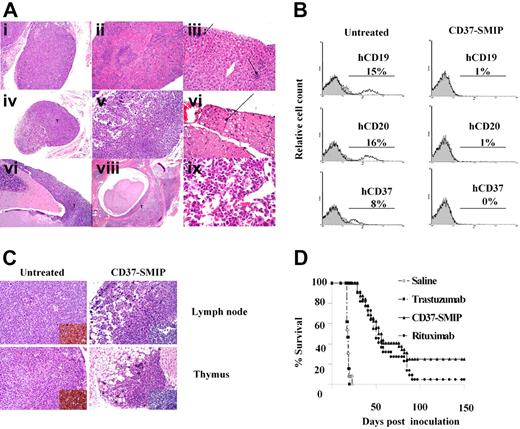
In vivo therapeutic evaluation of CD37-SMIP. A Raji cell-inoculated xenograft mouse model of disseminated leukemia/lymphoma was used to evaluate the therapeutic efficacy. (A) H&E staining of tissue sections from Raji cell–inoculated SCID mouse (placebo-treated). (i) Thymus infiltrated with neoplastic cells. (ii) Lymphoid atrophy/hypoplasia in spleen. (iii) Extramedullary hematopoiesis in liver (arrows). (iv) Atrophic lymph node partially replaced by tumor cells. (v) Markedly atrophic cervical lymph node. (vi) Spinal nerve showing rare swollen axons (arrow). (vii) Neoplastic cells in meninges over cerebellum. (viii) Neoplastic cells in vertebral bone marrow and within spinal canal. (ix) neoplastic cells in bone marrow. (B) Flow cytometric analysis of bone marrow cells from Raji cells– engrafted mice at day 17 after inoculation. Mice were treated with placebo (saline) or CD37-SMIP, and the percentage of human CD19+, human CD20+, or human CD37+ cells were analyzed by surface staining with PE-labeled antibodies. (C) Depletion of infiltrated human CD45+ cells in CD37-SMIP treated but not control mice. Histologic (H&E staining) analysis of thymus (top panels) and lymph node (bottom panels) from control untreated (left panels) or CD37-SMIP–treated (right panels) mice. Insert shows presence of human CD45+ cells in the untreated but not in CD37-SMIP–treated mice as detected by immunohistochemistry of the tissue sections. (D) Evaluation of therapeutic efficacy of CD37-SMIP in Raji cell–inoculated SCID mice. Raji cell–inoculated SCID mice were treated with saline (n = 22), negative control trastuzumab (n = 22), rituximab (n = 32), or CD37-SMIP (n = 32). Comparison among different groups was made by log-rank test (P < .001 between CD37-SMIP and trastuzumab, and P = .124 between CD37-SMIP and rituximab).
In vivo therapeutic evaluation of CD37-SMIP. A Raji cell-inoculated xenograft mouse model of disseminated leukemia/lymphoma was used to evaluate the therapeutic efficacy. (A) H&E staining of tissue sections from Raji cell–inoculated SCID mouse (placebo-treated). (i) Thymus infiltrated with neoplastic cells. (ii) Lymphoid atrophy/hypoplasia in spleen. (iii) Extramedullary hematopoiesis in liver (arrows). (iv) Atrophic lymph node partially replaced by tumor cells. (v) Markedly atrophic cervical lymph node. (vi) Spinal nerve showing rare swollen axons (arrow). (vii) Neoplastic cells in meninges over cerebellum. (viii) Neoplastic cells in vertebral bone marrow and within spinal canal. (ix) neoplastic cells in bone marrow. (B) Flow cytometric analysis of bone marrow cells from Raji cells– engrafted mice at day 17 after inoculation. Mice were treated with placebo (saline) or CD37-SMIP, and the percentage of human CD19+, human CD20+, or human CD37+ cells were analyzed by surface staining with PE-labeled antibodies. (C) Depletion of infiltrated human CD45+ cells in CD37-SMIP treated but not control mice. Histologic (H&E staining) analysis of thymus (top panels) and lymph node (bottom panels) from control untreated (left panels) or CD37-SMIP–treated (right panels) mice. Insert shows presence of human CD45+ cells in the untreated but not in CD37-SMIP–treated mice as detected by immunohistochemistry of the tissue sections. (D) Evaluation of therapeutic efficacy of CD37-SMIP in Raji cell–inoculated SCID mice. Raji cell–inoculated SCID mice were treated with saline (n = 22), negative control trastuzumab (n = 22), rituximab (n = 32), or CD37-SMIP (n = 32). Comparison among different groups was made by log-rank test (P < .001 between CD37-SMIP and trastuzumab, and P = .124 between CD37-SMIP and rituximab).
