Abstract
Acute graft-versus-host disease (GVHD) and leukemic relapse are serious complications of allogeneic stem-cell transplantation (SCT). Recruitment of activated T cells to host target tissues or sites of leukemic infiltration (graft-versus-leukemia [GVL]) is likely mediated by chemokine receptor–ligand interactions. We examined the contribution of donor cell CCR1 expression to the development of GVHD and GVL using a well-established murine SCT model (B6 → B6D2F1) and CCR1-deficient mice (CCR1−/−). Allo-SCT with CCR1−/− donor cells significantly reduced systemic and target organ GVHD severity, and CCR1 expression on both T cells and accessory cells contributed to GVHD mortality. Significant GVL activity was preserved following CCR1−/− SCT, but the survival advantage diminished with increasing tumor burden. We then explored the effects of CCR1 expression on allo-specific T-cell responses. Although cytolytic effector function was maintained on a per-cell basis, T-cell proliferation and IFNγ secretion were significantly reduced both in vivo and in vitro. T-cell function was partially dependent on interactions between CCR1 and CCL5. Collectively, these data demonstrate that CCR1 expression on donor cells contributes to the development of both GVHD and GVL, and suggest that CCR1/CCL5 receptor-ligand interactions modulate allo-specific T-cell responses occurring in this context.
Introduction
Allogeneic stem-cell transplantation (allo-SCT) is an important therapeutic option for a variety of malignant and nonmalignant disorders.1 Acute graft-versus-host disease (GVHD) is the most frequent and serious complication following allo-SCT and continues to limit the broader application of this therapy.2 In the context of hematologic malignancies, a delicate balance exists between the harmful consequences of GVHD and the beneficial effects incurred when donor T cells attack recipient malignant cells, a process referred to as the graft-versus-leukemia (GVL) effect.3,4 A better understanding of the mechanisms responsible for these graft-versus-host reactions may ultimately allow for directed therapies that promote GVL while reducing GVHD.5
The pathophysiology of acute GVHD is complex. Experimental and clinical data suggest that host antigen-presenting cells (APCs) present alloantigen to donor T cells and initiate a cascade of events resulting in the development of cytotoxic effectors and the release of inflammatory proteins including cytokines and chemokines.6 Activated donor leukocytes subsequently traffic to specific host tissues, where they, along with soluble effectors, cause end organ damage and dysfunction.
Leukocyte migration is characterized by an orchestrated process involving interactions between white blood cells (WBCs) and endothelial cells that are mediated by adhesion molecules, chemoattractants, and their receptors. Chemokines are a large family of chemotactic cytokines that interact with specific G-protein–coupled chemokine receptors.7 Chemokines are involved in both the innate and adaptive immune responses,8 and have well-defined roles in cellular movements by providing directional cues and indirectly by enhancing integrin expression and activation.9 In response to proinflammatory cytokines such as TNFα, LPS, or IL-1, the expression of specific chemokines and their receptors is up-regulated on a variety of hematopoietic and nonhematopoietic cells.10-12
CCR1 is a chemokine receptor that is expressed on neutrophils, monocytes, and T lymphocytes and binds several chemokine ligands, including CCL5/RANTES (regulated on activation, normal T expressed and secreted), CCL3/Mip-1α, and CCL4/Mip-1β.13 A role for CCR1 has been well characterized in several inflammatory conditions, such as arthritis,14,15 chronic kidney disease,16-18 immune-mediated hepatitis,19 tumor angiogenesis,20 ischemia-reperfusion injury,21 sepsis,22,23 and transplantation rejection.24,25 We have recently shown that CCL5 contributes to the development of lung injury and survival following allo-SCT.26 In this light, we sought to investigate the role of CCR1 in the development of systemic and target organ GVHD. We used a well-established murine SCT model, wherein GVHD develops following myeloablative conditioning and in the context of major and minor histocompatibility differences between donor and recipient. Our data demonstrate that the expression of CCR1 on donor cells significantly contributes to the development of acute GVHD. Surprisingly, we found that the expression of CCR1 on donor T cells is dominant and modulates T-cell alloreactivity both in vitro and in vivo via a mechanism that is dependent in part on receptor-ligand interactions with CCL5.
Materials and methods
Mice and SCT
Female B6D2F1 (H-2bxd), C57BL/6 (H-2b), BABL/C (H-2d), B6.C-H2 < bm1 > /ByJ (bm1; H-2b), and B6.C-H2 < bm12 > KhEg (bm12; H-2b) mice were purchased from the Jackson Laboratories (Jax; Bar Harbor, ME) or from the Frederick Cancer Research and Development Center (National Cancer Institute; Frederick, MD). B6 RANTES knock-out (B6 RANTES −/−; H-2b) and Balb/c RANTES (Balb RANTES −/−; H-2d) mice were kindly provided by Dr J. Serody (University of North Carolina, Chapel Hill). CCR1 knock-out (CCR1−/−; H-2b) mice on 129Sv × B6 (B6.129) background were generated from 129Sv strain embryonic stem cells using targeting vectors as previously described.27 Age-matched, littermate B6 × 129F2 (CCR1+/+; H-2b) mice were used as controls in all experiments. Both mouse strains were kindly provided by Dr S. Chensue. Animals used for SCT and in vitro experiments were between 10 and 20 weeks old. All experiments were approved by the University of Michigan Committee on the Use and Care of Animals.
Mice received transplants according to a standard protocol.28 Briefly, B6D2F1 recipients received 11 Gy of total body irradiation (TBI; 137Cs source) prior to transplantation followed by the infusion of 5 × 106 bone marrow (BM) cells and 2 × 106 splenic T cells from either syngeneic (B6D2F1), allogeneic CCR1+/+(wild-type), or CCR1−/− donors. T-cell purification was performed by magnetic-bead separation using CD4 and CD8 MicroBeads and the autoMACS system (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer's protocol, with more than 85% of cells obtained being positive for CD4 or CD8 surface antigens (data not shown). In some experiments, aliquots of BM and T cells from either CCR1+/+or CCR1−/− donors were “mixed.” A total of 4 possible combinations resulted in 5 total experimental groups: B6D2F1 → B6D2F1; CCR1+/+BM plus CCR1+/+T → B6D2F1; CCR1+/+ BM plus CCR1−/− T → B6D2F1; CCR1−/− BM plus CCR1−/− T → B6D2F1; and CCR1−/− BM plus CCR1+/+T → B6D2F1. In these experiments, the BM or “accessory cell” component was derived from T-cell–depleted BM using CD90 (Thy1.2) MicroBeads, and the T-cell component was obtained by AutoMACS-purified splenic T cells.
In experiments using the B6 → Balb/c system, Balb/c (wild-type, RANTES−/−, or chimera) recipient mice received 9 Gy TBI and 5 × 106 BM and 0.5 × 106 autoMACS-purified CD4+CD8+ (vs CD90+) T cells. BM chimeras were created with the Balb/c mice (wild-type → RANTES−/− or RANTES−/− → wild-type), wherein recipient mice received 8.5 Gy TBI and 8 × 106 BM cells. The chimeras were subsequently used as recipient mice in a second SCT experiment using either B6 or Balb/c donors.
Systemic and target organ GVHD
Mice that received transplants were ear punched, and individual weights were obtained and recorded on day 0 and weekly thereafter. Survival was monitored daily, and clinical GVHD was assessed weekly by the summation of 5 criteria scores: percentage of weight change, posture (hunching), activity, fur texture, and skin integrity (maximum index = 10) as described.29
Acute GVHD was also assessed by histopathologic analysis of the liver and small (ileum) and large (ascending) intestines. Specimens were harvested from animals on specified days, placed in 10% buffered formalin, embedded in paraffin, cut into 5-μm-thick sections, and stained with hematoxylin and eosin for histologic examination. Slides were coded without reference to mouse type or prior treatment status and examined systematically by a single pathologist (C.L.) for abnormalities known to be consistent with GVHD. Using previously described scoring systems,30,31 8 parameters each were scored for small bowel and large bowel, and 10 parameters were evaluated for hepatic inflammation. The scoring system for each parameter denoted 0 as normal, 0.5 as focal and rare, 1 as focal and mild, 2 as diffuse and mild, 3 as diffuse and moderate, and 4 as diffuse and severe. Scores for each parameter were then summed to give a pathology index for individual organs.
