Abstract
Allogeneic hematopoietic stem-cell transplantation (HSCT) remains an effective strategy for inducing durable remission in chronic myeloid leukemia (CML). Reduced-intensity conditioning (RIC) regimens extend HSCT to older patients and those with comorbidities who would otherwise not be suitable candidates for HSCT. The long-term efficacy of this approach is not established. We evaluated outcomes of 64 CML patients with advanced-phase disease (80% beyond first chronic phase), not eligible for myeloablative preparative regimens due to older age or comorbid conditions, who were treated with fludarabine-based RIC regimens. Donor type was matched related (n =30), 1 antigen-mismatched related (n =4), or matched unrelated (n =30). With median follow-up of 7 years, overall survival (OS) and progression-free survival (PFS) were 33% and 20%, respectively, at 5 years. Incidence of treatment-related mortality (TRM) was 33%, 39%, and 48% at 100 days, and 2 and 5 years after HSCT, respectively. In multivariate analysis, only disease stage at time of HSCT was significantly predictive for both OS and PFS. RIC HSCT provides adequate disease control in chronic-phase CML patients, but alternative treatment strategies need to be explored in patients with advanced disease. TRM rates are acceptable in this high-risk population but increase over time.
Introduction
Allogeneic hematopoietic stem-cell transplantation (HSCT) has been used most extensively and effectively in the treatment of chronic myeloid leukemia (CML), inducing durable remissions in the majority of patients.1-3 The discovery of tyrosine kinase inhibitors (TKIs) has expanded treatment options for this disease, and increasing safety and efficacy data support the use of TKIs in the upfront treatment of CML patients.4 However, HSCT remains an effective treatment strategy for patients with advanced-phase CML, and those in whom TKI therapies have failed
A major concern with conventional myeloablative HSCT has been the potential for increased toxicity and treatment-related mortality (TRM), especially in an older patient population or those with comorbidities. Reduced-intensity conditioning (RIC) and nonmyeloablative regimens are designed to be less myelosuppressive, but remain adequately immunosuppressive, allowing for successful engraftment with acceptable toxicity in more frail patients who otherwise would not be suitable candidates for HSCT. This approach relies on the graft-versus-leukemia (GVL) effect to eradicate the malignancy, an immunologic activity of allogeneic T cells against residual leukemia cells that survive the preparative regimen.5,6 The definition of a truly nonmyeloablative regimen is one that does not eradicate host hematopoiesis, and autologous hematopoietic recovery would occur within 28 days without hematopoietic transplantation. If used with HSCT, mixed chimerism is expected to be present early after transplantation.7 More intensive RIC regimens, involving combination of a purine analog with melphalan or busulfan, require stem cell support for hematologic recovery and usually produce prompt complete donor chimerism.8 Phase 2 studies have demonstrated that reliable and durable engraftment occurs with both RIC and nonmyeloablative regimens. The 1-year TRM is in the range of 10% to 15%.8-12 However, the long-term antitumor effect of this approach is less well established.
The effect of dose intensity on tumor control has been evaluated in acute myeloid leukemia. De Lima et al compared outcomes after a nonmyeloablative regimen of fludarabine 120 mg/m2, cytarabine 4 g/m2, and idarabucin 36 mg/m2 (FAI) versus the RIC regimen of fludarabine 100 to 150 mg/m2 and melphalan 140 or 180 mg/m2 (FM) in 94 patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS).13 While the more myelosuppressive FM regimen was associated with a higher incidence of TRM, it was also associated with a significantly lower relapse rate (61% vs 30%, P =.029), suggesting that FM provided better disease control, albeit at the cost of increased TRM, resulting in comparable overall survival between the 2 groups.13
The contribution of preparative regimen dose intensity to long-term disease control in CML is less well understood. Allogeneic stem cell transplantation is associated with a more potent GVL effect in CML than in acute leukemias, and cytoreduction from the preparative regimen may be less important than in acute leukemias. We analyzed the results of CML patients treated at the M. D. Anderson Cancer Center with nonmyeloablative and RIC transplantation preparative regimens in efforts to determine the impact of dose intensity within the realm of reduced-intensity HSCT. The majority of these patients had advanced-phase disease, and few had received prior TKI therapy.
Patients and methods
Patients
Patients were treated from June 1996 to April 2005 on 4 clinical trials that were approved by the institutional review board (IRB), and written informed consent was obtained in accordance with the Declaration of Helsinki. Patients were included in this analysis if they had CML in chronic, accelerated, or blast phase and had undergone an allogeneic HSCT from a related or unrelated donor with either a truly nonmyeloablative regimen of fludarabine, cytarabine, and idarubicin (FAI) or with a RIC conditioning regimen consisting of fludarabine in combination with melphalan (FM).
