Abstract
B-lymphocyte–induced maturation protein-1 (BLIMP1), encoded by the PRDM1 gene, is a transcriptional repressor considered a master regulator that is required and sufficient for plasma cell (PC) differentiation. BLIMP1 represses the PAX5 gene, coding for the B-cell lineage–specific activator protein (BSAP), which is required for B-cell identity and survival. Mutations in PAX5 gene as well as in PRDM1 gene have been recently implicated in lymphomas. In the present study, sequence analysis of PRDM1 gene revealed a binding site for BSAP transcription factor. By analyzing different human cell lines, we have found that a specific nuclear factor for B-cell lines binds to a site on the PRDM1 promoter. Electrophoretic mobility shift assays identified this factor as BSAP, and chromatin immunoprecipitation assays confirmed its binding in vivo to the human PRDM1 promoter. Moreover, by ectopically expressing BSAP, and using a PRDM1 promoter with the BSAP-binding site mutated, we demonstrated that this factor represses the expression of BLIMP1. Therefore, repression of PRDM1 by BSAP reveals an autoregulatory negative-feedback loop that could play a relevant role in controlling human PC differentiation.
Introduction
In multicellular organisms, cell development and cell differentiation into many cell types is under the control of transcriptional factors. In the vertebrate immune system, the B-lymphocyte developmental pathway represents a model for the analysis of genetic networks, which orchestrate cell fate specification and commitment. Plasma cell (PC) differentiation from B lymphocyte depends on the switch-on/-off balance between transcription factors.1,2 In this context, BSAP is a critical transcription factor required to establish and maintain B-cell lineage identity until the PC stage.3-5 BSAP is a bifunctional transcription factor that can, depending on the gene context, either activate transcription of genes involved in maintaining B-cell identity including CD19,6 Igα, BLNK, and CIITA4 or repress transcription of PC-associated gene commitment such as J chain, IgH, IgL, and XBP1.7-10 In the absence of PAX5, the gene coding for BSAP, progenitor B cells acquire the ability to differentiate into multiple hematopoietic lineages both in vitro and in vivo.11-13 On the other hand, overexpression of PAX5 in the late stage of B-cell lines and in PC lines leads to increased cell proliferation and suppression of Ig synthesis.14 Thus, repression of PAX5 is important for inhibiting B-lymphocyte functions and is required for PC functions.15,16 Moreover, recent studies demonstrate the oncogenic role of BSAP by mutations or alterations in the expression of PAX5 gene.17-21
Murine B-lymphocyte–induced maturation protein-1 (BLIMP1), or its human homologue positive regulatory domain I binding factor 1 (PRDI-BF1), is a transcription factor that has been demonstrated to act as a master regulator required10 and sufficient22 for the generation and for the prolonged maintenance of PCs.23 To differentiate into PCs, BLIMP1 reduces B-cell proliferation as a consequence of MYC repression,24 and decreases B-cell functions as a result of PAX5 repression.25 An essential gene for PC differentiation indirectly induced by BLIMP1, as well as by IRF4, is XBP1.25-27 Moreover, BLIMP-1 (PRDM1) has been implicated in cell growth control through p53 repression28 and in diffuse large B-cell lymphomas.29,30
BCL6 is another transcription factor that plays a role in the germinal center reaction and in the inhibition of terminal B-cell differentiation.31-33 BCL6 acts as repressor of multiple genes,34 including the PRDM1 gene coding for BLIMP1. Direct as well as indirect mechanisms for this repression have been reported.34-37 At the same time, BLIMP1 could repress the expression of BCL6.38 Therefore, BCL6 and BLIMP1 together form a reciprocal regulatory loop in which BCL6 and BLIMP1 antagonize each other's expression.
Recently, the results obtained using a PAX5-deficient chicken B-cell line (DT40) as knockout model showed that PAX5−/− cells differentiate to PC fate exhibiting an up-regulation in BLIMP1 and XBP-1 expression and a down-regulation for BCL6 expression.39 This study suggests that BLIMP1 is up-regulated as consequence of the low BCL6 levels because of PAX5 deficiency. However, a simultaneous study using a knockout mouse model where conditional PAX5 inactivation takes place only in mature B cells did not show PAX5-mediated BCL6 and XBP1 regulation but demonstrated BLIMP1 activation.13 This study proposes that PAX5 could be involved in the repression of BLIMP1 and the corresponding PC transcription program.
In the present study, we have identified a BSAP-binding site onto the first exon of the PRDM1 gene, which has been corroborated by supershift assays. Our results show, for the first time in vitro, the direct repression of PRDM1 by expression of BSAP transcription factor. Moreover, BSAP binds to the PRDM1 promoter in vivo, as demonstrated by chromatin immunoprecipitation (ChIP) assays.
Materials and methods
Sequence retrieval and analysis programs
Genomic sequence for PRDM1 genes was retrieved from http://www.ensembl.org (Wellcome Trust Sanger Institute) with accession numbers AL358952(Homo sapiens), AC027700.11 (Mus musculus), Contig-1099214663548 (Macaca mulata), Contig-RNOR03271653 (Rattus norvegicus), Contig-13435 (Bos taurus), Contig-203874 (Dasypus novemcinctus), Contig-414711 (Loxodonta Africana), Contig-367418 (Felix catus), Contig-564227 (Echinops telfairi), and Contig-0.215 (Gallus domesticus), and were compared by CLUSTALW (European Bioinformatics Institute, http://www.ebi.ac.uk/clustalw). For searching transcription factor–binding sites, the TFSEARCH program (National Institute of Advanced Industrial Science and Technology, http://www.cbrc.jp/research/db/TFSEARCH.html) was used.
Cell cultures
Daudi, Raji, NALM-6, Ramos, U266, RPMI-8226, IM-9, NCI-H929, and HEp-2 cell lines were cultured in RPMI-1640 medium; and HEK293 cell line, in Dulbecco modified Eagle medium, supplemented with 10% fetal calf serum, glutamine, and antibiotics (100 IU/mL penicillin and 100 μg/mL streptomycin).
