Abstract
For more than 60 years, heparin and coumarin have been mainstays of anticoagulation therapy. They are widely available, inexpensive, effective, and have specific antidotes but are regarded as problematic because of their need for careful monitoring. In addition, coumarin has a delayed onset of action, interacts with many medications, has a narrow therapeutic window, and is paradoxically prothrombotic in certain settings (ie, can precipitate “coumarin necrosis”). Heparin may require monitoring of its therapeutic effect and can also cause thrombosis (heparin-induced thrombocytopenia/thrombosis syndrome). These limitations have led to the development of new anticoagulants with the potential to replace current agents. These newer agents fall into 2 classes, based on whether they are antithrombin dependent (low-molecular-weight heparin, fondaparinux) or antithrombin independent (direct inhibitors of factor Xa and thrombin [factor IIa]). This paper addresses newer anticoagulants, reviewing their efficacy and limitations, and focuses on the risk of major bleeding that may complicate their use. In contrast to heparin and coumarin, none of these newer agents has a specific antidote that completely reverses its anticoagulant effect. Available data on the efficacy and safety of current and experimental agents for anticoagulant reversal are reviewed, and a plan for management of anticoagulant-induced bleeding is presented.
Introduction
Anticoagulant therapy is one of the most common forms of medical intervention. It is the mainstay of treatment and prevention of thrombosis in diverse clinical settings, including acute venous thromboembolism (VTE), atrial fibrillation, acute coronary syndrome (ACS), and in patients undergoing invasive cardiac procedures.1-4 Omission of appropriate anticoagulant prophylaxis is a widely recognized medical error.5
Bleeding is the primary complication of anticoagulant therapy, and is a risk of all anticoagulants,6,7 even when maintained within usual therapeutic ranges. Ironically, whereas unfractionated heparin (UFH) and coumarin, the oldest and most widely used anticoagulants, have specific antidotes for their anticoagulant effect, many of the newer agents currently undergoing clinical evaluation do not have specific antidotes; thus, the best ways to reverse their actions remain to be determined.7
Newer anticoagulants approved or undergoing clinical studies include both direct and indirect inhibitors of coagulation factors.7,8 The indirect (antithrombin-dependent) inhibitors include the low-molecular-weight heparins (LMWHs), such as enoxaparin, dalteparin, and tinzaparin; heparin-like compounds, such as danaparoid; as well as the selective factor Xa inhibitors fondaparinux and idraparinux. Like UFH, the LMWHs inhibit both factors Xa and IIa (thrombin), whereas fondaparinux and idraparinux primarily inhibit factor Xa. The direct thrombin inhibitors (DTIs), which include lepirudin, argatroban, and bivalirudin, directly bind to and inhibit thrombin. A variety of direct inhibitors of factor Xa are under development.9
If rapid reversal of the anticoagulant effect of these agents is required, either as a result of bleeding or need for an invasive procedure, a specific reversal agent would be valuable. The effects of UFH can be readily reversed with protamine sulfate, and vitamin K is a specific antidote for coumarin. Approximately 60% of the anticoagulant effect of LMWH can also be neutralized by protamine.10 In contrast, there are no specific reversal agents for any of the newer anticoagulants.8,11
The use of novel anticoagulants is further complicated by a lack of easily available laboratory tests to measure their levels and thereby optimize their clinical benefit and safety. Whereas UFH, LMWH, and warfarin therapy all can be monitored using either indirect or more specific quantitative assays, many of the novel anticoagulants are either not routinely monitored (eg, fondaparinux, idraparinux) or are usually monitored using indirect tests (eg, DTIs), such as the (activated) partial thromboplastin time and the ecarin clotting time. In the absence of routine monitoring, unexpected drug accumulation can occur, resulting in avoidable major or life-threatening hemorrhage.12 If indirect tests are used, misleading results can occur under certain circumstances (eg, underdosing of DTI therapy that results when warfarin coadministration, liver disease, or consumptive coagulopathy produces greater prolongation of the activated partial thromboplastin time than expected from the DTI itself).13
In this review, we evaluate the risk of bleeding associated with current and future anticoagulants, review the data available on current and experimental agents used for the reversal of anticoagulation, and provide recommendations for the management of major bleeding associated with anticoagulant use.