We next determined the therapeutic efficacy of CD37-SMIP relative to both trastuzumab and rituximab in this Raji cell-engrafted xenograft model. The depletion of transferred human B cells in the bone marrow was confirmed by flow cytometry using antibodies directed against human CD19, CD20, or CD37 molecules (Figure 6B). Consistent with this, hematoxylin-eosin staining and immunohistochemical analysis of tissue sections of thymus and lymph node with anti–human CD45 antibody revealed loss of human B cells in the CD37-SMIP treated but not untreated control groups (Figure 6C). Compared with placebo-treated (17 days; 95% CI, 16, 17; P < .001) or isotype-treated mice (17 days; 95% CI, 16, 17; P < .001), the median survival time for CD37-SMIP-treated mice (54 days, 95% CI, 45, 85) was significantly prolonged (Figure 6D). The hazard ratio and 95% confidence interval for rituximab versus CD37-SMIP (1.57; 95% CI, 0.87, 2.8; P = .137) suggest that CD37-SMIP is not inferior and could in fact be superior to rituximab. These data are in agreement with our in vitro data suggesting that CD37-SMIP may be comparable with or exceed rituximab efficacy.
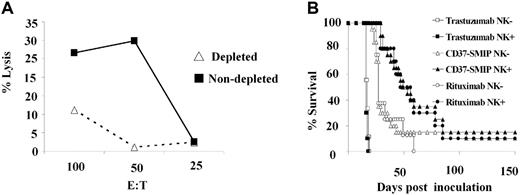
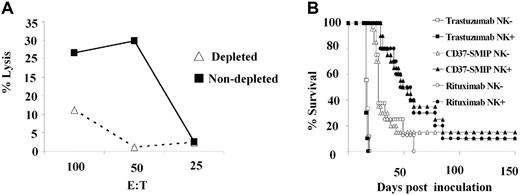
In vivo anti-lymphoma activity by CD37-SMIP was dependent on NK cells
Our in vitro studies suggested that NK cells, but not naive or activated monocytes, are critical mediators of CD37-SMIP–dependent ADCC. To determine the in vivo importance of NK cells, we evaluated the efficacy of CD37-SMIP or rituximab treatment in the above-described mouse model depleted of NK cells using anti-asialo GM1 antibody. Depletion of NK cells after anti-asialo GM1 antibody treatment, resulted in reduction of YAC-1 cell targeted cytotoxicity by the mouse splenocytes (Figure 7A). Consistent with this finding, immunostaining of peripheral blood mononuclear cells using mouse NK-cell– specific CD122 and DX5 antibodies also showed decreased NK cells (data not shown). Consistent with these in vitro results, the in vivo therapeutic efficacy of CD37-SMIP or rituximab was significantly compromised by depletion of NK cells (Figure 7B). The median survival time of CD37-SMIP–treated mice decreased from 51 days (95% CI, 38, 78) to 27 days (95% CI, 25, 37) (P = .017) with the depletion of NK cells. A similar result was also observed with the rituximab-treated group (49 days vs 25 days), which is consistent with an earlier report.34 The depletion of NK cells failed to exhibit significant effect to the trastuzumab control groups (16 days vs 17 days, P = .16). These data provide further support to the importance of NK cells in mediating CD37-SMIP efficacy in vivo.
In vivo anti-lymphoma activity by CD37-SMIP is dependent on NK cells. (A) Decreased ex vivo NK-cell activity in splenocytes from mice treated with anti-asialo GM1 antibody. Splenocytes from control or mice treated with NK-cell–depleting anti-asialo GM1 antibody were tested for their ability to mediate cytotoxicity against NK-cell target Yac-1 cells. Solid and broken lines represent percentage lysis mediated by cells from NK-undepleted and -depleted mice, respectively. (B) Depletion of NK cells reduced therapeutic efficacy of CD37-SMIP in vivo. In vivo therapeutic efficacy of trastuzumab, CD37-SMIP or rituximab were compared in control (NK+) or anti-asialo GM1 antibody–treated (NK−) Raji xenograft model. Survival is determined based on the paralysis time after inoculation. Log-rank test was applied for statistical analysis.