Isolation of the 5′ flanking region of the human PRDM1 gene and plasmid construction
The 5′-flanking region of the human PRDM1 gene (− 123/+ 138) was isolated by polymerase chain reaction (PCR). Two specific primer genes, 5′-GCTAGCAATCTGGGGGAAAG-3′ as sense (− 123 bp relative to the translation start site) and 5′-CTCGGCGGTCCCTCCTCG-3′ as antisense (+ 138 bp relative to the translation start site and including the entire exon 1), were selected on the basis of the sequence of the human PRDM1 genomic DNA accession number AL358952. The resulting PCR fragment was cloned and sequenced into the pGL3-Basic (pGL3-PRDM1) Firefly luciferase reporter vector (Promega, Madison, WI). For site-directed mutagenesis of the BSAP cis element in the region of the human PRDM1 promoter, we performed PCR amplification of pGL3-PRDM1 wild-type promoter with appropriate mutagenic primers (sense: 5′-TCGGCCCTCTTGTATTTCGGAGAGGCAAGAG-3′; antisense: 5′-GCTCTTGCCTCTCCGAAATACAAGAGGG-3′). PCR products were sequence confirmed and cloned and transferred to pGL3-Basic vector, obtaining a pGL3-PRDM1-BSAPmut vector.
A full-length human BSAP expression vector (pBSAP) was a gift provided by Dr M. Busslinger,40 and the BSAP cDNA was extracted from this vector and cloned into the pIRES2-EGFP (Clontech, Palo Alto, CA). To test the effect of the pBSAP-IRES2-EGFP expression vector, we transfected the same amount of a control plasmid consisting of pIRES2-EGFP empty vector.
Transient transfections and luciferase assays
For transfection experiments, Daudi cells were washed and resuspended at 107 cells/mL in serum-free RPMI-1640 medium supplemented with HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) 25 mM. One million cells (100 μL) were placed in a 0.4-cm electrode gap cuvette and, after addition of 10 μg pGL3-Basic or pGL3-Control plasmids or an equivalent molar amount of test plasmid along with 1 μg pRL-TK Renilla luciferase vector (Promega) (used as an internal control plasmid for differences in transfection efficiency), were subjected to electroporation using a Gene Pulser II apparatus (Biorad, Madrid, Spain). Electrical setting parameters were approximately 350 μF (τ∼20-30 ms) and 220 V for U266 or 240 V for Daudi cell lines. Immediately after the pulse, the cells were transferred to a well containing 900 μL antibiotic-free RPMI-1640 medium supplemented with 20% FCS and incubated at 37°C and 5% CO2. Cells were harvested approximately 48 hours later, washed with phosphate-buffered saline (PBS), and lysed, and the luciferase activity was measured using a de Dual luciferase reporter assay kit (Promega) and a Sirius luminometer (Berthold, Pforzheim, Germany).
For HEK293 cotransfection experiments, the Lipofectamine 2000 system (Invitrogen, Rockville, MD) was used. HEK293 cells were grown at 37°C with 5% CO2 with 10% serum and were seeded at 2 × 105 cells in 500 μL medium per well in 24-well plates. Plasmids pBSAP-IRES2-EGFP or empty pIRES2-EGFP (1 μg) and pGL3-PRDM1 (1 μg) and the pRL-TK reporter vector (0.01 μg) were mixed with 2 μL Lipofectamine 2000 reagent and added to the wells. HEK293 cells were harvested and the luciferase activity was measured 48 hours after transfection. Each transfection experiment was undertaken in triplicate, with at least 2 different plasmid preparations.
Western blotting
Total cell lysates from pBSAP-IRES2-EGFP– or pIRES2-EGFP–transfected HEK293 cells (∼ 105) or Daudi cells (∼ 106) were prepared in 2 × lysis buffer (125 mM Tris H-Cl, pH 7.5, β-mercaptoethanol 5%, SDS 4%, glycerol 10%), separated by 10% SDS–polyacrylamide gel electrophoresis and transferred to polyvinylidenefluoride (PVDF) membranes (Millipore, Billerica, MA). Blots were carried out as described.41 Briefly, PVDF membranes were blocked for 1 hour at 4°C with 5% dry nonfat milk/PBS and incubated overnight at 4°C with the anti-BSAP antibody clone 24 (BD Transduction Laboratories, Madrid, Spain) in the same blocking buffer (1:250). After 3 15-minute washes in TBST (25 mM Tris-HCl, pH 8, 500 mM NaCl, 25 mM KCl, 0.05% [wt/vol] Tween-20) and 1 wash with PBS, the membrane was incubated with secondary antibody in 5% dry nonfat milk/PBS at room temperature for 1 hour. After 3 more 15-minute washes with TBST, the specific protein was detected by the appropriate secondary alkaline phosphatase–conjugated antibody and a chromogenic reaction with NBT (nitroblue tetrazolium) and BCIP (5-bromo-4-chloro-3-indoyl phosphate p-toluidine salt) (Biorad).
Preparation of nuclear extracts and gel mobility shift assays
Nuclear extracts were prepared from cell lines by hypotonic lysis followed by high salt extraction of nuclei, as described.42 The protein concentrations of all the extracts were determined by the bicinchoninic acid protein assay (Pierce, Rockford, IL) with bovine serum albumin as the standard. Nuclear extracts were stored at − 70°C until used.
For probe + 1/+ 104, the corresponding human PRDM1 promoter DNA fragment was digested with HincII and XmaI and end-labeled by Klenow filling with [α-32P]dCTP. The human PRDM1 probe + 28/+ 59, as well the cold fragments for competitions, was obtained by annealing the complementary oligonucleotides leaving protruding ends, and labeled by Klenow filling with [α-32P]dCTP.