Methods
Our aim was to undertake a systematic evaluation of the literature available to guide the management of patients with anticoagulant-associated bleeding. Despite the frequency of this clinical complication and the potential for both major morbidity and death, the body of evidence to guide practice was extremely limited. As a result, we present the results of a narrative review designed to evaluate the following 2 questions: (1) What are the risks of bleeding associated with current and future anticoagulants? (2) Can evidence-based recommendations be provided for the management of bleeding in patients receiving anticoagulants?
What are the risks of bleeding associated with current and future anticoagulants?
Bleeding criteria
Bleeding severe enough to require significant medical intervention, such as transfusions or surgery, or that results in serious morbidity or mortality, is classified as major.6,14 Major bleeding generally requires stopping anticoagulant therapy, at least temporarily.7 When evaluated in real-time clinical practice, major bleeding is usually regarded as bleeding associated with a significant risk of death (such as intracerebral bleeding or catastrophic gastrointestinal bleeding), bleeding associated with long-term morbidity (such as intraocular bleeding or less severe intracerebral hemorrhage), or bleeding requiring transfusion therapy to maintain a hemoglobin value.
Risk for major bleeding with coumarin
Coumarin (a vitamin K antagonist) is commonly used in patients with ischemic stroke, prosthetic heart valves, atrial fibrillation, ischemic heart disease, and VTE. In randomized trials of vitamin K antagonists following an acute episode of ischemic cerebrovascular disease, the risk of major bleeding ranged from 2% to 13% during a mean duration of follow-up of 6 to 30 months.6 In randomized trials in patients with mechanical heart valves, vitamin K antagonists were associated with a risk of major bleeding that ranged from 1% to 8.3%.6 In randomized trials in patients with atrial fibrillation, the risk of major bleeding with vitamin K antagonists ranged from 0% to 6.6%.6 In randomized trials in patients with ischemic heart disease, the risk of major bleeding with vitamin K antagonists ranged from 0% to 19.3%.6 In randomized trials in patients with VTE, the risk of major bleeding with vitamin K antagonists ranged from 0% to 16.7%.6 The wide ranges of risk associated with vitamin K antagonists occur because the bleeding risk depends on several factors, including intensity of anticoagulant effect, patient characteristics (particularly age), the concomitant use of drugs that interfere with hemostasis, and the length of therapy.6
Risk for major bleeding with therapeutic-dose UFH and LMWH
In most recent trials involving the treatment of acute VTE using UFH, the risk of major bleeding was less than 3%.6,8 In 2 meta-analyses of studies comparing LMWH with UFH in treating acute VTE, LMWH was associated with less major bleeding than UFH.15,16 Likewise, in a major pooled analysis of 19 trials, LMWH was associated with a significantly reduced incidence of major hemorrhage compared with UFH (1.2% vs 2.0%, odds ratio = 0.57; 95% confidence interval [CI], 0.39-0.83).17 The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy concluded that LMWH was associated with less major bleeding than UFH for VTE but noted that more recent studies have failed to demonstrate any statistically significant differences.6 The reasons for this are not clear. A meta-analysis of recent trials comparing extended-duration LMWH with oral anticoagulants for VTE showed a nonsignificant reduction in major bleeding with LMWH.18 Major bleeding rates for several available anticoagulant agents are listed in Table 1.19-34
The standard of care in treating ACS includes anticoagulant therapy.21 A meta-analysis of 6 randomized controlled trials comparing UFH with the LMWH enoxaparin in patients with non–ST-elevation myocardial infarction (NSTEMI) concluded that there was no significant difference between the agents in major bleeding or need for transfusion after 7 days (Table 1).21 However, subsequent trials in patients with ACS have suggested that there may be a significantly higher risk for major bleeding in patients receiving enoxaparin than those receiving UFH if the TIMI (Thrombolysis In Myocardial Infarction) bleeding classification is used. TIMI criteria for major bleeding are intracranial hemorrhage, a more than or equal to 5 g/dL decrease in hemoglobin concentration, or a more than or equal to 15% absolute decrease in hematocrit. In a large study of nearly 4000 patients, the rate of major bleeding with enoxaparin was significantly greater than with UFH (0.9% vs 0.4%; P = .05) when the TIMI classification was used.35 In another large study of nearly 10 000 patients, both TIMI and GUSTO (Global Strategies for Opening Occluded Coronary Arteries) criteria were used to determine rates of major bleeding. GUSTO criteria for severe or life-threatening bleeding include either intracranial hemorrhage or bleeding that leads to hemodynamic compromise and requires intervention. Using the TIMI criteria for major bleeding, the rate with enoxaparin was 9.1% compared with 7.6% with UFH (P = .008). Using the GUSTO criteria for severe bleeding, the rate with enoxaparin was 2.7% compared with 2.2% with UFH (P = .08).36