Leukemia induction
P815 (H-2d CD45.2) is a mastocytoma cell line derived from DBA/2 mice. Injection of as little as 500 P815 cells into animals that cannot reject this tumor is uniformly lethal. In some animals, lower-limb paralysis (from spinal infiltration) is also observed and is very specific for death from leukemia.32 In GVL experiments, 129Sv × B6 CCR1+/+ (H-2b) and 129Sv × B6 CCR1−/− (H-2b) mice were used as allo-SCT donors, and 500 to 2000 P815 tumor cells (H-2d) were added to the BM inoculum on day 0. Survival was monitored daily, and the cause of each death after SCT was determined to be either GVHD or tumor by postmortem examination. Death from P815 was defined by enlargement of the liver and spleen with macroscopic tumor nodules or hind-limb paralysis, whereas GVHD death was defined as the absence of tumor and the presence of GVHD as determined by clinical and histopathologic scoring.
Cell-surface phenotype and T-cell expansion after SCT
Splenocytes were harvested from naive donor mice and transplant recipients at specified times following SCT and counted for individual animals. To analyze cell-surface phenotype, aliquots of cell suspensions were stained with fluorescein isothiocyanate (FITC)–conjugated monoclonal antibodies (MoAbs) to CD4 and CD8, PE-conjugated MoAbs to CD4, CD8, Gr-1, and CD11c, or allophycocyanin-conjugated MoAbs to CD4, CD8, and CD11b for flow cytometric analysis as previously described.33 All MoAbs were purchased from BD Biosciences Pharmingen (San Diego, CA). CCR1 staining was performed using a polyclonal antibody to CCR1 (Imgenex, San Diego, CA), FITC–goat anti-rabbit antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) and anti-FITC goat IgG, Alexa Fluor 488 conjugate (Molecular Probes, Invitrogen, Carlsbad, CA). A 3-color flow cytometric analysis was performed using a FACSCalibur (BD Biosciences, San Jose, CA). The FACScan was calibrated using FITC, PE, and allophycocyanin.
Cell culture, proliferative response, and CTL function
All culture media reagents were purchased from Gibco (Gaithersburg, MD). B6D2F1, B6.129 wild-type, and B6.129 CCR1−/− splenic T cells (0.2 × 105) were cultured in flat- or round-bottomed 96-well Falcon plates (BD Biosciences) for 96 hours in the presence of 20 × 104 irradiated (20 Gy) naive, splenic-derived, CD11c+ B6D2F1 dendritic cells (DCs) at 37°C in a humidified incubator supplemented with 7.5% CO2. After 72 hours, supernatant was obtained and subsequently analyzed for IFN-γ by enzyme-linked immunosorbent assay (ELISA). Proliferation was measured by a 1205 Betaplate reader (Wallac, Turku, Finland) after 96 hours by incorporation of [3H]thymidine (3.7037 × 104 Bq) for the last 24 hours of incubation. To measure proliferation of T cells in response to mitogen or T-cell receptor (TCR) costimulation (anti-CD28 and plate-bound anti-CD3), wild-type or CCR1−/− splenic T cells were cultured in flat-bottomed 96-well Falcon plates at 1 × 105 T cells with or without 1.5 μg/mL concanavalin A (ConA; Sigma, St Louis, MO) or with or without anti-CD3 and anti-CD28 (1 μg/mL; BD Biosciences Pharmingen). In some experiments, T-cell proliferation was also measured following the addition of monoclonal antibodies to CCL5/RANTES (25, 50, and 100 μg/mL; gift from Tom Lane, University of California, Irvine), CCL3/Mip-1α MoAb (25, 50, and 100 μg/mL; R&D Systems, Minneapolis, MN), and CCL4/Mip-1β MoAb (25, 50, and 100 μg/mL; R&D Systems). Cytotoxic T lymphocyte (CTL) assays were performed according to previously published methods for a 51Cr release assay.33 The P815 (H-2d) mouse mastocytoma cell line as well as the EL-4 cell line (H-2b) were used as allogeneic and syngeneic target cells, respectively, and percentages of specific lysis for different effector-target ratios were calculated as described.33
Measurement of cytokine protein levels in sera and cell supernatants by ELISA
Animals were exsanguinated on days 4 and 7 after SCT, and blood samples were collected in 1.5-mL Eppendorf tubes and centrifuged at 5000 rpm for 5 minutes, and serum was obtained for analysis. Cell-culture supernatants were harvested at various time points during mixed leukocyte reaction (MLR) experiments. Concentrations of specific cytokines and chemokines were measured by ELISA. Assays were performed according to the manufacturer's protocol for each of the following proteins: TNFα (Quantikine M; R&D Systems), IFNγ (OptEIA; BD Biosciences Pharmingen), and CCL5 (R&D Systems). Assay sensitivity was less than 7.5 pg/mL for IFNγ, less than 3.0 pg/mL (BioSource, Camarillo, CA), or less than 5.0 pg/mL (R&D Systems) for TNFα, and less than 2.0 pg/mL (R&D Systems) for CCL5. ELISA plates were read by microplate reader (Bio-Rad Laboratories, Hercules, CA).
RNase protection assay (RPA)
Determination of CCR1 mRNA expression was completed as previously described.26 Briefly, liver, spleen, and intestinal tissue were collected at various time points after SCT and stored at −80°C. mRNA was extracted from frozen samples using TRIzol following the manufacturer's protocol (GibcoBRL, Grand Island, NY) and quantitated by spectrophotometry. The multiprobe-template sets mCK-5b and mCR-5 were purchased from BD Biosciences Pharmingen. [32P]UTP-radiolabeled antisense riboprobes were synthesized according to the manufacturer's protocol and purified using G-25 Sephadex Quick Spin Columns (Roche, Indianapolis, IN). Expression of chemokine genes was quantified by RPA using RiboQuant RPA kits (BD Biosciences Pharmingen), according to the manufacturer's protocol. Protected RNA products were separated on a 5% polyacrylamide gel; the gel was exposed to a storage phosphor screen (Molecular Dynamics, Sunnyvale, CA) for quantification. Signal intensity was measured with ImageQuant (Molecular Dynamics) and standardized to the intensity of the L32 signal for each sample.
Statistical considerations
All values are expressed as the mean plus or minus standard error margin (SEM). Statistical comparisons between groups were completed using the parametric independent sample t test with 5 or more animals per group and using the Mann-Whitney test if the number of animals per group was less than 5. The Wilcoxon rank-test was used for analyzing survival data.