DNA-protein–binding assays were carried out in 20-μL reactions containing 12% glycerol, 60 mM KCl, 12 mM HEPES (pH 8), 4 mM Tris (tris(hydroxymethyl)aminomethane)-Cl (pH 8), 1 mM EDTA (ethylenediaminetetraacetic acid), 1 mM EGTA (ethyleneglycoltetraacetic acid), 1 mM DTT, 20 ng BSA, 1 to 5 μg nuclear extracts, and 1 μg poly(dA-dT)(dA-dT) as a nonspecific competitor (Sigma-Aldrich, Madrid, Spain). Reactions were incubated for 10 minutes at room temperature, the labeled probe (0.1-0.5 ng, ∼ 2 × 104 cpm) was added, and the incubation was continued for an additional 20-minute period. For competition studies, 10- to 100-fold molar excess competitor DNA was added to the reaction 10 minutes prior to the labeled probe. Supershift assays were performed by preincubating nuclear extracts with the antibody against BSAP (clone 24; BD Transduction Laboratories) or with a mouse IgG nonspecific antibody for 2 hours on ice before the probe addition. DNA-protein complexes were separated on 6% nondenaturing polyacrylamide gel in 0.5 × TBE buffer in a cold room. The gel was vacuum-dried and visualized by autoradiography.
ChIP assays
Chromatin immunoprecipitation (ChIP) procedure43 was performed as recommended in the manufacturer's protocol for Kit EpiQuik (Epigentek, Brooklyn, NY). Briefly, we incubated Daudi cells or NCI-H929 cells (∼ 106) with 1% formaldehyde for 10 minutes at room temperature. Chromatin was sheared by sonication (Branson Sonifier-150; (Brauson Ultrasonics, Danbury, CT) microtip probe, 6 × 15 seconds, output control 2, hold on ice 1 minute between pulses) performed to an average size of 200 to 1000 bp. After a spinning step to reduce debris, immunoprecipitates of cross-linked complexes were prepared using 3 μg anti-BSAP antibody (SC-1974X; Santa Cruz Biotechnology, Santa Cruz, CA). An unspecific antibody (mouse IgG) was carried out as a control for nonspecific interactions. After incubation at 25°C for 90 minutes on a rocking platform, the bound antibody-protein-DNA complexes (immunoprecipitates) were washed. After cross-linking reversal and proteinase K digestion, each individual immunoprecipitate was purified by elution. PCR reactions were performed with the corresponding specific primers (Table 1) for 38 to 42 cycles of amplification, and products were run on a 2% agarose gel and analyzed by ethidium bromide staining.
Results
Identification of cis elements in PRDM1 promoter
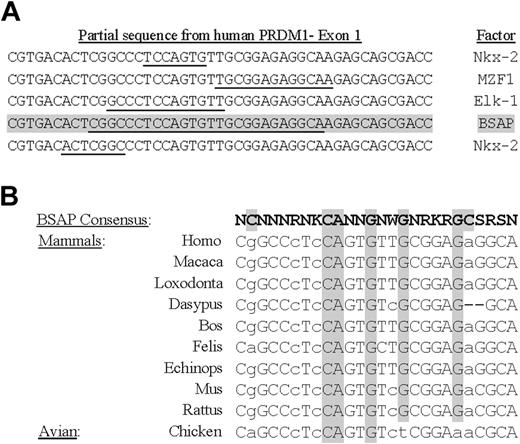
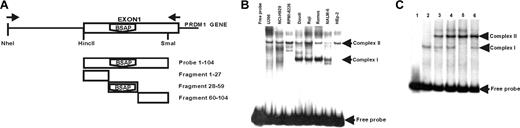
To study the promoter regulation of the human PRDM1 gene, a genomic fragment, containing approximately 2.3 Kbp upstream of the initiation start sites and the first exon extracted from sequence accession number AL358952, was analyzed with the TFSEARCH program.44 From the many theoretic binding sites for transcription factors, our attention was attracted to an intriguing site for BSAP located on the first exon coding for BLIMP1 (Figure 1A). Although the score matrix obtained for this factor was not too high (∼ 72.1), 2 recent works have shown that in PAX5-deficient B cells (in mouse as animal model or in a chicken B-cell line) the differentiation to PCs was stimulated.13,39 Moreover, the BLIMP1 transcript levels undergo a strong induction in PAX5-deficient mature B cells. Then, we decided to test if this possible binding site for BSAP was conserved through the evolution, by a phylogenetic analysis of the sequences of PRDM1 gene originating from different mammals and from chicken (avian). The program used was CLUSTALW and the result is shown in Figure 1B. As can be seen, the main nucleotide positions for the BSAP consensus-binding site were well preserved between the mammal species, and a satisfactory match exists with the theoretic consensus-binding site. However, although the chicken presents a good match in sequences compared with mammals, its consensus-binding sequence for BSAP differs much more and lacks 4 central conserved positions for possible binding. Then, on the basis of the theoretic BSAP-binding site in the human PRDM1 gene, we decided to test if it could, in fact, be functional in human mature B-cell fate.
BSAP cis element in exon 1 of human PRDM1 gene. (A) Location of binding sites for transcription factors in a partial sequence of human PRDM1 gene. Theoretic binding sites for the factor are underlined, and the score obtained with the TFMATRIX program for binding site matching is shown. Data for BSAP factor are shaded. (B) Comparison of phylogenetic sequences in exon 1 regions between mammals and avian species. The BSAP consensus-binding site region (shown in bold) was obtained with the TFMATRIX program. The most conserved positions are shaded. Capital and lowercase letters show nucleotides matching or not matching the theoretic BSAP-binding site, respectively. Note that chicken sequence lacks 4 essential positions against 2 lacking in mammals.
BSAP cis element in exon 1 of human PRDM1 gene. (A) Location of binding sites for transcription factors in a partial sequence of human PRDM1 gene. Theoretic binding sites for the factor are underlined, and the score obtained with the TFMATRIX program for binding site matching is shown. Data for BSAP factor are shaded. (B) Comparison of phylogenetic sequences in exon 1 regions between mammals and avian species. The BSAP consensus-binding site region (shown in bold) was obtained with the TFMATRIX program. The most conserved positions are shaded. Capital and lowercase letters show nucleotides matching or not matching the theoretic BSAP-binding site, respectively. Note that chicken sequence lacks 4 essential positions against 2 lacking in mammals.