LMWH should be used with care in patients with impaired renal function because it may bioaccumulate. Evidence exists that such bioaccumulation may cause bleeding, particularly with enoxaparin, which has the largest published experience in this setting.37 However, it has been reported that the risk of major bleeding in patients with impaired renal function is increased with both LMWH and UFH.38 The risk of bleeding may be reduced by either empiric dose reduction, dose modification based on anti-Xa heparin levels, the use of a LMWH less dependent on the kidneys for its elimination, or the use of lower doses.39-43
These observations suggest that major bleeding is uncommon with LMWH and UFH; however, in clinical practice, the risk of bleeding with any anticoagulant will be higher than that reported in studies where the rate is reduced by careful patient selection. Extending these observations, a pattern of bleeding risk may be inferred: bleeding is less common when anticoagulants are used at prophylactic doses than at therapeutic doses. The risk of bleeding may also vary depending on the clinical circumstances within which they are used; for example, LMWH may cause less bleeding than UFH in patients with venous disease, but more bleeding in patients with ACS.
Risk for major bleeding with selective factor Xa inhibitors
Fondaparinux is the only selective factor Xa inhibitor currently approved for the prevention and treatment of VTE. In a recent study, fondaparinux was compared with UFH for initial treatment of patients with an acute pulmonary embolism. Similar rates of major bleeding were recorded for fondaparinux and UFH (1.3% vs 1.1%; 95% CI, −0.7 to 1.1).44 In an acute deep vein thrombosis trial comparing fondaparinux with enoxaparin, similar rates of major bleeding were recorded for fondaparinux and enoxaparin (1.1% vs 1.2%; 95% CI, −1.0 to 0.8) (Table 1).25
Although fondaparinux has not been approved as antithrombotic therapy for patients with ACS, a recent trial involving more than 20 000 patients evaluated its use in this setting. In this study of patients with unstable angina or NSTEMI, fondaparinux administered at the dose usually reserved for orthopedic prophylaxis was compared with therapeutic-dose enoxaparin.26 The incidence of major bleeding after 9 days was significantly less with fondaparinux than with enoxaparin (2.2% vs 4.1%; P < .001) (Table 1). In addition, mortality rates at both 30 days and 6 months were reduced significantly with fondaparinux compared with LMWH; this difference was attributed almost entirely to the reduced incidence of major bleeding.26 In a second ACS trial evaluating more than 12 000 patients with ST-elevation myocardial infarction (STEMI), the rate of major bleeding observed with fondaparinux after 9 days was similar to that observed in the NSTEMI trial, as well as to that of the UFH group in the STEMI trial (UFH, 2.3% vs fondaparinux, 2.1%; P = .69).45
Risk for major bleeding with licensed DTIs
A meta-analysis of studies evaluating the safety of lepirudin (r-hirudin) in patients with heparin-induced thrombocytopenia (HIT) determined that after 35 days the incidence of major bleeding that required transfusion was significantly greater in patients receiving lepirudin than in historical controls (18.8% vs 7.1%; P = .02) (Table 1).27 In 2 similar studies evaluating the safety of argatroban in patients with HIT, the rates of major bleeding were lower in patients receiving argatroban (3.1% and 5.3%) than historical controls (8.2% and 8.6%, respectively), but the differences were not statistically significant (P = .078 and P = .27, respectively).28,46 However, the between-study rates of major bleeding for lepirudin and argatroban when used to treat thrombosis-complicating HIT, as reported on a per treatment-day basis, are fairly similar.47
There is now considerable evidence that the approved dosing regimen for lepirudin in HIT is too high30-33 ; the initial infusion rates should probably be approximately half of that approved (0.05-0.10 mg/kg per hour vs 0.15 mg/kg per hour). Similarly, recommendations for markedly reduced dosing of argatroban for treatment of HIT in settings of critical illness have also been made.48,49
A meta-analysis of 11 randomized controlled trials comparing DTIs with heparin in nearly 36 000 patients with ACS reported a reduced risk of major bleeding in patients treated with DTIs during the treatment period (DTIs, 1.9% vs heparin, 2.3%; P < .001) (Table 1); however, further statistical analysis revealed heterogeneity among the various DTIs, with a reduced risk of bleeding with bivalirudin but an increased risk with hirudin.50
Can evidence-based recommendations be provided for the management of bleeding in patients receiving anticoagulants?