In vivo anti-lymphoma activity by CD37-SMIP is dependent on NK cells. (A) Decreased ex vivo NK-cell activity in splenocytes from mice treated with anti-asialo GM1 antibody. Splenocytes from control or mice treated with NK-cell–depleting anti-asialo GM1 antibody were tested for their ability to mediate cytotoxicity against NK-cell target Yac-1 cells. Solid and broken lines represent percentage lysis mediated by cells from NK-undepleted and -depleted mice, respectively. (B) Depletion of NK cells reduced therapeutic efficacy of CD37-SMIP in vivo. In vivo therapeutic efficacy of trastuzumab, CD37-SMIP or rituximab were compared in control (NK+) or anti-asialo GM1 antibody–treated (NK−) Raji xenograft model. Survival is determined based on the paralysis time after inoculation. Log-rank test was applied for statistical analysis.
Discussion
Herein, we describe a new targeted therapeutic approach using the SMIP directed at the lineage-specific CD37 antigen in CLL and related B-cell malignancies. CLL is currently incurable, and available antibody therapies targeting CD20 (rituximab) or CD52 (alemtuzumab) are either less effective or highly immunosuppressive. CD37 therefore represents a potentially superior target for peptide-based therapy, based on either expression features relative to CD20 or selectivity relative to CD52. Our data herein against primary CLL cells demonstrate that CD37-SMIP is superior to both rituximab and alemtuzumab relative to direct cytotoxicity (in the presence of a cross-linker) and ADCC efficacy. In addition, CD37 targeted SMIP therapy has B-cell selectivity that contrasts with alemtuzumab.37
CD37-SMIP differentiates itself from several other therapies used in CLL by mediating apoptosis through a novel caspase-independent pathway not used by other therapies used in this disease. Our data demonstrates that apoptosis by CD37-SMIP is directly proportionate to CD37 surface antigen expression and dependent in part on signal transduction and tyrosine phosphorylation of specific proteins. Disruption of CD37-SMIP–mediated tyrosine phosphorylation results in protection against apoptosis, a finding that contrasts with rituximab where herbimycin actually enhances apoptosis.38 Identification of these specific proteins phosphorylated by CD37-SMIP is currently under active investigation by our group. A difference in CD37-SMIP mechanism of action compared with rituximab is further supported by a preliminary report demonstrating synergy between rituximab and CD37-SMIP in lymphoma xenograft models.41
How CD37-SMIP cross modulates other cell-surface molecules, such as CD20 and CD52, is an important question to be addressed for potential combination of CD37-SMIP with other therapeutic antibodies currently being used for B-cell malignancies. We have conducted studies in both Raji cell lines and CLL cells, examining the effect of CD37-SMIP treatment toward the expression level of CD19, CD20, CD52, CD40, CD86, and HLA-DR. So far, our preliminary results have failed to reveal significant changes in expression of CD19, CD52, CD40, or CD86 in response to treatment with CD37-SMIP (with or without cross-linker). However, detectable modulation of CD20 expression as evidenced by decreased mean fluorescence intensity with minimal decrease in CD20+ cells have been variably observed. In contrast, both Raji cells and CLL cells seemed to have diminished binding affinity toward mouse anti–human CD37 monoclonal antibody, which may indicate either blocking of binding by CD37-SMIP or shedding of CD37 molecule from the cell surface. It will be a valuable approach to investigate whether combination of CD37-SMIP with other therapeutic antibodies currently used for B-cell malignancies therapy will bring synergistic effect.