Specific DNA-protein complexes are observed with B-cell nuclear extracts
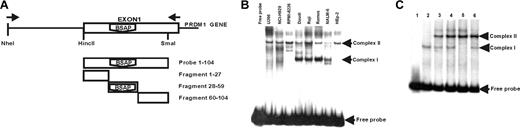
To identify if BSAP could bind to the PRDM1 promoter region, we performed an electrophoretic mobility shift assay (EMSA) with nuclear extracts from Daudi, Raji, Ramos, and NALM-6 (as B-cell lines), from U266, NCI-H929, RPMI-8226 (as PC cell lines), and HEp-2 (an epithelial cell line). For this purpose, we have used as probe a human PRDM1 gene fragment running from a HincII site up to a SmaI site, both located in the first exon (Figure 2A). This probe contains the theoretic BSAP-binding site identified. The EMSA results (Figure 2B) show a different pattern of protein complexes depending on the nuclear extract analyzed, with 2 principal retarded complexes observed. One complex, denoted as complex II, appears in all the nuclear extracts tested (lymphoid and nonlymphoid cell lines), indicating an unspecific binding or a general binding factor. The other, denoted complex I, was observed in nuclear extracts from B-cell line but not in those from PC or HEp-2 cell lines. This preliminary result suggested to us that probably several different factors can bind to the PRDM1 promoter and that at least one of them is specific in B-cell nuclear extracts.
EMSA comparing nuclear extracts from B lymphocytes and PC lines for specific binding to exon 1 of the human PRDM1 gene. (A) Schematic representation of the fragment used as probe and the competitive fragments for EMSA. A fragment corresponding to the entire exon 1 (+ 1/+ 104) was used as probe. Three cold fragments, divisions of exon 1 (+ 1/+ 27), (+ 28/+ 59), (+ 60/+ 104), were used for competition experiments. (B) EMSA with probe (+ 1/+ 104) and nuclear extracts from human PC lines: U266 (myeloma), NCI-H929 (myeloma), RPMI-8226 (myeloma); from human B-cell lines: Daudi (Burkitt lymphoma), Raji (Burkitt lymphoma), Ramos (Burkitt lymphoma), NALM-6 (pre-B-cell leukemia); or from the epithelial cell line: HEp-2 (laryngeal carcinoma). Lane marked as free probe was nuclear-extract–free. (C) Competition experiments using the probe (+ 1/+ 104) and Daudi nuclear extracts. (Lane 1) Probe-free. (Lanes 2,3) Probe with nuclear extract without and with Zn++ (0.5 mM) in the binding buffer, respectively. Competition assays with the corresponding cold fragments (100 ×) are shown: (lane 4) + 1/+ 27; (lane 5) + 28/+ 59; and (lane 6) + 60/+ 104.
EMSA comparing nuclear extracts from B lymphocytes and PC lines for specific binding to exon 1 of the human PRDM1 gene. (A) Schematic representation of the fragment used as probe and the competitive fragments for EMSA. A fragment corresponding to the entire exon 1 (+ 1/+ 104) was used as probe. Three cold fragments, divisions of exon 1 (+ 1/+ 27), (+ 28/+ 59), (+ 60/+ 104), were used for competition experiments. (B) EMSA with probe (+ 1/+ 104) and nuclear extracts from human PC lines: U266 (myeloma), NCI-H929 (myeloma), RPMI-8226 (myeloma); from human B-cell lines: Daudi (Burkitt lymphoma), Raji (Burkitt lymphoma), Ramos (Burkitt lymphoma), NALM-6 (pre-B-cell leukemia); or from the epithelial cell line: HEp-2 (laryngeal carcinoma). Lane marked as free probe was nuclear-extract–free. (C) Competition experiments using the probe (+ 1/+ 104) and Daudi nuclear extracts. (Lane 1) Probe-free. (Lanes 2,3) Probe with nuclear extract without and with Zn++ (0.5 mM) in the binding buffer, respectively. Competition assays with the corresponding cold fragments (100 ×) are shown: (lane 4) + 1/+ 27; (lane 5) + 28/+ 59; and (lane 6) + 60/+ 104.
To verify that probability and to locate more accurately where the protein complexes bind, we decided to carry out competition assays. To do this, we divided the fragment corresponding to the probe into 3 smaller fragments, fragment + 1 + 27, fragment + 28 + 59, and fragment + 60 + 104 (Figure 2A), and these were assayed in competition EMSAs with the intact probe (+ 1 + 104). Daudi nuclear extracts were used. As shown in Figure 2C, the complex II formation was Zn++ dependent (compare lanes 2, 3), and it was not ablated by any of the competitive fragments. The same result was obtained in competition experiments for this complex II, when fragment + 1 + 59 or + 28 + 104 (data not shown), suggesting that the binding site for this complex formation was not located on the excision sequences between smaller fragments. In contrast, complex I was apparently Zn++ independent, and the fragment + 28 + 59 showed an obvious competition (Figure 2C lane 5), although the other 2 fragments, + 1 + 27 and + 60 + 104, did not (Figure 2C lanes 4, 5, respectively). The combined observations that the fragment + 28 + 59 included the predicted BSAP-binding sequence, and that only complex I was present in B-cell lines nuclear extracts, suggest that BSAP factor could be involved in the formation of complex I.