Results
mRNA and protein expression of CCR1 is increased following allo-SCT
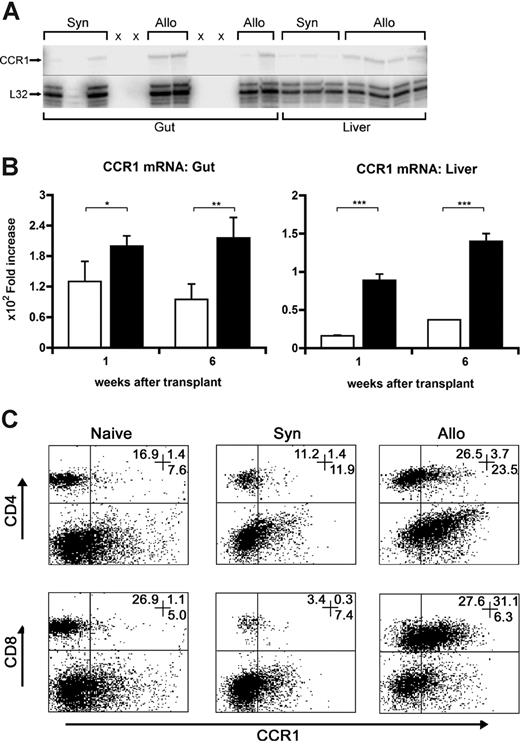
CCR1 is expressed on monocytes, macrophages, and activated T-cell subsets,34 and contributes to the recruitment of these cells to sites of inflammation in various models of disease.14-25 We hypothesized that expression of CCR1 would be enhanced in the intestines and liver following allo-SCT and would correlate with the influx of donor leukocytes into these organs during the development of GVHD. RNA was isolated from the intestines and liver at weeks 1, 4, and 6 after transplantation, and mRNA expression of CCR1 was determined using the RPA. CCR1 mRNA expression was significantly elevated in allogeneic transplant recipients at each time point compared with syngeneic controls (Figure 1A,B; data not shown), and ultimately correlated with increases in intestinal and hepatic histopathology scores as shown in Figures 2 and 4. We next determined CCR1 mRNA and protein expression in the spleen following allo-SCT. mRNA (not shown) and the cell-surface expression of CCR1 were significantly up-regulated and correlated with the expansion of both CD4 and CD8 T cells on days 7 and 11 following allo-SCT compared with both syngeneic and naive controls (Figure 1C; Table 1). Moreover, the percentage of CCR1+CD4+ cells increased more than 100% from day 7 to day 11 in allogeneic transplant recipients, consistent with the expected expansion of CD4+ T cells in this SCT system.32 The cell-surface expression of CCR1 was also increased on both CD11b+ cells (31.1% ± 2.0% vs 7.9% ± 2.1%; P < .05) and Gr-1+ cells (36.1% ± 2.3% vs 23.6% ± 3.0%; P < .05) 7 days after allo-SCT.
CCR1 expression is increased in GVHD target organs and the spleen after allogeneic SCT. Lethally irradiated B6D2F1 mice received SC transplants from either syngeneic B6D2F1 (□) or allogeneic B6 donors (■) as described in “Materials and methods.” (A,B) RNA was isolated from the intestines and liver of SC transplant recipients on weeks 1, 4, and 6 and CCR1 expression was determined by the RPA. Shown is a representative gel from week 4 (A). L32 is a ribosomal protein used as an mRNA loading standard. A line has been inserted to indicate where the gel was cut. The gels came from the same experiment but the intermediate area was cut. CCR1 mRNA expression was significantly increased after allo-SCT at all time points when compared with syngeneic controls (B) (data not shown). *P < .04; **P < .08; ***P < .001. (C) Splenic CD4+ and CD8+ T-cell expression of CCR1 was analyzed on days 7 and 11 and was found to be increased after allo-SCT when compared with syngeneic and naive controls. Data are shown for naive, syngeneic, and allogeneic splenic T cells stained for CD4+ or CD8+ (PE) and CCR1+ (FITC) on day 7. Numbers in the upper right corner of each graph represent the percentage of events in the corresponding quadrants.
CCR1 expression is increased in GVHD target organs and the spleen after allogeneic SCT. Lethally irradiated B6D2F1 mice received SC transplants from either syngeneic B6D2F1 (□) or allogeneic B6 donors (■) as described in “Materials and methods.” (A,B) RNA was isolated from the intestines and liver of SC transplant recipients on weeks 1, 4, and 6 and CCR1 expression was determined by the RPA. Shown is a representative gel from week 4 (A). L32 is a ribosomal protein used as an mRNA loading standard. A line has been inserted to indicate where the gel was cut. The gels came from the same experiment but the intermediate area was cut. CCR1 mRNA expression was significantly increased after allo-SCT at all time points when compared with syngeneic controls (B) (data not shown). *P < .04; **P < .08; ***P < .001. (C) Splenic CD4+ and CD8+ T-cell expression of CCR1 was analyzed on days 7 and 11 and was found to be increased after allo-SCT when compared with syngeneic and naive controls. Data are shown for naive, syngeneic, and allogeneic splenic T cells stained for CD4+ or CD8+ (PE) and CCR1+ (FITC) on day 7. Numbers in the upper right corner of each graph represent the percentage of events in the corresponding quadrants.
CCR1 expression is increased on CD4+ and CD8+ T cells following allo-SCT
| . | Day 7 . | Day 11 . | ||||
|---|---|---|---|---|---|---|
| Naive | Syn | Allo | Naive | Syn | Allo | |
| CD4+ | 6.9 | 8.6 ± 1.6 | 16.5 ± 2.6 | 3.0 | 8.6 ± 2.3 | 33.6 ± 1.8* |
| CD8+ | 3.5 | 7.3 ± 1.8 | 42.1 ± 2.2* | 2.5 | 10.3 ± 0.9 | 52.3 ± 1.6* |
| . | Day 7 . | Day 11 . | ||||
|---|---|---|---|---|---|---|
| Naive | Syn | Allo | Naive | Syn | Allo | |
| CD4+ | 6.9 | 8.6 ± 1.6 | 16.5 ± 2.6 | 3.0 | 8.6 ± 2.3 | 33.6 ± 1.8* |
| CD8+ | 3.5 | 7.3 ± 1.8 | 42.1 ± 2.2* | 2.5 | 10.3 ± 0.9 | 52.3 ± 1.6* |
The percentage of the total CD4+ and CD8+ cells expressing CCR1: (CD4+CCR1+)/[CD4+ + (CD4+ CCR1+)] and (CD8+ CCR1+)/[CD8+ + (CD8+CCR1+)] was increased at each time point following allo-SCT. Data are presented as means plus SEM and represent a combination of 2 similar experiments.
Syn indicates syngeneic.
P < .001 (allo-SCT compared with syngeneic controls).
Allo-SCT with CCR1-deficient donor cells reduces the severity of GVHD
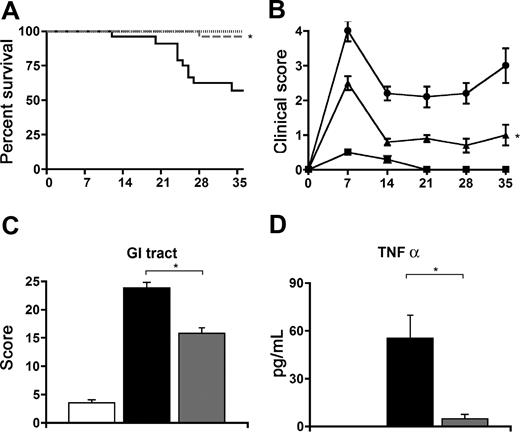
We next determined the contribution of CCR1 expression on donor cells to the development of GVHD. Lethally irradiated B6D2F1 mice received transplants of BM and T cells from either syngeneic, allogeneic CCR1+/+ or allogeneic CCR1−/− donors and were monitored for GVHD. As depicted in Figure 2, allo-SCT with CCR1−/− donors resulted in a reduction in the severity of systemic GVHD as measured by survival (96% vs 57%), and clinical score at each time point assessed (Figure 2A,B) when compared with allogeneic CCR1+/+ controls. Reductions in systemic GVHD were associated with significantly less GI-tract GVHD at 7 (Figure 2C) and 14 days (23.8 ± 1.0 vs 15.8 ± 1.8; P < .05) after SCT and with lower serum TNFα levels on day 7 (Figure 2D). The time points of our analyses of serum cytokines and target organ pathology were selected based upon our prior experience using this SCT model.32,33
SCT with CCR1-deficient donor cells is associated with a significant decrease in GVHD severity. Lethally irradiated B6D2F1 mice received transplants from syngeneic B6D2F1 (- - -, ■), and allogeneic B6.129 CCR1+/+ (—, ●) or B6.129 CCR1−/− (dashed gray line, ▴) donors as described in “Materials and methods.” Transplant recipients were monitored daily for survival (A), and GVHD clinical scores were assessed weekly (B). *P < .01 (B6.129 CCR1−/− vs B6.129 CCR1+/+). Intestinal histopathology (C) and serum TNFα levels were assessed on day 7 (syngeneic, □; allo CCR1+/+, ■; allo CCR1−/−,  ). *P < .01. Data are expressed as means plus or minus SEM (B-D) and are combined from 2 comparable experiments; n = 10 to 20 per group for survival data and 8 to 12 per group for pathology and TNFα data.
