To the editor:
Descriptions of chromosomally abnormal Philadelphia-negative (Ph−) clones in chronic myeloid leukemia (CML) patients treated with chemotherapy,1 interferon-alpha,2 and, most recently, imatinib3,4 suggest that such events arise indirectly as a result of suppression of the BCR-ABL–positive cell population. However, the incidence and significance of karyotypic abnormalities in Ph− cells of patients receiving alternative tyrosine kinase inhibitors (TKIs) to imatinib have not yet been explored. We assessed a series of 35 patients with imatinib-resistant CML who then received dasatinib, for the emergence of cytogenetic abnormalities in Ph− cells. After commencing dasatinib treatment, 3 (8.6%) of the 35 patients acquired cytogenetic abnormalities in Ph− cells, namely trisomy 8, disomy Y, and ins(22;3)(q11;q26q21) (Table 1 patients 1 to 3, respectively). G-band data and retrospective interphase fluorescence in situ hybridization (FISH) analysis of fixed bone marrow preparations from these 3 patients excluded the presence of the Ph− clones prior to dasatinib treatment.
Patients 1 to 3 are the first reported cases of de novo clonal cytogenetic aberrations arising in Ph− cells during dasatinib treatment of CML. The incidence and type of karyotypic abnormalities in Ph− cells after dasatinib were similar to those reported in patients responding to imatinib, in which the majority of aberrations resemble recurrent primary cytogenetic abnormalities of other myeloid malignancies.3,4 The ins(22;3)(q11;q26q21) in patient 3 resulted in rearrangement of the EVI1 oncogene, demonstrable by FISH (Figure 1). Importantly, acquisition of EVI1-associated chromosome rearrangement in Ph− cells has previously been reported in 2 CML patients with a t(3;21) who rapidly developed Ph− myelodysplastic syndrome (MDS).4,5 The absence of myelodysplastic features in patient 3 demonstrates that, despite being a poor prognostic indicator in other myeloid malignancies, EVI1 rearrangement can exist in an apparently benign clone in CML patients without overt progression to secondary neoplasia.
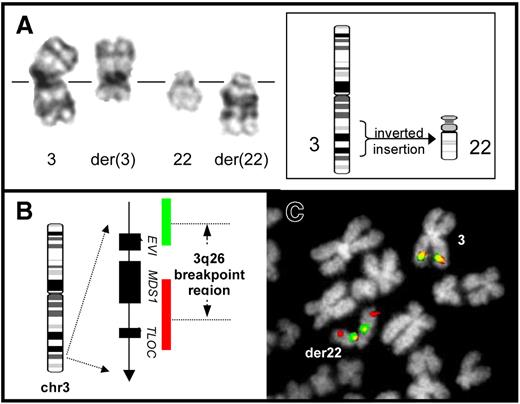
G-band and FISH analysis of an ins(22;3) in Ph− cells of a CML patient undergoing dasatinib treatment (patient 3). (A) Partial G-band karyotype showing the products of the ins(22;3)(q11;q26q21) (right) beside the normal chromosome homologues (left). Part of the q-arm of chromosome 3 is seen, inverted, within the structure of the abnormal chromosome 22. A simplified diagrammatic representation of the structural rearrangement resulting in this abnormality is shown (inset). (B) Structure of the EVI1 locus together with the composition and relative coverage of the dual-color EVI1 FISH probe (Kreatech Biotechnology, Amsterdam, The Netherlands). The probe system employs 2 separate components specific for the 5′ and 3′ portions of the locus, labeled in red and green, respectively. In cells without a 3q26 rearrangement, 2 red/green fusion signals are produced. In contrast, physical separation of 5′ and 3′ hybridization signals, or splitting of 1 of these component signals, is visible in cells with rearrangement of this region. (C) Analysis of an ins(22;3)–positive bone marrow metaphase cell from patient 3 using the dual-color EVI1 probe. One intact red/green fusion signal is observed on the normal chromosome 3 homologue, marking the unrearranged EVI1 locus. In contrast, the second EVI1 hybridization signal reveals relocation and rearrangement of this region, with part of the red 5′ signal hybridized toward the distal end of the der(22), whereas the remains of the red 5′ signal together with the green 3′ signal are present toward the centromere of this marker. This finding is consistent with an inversion of chromosome 3 involving a break distal to EVI1 and a subsequent insertion of 3q material, including the inverted region, into chromosome 22.