BSAP binds to the PRDM1 gene exon 1 region
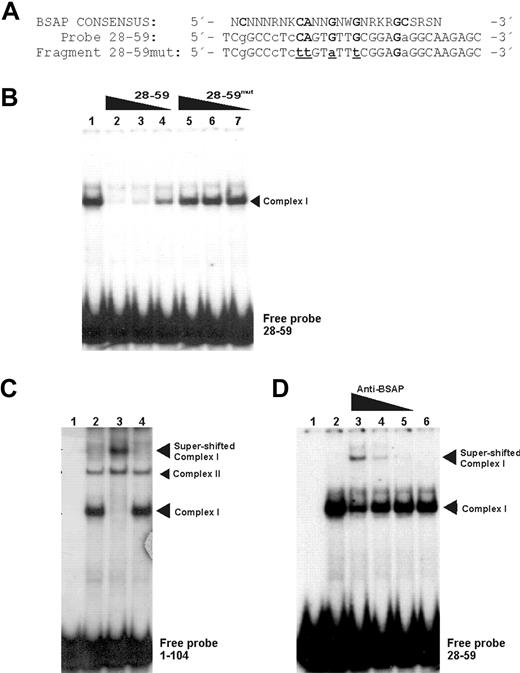
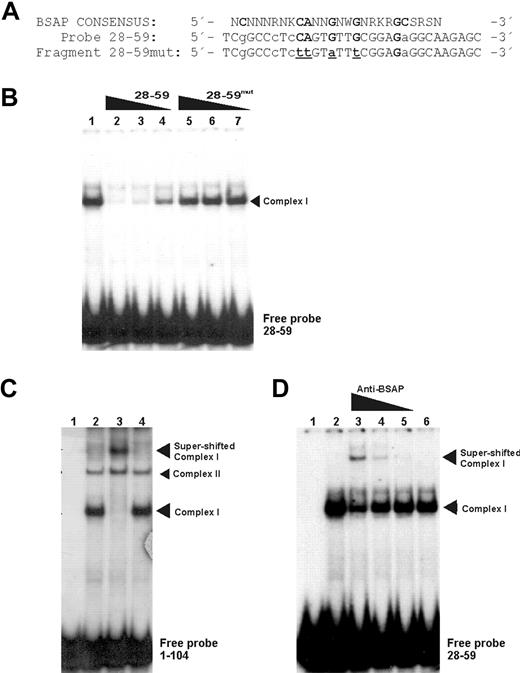
To support the last assumption and to identify the protein responsible for complex I, we performed EMSA experiments, but this time with a probe corresponding to the fragment + 28 + 59. In addition, the same fragment + 28 + 59, but mutated (+ 28 + 59mut) in the nucleotides most conserved for BSAP-binding site (Figure 3A), was used in competition experiments. As can be seen in Figure 3B, the + 28 + 59 probe showed a predominant retarded band (lane 1) corresponding to complex I obtained with the probe + 1 + 104. As expected, the cold fragment + 28 + 59 displaced this complex in a concentration-dependent manner (Figure 3B lanes 2-4). However, no competition was obtained when the + 28 + 59mut fragment for BSAP-binding site was added to the assays (Figure 3B lanes 5-7). This result demonstrated that complex I is caused by the BSAP-binding site. However, the crucial result confirming that the BSAP factor was able to bind to the PRDM1 promoter region and to produce complex I required supershift assays to be performed. These experiments, performed with the + 1 + 104 probe or with the + 28 + 59 probe, are shown in Figure 3C and 3D, respectively. A supershifted band was observed by an antibody against BSAP (Figure 3C lane 3). Moreover, the BSAP supershift specificity for complex I could be observed when compared with complex II (not shifted). The supershift specificity was confirmed when the smaller probe (+ 28 + 59) was used, and the antibody anti-BSAP was included in a dose-dependent manner (Figure 3D). No superretarded complexes appeared when a control nonrelated antibody was used (Figure 3C lane 4 or Figure 3D lane 6). Moreover, an attenuation of complex I was observed, thus demonstrating that it contained BSAP.
BSAP factor identification by specific binding site mutations and supershift EMSA. (A) Sequence comparison between the BSAP consensus-binding site and the wild-type fragment (+ 28 + 59) or mutated fragment (+ 28 + 59mut) sequences corresponding to abolish the possible BSAP-binding site. Bold capital letters represent the most conserved nucleotides. Capital and lowercase letters represent the nucleotides matched and unmatched to the consensus-binding site, respectively. Underlined letters are the mutated ones. (B) EMSA with Daudi nuclear extracts and the fragment (+ 28 + 59) used as probe (lane 1). Competition experiments were performed with the cold wild-type fragment (+ 28 + 59) (lanes 2-4) or mutated fragment (+ 28 + 59mut) (lanes 5,6). Molar ratios of the cold fragment were 100 × (lanes 2,5), 50 × (lanes 3,6), and 10 × (lanes 4,7) (C) Supershift experiment with Daudi nuclear extract and probe + 1/+ 104. (Lanes 1 and 2) Probe without and with extracts, respectively. Antibody against BSAP (1 μL) was added to the assays (lane 3). A nonspecific antibody control (1 μg/μL) was used (lane 4). (D) Same as panel C, except probe + 28 + 59 was used and the antibody against BSAP was added to the assays in descending concentration (0.1, 0.05, and 0.01 μL) from lane 3 to lane 5. Control nonspecific antibody (lane 6).
BSAP factor identification by specific binding site mutations and supershift EMSA. (A) Sequence comparison between the BSAP consensus-binding site and the wild-type fragment (+ 28 + 59) or mutated fragment (+ 28 + 59mut) sequences corresponding to abolish the possible BSAP-binding site. Bold capital letters represent the most conserved nucleotides. Capital and lowercase letters represent the nucleotides matched and unmatched to the consensus-binding site, respectively. Underlined letters are the mutated ones. (B) EMSA with Daudi nuclear extracts and the fragment (+ 28 + 59) used as probe (lane 1). Competition experiments were performed with the cold wild-type fragment (+ 28 + 59) (lanes 2-4) or mutated fragment (+ 28 + 59mut) (lanes 5,6). Molar ratios of the cold fragment were 100 × (lanes 2,5), 50 × (lanes 3,6), and 10 × (lanes 4,7) (C) Supershift experiment with Daudi nuclear extract and probe + 1/+ 104. (Lanes 1 and 2) Probe without and with extracts, respectively. Antibody against BSAP (1 μL) was added to the assays (lane 3). A nonspecific antibody control (1 μg/μL) was used (lane 4). (D) Same as panel C, except probe + 28 + 59 was used and the antibody against BSAP was added to the assays in descending concentration (0.1, 0.05, and 0.01 μL) from lane 3 to lane 5. Control nonspecific antibody (lane 6).