The FAI regimen was intended as a treatment for older patients, and those with major comorbidities, prohibiting a more intense conditioning regimen. Eligibility criteria included age up to 75 years and Zubrod performance status less than 3. Patients aged 55 years or older with more advanced disease, without vital organ compromise, were eligible for the FM protocols to provide higher dose intensity in the preparative regimen. Younger patients with organ dysfunction that made them ineligible for myeloablative treatment protocols were also eligible.
Donors
HLA typing for class I antigens was performed using serologic or low-resolution molecular techniques. Low-resolution molecular typing using hybridization techniques, followed by high-resolution molecular typing using polymerase chain reaction, was performed for class II alleles and as needed for selected class I loci. After January 2002, high-resolution molecular typing of class I and II antigens was performed for all unrelated donor transplants. Donors were human leukocyte antigen A (HLA-A), HLA-B, and HLA-DR compatible with the patients in 61 cases; 4 patients had 1 antigen-mismatched related donor.
Peripheral blood progenitor cells were obtained from donors using standard mobilization protocols and apheresis techniques; bone marrow was used if peripheral blood could not be used. Stem cells from all related donors were collected at M. D. Anderson Cancer Center. None of the grafts were depleted of T lymphocytes. Bone marrow or peripheral blood progenitor cells procured from unrelated donors were obtained through the National Marrow Donor Program.
Conditioning regimens
Patients received 1 of 4 preparative regimens. The truly nonablative regimen consisted of a combination of fludarabine 30 mg/m2 given intravenously daily for 4 days followed 4 hours later by an infusion of cytarabine 2 g/m2. In addition to these 2 agents, patients also received idarubicin 12 mg/m2 intravenously daily for 3 days. Patients receiving the reduced-intensity conditioning regimens received fludarabine 25 to 30 mg/m2 for 4 to 5 days in combination with melphalan 140 mg/m2 (FM140), melphalan 180 mg/m2 (FM180), or melphalan 140 mg/m2 plus cytarabine 2 g/m2 (FAM140).
In the absence of any active graft-versus-host disease (GVHD), patients treated on the FM180 protocol were scheduled to receive 0.5 × 108 CD3+ cells/kg donor lymphocyte infusions (DLIs) at 3 and 6 months following HSCT to prevent disease relapse.
Supportive care
GVHD prophylaxis consisted of a combination of tacrolimus and methotrexate 5 mg/m2 intravenously on days 1, 3, 6, and 11 after transplantation (n =58), or cyclosporine and steroids (n =6). Seven patients with mismatched related or matched unrelated donors received antithymocyte globulin (15 mg/kg). Tacrolimus levels were maintained at blood levels of 5 to 15 ng/mL and cyclosporine levels were kept at 150 to 300 ng/mL and tapered after day 60 at the discretion of the primary physician. Patients who experienced grade II or higher acute GVHD received intravenous methylprednisolone at a dosage of 2 mg/kg and, when possible, were enrolled in treatment protocols for GVHD.
Institutional transplant guidelines for antimicrobial, antifungal, and antiviral prophylaxis were followed. Specifically, prophylaxis consisted of trimethoprim and sulfamethoxazole for Pneumocystis carinii and acyclovir or valacyclovir for herpes simplex virus. Surveillance cytomegalovirus (CMV) antigenemia testing was performed for all patients, and a positive test triggered the preemptive use of ganciclovir or foscarnet. All patients received 5 μg/kg filgrastim subcutaneously daily from day + 7 until their absolute neutrophil count was 1.5 × 109/L or higher for 3 consecutive days. Immunoglobulins at dose of 200 mg/kg were infused weekly until day 100 following transplantation in patients receiving unrelated donor grafts. Packed red blood cells were administered to maintain hemoglobin levels of 80 g/L (8 g/dL) or higher. Platelet transfusions were administered to keep platelet counts of 10 × 109/L or higher. All blood products were filtered and irradiated.