Unfortunately, there is little evidence to guide the management of the anticoagulated and bleeding patient. Treatments include the specific reversal agent protamine sulfate and blood product transfusions. There are also several other experimental agents that are under investigation for the management of bleeding associated with anticoagulation. Given the emergent nature of these events and their inherent unpredictability, it is unlikely that randomized trials or even large cohort studies will be performed. More likely, recommendations will continue to be based on case series and anecdotal experience, making the strength of recommendations weak. A summary of recommendations of specific agents and blood products is included in Table 2.8,11,51-59
Protamine sulfate for LMWH
Although protamine sulfate is a specific and effective reversal agent for UFH, it only partially neutralizes the anticoagulant effect of LMWH. The reduced neutralization is the result of reduced sulfate charge densities of LMWH compared with UFH. The different LMWHs have different sulfate charge densities; thus, their degree of neutralization varies. There is a strong correlation between the degree of antifactor Xa neutralization and the total sulfate content of the LMWH, expressed per saccharide unit (r2 = .92).10 Based on these observations, tinzaparin is likely to be more neutralizable than dalteparin, which should be more neutralizable than enoxaparin.
Clinically, this reduced neutralization effect of protamine for LMWH may result in failure to terminate bleeding.8 In a small case series in patients undergoing cardiac surgery using LMWH,60 3 of 15 patients developed abnormal bleeding. Protamine sulfate was administered to the patients, but bleeding was stopped in only 1 of the patients after a second dose of protamine. Bleeding was not controlled in the other 2 patients. When used to reverse LMWH, protamine sulfate should be administered intravenously at doses estimated to be equivalent to the remaining anti-Xa effect of the LMWH. LMWH has a longer half-life than UFH requiring repeated doses in the case of ongoing bleeding after large subcutaneous doses.11,61
Adverse effects are associated with the use of protamine sulfate, including allergic reactions, respiratory problems, and serious cardiovascular reactions, such as hypotension and bradycardia.62 In a study of patients undergoing cardiovascular bypass surgery, 10.7% of the patients experienced adverse reactions immediately after protamine sulfate administration.62 A significant drop in blood pressure developed in 9.9%, whereas 1.6% experienced a precipitous drop in blood pressure. Slow administration (≤ 5 mg/min) is advised to reduce the risk of protamine-induced hypotension and bradycardia.11,51,61
Blood product transfusion
Transfusion of blood products, usually fresh frozen plasma (FFP), is the most commonly used method in North America for rapidly reversing coumarin.11 The usual dose of FFP for anticoagulant reversal is 15 mL/kg of body weight.11 When urgent reversal of a therapeutic (rather than supratherapeutic) international normalized ratio (INR) is required, a lower dose of 5 to 8 mL/kg may be appropriate. Complete correction of the INR is rarely seen when FFP is used without vitamin K to correct the INR irrespective of either the dose of FFP or the degree of prolongation of the INR. Transfusion of FFP or related products to patients with anticoagulant-associated hemorrhage should be confined to patients who have a defined coagulation factor deficiency (for example, resulting from coumarin or dilutional coagulopathy), as these products do not specifically neutralize anticoagulants. Concerns associated with the use of FFP or cryosupernatant plasma for the treatment of anticoagulant-associated bleeding include time delays associated with preparation, delivery, and administration,11,63 volume overload, and a very small risk of transmissible viral infection.8,64 Other adverse events include failure to completely reverse the coagulopathy, passive alloimmune thrombocytopenia (platelet-reactive alloantibodies within donor plasma),65 anaphylactoid reactions (in IgA-deficient recipients), and septicemia (bacterial contamination of the plasma).11