BSAP represses the PRDM1 promoter activity
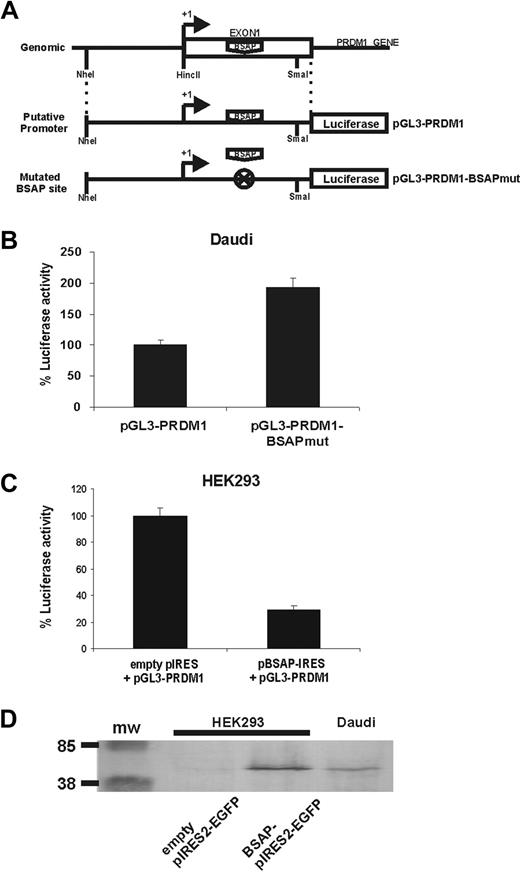
Luciferase reporter assays were used to determine whether BSAP could be functional for PRDM1 promoter activity. The genomic fragment − 123 + 138, corresponding to the PRDM1 minimal promoter encompassing a small fragment of the 5′ upstream of exon 1 and the entire exon 1, was cloned upstream of the luciferase gene and used in our reporter assays (Figure 4A). Two constructs, the putative minimal promoter luciferase construct (pGL3-PRDM1) and another in which the BSAP-binding site was mutated (pGL3-PRDM1-BSAPmut, with the same changes used in the competition experiment, Figure 3A), were transfected into the Daudi cells. The promoter activity was much lower (Figure 4B) in Daudi cells with the putative BSAP promoter than with the mutated BSAP promoter, suggesting that endogenous BSAP expressed by Daudi cells (Figure 4D) was repressing the BLIMP1 expression. In addition, the construct pGL3-PRDM1 was cotransfected with a BSAP-expressing vector in HEK293 cells (as BSAP-negative cells). Figure 4C shows that the transcriptional activity of the basal PRDM1 promoter was repressed. To demonstrate that BSAP protein was properly expressed in HEK293-transfected cells, and hence that it could be responsible for the repression observed, total extracts of Daudi cells and of HEK293 cells transfected with or without BSAP encoding cDNA were studied by Western blot. Figure 4D shows that, except for the enhanced expression, the BSAP protein expressed in HEK293 BSAP-transfected cells was similar to the endogenous BSAP in Daudi cells. No BSAP expression was observed in mock HEK293-transfected cells. Thus, all these results show that BSAP plays a repressor role in the regulation of PRDM1 gene expression.
BSAP represses PRDM1 through the binding site onto the exon 1. (A) Schematic representation showing the construction of reporter plasmids containing the putative minimal promoter of PRDM1 (pGL3-PRDM1) or with the mutated BSAP-binding site (pGL3-PRDM1-BSAPmut). (B) Daudi cells were transiently transfected with the pGL3-PRDM1 or pGL3-PRDM1-BSAPmut luciferase reporters. Relative luciferase activity was normalized to pGL3-PRDM1. Error bars represent SEM from at least 4 experiments. (C) HEK293 cells were transiently cotransfected with the pGL3-PRDM1 luciferase reporter and with the empty pIRES2-GFP or pBSAP-IRES2-GFP expression vectors. The relative luciferase activity from the empty pIRES2-GFP was used as 100%. Error bars represents SEM from at least 4 experiments. (D) A Western blot from cotransfected HEK293 cells (without endogenous BSAP expression) and Daudi cell as control of endogenous BSAP expression is shown. A total of 105 and 106 of HEK293 and Daudi cells per well were loaded, respectively.
BSAP represses PRDM1 through the binding site onto the exon 1. (A) Schematic representation showing the construction of reporter plasmids containing the putative minimal promoter of PRDM1 (pGL3-PRDM1) or with the mutated BSAP-binding site (pGL3-PRDM1-BSAPmut). (B) Daudi cells were transiently transfected with the pGL3-PRDM1 or pGL3-PRDM1-BSAPmut luciferase reporters. Relative luciferase activity was normalized to pGL3-PRDM1. Error bars represent SEM from at least 4 experiments. (C) HEK293 cells were transiently cotransfected with the pGL3-PRDM1 luciferase reporter and with the empty pIRES2-GFP or pBSAP-IRES2-GFP expression vectors. The relative luciferase activity from the empty pIRES2-GFP was used as 100%. Error bars represents SEM from at least 4 experiments. (D) A Western blot from cotransfected HEK293 cells (without endogenous BSAP expression) and Daudi cell as control of endogenous BSAP expression is shown. A total of 105 and 106 of HEK293 and Daudi cells per well were loaded, respectively.