Definitions
Criteria for complete response included normal cytogenetics, the absence of circulating blasts, less than 5% marrow blasts, and a platelet count of 100 × 109/L or higher. Standard morphologic criteria, conventional cytogenetic analysis by G-banding, or both were used to diagnose recurrent disease. The disease phase at transplantation was defined using established criteria.14 Response was documented as best response occurring after day 30 following HSCT. Molecular response measured by quantitative polymerase chain reaction analysis for BCR-ABL rearrangement was obtained when possible. Hematologic recovery was defined on the date that the patient had an absolute neutrophil count of 0.5 × 109/L or higher for 3 consecutive days. Platelet recovery was defined as occurring on the first of 7 consecutive days with a platelet count of 20 × 109/L or higher without transfusion support. Failure to engraft by day +30 was considered primary engraftment failure. Hematopoietic chimerism was evaluated in bone marrow by restriction fragment length polymorphisms at the AY-29 or YNH24 loci, by conventional cytogenetic analysis by G-banding, or by fluorescence in situ hybridization studies in sex-mismatched cases for the Y chromosome, to determine donor engraftment.
Overall survival (OS) was defined as the time from the date of HSCT until date of death from any cause, and patients still alive at last follow-up were considered censored. Progression-free survival (PFS) was defined as the date of transplantation until date of progression or death from any cause, and patients alive at last follow-up were considered censored. TRM was defined as death from any cause other than disease progression or relapse. The diagnosis of GVHD was confirmed by biopsy when feasible but was ultimately determined by clinical presentation. Acute GVHD (aGVHD) was clinically graded as 0 to IV based on standard criteria15 ; chronic GVHD (cGVHD) was classified as none, limited, or extensive.16 Acute GVHD, which persisted or progressed after day 100, was also scored as cGVHD in this study.
Toxicity was scored using the modified National Cancer Institute Common Toxicity Criteria version 3.0 (Bethesda, MD). Adverse events and hematologic parameters were monitored daily and clinical chemistry parameters at least twice weekly during the initial hospitalization and then at increasing intervals up to day +100. Subsequently, patients were followed up at least quarterly during the first year with physical examinations, assessments for GVHD, blood counts, and bone marrow aspiration and biopsy with chimerism analysis.
Statistical methods
The major end points of this analysis were to compare the RIC regimens with the truly nonmyeloablative transplant regimen in CML patients with respect to OS, PFS, and TRM. Relapse was defined as either cytogenetic or hematologic relapse. Kaplan-Meier17 curves were used to assess OS and PFS in years. Log-rank tests were used to test for a difference in survival between strata. A Cox18 proportional hazards model was created to determine multivariable models for OS and PFS outcomes. The variables included in the model were those that were marginally significant, P ≤ .10, in the univariate Kaplan-Meier analyses. A backwards selection procedure was used to determine the final model. Chronic GVHD was evaluated as a time-dependent covariate. Hazard ratios and corresponding 95% confidence intervals are reported for each prognostic factor in the final model. Nonrelapse-related mortality and relapse-related mortality were considered competing risks for TRM. Cumulative incidence curves were calculated using the method of Gooley et al,19 which took into account the presence of the competing risk of nontreatment-related death. The comprisk SAS macro created by Erik Bergstralh of the Mayo Clinic was used to compute cumulative incidence. Test statistics were computed in R using the method of Gray.20 Multivariable competing risks regression models were computed using the method of Fine21 and Gray20 and the R library cmprsk. Descriptive statistics were used to summarize patient demographics. Continuous variables were presented with their corresponding means, standard deviations, and ranges. For categoric variables, frequencies and percentages were generated. When comparing means for continuous variables, a 2-sample t test was used. If the data were not normally distributed, a Wilcoxon rank sum test was implemented. All P values presented are 2-sided and significance was assessed at the 5% level. Statistical analyses were performed using SAS version 8.2 and S-Plus version 6.1 (SAS Institute, Cary, NC).
Results
Patient and treatment characteristics
Patient demographics and baseline disease characteristics are listed in Table 1. Sixty-four patients with median age of 52 years (range, 17-72 years) were evaluated in this study. The median time to transplantation was 2.6 years (range, 0.2-18.1 years), with the majority of patients beyond first chronic phase at time of HSCT (80%). The median number of prior treatment regimens was 3, with 14% of patients having been treated with TKIs, 5% having had a prior autologous HSCT, and 11% having had a prior allogeneic HSCT. The majority of patients received the FM regimen (81%) versus the FAI regimen (19%). Finally, the majority of patients received tacrolimus and minidose methotrexate for GVHD prophylaxis (n =58).