). *P < .01. Data are expressed as means plus or minus SEM (B-D) and are combined from 2 comparable experiments; n = 10 to 20 per group for survival data and 8 to 12 per group for pathology and TNFα data.
SCT with CCR1-deficient donor cells is associated with a significant decrease in GVHD severity. Lethally irradiated B6D2F1 mice received transplants from syngeneic B6D2F1 (- - -, ■), and allogeneic B6.129 CCR1+/+ (—, ●) or B6.129 CCR1−/− (dashed gray line, ▴) donors as described in “Materials and methods.” Transplant recipients were monitored daily for survival (A), and GVHD clinical scores were assessed weekly (B). *P < .01 (B6.129 CCR1−/− vs B6.129 CCR1+/+). Intestinal histopathology (C) and serum TNFα levels were assessed on day 7 (syngeneic, □; allo CCR1+/+, ■; allo CCR1−/−,  ). *P < .01. Data are expressed as means plus or minus SEM (B-D) and are combined from 2 comparable experiments; n = 10 to 20 per group for survival data and 8 to 12 per group for pathology and TNFα data.
). *P < .01. Data are expressed as means plus or minus SEM (B-D) and are combined from 2 comparable experiments; n = 10 to 20 per group for survival data and 8 to 12 per group for pathology and TNFα data.
Expression of CCR1 on both donor T and accessory cells contribute to GVHD following allo-SCT
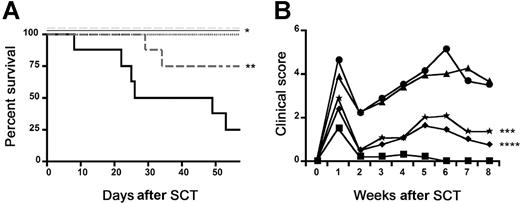
CCR1 expression is up-regulated on cells of both lymphoid and myeloid lineage after allo-SCT. To investigate the relative contributions of CCR1 expression on donor T and accessory cells to the development of GVHD, we completed “mixing” experiments wherein the admixture of various combinations of CCR1−/− and CCR1+/+ BM and T cells resulted in 4 possible allogeneic groups. As shown in Figure 3, the greatest reduction in GVHD severity was seen in recipients of CCR1−/− donor T cells, regardless of the phenotype of the coadministered BM cells. While allogeneic recipients of CCR1−/− BM plus CCR1+/+ T cells had a significant improvement in survival compared with recipients of CCR1+/+ BM and T cells, clinical GVHD scores did not differ between groups. These data demonstrate that while the expression of CCR1 on both donor accessory cells and T cells contributes to the development of GVHD, the impact of donor lymphocyte expression is dominant.
Absence of CCR1 expression on both donor T cells and accessory cells contributes to improved survival following allo-SCT. Lethally irradiated B6D2F1 mice underwent transplantation as described in Figure 2 (syngeneic: · · ·, ■; allogeneic CCR1+/+: —, ●; allogeneic CCR1−/−: solid gray line, *). Two additional allogeneic groups were evaluated in this “mixing” experiment as described in “Materials and methods”: allogeneic CCR1+/+ bone marrow cells mixed with allogeneic CCR1−/− T cells (light gray dashed line, ◆) and allogeneic CCR1−/− bone marrow cells mixed with allogeneic CCR1+/+ T cells (dark gray dashed line, ▴). Survival was monitored daily, and GVHD clinical scores were assessed weekly; n = 8 to 10 per group for survival and GVHD clinical score data; *P < .002 (allogeneic CCR1−/− and allogeneic CCR1+/+ BM + CCR1−/− T vs allogeneic CCR1+/+); **P < .03 (allogeneic CCR1−/− BM + CCR1+/+ T vs allogeneic CCR1+/+); ***P = .01 (allogeneic CCR1−/− vs allogeneic CCR1+/+); ****P = .001 (allogeneic CCR1+/+ BM + CCR1−/− T vs allogeneic CCR1+/+).
Absence of CCR1 expression on both donor T cells and accessory cells contributes to improved survival following allo-SCT. Lethally irradiated B6D2F1 mice underwent transplantation as described in Figure 2 (syngeneic: · · ·, ■; allogeneic CCR1+/+: —, ●; allogeneic CCR1−/−: solid gray line, *). Two additional allogeneic groups were evaluated in this “mixing” experiment as described in “Materials and methods”: allogeneic CCR1+/+ bone marrow cells mixed with allogeneic CCR1−/− T cells (light gray dashed line, ◆) and allogeneic CCR1−/− bone marrow cells mixed with allogeneic CCR1+/+ T cells (dark gray dashed line, ▴). Survival was monitored daily, and GVHD clinical scores were assessed weekly; n = 8 to 10 per group for survival and GVHD clinical score data; *P < .002 (allogeneic CCR1−/− and allogeneic CCR1+/+ BM + CCR1−/− T vs allogeneic CCR1+/+); **P < .03 (allogeneic CCR1−/− BM + CCR1+/+ T vs allogeneic CCR1+/+); ***P = .01 (allogeneic CCR1−/− vs allogeneic CCR1+/+); ****P = .001 (allogeneic CCR1+/+ BM + CCR1−/− T vs allogeneic CCR1+/+).
CCR1 expression modulates GVL activity following allo-SCT
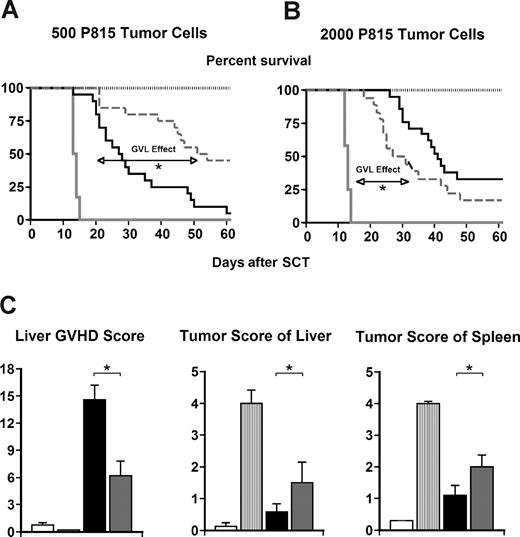
Alloreactive donor T cells are directly associated with GVHD, but also mediate GVL effects that are critical following allo-SCT for malignant disease.3,4,35 We anticipated that the reduction in GVHD severity observed after CCR1−/− SCT was secondary to impairment of donor cell recruitment to GVHD target organs, and therefore hypothesized that this observation would be associated with preservation of GVL activity. B6D2F1-recipient mice received syngeneic transplants, or allogeneic transplants with CCR1+/+ or CCR1−/− donors as before, and 500 host-type (H-2d) P815 tumor cells were added to the BM inoculum on day 0. Transplant recipients were subsequently monitored for survival. All syngeneic transplant recipients uniformly died of disseminated P815 tumor cell infiltration by day 14, whereas recipients of CCR1+/+ allogeneic transplants effectively rejected their tumor but died of GVHD. By contrast, CCR1−/− SCT resulted in a consistent reduction in GVHD severity, preservation of GVL activity, and improved leukemia-free survival (Figure 4A). To better ascertain the potency of these GVL effects, we increased the tumor burden at the time of SCT by 4-fold. Allo-SCT with CCR1−/− still resulted in significant antitumor activity, but the survival advantage was lost; CCR1−/− transplant recipients died at a rate similar to that observed in CCR1+/+ controls (Figure 4B). The cause of death was assessed for individual mice as described, and we found that the reduction of clinical and target organ GVHD was offset in part by an increase in tumor burden at this higher dose of P815 (Figure 4C). Taken together, these data suggest that CCR1 expression modulates donor T cell alloreactivity that is responsible for both GVHD and GVL effects in vivo.