In vivo association of human BSAP with PRDM1 exon 1
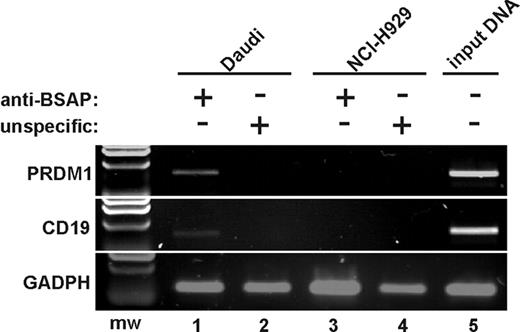
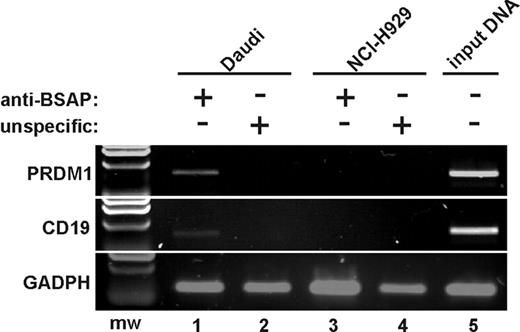
To examine whether BSAP binds to PRDM1 promoter in vivo, ChIP assays were performed. Human cell lines expressing (Daudi cells) or not expressing (NCI-H929 cells) BSAP were used for these analyses. As can be seen (Figure 5), PCR results indicated an enrichment for the PRDM1 gene, as well as for human CD19 promoter used as positive control, in Daudi cell samples (but not in NCI-H929 samples) immunoprecipitated with an antibody anti-BSAP. Thus, we conclude that BSAP binds to PRDM1 exon 1 in vivo, providing strong evidence that BSAP participates in the transcriptional repression of PRDM1.
BSAP binds to the exon 1 of PRDM1 in vivo. Chromatin was prepared from Daudi cells as a B-cell line expressing BSAP factor (lanes 1-2,5), and from NCI-H929 cells as a PC cell line not expressing BSAP (lanes 3-4). ChIP analysis was performed using a specific anti-BSAP antibody (lanes 1,3) or an unspecific antibody isotype as negative control (lanes 2,4). Products from final PCR analysis using primers specific to the PRDM1 promoter, CD19 promoter (as BSAP binding positive control), and exon 4 from GADPH (as BSAP binding negative control) were resolved by agarose gel. The input DNA for Daudi was amplified in lane 5. A representative experiment from 3 ChIP experiments is shown. mw indicates molecular weight marker.
BSAP binds to the exon 1 of PRDM1 in vivo. Chromatin was prepared from Daudi cells as a B-cell line expressing BSAP factor (lanes 1-2,5), and from NCI-H929 cells as a PC cell line not expressing BSAP (lanes 3-4). ChIP analysis was performed using a specific anti-BSAP antibody (lanes 1,3) or an unspecific antibody isotype as negative control (lanes 2,4). Products from final PCR analysis using primers specific to the PRDM1 promoter, CD19 promoter (as BSAP binding positive control), and exon 4 from GADPH (as BSAP binding negative control) were resolved by agarose gel. The input DNA for Daudi was amplified in lane 5. A representative experiment from 3 ChIP experiments is shown. mw indicates molecular weight marker.
Discussion
In this study, we have characterized the transcriptional repression of the PRDM1 gene by the BSAP transcription factor. Using specialized software to analyze the human PRDM1 gene, we recognized a probable BSAP-binding site present on its promoter. Considering that BLIMP1 has an essential role in leading B lymphocytes to differentiate into PCs,22,23,45,46 we decided to study in more detail the possible role of this binding site in the human PRDM1 promoter. Despite continuous improvements in sensitivity and accuracy, the computational methods for predicting transcription factor–binding sites in gene promoter regions often give numerous false-positive hits.47,48 The matrix score for the consensus-binding sequence for BSAP is highly variable; the definition of this sequence is as follows: NCNNNRNKCANNGNWGNRKRGCSRSN, where the italic letters are the most conserved positions and the bold letters are the most conserved ones for the PRDM1 gene in all mammals tested (Figure 1B). The phylogenetic sequence analysis indicates that, in mammals, the region encompassing the BSAP-binding site is especially preserved compared with the adjacent sequences. This suggests that this binding site could be functionally significant, and accordingly, the possibility that BSAP binds to and represses the human PRDM1 promoter was examined.
The use of a PRDM1 exon 1 probe that includes the putative BSAP-binding site in EMSA revealed that nuclear extracts from B-cell lines showed a retarded pattern different from that exhibited by PC and HEp-2 cell lines. A slow retarded complex (complex II) was present in all nuclear extracts tested and suggests that it is composed of nonlineage-dependent common factors. It is possible that this complex II (unidentified) takes part in the basic regulatory transcriptional activity for the PRDM1 gene, and its identification requires further analysis. Nevertheless, based on the finding that the BSAP is specific and necessary for B-cell lineage activation5,11,16 and the presence of the complex I retarded only in B-cell lines, we wished to confirm that BSAP was binding to the PRDM1 promoter. This assumption was demonstrated by competition as well as by supershift experiments in EMSA. Other evidence indicates that BSAP repressed the PRDM1 by binding to the consensus sequence located in the exon 1. First, ChIP assays revealed that BSAP binds to the PRDM1 promoter in Daudi cell line (a BSAP-positive cell line). Secondly, when this cell line was transfected with the mutated BSAP-binding site PRDM1 promoter, a 100% increase in the luciferase activity was observed. Finally, we performed cotransfection experiments using the HEK293 cell line with a reporter gene containing the PRDM1 minimal promoter and a human BSAP expression vector. In these assays, a luciferase activity reduction of around 75% was observed. All these data together demonstrate that the human PRDM1 promoter contains a binding site for BSAP and that, functionally, it acts as a repressor of transcription. This is in disagreement with previous data obtained in mouse LPS-treated splenic B cells where ectopic expression of BSAP did not alter the BLIMP1 mRNA levels.25 The discrepancy between these results could be explained either by the different cellular systems used or by differences in the regulatory transcription of the PRDM1 gene between murine and human species.