Graft content and engraftment
Stem cell graft characteristics and engraftment data are listed in Table 2. Thirty patients received a matched related graft, 30 received grafts from unrelated donors, and 4 received a mismatched related donor. All except for one patient underwent an unrelated donor transplantation before 2002 and, therefore, had no HLA class I molecular typing. The source of stem cells was bone marrow for 38 patients and peripheral blood for 26 patients. The median total nucleated cell counts and CD34+ cell doses were 2.3 × 108/kg (range, 0.5-3.9 × 108/kg) and 3.4 × 106/kg (range, 0.4-8.6 × 106/kg), respectively, for BM, and 5.8 × 108/kg (range, 2.6-15.5 × 108/kg) and 4.4 × 106/kg (range, 2.3-6.0 × 106/kg), respectively, for peripheral blood.
The median days to neutrophil and platelet recovery were 14 (range, 9-46 days) and 18 (range, 9-118 days), respectively. Although median days to neutrophil recovery were similar for patients receiving bone marrow (14 days) and peripheral blood stem cells (PBSCs; 13 days), time to platelet recovery was longer for bone marrow (21 days) compared with PBSC (15 days). Chimerism data were evaluable in 59 patients; 5 patients were not evaluable due to early death. Eighty-three percent of patients (n =49) achieved 100% donor chimerism, 6 patients remained with mixed chimerism (8% after FM [n =5] and 2% after FAI [n =1]), and 4 patients had graft failure with autologous reconstitution (5% after FM [n =3] and 2% after FAI [n =1]).
Treatment toxicity
Incidence of aGVHD was 45% (n =29): 14% developed grade I (n =9); 15%, grade II (n =10); 5%, grade III (n =3); and 11%, grade IV GVHD (n =7) (Table 2). Acute GVHD, grades II to IV, occurred in 25% (n =16) of patients treated with FM versus 6% (n =4) of patients treated with FAI, and aGVHD, grades III to IV, occurred in 11% (n =7) of patients treated with FM versus 5% (n =3) of patients treated with FAI. These differences were not significant. Chronic GVHD was evaluable in 55 patients surviving beyond 100 days (FM =46, FAI =9), of whom 31% developed limited plus extensive cGVHD (n =17), and 20% developed extensive cGVHD only (n =11) (Table 2). Extensive cGVHD occurred in 19% of FM patients (n =10) and 2% of FAI patients (n =1). In addition, among the 34 mismatched-related or matched unrelated transplants, 67% of patients had been treated with FAI (n =8) versus 50% treated with FM (n =26).
The cumulative incidence of TRM at 100 days, and 1, 2, and 5 years was 33%, 38%, 39%, and 48%, respectively. Only the use of a female donor into male recipient, bone marrow as the source of stem cells, and donor type other than matched related were associated with increased TRM in univariate analysis (Table 3). Use of a female donor into male recipient and bone marrow as the source of stem cells remained significant in a multivariate analysis, P =.001 and P =.006, respectively. Disease recurrence accounted for 12 of 42 deaths (n =3 first chronic phase [CP1]/second chronic phase [CP2], n =9 accelerated phaes [AP]/blast phase [BP]). The remaining deaths were due to following: graft rejection (n =1), infection (n =3), GVHD (n =17), hemorrhage (n =5), and organ failure (n =4).
Response, relapse, and progression-free survival
Overall complete remission rate including both bone marrow morphology and cytogenetic analysis was 77% (n =49) of patients. Cytogenetic complete response was noted in 77% (n =49) of patients. Molecular analysis was available for 41 patients. A complete molecular remission was attained by 78% (n =32) of evaluable patients. Among 24 patients with chronic-phase disease, 83% (n =20) of patients achieved complete remission compared with 40 patients with accelerated- or blast-phase disease, among whom 75% (n =30) achieved complete remission.
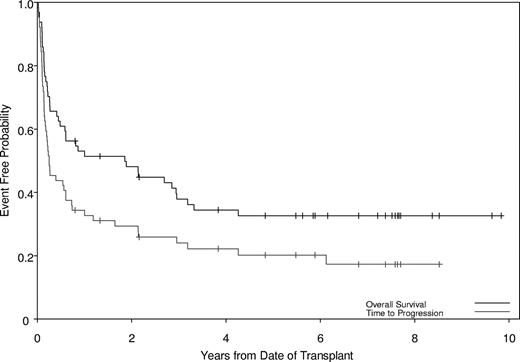
Twenty-two patients relapsed. PFS at 2 and 5 years was 29% and 20%, respectively (Figure 1) for the entire group; among chronic-phase patients, PFS at 2 and 5 years was 47% and 31%, respectively, compared with 15% and 11%, respectively, for patients with accelerated- or blast-phase disease (P =.03). Of note, 6 of 8 patients who did not achieve a molecular response and all patients who achieved less than a complete cytogenetic response were in the group that relapsed. The use of TKI therapy prior to transplantation did not affect response rates. Factors associated with increased relapse in a univariate model were advanced disease stage at time of HSCT, the use of a donor other than matched related, and the absence of any aGVHD (Table 3). The factors that retained significance on multivariate analysis for PFS were disease stage at time of HSCT, donor type, and development of aGVHD (Table 4). Patients who underwent transplantation in advanced-phase disease, receiving the FAI regimen or receiving mismatched-related or matched-unrelated donor transplants, were more likely to progress, while patients who developed some aGVHD had a decreased likelihood of progression (Table 4).