These limitations have led to recommendations that prothrombin complex concentrates (PCCs) be used in place of FFP for the treatment of anticoagulant-associated coagulation factor deficiencies. Although PCCs are prepared from plasma pooled from hundreds or thousands of donors, they are virally inactivated, and thus, even less likely than plasma to transmit infection.11 The different available PCCs contain the vitamin K-dependent factors in various amounts: those containing therapeutic quantities of factors II, IX, and X are referred to as “3-factor concentrates,” whereas those that additionally contain therapeutic amounts of factor VII are termed “4-factor concentrates.”11,52 Because of the lack of comparative studies, the clinical differences between 3- and 4-factor concentrates is not clear, although 3-factor concentrates should less effectively correct the lNR as a result of their reduced concentration of factor VII.52 PCCs reverse laboratory markers of coagulopathy resulting from coumarin overcoagulation more quickly and effectively than FFP.66,67 These agents are considered “standard of practice” in many centers in Europe.52,68 The optimal dose of PCCs for reversal of anticoagulation has not been established; however, the recommended dose ranges from 25 to 100 U/kg depending on the product used.11,52
Irrespective of the blood product administered to reverse coumarin anticoagulation, the patient should also receive intravenous vitamin K at a dose of 2.5 to 5 mg administered over 30 minutes; otherwise, the INR is unlikely to completely correct and a “rebound coagulopathy” may develop after the transfused factors are cleared.69 Intravenous administration of vitamin K has a small risk of severe anaphylactoid reaction and should only be given by slow intravenous infusion (over 20-30 minutes) and oral vitamin K should be used whenever possible.11 Because more rapid reversal is achieved with intravenous administration, it is preferred in cases when urgent reversal is required, such as life-threatening bleeding or reversal of coumarin effect in a patient diagnosed with acute HIT.34
Experimental agents for the reversal of newer anticoagulants
Recombinant factor VIIa
Recombinant factor VIIa (rFVIIa) is a synthetic product of recombinant biotechnology, structurally similar to native FVIIa present within human plasma. Evidence supporting the effectiveness of rFVIIa for the treatment of coumarin-associated bleeding is confined to case reports and small case series. rFVIIa rapidly corrected the INR in coumarin-treated healthy volunteers53,70 and in case studies appeared to prevent and arrest bleeding.53-55 Given its potential to generate thrombin in vivo even in the absence of tissue factor,71 rFVIIa is being investigated as a possible reversal agent for newer anticoagulants.72 Thrombosis complicates rFVIIa use in up to 7% of cases.73,74
Although neither fondaparinux (approved) nor idraparinux (investigational) has an antidote, recent studies suggest that rFVIIa may be beneficial in reversing their effects. In vitro, rFVIIa accelerates thrombin generation in the presence of fondaparinux.75 Moreover, rFVIIa reversed in vitro and ex vivo fondaparinux effects as measured by coagulation tests.76 In healthy volunteers given fondaparinux, rFVIIa normalized coagulation times and thrombin generation within 1.5 hours, with sustained effect for 6 hours.56 In healthy volunteers who received idraparinux, rFVIIa decreased the inhibitory effects of idraparinux on coagulation parameters.57 The clinical utility of rFVIIa or other agents in the treatment of bleeding associated with these indirect factor Xa inhibitors remains to be determined.