Recent studies, using the chicken DT40 B-cell line as a knockout model,39 show that PAX5−/− cells exhibit an increased BLIMP1 and XBP-1 expression and a BCL6 down-regulation. The XBP-1 up-regulation and the BCL6 down-regulation are both postulated to be consequences of a direct BSAP deficiency. This result is in accordance with that obtained by others, where a BSAP overexpression reduced the XBP-1 minimal promoter activity in B cells,9 but in disagreement with the observation it remains unaffected upon conditional short-term loss of PAX5 in mouse mature B cells.13 Shaffer et al38 were the first to postulate that a reciprocal regulatory loop might exist whereby BCL6 and BLIMP1 antagonize the expression of each other. Nera et al39 have suggested that PAX5 is not needed for the suppression of BLIMP1, and that the up-regulation observed for BLIMP1 expression is due to a drop in the BCL6 expression caused by BSAP deficiency. Simultaneous results, obtained in a conditional knockout mouse model,13 are in contrast with those obtained with the chicken DT40 B-cell model.39 The study described by Delogu et al13 in a mouse model did not reveal a role for PAX5 in the regulation of BCL6 and XBP-1. However, an up-regulation of BLIMP1 expression was observed only in mature B cells but not in pro-B cells. They suggest that both BSAP and BCL6 appear to be involved in the repression of BLIMP1 and the subsequent PC transcription program. The discrepancy in results observed between these studies13,39 has been considered to be due to the PAX5 deficiency being originated in different maturational states of B cells.15 However, the possibility of a difference in the regulation of B-cell activation between species cannot be excluded. In this context, the comparison of the BSAP-binding sequence of the PRDM1 promoter between human (or mouse) and chicken revealed a significantly reduced matching of the chicken sequences (CAGCCCTCCAGTGTCTCGGAAAGGCA) with respect to the BSAP-binding consensus sequence (NCNNNRNKCANNGNWGNRKRGCSRSN). This observation may mean that differences exist between mammals and avian species in the mechanism regulating the B cell to PC differentiation process.
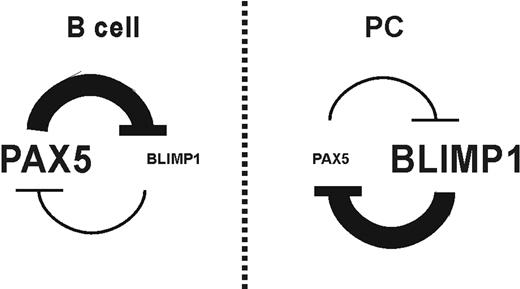
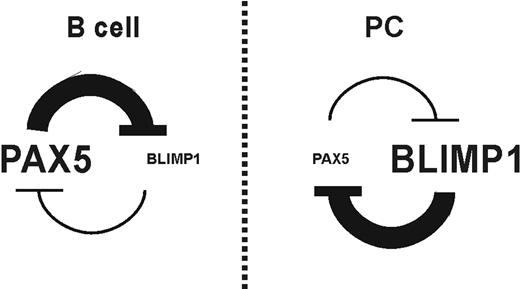
At the level of the germinal center reaction, the relationship between the B lymphocytes and PC transcriptional programs is one of mutual exclusion. At least 3 master regulators, BLIMP1,10 XBP-1,49 and IRF4,27 are required for PC differentiation. Moreover, the simultaneous absence of BCL635 and PAX525 is required too. Considering that, in germinal center mature B cells, PAX5 and BCL6 are positive, a repression of these factors, or alternatively, an induction in the expression of BLIMP1, must occur to allow the PC differentiation program. A loop between BLIMP1 and BCL6 has been suggested in which each one must suppress to the other,36-38 even though, in disagreement with that, an induction of both factors has been described in human B lymphocytes after IL-21 treatment.50 BLIMP1 directly represses transcription of PAX525 and increased transcription of PRDM1 takes place in PAX5-deficient B cells.13,39 Present data reveal for the first time that BSAP directly mediates BLIMP1 repression. In line with our findings, in B cells from multiple myeloma patients, the low PAX5 expression is associated with increased BLIMP1 expression.51 Before now, the mutually excluding expression patterns observed between BSAP and BLIMP1 were explained by BLIMP1-dependent repression of PAX5.25 From now on, we conclude that a reciprocal regulatory loop may exist whereby BSAP and BLIMP1 antagonize the expression of each other, at least in mature B cells.13 In this loop, BLIMP1 represses the crucial transcription factors for B cells, BCL6 and PAX5, which in turn repress BLIMP1, giving rise to a double-negative–feedback loop ensuring irreversible changes in cell fate (Figure 6). The transcriptional functions of these master regulators are complex, involving both gene activation and repression.
Negative-feedback loop between BSAP and BLIMP1. Schematic interaction loop that must exist between BSAP and BLIMP1 factors in the B-lymphocyte differentiation process.
Negative-feedback loop between BSAP and BLIMP1. Schematic interaction loop that must exist between BSAP and BLIMP1 factors in the B-lymphocyte differentiation process.
The results obtained in this work provide evidence of human PRDM1 gene transcriptional regulation that ensures B cells achieve the correct PC differentiation. A recent work had revealed a phase prior to BLIMP-1 expression in which several genes normally repressed by BSAP are re-expressed, suggesting that plasma-cell differentiation is initiated by the inhibition of PAX5 function.46 Here, we demonstrated that PRDM1 gene is directly repressed by BSAP and therefore PAX5 repression is required for BLIMP-1 expression and full plasma cell differentiation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from Fondo de Investigaciones Sanitarias-Ministerio de Sanidad y Consumo (G03/136 and PI052357) and by grants from Consejería de Salud-Junta de Andalucía (201/03 and 203/00).
The authors thank Dr Meinrad Busslinger (Research Institute of Molecular Pathology, Vienna, Austria) for providing the human BSAP full-length cDNA.
Authorship
Contribution: F.M.-L. designed research, did experiments, and analyzed data; E.R. prepared samples and performed experiments; A.C.-C. and J.A.B. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonio Campos-Caro, Unidad de Investigación, 9a Planta, Hospital Universitario Puerta del Mar, Avda. Ana de Viya, 21, 11009 Cádiz, Spain; e-mail: antonio.campos.exts@juntadeandalucia.es.