GVL activity is maintained following CCR1−/− SCT. Lethally irradiated B6D2F1 mice received transplants from syngeneic B6D2F1 (gray solid line;  ) and allogeneic CCR1+/+ (— ■), or CCR1−/− (gray dashed line; ▩) donors as described in Figure 2. P815 (H2Kd) tumor cells were added to the BM inoculum on day 0 as described in “Materials and methods.” A second syngeneic group without P815 tumor cells served as the negative control (gray dotted line; □). (A,B) GVL activity is maintained in CCR1−/− transplant recipients at 500 (A) and 2000 (B) P815 tumor cells, but the survival advantage compared with allogeneic CCR1+/+ transplant recipients is lost at the higher tumor burden. (*P < .001; allo-CCR1−/− vs allo-CCR1+/+ in panel A and allo-CCR1−/− vs syngeneic tumor in panel B). (C) Histopathology of the liver and spleen was assessed for tumor infiltration and GVHD severity. Shown are data represented from experiments in which 2000 P815 tumor cells were administered. Data are expressed as means plus or minus SEM and are combined from at least 2 comparable experiments at each tumor dose, n = 8 to 20 per group for survival data and 6 to 18 per group for pathology (*P < .01; allo-CCR1−/− vs allo-CCR1+/+).
) and allogeneic CCR1+/+ (— ■), or CCR1−/− (gray dashed line; ▩) donors as described in Figure 2. P815 (H2Kd) tumor cells were added to the BM inoculum on day 0 as described in “Materials and methods.” A second syngeneic group without P815 tumor cells served as the negative control (gray dotted line; □). (A,B) GVL activity is maintained in CCR1−/− transplant recipients at 500 (A) and 2000 (B) P815 tumor cells, but the survival advantage compared with allogeneic CCR1+/+ transplant recipients is lost at the higher tumor burden. (*P < .001; allo-CCR1−/− vs allo-CCR1+/+ in panel A and allo-CCR1−/− vs syngeneic tumor in panel B). (C) Histopathology of the liver and spleen was assessed for tumor infiltration and GVHD severity. Shown are data represented from experiments in which 2000 P815 tumor cells were administered. Data are expressed as means plus or minus SEM and are combined from at least 2 comparable experiments at each tumor dose, n = 8 to 20 per group for survival data and 6 to 18 per group for pathology (*P < .01; allo-CCR1−/− vs allo-CCR1+/+).
GVL activity is maintained following CCR1−/− SCT. Lethally irradiated B6D2F1 mice received transplants from syngeneic B6D2F1 (gray solid line;  ) and allogeneic CCR1+/+ (— ■), or CCR1−/− (gray dashed line; ▩) donors as described in Figure 2. P815 (H2Kd) tumor cells were added to the BM inoculum on day 0 as described in “Materials and methods.” A second syngeneic group without P815 tumor cells served as the negative control (gray dotted line; □). (A,B) GVL activity is maintained in CCR1−/− transplant recipients at 500 (A) and 2000 (B) P815 tumor cells, but the survival advantage compared with allogeneic CCR1+/+ transplant recipients is lost at the higher tumor burden. (*P < .001; allo-CCR1−/− vs allo-CCR1+/+ in panel A and allo-CCR1−/− vs syngeneic tumor in panel B). (C) Histopathology of the liver and spleen was assessed for tumor infiltration and GVHD severity. Shown are data represented from experiments in which 2000 P815 tumor cells were administered. Data are expressed as means plus or minus SEM and are combined from at least 2 comparable experiments at each tumor dose, n = 8 to 20 per group for survival data and 6 to 18 per group for pathology (*P < .01; allo-CCR1−/− vs allo-CCR1+/+).
) and allogeneic CCR1+/+ (— ■), or CCR1−/− (gray dashed line; ▩) donors as described in Figure 2. P815 (H2Kd) tumor cells were added to the BM inoculum on day 0 as described in “Materials and methods.” A second syngeneic group without P815 tumor cells served as the negative control (gray dotted line; □). (A,B) GVL activity is maintained in CCR1−/− transplant recipients at 500 (A) and 2000 (B) P815 tumor cells, but the survival advantage compared with allogeneic CCR1+/+ transplant recipients is lost at the higher tumor burden. (*P < .001; allo-CCR1−/− vs allo-CCR1+/+ in panel A and allo-CCR1−/− vs syngeneic tumor in panel B). (C) Histopathology of the liver and spleen was assessed for tumor infiltration and GVHD severity. Shown are data represented from experiments in which 2000 P815 tumor cells were administered. Data are expressed as means plus or minus SEM and are combined from at least 2 comparable experiments at each tumor dose, n = 8 to 20 per group for survival data and 6 to 18 per group for pathology (*P < .01; allo-CCR1−/− vs allo-CCR1+/+).
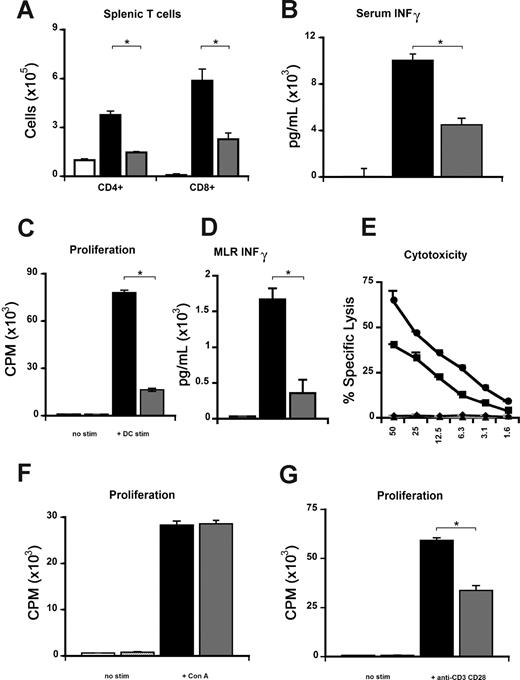
Alloantigen-specific proliferation and cytokine secretion but not CTL function are reduced in the absence of CCR1
We further evaluated the contribution of CCR1 to allo-specific T-cell responses both in vivo and in vitro. First, we found that the expansion of donor T cells was significantly reduced in the spleen on day 7 following allogeneic CCR1−/− SCT compared with CCR1+/+ controls (Figure 5A). The reduction in T-cell numbers was associated with significantly decreased serum IFNγ levels on day 7 (Figure 5B). This in vivo observation was reproduced in vitro; naive CCR1−/− T cells showed significantly diminished proliferation and IFNγ production compared with CCR1+/+ cells when stimulated in a MLR with host-type (B6D2F1) alloantigen (Figure 5C,D). We next tested the effect of CCR1 deficiency on cytolytic activity and found that both wild-type and CCR1−/− CTLs were equally capable of lysing allogeneic targets (Figure 5E).
CCR1-deficient T cells demonstrate impaired allo-specific responses but maintain cytolytic function. To assess donor T-cell function in vivo, B2D2F1 mice received transplants from syngeneic (□) and allogeneic CCR1+/+ (■), or CCR1−/− (▩) donors as described in Figure 2. (A,B) Absence of CCR1 on donor cells reduces splenic T-cell expansion (A) and serum INFγ levels (B) at day 7 following SCT. (C,D) Allospecific proliferation (C) and INFγ production (D) were also reduced in vitro during a MLR with wild-type (■) or CCR1−/− (▩) T cells and allogeneic B6D2F1 stimulators or with control media (■ or  , respectively), as described in “Materials and methods.” (E) Cytotoxic function of splenic T cells after in vitro priming was determined by a chromium release assay using P-815 (H2d) and EL-4 (H2b) target cells as previously described (■, wild-type → P-815; ●, CCR1−/− → P-815; ▴, wild-type → EL-4; and ◆, CCR1−/− → EL-4). (F,G) No differences in proliferation between CCR1+/+ (■) and CCR1−/− (▩) T cells are evident following stimulation with ConA (F), whereas proliferation of CCR1−/− T cells was significantly reduced following anti-CD3–CD28 stimulation; *P < .05 (G). Data are from 1 of at least 3 similar experiments and are presented as means plus or minus SEM.