Desmopressin
Desmopressin acetate (DDAVP) is a nonspecific prohemostatic agent that stimulates the release of factor VIII and VWF from the endothelium into the plasma.7,77 It is used for the treatment or prevention of bleeding in patients with mild hemophilia A, type I von Willebrand disease, and congenital platelet dysfunction.58,77
DDAVP reduced the anticoagulant effect of the DTI hirudin in an in vitro model using blood from healthy volunteers who had received this agent.59 Rabbits receiving hirudin had significantly reduced duration of spontaneous rebleeding after DDAVP administration.78 DDAVP is a synthetic analog of vasopressin and, as such, retains many of the side effects associated with vasopressin (headaches, palpitations, facial flushing). It also mimics the antidiuretic actions of vasopressin and can produce clinically significant hyponatremia if fluids are not restricted.77
Antifibrinolytic therapy
Tranexamic acid, ϵ-aminocaproic acid, and aprotinin are antifibrinolytic agents, although their mechanisms of action differ.77 Tranexamic acid and ϵ-aminocaproic acid block the proteolytic site of plasmin and inhibit plasminogen activator incorporation into the nascent fibrin clot. Aprotinin directly inactivates plasmin, among several other serine proteases. These agents benefit selected patients undergoing orthopedic, urologic, or cardiac surgery.77,79,80 In October 2007, a large randomized controlled trial comparing antifibrinolytics in patients undergoing cardiac surgery was stopped after a preliminary analysis suggested a trend toward an increase in all-cause 30-day mortality associated with aprotinin. Subsequently, the manufacturer of aprotinin temporarily suspended marketing and halted all shipment of aprotinin on a worldwide basis.81
Hemodialysis, hemofiltration, and plasmapheresis
A variety of mechanical strategies to remove anticoagulants might be considered in patients with life-threatening bleeding. Hemodialysis removes selected small-molecule anticoagulants able to cross the dialysis membrane. Hirudin can be cleared through dialysis with high-flux membranes (certain low-flux hemodialyzers are r-hirudin-impermeable). Hirudin might also be slowly removed by hemofiltration, although the rate of drug removal is low.82 Plasmapheresis and hemoperfusion have both been used in cases of overdose, although neither intervention is supported by good-quality evidence.
Management strategies in the event of a major bleed
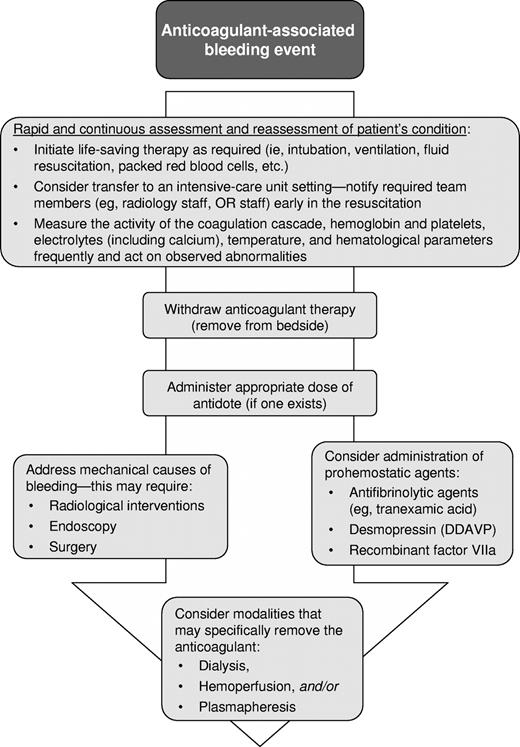
Although there are few high-quality data comparing different strategies for the management of bleeding associated with anticoagulation and thus evidence-based recommendations are not possible, there are basic management principles that can be applied. When faced with an actively bleeding patient, it is important to quickly implement the fundamentals of bleeding management (Figure 1). Initial patient evaluation requires rapid and careful assessment of the source, cause, and severity of bleeding. If the bleeding is determined to be minor, packing or dressing are appropriate measures to mechanically control bleeding. Reduction of the dose or frequency of the anticoagulant agent may be necessary. In all cases, the patient's vital status should be monitored continuously and transfer to an appropriately intensive setting should be considered.
In the presence of major bleeding, a more aggressive approach is required. Often more than 1 individual is required to manage effectively a major bleeding event. Life-saving therapies, such as mechanical ventilation and vasopressors, should be used when appropriate. Determining the mechanical causes of the bleeding may require invasive approaches, including endoscopy, surgery, or invasive radiologic procedures; in general, the risks associated with such procedures (such as local bleeding even in a fully anticoagulated patient) will be much lower than the risks associated with untreated hemorrhage. A specific reversal agent should be administered if one is available, including vitamin K and FFP/PCC for coumarin reversal and protamine sulfate for the neutralization of heparin and LMWH (Table 2).