Probabilities of survival and progression. The median survival time was 1.86 years (95% CI, 0.49-3.19), with overall survival of 48% and 33% at 2 and 5 years, respectively. The median progression-free time was 0.25 years (95% CI, 0.17-0.61), with progression-free survival of 29% and 20% at 2 and 5 years, respectively.
Probabilities of survival and progression. The median survival time was 1.86 years (95% CI, 0.49-3.19), with overall survival of 48% and 33% at 2 and 5 years, respectively. The median progression-free time was 0.25 years (95% CI, 0.17-0.61), with progression-free survival of 29% and 20% at 2 and 5 years, respectively.
Overall survival
Median follow-up of survivors was 7 years (range, 0.8-9.8 years). OS was 48% and 33% at 2 and 5 years, respectively (Figure 1); among chronic-phase patients, OS at 2 and 5 years was 66% and 48%, respectively, compared with 32% (2-year) and 19% (5-year) for patients with accelerated- or blast-phase disease, respectively (P =.03). Only disease stage at time of HSCT was a significant predictor for OS in univariate (Table 3) and multivariate (Table 4) analyses. Patients with advanced disease had worse OS (HR, 2.26; 95% CI, 1.18-4.34; P =.014) than patients in chronic phase.
Donor lymphocyte infusion
Four patients received a DLI on the FM180 protocol at a median of 3 months (range, 3-9 months) following HSCT. At time of DLI, 3 patients had persistent chronic-phase disease following HSCT and had no response to DLI; one patient had relapsed chronic-phase CML and had initial control of disease, and received 2 subsequent DLIs, but eventually relapsed at 1 year from time of relapse. Imatinib was used in conjunction with DLI, and one patient remains alive in hematologic and cytogenetic remission following continued tyrosine kinase therapy.
Second HSCT following relapse
Three patients underwent a second HSCT using fludarabine-based RIC regimens at median of 10 months (range, 3-48 months) following HSCT. All 3 patients relapsed at a median of 3 months (range, 1-5 months) following the second HSCT, with 2 patients in blast crisis and 1 patient in chronic phase at time of HSCT. No patient survived. Imatinib was used alone or in conjunction with HSCT at time of relapse in all patients.
Discussion
With a median follow-up time of 7 years, this is one of few published series to assess the long-term outcome of RIC and nonmyeloablative transplants in CML patients. These were first-generation nonmyeloablative and reduced-intensity regimens.
When compared with our myeloablative, radiation- or busulfan-based transplants, survival and toxicity rates were comparable.22,23 Giralt et al reported 2-year OS rates of 56% and 35% for patients undergoing matched related HSCT for chronic- and advanced-phase CML, respectively, with 100-day TRM of 30%.22 In our study, 13 patients in chronic-phase (CP) and 18 patients in accelerated-phase (AP) or blast-phase (BP) disease underwent matched related HSCT; 8 CP and 5 AP/BP patients remain alive. Of note, patients receiving the myeloablative regimen were younger with a median age of 38 years, and had a shorter time to transplantation at a median of 1.9 years compared with patients in this study. Improved results have been reported by ourselves and others using busulfan-fludarabine and melphalan-fludarabine combinations. Kerbauy et al have also reported improved OS rates of 54% using low-dose total-body irradiation with or without fludarabine.24
In this study, we found that dose intensity between the 2 preparative regimens used did not make a difference with regard to progression-free or overall survival for patients in all stages of disease. This is in contrast to what was reported by de Lima et al13 in AML, where the more myelosuppressive FM regimen was associated with a significantly lower relapse rate when compared with FAI. However, our comparison in this study may be limited by the small number of patients who received the FAI regimen compared with the FM.