, respectively), as described in “Materials and methods.” (E) Cytotoxic function of splenic T cells after in vitro priming was determined by a chromium release assay using P-815 (H2d) and EL-4 (H2b) target cells as previously described (■, wild-type → P-815; ●, CCR1−/− → P-815; ▴, wild-type → EL-4; and ◆, CCR1−/− → EL-4). (F,G) No differences in proliferation between CCR1+/+ (■) and CCR1−/− (▩) T cells are evident following stimulation with ConA (F), whereas proliferation of CCR1−/− T cells was significantly reduced following anti-CD3–CD28 stimulation; *P < .05 (G). Data are from 1 of at least 3 similar experiments and are presented as means plus or minus SEM.
CCR1-deficient T cells demonstrate impaired allo-specific responses but maintain cytolytic function. To assess donor T-cell function in vivo, B2D2F1 mice received transplants from syngeneic (□) and allogeneic CCR1+/+ (■), or CCR1−/− (▩) donors as described in Figure 2. (A,B) Absence of CCR1 on donor cells reduces splenic T-cell expansion (A) and serum INFγ levels (B) at day 7 following SCT. (C,D) Allospecific proliferation (C) and INFγ production (D) were also reduced in vitro during a MLR with wild-type (■) or CCR1−/− (▩) T cells and allogeneic B6D2F1 stimulators or with control media (■ or  , respectively), as described in “Materials and methods.” (E) Cytotoxic function of splenic T cells after in vitro priming was determined by a chromium release assay using P-815 (H2d) and EL-4 (H2b) target cells as previously described (■, wild-type → P-815; ●, CCR1−/− → P-815; ▴, wild-type → EL-4; and ◆, CCR1−/− → EL-4). (F,G) No differences in proliferation between CCR1+/+ (■) and CCR1−/− (▩) T cells are evident following stimulation with ConA (F), whereas proliferation of CCR1−/− T cells was significantly reduced following anti-CD3–CD28 stimulation; *P < .05 (G). Data are from 1 of at least 3 similar experiments and are presented as means plus or minus SEM.
, respectively), as described in “Materials and methods.” (E) Cytotoxic function of splenic T cells after in vitro priming was determined by a chromium release assay using P-815 (H2d) and EL-4 (H2b) target cells as previously described (■, wild-type → P-815; ●, CCR1−/− → P-815; ▴, wild-type → EL-4; and ◆, CCR1−/− → EL-4). (F,G) No differences in proliferation between CCR1+/+ (■) and CCR1−/− (▩) T cells are evident following stimulation with ConA (F), whereas proliferation of CCR1−/− T cells was significantly reduced following anti-CD3–CD28 stimulation; *P < .05 (G). Data are from 1 of at least 3 similar experiments and are presented as means plus or minus SEM.
Finally, similar findings with respect to proliferation and IFNγ production were also observed when CCR1−/− T cells were stimulated with DCs from either major histocompatibility complex (MHC) class II (bm12) or MHC class I (bm1) disparate mice (data not shown), and when naive CCR1−/− T cells were stimulated through the T-cell receptor in the presence of costimulation with anti-CD3e plus anti-CD28 (Figure 4G). However, T-cell responses to the mitogen ConA were intact (Figure 5F). These findings confirm that T-cell proliferation and IFNγ production to allo-antigen stimulation are significantly altered in the absence of CCR1 both in vitro and in vivo.
Reduction in T-cell alloreactivity depends in part on interactions between CCR1 and CCL5
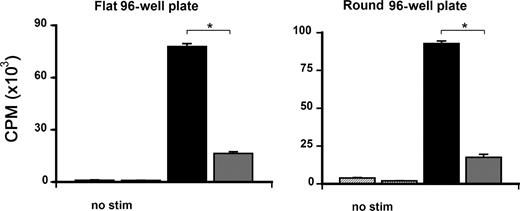
To investigate whether the reduction in proliferation of CCR1-deficient T cells may be secondary to a migration defect in vitro, CCR1+/+ and CCR1−/− T cells were stimulated in vitro in a MLR using both flat- and round-bottom 96-well plates as described by Benvenuti et al.36 As shown in Figure 6A, naive CCR1−/− T cells showed significantly diminished proliferation to alloantigen, regardless of culture conditions, suggesting that the reduction in proliferation was not due to a migration defect per se. In order to determine whether the reduction in allo-specific proliferation seen with CCR1−/− T cells was due to interactions between CCR1 and its ligands, we next completed MLRs in the presence of MoAbs to CCL3 (Mip-1α), CCL4 (Mip-1β), and CCL5 (RANTES) individually. A significant reduction in proliferation was observed following the addition of anti-CCL5 MoAb to wild-type T cells compared with its isotype control, whereas the addition of anti-CCL3 or anti-CCL4 MoAbs had no effect (Table 2). These findings suggest that allospecific T-cell proliferation is dependent in part on interactions between CCR1 and CCL5.
Alloantigen-specific proliferation is dependent on interactions between CCR1 and CCL5. Allospecific proliferation was assessed in vitro during a mixed lymphocyte reaction in either flat-bottom or round-bottom plates using CCR1+/+ (■) or CCR1−/− (▩) T cells with B6D2F1 stimulators or control media as described in Figure 5. Data are presented as means plus or minus SEM and are from 1 of 3 similar experiments; *P < .001.
Alloantigen-specific proliferation is dependent on interactions between CCR1 and CCL5. Allospecific proliferation was assessed in vitro during a mixed lymphocyte reaction in either flat-bottom or round-bottom plates using CCR1+/+ (■) or CCR1−/− (▩) T cells with B6D2F1 stimulators or control media as described in Figure 5. Data are presented as means plus or minus SEM and are from 1 of 3 similar experiments; *P < .001.
MoAb to CCL5 reduces T-cell proliferation in vitro
| . | CCL5* . | CCL3† . | CCL4† . |
|---|---|---|---|
| Absolute reduction, cpm × 103 | 48.9 | −4.2 | −5.2 |
| Reduction, % | 77.7 | −13.8 | −17.1 |
| . | CCL5* . | CCL3† . | CCL4† . |
|---|---|---|---|
| Absolute reduction, cpm × 103 | 48.9 | −4.2 | −5.2 |
| Reduction, % | 77.7 | −13.8 | −17.1 |
The addition of anti-CCL5 MoAb significantly reduced allospecific proliferation of CCR1+/+ wild-type T cells in vitro compared with isotype control (P < .05), whereas anti-CCL3 and anti-CCL4 MoAb did not. Data are presented as means plus or minus SEM and are from 1 of 3 similar experiments.
Based on reference T + DC + IgG of 62.9 ± 6.1 cpm × 103.
Based on reference T + DC + IgG of 30.4 ± 1.3 cpm × 103.
Absence of CCL5 in host APCs results in improved survival following allo-SCT
Our data suggesting a role for CCR1/CCL5 interactions in T-cell allo-activation was intriguing and lead us to examine the role of CCL5 production from host cells in the induction of GVHD. B6D2F1 mice underwent SCT from either B6 or B6D2F1 donors, as described in Figure 1, and CCL5 levels were measured in the serum at days 4 and 7 after SCT. CCL5 levels were increased at both time points in transplant recipients compared with syngeneic controls (Figure 7A). Next, we used CCL5−/− animals as recipients in a second, MHC-disparate SCT system (B6 → Balb/c), and found that the inability of host cells to secrete CCL5 resulted in a significant survival advantage following allo-SCT compared with CCL5+/+ controls (Figure 7B). In a final set of experiments, we tested the hypothesis that the activity of host hematopoietic cells (specifically APCs) was critical to the survival advantage observed in Figure 7B. CCL5 chimeras were generated, and these mice (wild-type → CCL5−/− and CCL5−/− → wild-type) were subsequently used as recipients in a tandem SCT experiment. Our data show that reconstitution of CCL5+/+ mice with CCL5−/− hematopoietic cells also results in a significant reduction in GVHD mortality following allo-SCT (Figure 7C).