It is important to maintain an optimal body temperature, blood pH, and electrolyte balance, and to monitor coagulation tests and the complete blood count closely. Because prophylactic transfusions of FFP, cryosupernatant plasma, or cryoprecipitate have not been shown to be beneficial, they should not be given as a “reflex action,” but only if a severe deficiency of one or more relevant hemostasis factors is evident. Their use may lead to an unreasonable expectation of efficacy and delay the use of other, more appropriate, prohemostatic therapies.7 In addition, hypovolemia and anemia (and, perhaps, dilutional thrombocytopenia) often require appropriate blood product replacement. In patients treated with newer anticoagulants without specific reversal agents (ie, fondaparinux, DTIs), desmopressin or antifibrolytic agents may be beneficial. PCC or rFVIIa should be considered as a last resort in instances of life-threatening bleeding when conventional treatment methods have failed or are unavailable.
Several common errors can occur during the resuscitation of an acutely bleeding patient; care should be taken to avoid these. Although establishing wide-bore intravenous access is critical, indwelling capped central venous catheters should not be used for fluid resuscitation until the indwelling fluid has been withdrawn along with an adequate blood discard (minimum of 10 mL); if this is not completed, the anticoagulant dwelling in the line will be flushed into the patient. Anticoagulant-containing intravenous solutions should be removed from the patient's bedside to avoid inadvertent administration should a rapid intravenous bolus of fluid be required. A supply of protamine, DDAVP, and antifibrinolytics should be easily accessible in at least 1 location in the hospital to avoid the need to contact employees to obtain a supply of medication should bleeding occur after usual pharmacy hours. Finally, guidelines for the treatment of major or life-threatening bleeding should be developed and be easily accessible at each hospital because “expert” advice is not likely to be immediately available at most sites where these relatively unusual events are likely to occur.
Conclusions
Anticoagulant therapy is one of the most widely used medical therapies. The classic anticoagulants, UFH and coumarin, have reasonable efficacy and safety, and specific antidotes, but have certain undesirable features. Ironically, newer anticoagulants developed to overcome these limitations do not have specific antidotes, requiring careful patient selection and dosing to optimize their therapeutic benefits. This group includes LMWHs, specific factor Xa inhibitors, and DTIs. There is preliminary evidence that nonspecific and experimental reversal agents can be effective (to various degrees) for reversing the effects of the newer anticoagulant therapies. This evidence largely stems from individual case studies, controlled evaluations of healthy volunteers, or animal or in vitro studies. Additional research on reversal agents or techniques for the newer anticoagulants is needed, including adequately powered clinical studies. A strategy for the management of bleeding complications in patients undergoing anticoagulation therapy, including that with the newer anticoagulants, has been proposed. This strategy emphasizes the necessity for awareness of the basic principles of bleeding management, including rapid assessment of the source, cause, and severity of bleeding, and prompt appropriate action, both mechanical and systemic, to control the bleeding. In the setting of major bleeding, this includes the cessation of anticoagulation therapy and, if possible, reversal of anticoagulation effects, using available, specific reversal agents. In some cases, the use of specific blood products may be necessary.
Acknowledgments
The authors thank Dr Jennifer Power for her assistance with the literature review, data extraction, reference formatting, and other editorial and formatting considerations during the development of the manuscript.
Authorship
Contribution: M.A.C. and T.E.W. are responsible for the content, style, and composition of the manuscript.
Conflict-of-interest disclosure: Both authors have received consultancies, research grants, and/or appointments to advisory panels for a variety of anticoagulant medications that are mentioned in the manuscript; however, none of these payments was related to the content of this manuscript (managing bleeding complications). M.A.C. sits on the advisory board for a manufacturer of a prothrombin complex concentrate.
Correspondence: Mark A. Crowther, St Joseph's Hospital, 50 Charlton Avenue East, Room L208, Hamilton, ON, Canada L8N 4A6; e-mail: crowthrm@mcmaster.ca.