Disease stage at time of HSCT was a significant predictor for both OS (HR, 2.26) and PFS (HR, 1.85) in multivariate analysis. When patients were stratified by disease phase at time of HSCT, the 2-year OS and PFS were 66% and 47%, respectively, for patients in first chronic phase (CP1) or second chronic phase (CP2) compared with 32% and 15%, respectively, for patients with AP or BP disease. Our results are corroborated by similar findings from the Chronic Leukemia Working Party registry of the European Group for Blood and Marrow Transplantation (EBMT). In this retrospective analysis of RIC regimens in CML patients, those undergoing HSCT in CP1 had significantly better OS (69%, P < .001) and PFS (45%, P < .001) than those with more advanced disease.25
The cumulative incidence of TRM at 100 days was 33%, and continued to rise to 48% at 5 years. There was no observed significant difference for TRM based on preparative regimen: The 5-year TRM rate for patients receiving the nonmyeloablative regimen was 45% versus 58% for the RIC regimen (Table 3). In a multivariate analysis, use of a female donor into male recipient, and bone marrow as the source of stem cells were significantly associated with TRM. A higher TRM rate with use of bone marrow in advanced-phase CML was also found in the retrospective analysis performed by the International Bone Marrow Transplant (IBMTR) and EBMT registries.26 It should be noted that the analysis for donor-recipient sex match may be limited by the small numbers of male patients receiving a female donor. In addition, CP1 and CP2 were combined for analysis with the majority of patients in CP2 at time of HSCT, perhaps obscuring the advantage of having very early disease at time of HSCT.
The TRM rate is higher than that reported by the EBMT.25 However, it should be noted that there was a greater percentage of matched related donors and patients in chronic phase at time of HSCT in the EBMT study. In our study, the 5-year TRM for patients who received a matched related allograft was 32% versus 63% for those who received a matched unrelated allograft (P =.02, Table 3). Furthermore, TRM was noted in only 1 of 6 patients who received a transplant from a matched sibling in CP1. The impact of allotype relation on TRM is well established.27 In a recent analysis of myeloid leukemia patients who received unrelated donor allografts matched at the standard HLA-A, -B, -C, -DRB1, and -DQBI loci, TRM was noted to be lower in those patients who were matched additionally at the DPB1 loci,28 underscoring the importance of allotype matching. Thus, the data suggest that disease stage and allotype, rather than the dose intensity of the preparative regimen, may have the greatest impact on TRM. Finally, 14% of our patients received TKI therapy prior to HSCT. There did not appear to be an increased risk for regimen-related toxicity or TRM, or an increased risk of relapse after transplantation. These findings are similar to other retrospective studies that also did not find any negative impact with respect to TKI use prior to HSCT.29,30
These data are especially relevant in the current era of effective, nontransplantation therapy, namely TKIs, for these patients. Tyrosine kinase inhibitors are now the preferred treatment for newly diagnosed chronic-phase CML patients, resulting in a marked decrease in the number of transplantations performed currently for CML in CP1.31 However, while these agents have produced prolonged disease control, minimal residual disease is still generally present,14,32 and patients require life-long monitoring and continued drug therapy. For patients who progress on this treatment or present with advanced disease, HSCT remains a viable option. Furthermore, for patients living in countries with limited healthcare budgets, the financial aspects of treatment may be a consideration in the decision of a one-time, potentially curative option versus life-long drug therapy.33,34
RIC and nonmyeloablative regimens have been embraced because of generally noted decreased regimen-related toxicity around the time of transplantation. However, the trade-off with respect to disease control continues to be investigated. The data from this study suggest that reduced-intensity regimens provide adequate disease control for patients with chronic-phase disease, but alternative strategies are needed for patients with advanced CML. Incorporation of TKIs into therapy may downstage patients prior to HSCT, and their use as maintenance following HSCT may help to prevent relapse. Finally, TRM rates are acceptable in this high-risk population but continue to increase over time. Thus, in addition to the preparative regimen, appropriate patient and donor selection are necessary to obtain optimal HSCT outcome.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.G., D.C., I.F.K., P.A., C.H., R.E.C, and M.L. were involved in the design of the protocols, patient accrual, and paper review; M.L., A. Alousi, A. Anagnostopoulos, and A.C.-Y. were involved in obtaining the data, data analysis, and paper review; P.K. was involved in obtaining the data, data analysis, and writing the paper; M.A.D. performed the statistical analyses for the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Partow Kebriaei, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 423, Houston, TX 77030; e-mail: pkebriaei@mdnaderson.org.