CCL5 production by host cells contributes to GVHD after allo-SCT. B6D2F1 mice received transplants from either syngeneic B6D2F1 (□) or allogeneic B6 donors (■) as described in Figure 1, and serum concentrations of CCL5 were measured by ELISA on days 4 and 7 after SCT (A). Data are presented as means plus or minus SEM; n = 4 to 6 mice per group at each time point; *P < .002. Lethally irradiated CCL5+/+ Balb/c mice received transplants from either syngeneic (gray dotted line) or allogeneic (—,) B6 donors, and CCL5−/− Balb/c mice received transplants (– – –) as described in “Materials and methods.” Complete absence of CCL5 in transplant recipient mice results in improved survival after allo-SCT (B). Data are combined from 2 similar experiments; n = 10 to 20 per group; *P < .04 (allo-CCL5−/− vs allo-CCL5+/+). CCL5 chimeric balb/c mice (wild-type [WT] → CCL5−/− [gray solid line] or CCL5−/− → WT [– – –]) were generated as described in “Materials and methods” and were subsequently used as transplant recipients in the second of 2 tandem experiments. The absence of CCL5 in host APCs also results in a significant survival advantage following allo-SCT. Data are from 1 experiment; n = 4 to 10 per group; **P = .01 (CCL5−/− → WT vs WT → CCL5−/−).
CCL5 production by host cells contributes to GVHD after allo-SCT. B6D2F1 mice received transplants from either syngeneic B6D2F1 (□) or allogeneic B6 donors (■) as described in Figure 1, and serum concentrations of CCL5 were measured by ELISA on days 4 and 7 after SCT (A). Data are presented as means plus or minus SEM; n = 4 to 6 mice per group at each time point; *P < .002. Lethally irradiated CCL5+/+ Balb/c mice received transplants from either syngeneic (gray dotted line) or allogeneic (—,) B6 donors, and CCL5−/− Balb/c mice received transplants (– – –) as described in “Materials and methods.” Complete absence of CCL5 in transplant recipient mice results in improved survival after allo-SCT (B). Data are combined from 2 similar experiments; n = 10 to 20 per group; *P < .04 (allo-CCL5−/− vs allo-CCL5+/+). CCL5 chimeric balb/c mice (wild-type [WT] → CCL5−/− [gray solid line] or CCL5−/− → WT [– – –]) were generated as described in “Materials and methods” and were subsequently used as transplant recipients in the second of 2 tandem experiments. The absence of CCL5 in host APCs also results in a significant survival advantage following allo-SCT. Data are from 1 experiment; n = 4 to 10 per group; **P = .01 (CCL5−/− → WT vs WT → CCL5−/−).
Discussion
Allo-SCT is the only curative therapy for many patients with leukemia and lymphoma, but the broader application of this therapy is limited by the development of GVHD and recurrence of underlying disease. The development of GVHD involves soluble and cellular components of both the adaptive and innate immune response.37-39 Myeloid and lymphoid cellular effectors synergize to cause target organ damage, and the contribution of donor myeloid or “accessory” cells is primarily through the secretion of inflammatory proteins, including cytokines and chemokines.40 The development of GVHD can be divided into discrete pathophysiologic stages. The first stage focuses on the effects of SCT conditioning regimens and the resultant proinflammatory milieu that sets the stage for early leukocyte activation and infiltration. Stage 2 is fundamentally dependent upon the activation of donor T cells.41 Alloantigen-specific donor T-cell clones expand, migrate to GVHD target organs, and ultimately secrete soluble factors that recruit other effector cells. Additional inflammatory cytokine release, in combination with direct cell-mediated killing, each contributes to the third and final phase of GVHD.
The migration of leukocytes to and from secondary lymphoid tissues is therefore an essential component of GVHD, but the mechanisms by which activated WBCs traffic to target organs and cause inflammation remain unresolved. Chemokines are extracellular messengers that facilitate the migration of WBCs to sites of inflammation.11 They also modulate the immune response via their ability to act as costimulatory molecules for a variety of immune cells. Like cytokines, a chemokine response (1) can be elicited by nearly any stimulus that disrupts immunologic homeostasis, and (2) results in a robust recruitment of inflammatory cells. When that stimulus is potent and long lasting, an injurious rather than protective chemokine response may ensue and foster progressive leukocyte-mediated tissue damage and organ dysfunction. Despite the complexity of the chemokine microenvironment at sites of tissue injury, emerging data suggest that chemokines play a significant role in all 3 stages of GVHD.
In the present study, we examined the contribution of CCR1 expression on donor cells to both GVHD and GVL effects following allo-SCT. We hypothesized that systemic inflammation engendered early in the time course of allo-SCT and the subsequent recruitment of donor cells into GVHD target organs are mediated in part through interactions with CCR1. We first established that CCR1 mRNA expression is up-regulated in the liver, gut, and spleen in allogeneic transplant recipients, and that these increases corresponded with enhanced cell-surface expression on lymphocytes, monocyte/macrophages, and neutrophils early after SCT. Allo-SCT with CCR1−/− donor cells significantly reduced the severity of systemic and target organ GVHD. Importantly, the absence of CCR1 reduced the recruitment of mononuclear cells and neutrophils into both the gut (7.0 ± 0.6 vs 11.5 ± 1.0; P < .05) and liver (3.9 ± 1.0 vs 8.3 ± 0.8; P < .01) as measured by the corresponding parameters of cellular infitration in our semiquantitative scoring systems. Since CCR1 is expressed on both lymphoid and myeloid cells,13 it was necessary to determine the contribution of each cell type to GVHD. Mixing experiments revealed that CCR1 expression on both T cells and accessory cells contributed to GVHD mortality, but CCR1 expression on lymphocytes was dominant. Interestingly, recipients of CCR1−/− BM plus wild-type T cells had a significant improvement in survival compared with wild-type BM plus wild-type T cells, while clinical GVHD scores did not differ between the groups. This suggests that CCR1 expression on donor accessory cells may also play a role in leukocyte recruitment and target organ GVHD; CCR1 expression is increased on CD11b+ and Gr-1+ cells after allo-SCT, and SCT using CCR1−/− donors results in the reduction in mononuclear and neutrophilic infiltrates in both the gut and liver in the allogeneic recipients. These data may indicate a possible positive feedback loop wherein hematopoietic myeloid cells contribute to chemokine production (eg, CCL5) and facilitate further T-cell recruitment.26 Alternatively, as described by Matte et al,42 while donor T cells are essential to the induction of GVHD through interactions with host APCs, GVHD is intensified by donor-derived APCs. Thus, in the absence of CCR1 on donor accessory cells, GVHD may potentially be attenuated via a donor APC-related mechanism.
GVL activity was also preserved following CCR1−/− SCT, but the survival advantage was lost (compared with mice that underwent CCR1+/+ SCT) when the tumor burden was increased. These findings lead us to explore the effects of CCR1 expression on allo-specific T-cell responses in greater detail. Surprisingly, although cytolytic effector function was maintained on a per-cell basis, T-cell proliferation and IFNγ secretion was significantly reduced both in vivo and in vitro in several MHC-disparate conditions. Alterations in T-cell function did not appear to be due to a migration defect, but were dependent upon interactions between CCR1 and CCL5; antibody neutralization of CCL5 reduced proliferation of CCR1+/+ T cells to an extent comparable with that observed when CCR1 was absent. In addition, allo-SCT using CCL5-deficient recipients or chimeric mice with APCs incapable of secreting CCL5 resulted in improved survival in a second, MHC-disparate model. Taken together, our data demonstrate that CCR1 expression on donor cells contributes to the development of both GVHD and GVL, and suggest that CCR1/CCL5 receptor-ligand interactions play a role in allo-specific T-cell responses that occur in this context. Thus, while we were initially interested in specifically understanding how CCR1 contributed to the recruitment of donor leukocytes in the third and final stage of GVHD, we ultimately found that CCR1 interactions also contribute to T-cell allo-activation, which is a focal point of stage 2 of this process.