The success of therapeutic antibodies to eliminate both normal B cells and NHL tumor cells seems to be greatly influenced by ability to mediate ADCC as demonstrated by both preclinical in vivo studies42,–44 and clinical trials in NHL where high affinity FcγRIIIa polymorphisms associated with enhanced ADCC are linked to superior response to rituximab.45,,–48 The critical role of FcγR in in vivo effects of therapeutic antibodies has also been supported by studies in mice deficient of FcγR.42,44 In CLL cells, however, rituximab mediates relatively poor ADCC, possibly explaining diminished efficacy of this antibody. Our data provide evidence that CD37-SMIP mediates significantly greater selective ADCC against CLL cells and related B-cell lymphoma cell lines compared with rituximab and alemtuzumab ADCC mediated by CD37-SMIP in CLL cells occurs predominantly through human NK cells but not monocytes. In contrast to what was observed in human systems, murine effector cells mediate less effective ADCC with CD37-SMIP compared with rituximab and alemtuzumab (data not shown). We nonetheless pursued in vivo studies in a highly aggressive Raji-disseminated leukemia/lymphoma model in which the therapeutic efficacy of CD37-SMIP may be superior than that observed with rituximab. A subsequent experiment demonstrated the importance of NK cells to the effectiveness of CD37-SMIP therapy and possibly rectified why only equivalent efficacy to rituximab was observed in this murine model of lymphoma. The ultimate test of CD37 SMIP efficacy will require pursuit of clinical studies in patients with CLL and related diseases. Despite macrophage/monocytes-mediated ADCC activity being observed with several antibodies,49,50 we failed to demonstrate that CD37-SMIP is associated with phagocytic efficacy in our studies. How the absence of CD37-SMIP–induced phagocytosis will differentiate this engineered protein from other Ab-based therapeutics will be interesting to further define the in vivo function of this molecule. Future studies on the importance of FcγRIIIa polymorphisms toward SMIP-based therapeutics will also be important to elucidate the clinical activity for this class of SMIP molecules compared with existing Ab therapeutics. The ultimate test of CD37 SMIP efficacy will require pursuit of clinical studies in patients with CLL and related diseases.
The development of targeted peptide therapies that depend on signaling and ADCC has been unsuccessful so far because of limited production capability and poor pharmacokinetic features relative to therapeutic antibodies. Preclinical development of the first CD20-directed SMIP Tru15 has demonstrated absence of immunogenicity in cynomolgus monkeys, and superior B-cell depletion compared with rituximab.51 Preliminary results of the first phase I study with Tru15 demonstrated it to be clinically well tolerated, and successful at depleting B-cells in a dose-dependent manner with an extended serum half-life (12-19 days).52 Thus, development of CD37-SMIP for clinical investigation in CLL based on our data herein represents an obvious extension of this exciting targeted therapeutic class of agents directed at a B-cell–selective antigen not yet pursued. The unique engineered features of SMIP-based therapies may offer an advantage over classic antibody based-therapies, including improved effector function, target affinity, and apoptotic signaling. Detection of immunogenicity-related neutralizing antibody response against CD37-SMIP after multiple injections at various frequencies in cynomolgus monkeys will be valuable to further investigate the immune response toward this class of engineered proteins with modified Fc region. In addition, pharmacokinetic studies to define the plasma half-life, and biodistribution studies to define the targeting effect will also be important to determine the clinical application of this novel targeted therapy in CD37+ lymphoid malignancies. Based on these exciting preclinical data, further clinical development of CD37-SMIP is warranted.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Cancer Institute (P01-CA95426), the Leukemia and Lymphoma Society of America, and the D. Warren Brown Foundation. J.C.B. is a clinical scholar of the Leukemia and Lymphoma Society of America.
Authorship
Contribution: X. Z. performed research, analyzed data, and wrote the manuscript; R.L., T.J., C.C., and A.G. performed research; M.S.H-L. and P.R.B. contributed vital new reagents, including construction of CD37-SMIP; T.S.L. contributed clinical patient samples; D.J. and A.L. performed statistical analysis; D.K. directed mouse pathology analysis; R.J.L. advised students and reviewed the manuscript; M.A.C. directed the program and contributed in raising funds; S.T. advised students and reviewed the manuscript; N.M. designed and supervised research and wrote the manuscript, J.C.B., the senior author, designed and supervised research and wrote the manuscript.
Conflict-of-interest disclosure: M.S.H-L. and P.R.B. are employees of Trubion Pharmaceuticals and have a financial interest in CD37-SMIP. The remaining authors declare no competing financial interests.
Correspondence: John C. Byrd, MD, B302 Starling Loving Hall, The Ohio State University, 320 West 10th Avenue, Columbus, Ohio 43210; e-mail:john.byrd@osumc.edu.