G-band and FISH analysis of an ins(22;3) in Ph− cells of a CML patient undergoing dasatinib treatment (patient 3). (A) Partial G-band karyotype showing the products of the ins(22;3)(q11;q26q21) (right) beside the normal chromosome homologues (left). Part of the q-arm of chromosome 3 is seen, inverted, within the structure of the abnormal chromosome 22. A simplified diagrammatic representation of the structural rearrangement resulting in this abnormality is shown (inset). (B) Structure of the EVI1 locus together with the composition and relative coverage of the dual-color EVI1 FISH probe (Kreatech Biotechnology, Amsterdam, The Netherlands). The probe system employs 2 separate components specific for the 5′ and 3′ portions of the locus, labeled in red and green, respectively. In cells without a 3q26 rearrangement, 2 red/green fusion signals are produced. In contrast, physical separation of 5′ and 3′ hybridization signals, or splitting of 1 of these component signals, is visible in cells with rearrangement of this region. (C) Analysis of an ins(22;3)–positive bone marrow metaphase cell from patient 3 using the dual-color EVI1 probe. One intact red/green fusion signal is observed on the normal chromosome 3 homologue, marking the unrearranged EVI1 locus. In contrast, the second EVI1 hybridization signal reveals relocation and rearrangement of this region, with part of the red 5′ signal hybridized toward the distal end of the der(22), whereas the remains of the red 5′ signal together with the green 3′ signal are present toward the centromere of this marker. This finding is consistent with an inversion of chromosome 3 involving a break distal to EVI1 and a subsequent insertion of 3q material, including the inverted region, into chromosome 22.
The biologic mechanism behind the genesis of Ph− clones remains elusive. It has been suggested that the karyotypic abnormalities provide evidence of a 2-step process of CML pathogenesis in which a monoclonal preleukemic stage exists that favors the acquisition of either the Philadelphia rearrangement or other chromosome aberrations. This notion has gained support from the recent observation that some patients responding to imatinib retain specific cytogenetic abnormalities other than the Ph chromosome that were present originally in Ph+ cells.6 Alternatively it is possible that Ph− clones arise as a result of increased pressure on normal hematopoietic stem cells to expand rapidly to replace the BCR-ABL–positive population. Such an environment would favor any BCR-ABL–negative cell that acquired a mechanism of selective advantage such as one that might be conferred by a cytogenetic abnormality. It is tempting to speculate that the latter effect might be compounded in those patients experiencing cytopenias, a side effect observed in around 50% of CML patients undergoing TKI therapy.7 However, of the 3 patients described here, cytopenia was detected only in patients 1 and 3 prior to the acquisition of the Ph− aberrant clone, thus excluding a consistent link between dasatinib-induced myelosuppression and Ph− clonal chromosome abnormalities.
Our findings demonstrate that Philadelphia-negative clonal hematopoiesis is not only a feature of imatinib therapy but also likely to be a general phenomenon associated with TKI-induced suppression of BCR-ABL–positive cells. These data emphasize the importance of conventional cytogenetic analysis as part of routine patient management for CML patients undergoing TKI therapy, even after induction of complete cytogenetic remission.
Authorship
Conflict of interest: The authors declare no competing financial interests.
Correspondence: Alistair G. Reid, Department of Haematology, Imperial College London, Hammersmith Hospital Campus, Du Cane Road, London W12 0NN, UK; e-mail: a.reid@imperial.ac.uk.