Although a recent clinical study reported a correlation between the expression of several chemokine receptors, including CCR1, and acute GVHD after allo-SCT,43 to our knowledge, the data presented herein represent the first to demonstrate a mechanistic role for CCR1 in the development of GVHD using animal models. In mice, CCR1 is found on both CD4 and CD8 T cells and also on monocytes, macrophages, and neutrophils.44 Consistent with our data, CCR1 has been shown to be highly expressed on T helper 1 (Th1) but not Th2 lymphocytes.25 The expression pattern of CCR1 on cellular components of both the adaptive and innate immune response is consistent with reports that CCR1 contributes to a number of inflammatory conditions and to tissue injury that accompanies solid organ rejection.14-25 Allograft rejection models, though different than those used to study GVHD, have provided insight as to how chemokines and their receptors may affect graft-versus-host reactions. Gao and colleagues identified a key role for CCR1 during the development of cardiac allograft rejection. CCR1 mRNA and protein were expressed by most graft-infiltrating mononuclear cells, and CCR1−/− mice receiving MHC-mismatched grafts showed a doubling of survival time25 and accepted their allografts permanently when low-dose cyclosporine was added.
CCL5 is one of the primary ligands for CCR1, and the expression pattern of CCL5 has been studied in several disease models.44,45 CCL5 is produced by a variety of cell types, including macrophages, DCs, lymphocytes, and endothelial cells (ECs). CCL5 can be expressed by nonhematopoietic cells within minutes of stimulation by TNFα and LPS, and also by activated T lymphocytes.46 CCL5 can attract a wide range of immune cells, including activated T cells, monocytes, macrophages, immature DCs and neutrophils.47-49 Studies targeting the biologic effects of CCL5 in vivo by using neutralizing antibodies or receptor antagonists have revealed a role for CCL5 in lymphocyte and macrophage recruitment to sites of inflammation and allograft rejection.24,50-62 In addition, we have recently reported that CCL5 expression is up-regulated in the lung after allo-SCT, is associated with elevations in mRNA levels for CCR1, and significantly contributed to the influx of donor cells that characterizes pulmonary injury during the development of idiopathic pneumonia syndrome.26
In addition to modulating leukocyte recruitment, our data suggest that interactions between CCR1 and CCL5 also contribute to T-cell activation. CCL5 has been shown to play a significant role in both antigen-specific activation of helper and cytotoxic T cells and their subsequent recruitment to sites of inflammation.45,63 In the presence of anti-CD3 MoAb, CCL5 can co-stimulate T cells and T-cell clones to proliferate and produce IL-2, and neutralization of CCL5 abrogates these effects.63 Moreover, CCL5-deficient mice have impaired T-cell function in vivo and in vitro.64
CCL5 binds to at least 3 receptors, CCR1, CCR3, and CCR5. Met-RANTES, a soluble antagonist to both CCR1 and CCR5 that reduces inflammation associated with models of chronic kidney rejection, colitis, and cardiac allograft vasculopathy24,58,65,66 also reduced T-cell proliferation when added to an MLR across MHC antigens.24 In combination with the results of Yun and colleagues,24 the data presented herein along with our unpublished observation that T cells lacking CCR5 proliferate normally in response to allo-antigen, support a more specific role for CCR1 in this context. Thus, while CCL5 and CCR1 have been individually shown to contribute to T-cell activation, our data implicate for the first time a direct role for CCR1/CCL5 receptor-ligand interactions in allo-immune responses that characterize the development of GVHD and GVL.
GVHD remains the major obstacle to successful outcomes following allo-SCT. T-cell activation by host allo-antigens and the subsequent recruitment of lymphoid and myeloid effectors into target tissues are essential components of this process. The specificity for discrete organ systems in GVHD suggests that the mechanisms by which donor leukocytes migrate to target tissues may represent novel therapeutic opportunities to reduce the severity of GVHD. We demonstrate that CCR1 expression is up-regulated on donor cells early after SCT and contributes to leukocyte migration into GVHD target organs. Our data also show that CCR1 directly modulates allo-immune responses both in vitro and in vivo, and although the molecular basis for this effect is under investigation, results support CCL5/CCR1 binding as a critical upstream event. Collectively, these findings suggest that disrupting CCR1/CCL5 receptor-ligand interactions in combination with standard immunoprophylaxis may provide a novel approach to diminishing the adverse affects of GVHD following allo-SCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Drs James L. M. Ferrara and Pavan R. Reddy for their thoughtful discussions and review of this manuscript.
This work was supported by National Institutes of Health grant R01 5HL072258–04 and the Walther Cancer Institute. G.C.H. is a Deutsche Krebshilfe e.V. Scholar. K.R.C. is an Amy Strelzer-Manasevit Scholar of the National Marrow Program, a Clinical Scholar of the Leukemia and Lymphoma Society, and the Recipient of a Translational Research Award from the Leukemia and Lymphoma Society.
National Institutes of Health
Authorship
Contribution: S.W. Choi designed the research, performed research, analyzed data, and wrote the paper, G.C.H. designed the research, performed research, analyzed data, and contributed to the writing of the paper. K.M.O. performed research and analyzed data. D.A.H. analyzed data and assisted with manuscript preparation. M.N.C. performed research and analyzed data; I.A.S. performed research and analyzed data. C.E.R. performed research and analyzed data. D.D. performed research. J.M.F. performed research. C.L. analyzed pathology data. D.A. analyzed data. S.W. Chensue contributed vital new reagents. K.R.C. designed the research, analyzed data, and assisted with manuscript preparation.
S.W. Choi and G.C.H. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kenneth R. Cooke, Case Western Reserve University School of Medicine, Wolstein Research Bldg, Rm 6524, 2103 Cornell Rd, Cleveland, OH 44106-7288; e-mail: kenneth.cooke@uhhospitals.org.

![Figure 7. CCL5 production by host cells contributes to GVHD after allo-SCT. B6D2F1 mice received transplants from either syngeneic B6D2F1 (□) or allogeneic B6 donors (■) as described in Figure 1, and serum concentrations of CCL5 were measured by ELISA on days 4 and 7 after SCT (A). Data are presented as means plus or minus SEM; n = 4 to 6 mice per group at each time point; *P < .002. Lethally irradiated CCL5+/+ Balb/c mice received transplants from either syngeneic (gray dotted line) or allogeneic (—,) B6 donors, and CCL5−/− Balb/c mice received transplants (– – –) as described in “Materials and methods.” Complete absence of CCL5 in transplant recipient mice results in improved survival after allo-SCT (B). Data are combined from 2 similar experiments; n = 10 to 20 per group; *P < .04 (allo-CCL5−/− vs allo-CCL5+/+). CCL5 chimeric balb/c mice (wild-type [WT] → CCL5−/− [gray solid line] or CCL5−/− → WT [– – –]) were generated as described in “Materials and methods” and were subsequently used as transplant recipients in the second of 2 tandem experiments. The absence of CCL5 in host APCs also results in a significant survival advantage following allo-SCT. Data are from 1 experiment; n = 4 to 10 per group; **P = .01 (CCL5−/− → WT vs WT → CCL5−/−).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/9/10.1182_blood-2007-05-087403/7/m_zh80220708990007.jpeg?Expires=1769164204&Signature=ft0OdStn52kL5u5XN~s3Qgut6zlQV91j52xz3RjaJWWf28kqslmUqLpex~p0GIikVNnbjnxeWW6CkrGtozPipIqwoTnwh7F2VRlWR8TXbH30unzrVvIBJEf3sl2Ehv9FkR8aqi6i8MHN8vuE4NKJacxn6fVLaIkhJb7JFRgLe7AztDBhho6n8WO5Y6Ok7LqFgXAP~WfR-10HCYXFxm92YWFvw0hJtpYBaOJ1gHVz~kaHC3IIsy3cnEe-5oQRgZdh4tBvYwGkReP-kFGVUFBBZIoiZRtsBP3QxQ0eQ9FfwNsJiv0HThLbtMNlRFCGhW~iYUgLfnKWnCUq5NJKMhxcMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal