Abstract
Human leukocyte antigen G (HLA-G) is a nonclassic major histocompatibility complex (MHC) class I molecule that functions as an immunomodulatory molecule capable of protecting fetal tissues from the maternal immune system. The relevance of HLA-G in other contexts was investigated soon afterward. Numerous studies have sought (and some have shown) the relevance of HLA-G in pathologic conditions, such as transplantation, autoimmunity, and cancer and hematologic malignancies. One of the main goals of the current research on HLA-G is now to use it in the clinic, either for diagnosis or as a therapeutic tool/target. For this, precise knowledge on the nature and functions of HLA-G is critical. We highlight here what we consider are recent key basic findings on the immunomodulatory function of HLA-G. These strengthen the case for considering HLA-G as clinically relevant.
Introduction
Human leukocyte antigen G (HLA-G) is a nonclassic HLA class I molecule that was first identified as selectively expressed by choriocarcinoma cells.1-3 HLA-G has since then been called “nonclassic” because it differs from classic HLA class I molecules by its genetic diversity, expression, structure, and functions. Indeed, HLA-G has a very low amount of polymorphism, having only 8 protein variants (vs 462 for HLA-A and 789 for HLA-B). Second, HLA-G expression is highly tissue-restricted: besides being expressed in fetal tissues, such as trophoblast cells,4 HLA-G constitutive expression was found only in adult thymic medulla,5 cornea,6 pancreatic islets,7 and erythroid and endothelial-cell precursors.8 However, HLA-G expression can be induced in cancers,9,10 transplantation,11 multiple sclerosis,12 inflammatory diseases,12-14 and viral infections.15,16
The gene structure of HLA-G is homologous to that of other HLA class I genes, but HLA-G primary transcript generates 7 alternative mRNAs that encode membrane-bound (HLA-G1, G2, G3, G4) and soluble (HLA-G5, G6, G7) protein isoforms17 (Figure 1A,B). Alternate splicing of HLA-G primary transcript stands out because 1) it leads to soluble and truncated protein production, and 2) it can be regulated, as indicated by the fact that depending on the cell type and the situation some isoforms might be expressed, whereas others are not.11,18-20 The “complete” HLA-G1 molecule and its soluble counterpart HLAG5 are those that have been studied the most. They have an identical extracellular structure, which is classic HLA class I-like: a heavy chain of 3 globular domains noncovalently bound to β2-microglobulin and a nonapeptide. The other isoforms are likely to be of simpler structure: lacking one or 2 globular domains, they are smaller, and should not bind β2-microglobulin and present peptides.
HLA-G mRNA, protein isoforms, and receptors. (A) This HLA-G primary transcript is homologous to that of classic HLA class I molecules but contains a stop codon in exon 6, shortly after the coding sequence for the transmembrane domain. (B) Alternative splicing of the primary transcript yields 7 protein isoforms: truncated isoforms are generated by excision of one or 2 exons encoding globular domains, whereas translation of intron 4 or intron 2 yields soluble isoforms that lack the transmembrane domain. (C) HLA-G is well known to act through binding of inhibitory receptors, such as immunoglobulin-like transcript 2 (ILT2), ILT4, and killer-cell immunoglobulin-like receptor, 2 domains, long cytoplasmic tail, 4 (KIR2DL4), that are differentially expressed by immune cells, but binding to CD8 has also been reported.88
HLA-G mRNA, protein isoforms, and receptors. (A) This HLA-G primary transcript is homologous to that of classic HLA class I molecules but contains a stop codon in exon 6, shortly after the coding sequence for the transmembrane domain. (B) Alternative splicing of the primary transcript yields 7 protein isoforms: truncated isoforms are generated by excision of one or 2 exons encoding globular domains, whereas translation of intron 4 or intron 2 yields soluble isoforms that lack the transmembrane domain. (C) HLA-G is well known to act through binding of inhibitory receptors, such as immunoglobulin-like transcript 2 (ILT2), ILT4, and killer-cell immunoglobulin-like receptor, 2 domains, long cytoplasmic tail, 4 (KIR2DL4), that are differentially expressed by immune cells, but binding to CD8 has also been reported.88
Unlike classic HLA class I molecules, HLA-G does not seem to possess significant immune stimulatory functions, and even responses directed against allogeneic HLA-G have not been reported. HLA-G, however, possesses the capability common to HLA class I molecules, to bind inhibitory receptors (Figure 1C). Three HLA-G receptors have been described: ILT2/CD85j/LILRB1 (ILT2), ILT4/CD85d/LILRB2 (ILT4), and KIR2DL4/CD158d (KIR2DL4).21-23 ILT2 is expressed by B cells, some T cells, some NK cells, and all monocytes/dendritic cells,21 but ILT4 is myeloid-specific and only expressed by monocytes/dendritic cells.22 Concerning KIR2DL4, its expression is mainly restricted to the CD56bright subsets of NK cells,24,25 which constitute a minority of peripheral NK cells, but a majority of uterine NK cells.26 Nevertheless, through these differentially expressed receptors, HLA-G can interact with B cells, T cells, NK cells, and antigen-presenting cells (APCs; Figure 1C).
ILT2 and ILT4 are clearly inhibitory receptors, whereas the situation is not as simple for KIR2DL4. Indeed, KIR2DL4 seems to be able to send inhibitory as well as activatory signals, having a single immunoreceptor tyrosine-based inhibitory motif in its cytoplasmic tail, and a positively charged arginine in the transmembrane region.27,28 Another difference between the ILTs and KIR2DL4 is that ILT2 and ILT4 bind classic HLA molecules,21,22 whereas HLA-G is the sole ligand of KIR2DL4.23 HLA-G is nonetheless the ligand of highest affinity for ILT2 and ILT4,29 and as will be discussed later, ILT2 and ILT4 have an even higher affinity for HLA-G multimers.30 Finally, ILT2 and ILT4 differ at the level of the HLA-G structures they recognize: ILT2 is a receptor for HLA-G associated with β2-microglobulin, whereas ILT4 also recognizes HLA-G free heavy chains.30,31
Functionally, HLA-G1 inhibits the cytolytic function of uterine and peripheral blood NK cells,32,33 the antigen-specific cytolytic function of cytotoxic T lymphocytes,34 the alloproliferative response of CD4+ T cells,35,36 the proliferation of T cells and peripheral blood NK cells,37-39 and the maturation and function of dendritic cells40,41 (Table 1). Soluble HLA-G5 or soluble HLA-G1, which is generated by proteasomal cleavage from the cell membrane, has similar functions. The other HLA-G isoforms have been less well studied, and little is known about their function except that membrane-bound HLA-G2, HLA-G3, and HLA-G4 can inhibit NK-cell and cytotoxic T lymphocyte cytolysis in vitro.34 Even though truncated HLA-G isoforms are comparatively less expressed than the “complete” HLA-G1 and HLA-G5 molecules, they were proven to be significant in several instances. The first of these instances is in individual bearing the HLA-G “null” allele HLA-G*0105N. This allele is called null because of a point mutation leading to an early stop codon in exon 3 (alpha2 domain), which prevents the production of HLA-G1, HLA-G5, and HLA-G4, but not that of HLA-G2 and HLA-G6. The capability of this allele to protect cells against NK cytolysis was demonstrated,42 and the very existence of individuals homozygous for HLA-G*0105N43 might constitute the physiologic demonstration of the importance of HLA-G isoforms, and not the demonstration that HLA-G is of not important for pregnancy. In addition, soluble HLA-G6 was detected in the circulation of pregnant women44 and was also detected in the circulation of transplanted heart patients and associated with better graft acceptance.11,45
HLA-G functions
| Effector cell, HLA-G function . | Receptors involved . | Reference . |
|---|---|---|
| NK cells | ||
| Inhibition of cytotoxic function | ILT2, KIR2DL4 | 32, 33 |
| Indirect inhibition of cytotoxic function through stabilization of cell-surface HLA-E | CD94/NKG2A (interacts w/HLA-E) | 86 |
| Inhibition of proliferation | ILT2 | 39 |
| Up-regulation of inhibitory receptors | — | 87 |
| Apoptosis | CD8 | 88, 89 |
| Increased proliferation and IFNγ production | KIR2DL4 | 46, 90 |
| Increased secretion of pro-angiogenic factors | Internalized KIR2DL4 | 47 |
| Inhibition of transendothelial migration | ILT2 | 91 |
| CD8+T cells | ||
| Inhibition of cytotoxic function | — | 34, 92 |
| Inhibition of proliferation | ILT2 | 37 |
| Generation of CD8low regulatory T cells | — | 55 |
| Apoptosis | CD8 | 89 |
| CD4+T cells | ||
| Inhibition of alloreactivity | ILT2, ILT457 | 35, 82 |
| Inhibition of proliferation | ILT2 | 37, 38 |
| Up-regulation of inhibitory receptors | — | 87 |
| Generation of regulatory T cells, which include CD4low T cells | — | 55, 82 |
| APC | ||
| Inhibition of DC maturation, antigen presentation, trafficking, and induction of regulatory T cells | ILT4 | 40 |
| Up-regulation of inhibitory receptors | — | 87 |
| PBMC: secretion of Th2 cytokines | CD160 | 93 |
| Endothelial cells: apoptosis | — | 94 |
| Effector cell, HLA-G function . | Receptors involved . | Reference . |
|---|---|---|
| NK cells | ||
| Inhibition of cytotoxic function | ILT2, KIR2DL4 | 32, 33 |
| Indirect inhibition of cytotoxic function through stabilization of cell-surface HLA-E | CD94/NKG2A (interacts w/HLA-E) | 86 |
| Inhibition of proliferation | ILT2 | 39 |
| Up-regulation of inhibitory receptors | — | 87 |
| Apoptosis | CD8 | 88, 89 |
| Increased proliferation and IFNγ production | KIR2DL4 | 46, 90 |
| Increased secretion of pro-angiogenic factors | Internalized KIR2DL4 | 47 |
| Inhibition of transendothelial migration | ILT2 | 91 |
| CD8+T cells | ||
| Inhibition of cytotoxic function | — | 34, 92 |
| Inhibition of proliferation | ILT2 | 37 |
| Generation of CD8low regulatory T cells | — | 55 |
| Apoptosis | CD8 | 89 |
| CD4+T cells | ||
| Inhibition of alloreactivity | ILT2, ILT457 | 35, 82 |
| Inhibition of proliferation | ILT2 | 37, 38 |
| Up-regulation of inhibitory receptors | — | 87 |
| Generation of regulatory T cells, which include CD4low T cells | — | 55, 82 |
| APC | ||
| Inhibition of DC maturation, antigen presentation, trafficking, and induction of regulatory T cells | ILT4 | 40 |
| Up-regulation of inhibitory receptors | — | 87 |
| PBMC: secretion of Th2 cytokines | CD160 | 93 |
| Endothelial cells: apoptosis | — | 94 |
— indicates not available.
Recently, it was reported that through KIR2DL4, membrane-bound HLA-G induced proliferation and interferon-γ (IFN-γ) production by uterine NK cells46 and that soluble HLA-G (sHLA-G) also induced the secretion of other cytokines by polyclonal NK cells.47 Thus, it seems that, depending on the NK cell subset and also on the pattern of HLA-G receptors they express, HLA-G may act as inhibitory or activatory. In particular, it is interesting to consider the possibility that HLA-G may trigger the immunoregulatory functions of uterine CD56bright NK cells, which have low cytolytic function and express high levels of KIR2DL4 but inhibit the cytolytic functions of peripheral CD56dim NK cells, which do not express KIR2DL4. Other functions for the HLA-G molecules have been described, which are reported in Table 1. This review will however focus primarily on the inhibitory functions of HLA-G.
Given HLA-G expression patterns, its prime physiologic relevance is likely to be at the fetal-maternal interface (reviewed by Hviid48 ), as a key contributor to the tolerance of the fetus by the immune system of the mother. Indeed, HLA-G can protect fetal trophoblast cells from maternal NK cells through interaction with their inhibitory receptors,33,49 and HLA-G expression by embryos seems to be a prerequisite to their implantation and the subsequent pregnancy.50,51 However, because of this broad inhibitory function, capable of targeting multiple immune cell subsets, much effort has been put into determining whether HLA-G is pathologically relevant and whether it can be used as a diagnostic tool or as a therapeutic tool and/or target.
Transplantation and oncology are 2 particularly clear situations. In the context of transplantation, HLA-G expression might be beneficial and promote tolerance to grafts. To date, the expression of HLA-G was studied in more than 1000 patients after heart,11,52,53 kidney,54 liver,55 and liver-kidney55-57 transplantation, with those expressing HLA-G in the graft and/or the plasma exhibiting significantly better graft acceptance. Thus, in transplanted patients, titration of HLA-G might be used as a monitoring tool to determine and follow tolerance status, which could then be used to adjust immunosuppressive therapies. In this context, patients with high HLA-G titers could be candidates for a reduction in immunosuppressive treatment, whereas HLA-G–negative patients would have a comparatively higher risk of rejection. Furthermore, HLA-G itself might be used as therapeutic tolerogenic agent, exogenously provided to HLA-G–negative patients as complementary and/or alternative therapy.
In the context of oncology, studies on more than 1000 malignant lesions confirmed our first study on melanoma,9 which showed that HLA-G transcription and protein expression may be switched on in tumor lesions and protect them from NK cytolysis. It was later shown that HLA-G expression by tumor lesions protected against cytolysis58-60 correlated with malignancy in ovarian and breast carcinomas,61 as well as in melanocytic lesions,62 with unfavorable outcome in chronic lymphocytic leukemia,63 and gastric and colorectal cancers.64 High HLA-G plasma levels were also recently observed in patients with neuroblastoma and correlated with relapse.65
Expression of HLA-G has been evidenced in different malignant hematopoietic diseases, but most particularly in acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and B-chronic lymphocytic leukemia (B-CLL).
In AML, expression of HLA-G was first shown to be dependent on IFN-γ.66 In this study, 28 samples from AML patients were analyzed, but none of them expressed HLA-G. However, a prior incubation of AML cell samples with IFN-γ induced HLA-G expression in 21% of AML patients. It has to be noted that in this study detection of HLA-G was performed by flow cytometry, which does not allow detecting soluble forms of HLA-G. In another study on HLA-G expression in AML cells, Yan et al observed an expression of HLA-G in 18.5% of 54 patients with AML.67 Moreover, ex vivo cytotoxic experiments showed that HLA-G expression by AML cells could directly inhibit NK cell cytolysis, suggesting the possible use of HLA-G as a therapeutic target in AML treatment. Finally, Polakova et al68 also analyzed HLA-G expression in 25 AML patients but did not find HLA-G expression. No explanation has been proposed for such discrepancy; however, such a difference in HLA-G expression could be the result of the influence of treatment given to the different patient who could favor or repress expression of HLA-G.
HLA-G protein expression has also been investigated in B-CLL.63 In this study, HLA-G expression at the surface of circulating B-CLL cells was assessed by flow cytometry in 47 patients. Expression of HLA-G on B-CLL cell population varied from 1% to 54%, depending on patients. Moreover, patients with 23% or fewer HLA-G–positive cells had a significantly longer progression-free survival time than patients with more than 23% positive cells, suggesting a possible role of HLA-G in tumoral escape from immune survey in B-CLL. In agreement with these data, HLA-G transcription and protein expression in CLL patients were demonstrated to protect tumor cells from autologous NK lysis.59 Finally, the presence of sHLA-G in patients with ALL has been investigated.69 Expression of sHLA-G by ALL cells was determined by enzyme-linked immunosorbent assay and showed an elevated secretion of sHLA-G that was even higher in the presence of granulocyte-macrophage colony-stimulating factor or IFN-γ.
Thus, HLA-G expression would favor tumor development by impairing antitumor immunity. Here again, HLA-G titration in peripheral blood might be used for diagnosis and/or monitoring, but in this context, high titers of HLA-G would represent a negative factor.61,63 In HLA-G–positive patients, HLA-G itself might finally constitute a therapeutic target: if expressed as a membrane-bound protein, as observed in some hematologic malignancies,63 HLA-G could be used as a tumor marker to deliver therapy. Alternatively, HLA-G could be blocked or deleted as a contributor to tumor immunosuppression and/or tumoral escape.70
Recently, new aspects of HLA-G biology have been reported that are critical to HLA-G pathologic relevance and should help design HLA-G–based diagnosis and therapeutic strategies. First is the description of HLA-G multiple structures and the demonstration that HLA-G multimers carry most, if not all, of HLA-G inhibitory function. These data imply that the pathologic relevance of HLA-G might have been underestimated for lack of a dimer-specific detection method. Second is the demonstration that HLA-G is not only a shield against immune aggression but can also have a long-term inhibitory function through regulatory cells. Third is the demonstration that HLA-G can transfer from cell to cell, cause effector cells to behave as regulatory cells, and so spread HLA-G inhibitory function beyond the reach of HLA-G–expressing cells. These data reinforce the significance of even a few HLA-G–expressing cells.
HLA-G structural features and functions
HLA-G exists in 7 structural isoforms because of alternative splicing of its primary transcript (Figure 1). Recent crystallography studies71 have confirmed that the structure of HLA-G1 is class I–like (heterotrimer of heavy chain, β2-microglobulin, and peptide, herein referred to as monomers), but have also shown that it differs at the α3 domain, which is more hydrophobic and may explain why HLA-G has a higher affinity for ILT2 than classic HLA class I molecules. In addition, HLA-G1 and HLA-G5 can also be found as β2-microglobulin–free heavy chains,72 and more importantly, as disulphide-bonded homodimers.73,74
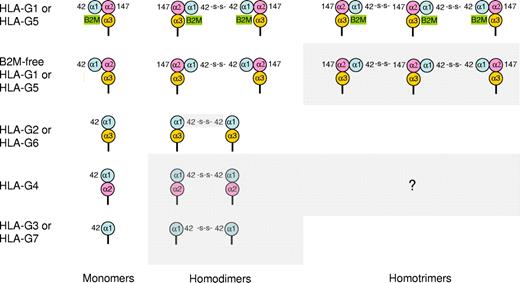
The existence of HLA-G dimers was first hypothesized after sequence analysis of HLA-G275 but first observed for HLA-G173 and then crystallized.30 Dimerization of HLA-G occurs through the creation of disulphide bonds between 2 unique cysteine residues at positions 42 (Cys42-Cys42 bonds) and 147 (Cys42-Cys147 bonds) of HLA-G heavy chains,73,74 and commonly observed using electrophoresis in nonreducing conditions.44 Such interactions can also generate HLA-G homotrimers74 (Figure 2). HLA-G1 homodimers are joined head-to-tail by Cys42-Cys42 disulphide bonds, and dimerization does not induce significant structural changes to the main backbone of the monomers.30 However, HLA-G1 homodimers have an oblique orientation that exposes the ILT2- and ILT4-binding sites of the α3 domain upwards, making them more accessible to the receptors.
Homomultimeric structures of HLA-G. HLA-G molecules can form homomultimers through the generation of Cys42-Cys42 or Cys42-Cys147 disulphide bonds. Possible monomeric and multimeric structures are shown. Gray boxes indicate hypothetical structures that have not been reported: β2m-free HLA-G1 or HLA-G5 homotrimers, HLA-G4 homodimers and homotrimers, and HLA-G3 or HLA-G7 homodimers. HLA-G2 or HLA-G6 homodimers have been reported but their generation through Cys42-Cys42 disulphide bonds has not, although it is likely. HLA-G2 or HLA-G6 and HLA-G3 or HLA-G7 homotrimers do not seem to be possible as these isoforms lack Cys147 of α2 domain, and the structure of HLA-G4 homotrimers is open to speculation.
Homomultimeric structures of HLA-G. HLA-G molecules can form homomultimers through the generation of Cys42-Cys42 or Cys42-Cys147 disulphide bonds. Possible monomeric and multimeric structures are shown. Gray boxes indicate hypothetical structures that have not been reported: β2m-free HLA-G1 or HLA-G5 homotrimers, HLA-G4 homodimers and homotrimers, and HLA-G3 or HLA-G7 homodimers. HLA-G2 or HLA-G6 homodimers have been reported but their generation through Cys42-Cys42 disulphide bonds has not, although it is likely. HLA-G2 or HLA-G6 and HLA-G3 or HLA-G7 homotrimers do not seem to be possible as these isoforms lack Cys147 of α2 domain, and the structure of HLA-G4 homotrimers is open to speculation.
HLA-G1 homodimers have been shown to be expressed at the surface of normal trophoblasts in vitro31,76 and at the surface of human extravillous trophoblast cells in vivo.76 sHLA-G5 homodimers have also been described in vitro.76 HLA-G1 and HLA-G5 β2-microglobulin–free heavy chains, which can be detected at the cell surface or in culture supernatants of HLA-G–expressing cells,72 also form homodimers.31,77 Indeed, such heavy-chain homodimers may be the main HLA-G5 structure produced by human villous trophoblast cells.77
Functional importance of HLA-G multimers
All these various structures would not matter if monomers and dimers had equivalent inhibitory functions. However, recent data show that the ILT2 and ILT4 binding sites of HLA-G dimers are more accessible than those of HLA-G monomers.30 Consequently, HLA-G dimers bind 2 ILT receptors, and with a higher affinity and slower dissociation rates than monomers (Kd of monomers vs dimers were calculated at 3.5 μM vs 0.0067 μM for ILT2, and 15 μM vs 0.75 μM for ILT4).30 Biochemical data further showed that ILT2 bound mostly cell surface dimers in vitro74,76 and in vivo76 and that dimers signaled through ILT2 more efficiently than monomers.30 It is therefore not surprising that the inhibitory function of HLA-G is mostly the result of dimers, not monomers.74,76
These observations represent a turning point in the field of HLA-G research because, until recently, the possibility of functional heterogeneity of the HLA-G1/HLA-G5 pool was not considered. Even now that this is established, the full extent of HLA-G structural diversity is unknown. Indeed, because all HLA-G isoforms have the Cys42 responsible for dimerization, all translated isoforms could form membrane-bound homodimers, soluble homodimers, β2-microglobulin–free homodimers, and possibly homotrimers (with or without associated β2-microglobulin; Figure 2). Finally, functional heterodimers could also form between different monomeric HLA-G isoforms.
Current HLA-G analysis strategies were designed to detect most of the HLA-G pool, not to discriminate between monomers and dimers. Depending on the pathologic context, dimers might have been detected, or monomers, or mixtures of both. Because of the prominent function of HLA-G multimers, we think it is possible that their specific titration might bear higher significance than that of monomers. For this reason, HLA-G pathologic relevance might actually have been underestimated by using titration methods that detect monomers and dimers indiscriminately, hence set to detect sHLA-G but not functional sHLA-G. Indeed, in instances where only HLA-G dimers were present, nondiscriminative titration methods should be sufficient and provide the true significance of sHLA-G titration. This situation might for be that of liver-kidney transplanted patients, in which HLA-G expression is pathologically highly significant.56,78 However, in cases where monomers are present, results should yield results similarly positive but not pathologically significant for lack of functional relevance of monomers, and this might account for numerous reports of lack of association between sHLA-G and pathologic status (eg, Sebti et al70 ). It is now clear that the relationship between HLA-G structure and its functional relevance needs to be established beyond doubt in vivo, and that for this, new analytical tools have to be developed to specifically detect and study the most active HLA-G structures. This would allow a reanalysis of data, which might greatly strengthen the pathologic significance of HLA-G.
Relevance of a few HLA-G–expressing cells: immune regulation by trogocytosis of HLA-G
We recently reported how a few HLA-G–expressing cells could protect a comparatively larger number of HLA-G–negative cells. These reports show that transfer of membrane patches containing HLA-G molecules from HLA-G–expressing cells to activated T cells and NK cells (also called “trogocytosis”79 ) might be a mechanism of immune inhibition and of protection for HLA-G–negative tumor cells that are in the vicinity of HLA-G–positive tumor cells.38,39
Trogocytosis (reviewed by Davis80 ) is a fast, cell-to-cell contact-dependent uptake of membranes and associated molecules by a cell from another. Trogocytosis is a transfer of membrane fragments, not individual molecules. Consequently, during trogocytosis, all molecules contained within a certain membrane area are transferred, whether they are involved in cell-to-cell cross-talk or not. Molecules whose transfers have been most studied include but are not restricted to MHC-I, MHC-II, CD54, CD80, CD86, and NK receptors. What makes these transfers important is that they are fast, the half-life of the transferred molecules is limited, and the transferred membrane patches may temporarily endow the acceptor cell with some functions of the donor cells.
In 2 recent studies,38,39 we showed that activated T cells (both CD4+ and CD8+ T cells) and activated NK cells can acquire HLA-G1–containing membrane fragments from HLA-G1–expressing APCs and tumor cells, respectively. This acquisition is through trogocytosis and, as such, is cell-contact dependent, fast (a few minutes), temporary (a few hours), and concerns not only HLA-G1 but all molecules contained within the transferred membrane patch. HLA-G interaction with its receptors is not required for transfer, meaning that cells devoid of HLA-G receptors may acquire it. The molecules responsible for HLA-G transfer in this case remain undetermined. On acquisition of HLA-G1–containing membranes from APCs, effector CD4+ T cells stop proliferating, stop responding to stimulation, and behave as regulatory T cells that are capable of inhibiting the reactivity of autologous T cells in vitro.38 Similarly, on acquisition of HLA-G1–containing membranes from tumor cells, effector NK cells stop proliferating, stop being cytotoxic toward legitimate targets, and behave as regulatory cells that are capable of inhibiting the cytotoxic functions of other NK cells.39 This immediate functional inversion from effector cell to regulatory cell is directly the result of acquired cell-surface HLA-G1, as shown by specific blocking of HLA-G1–ILT2 interactions. So, effector cells that have acquired HLA-G1 by trogocytosis act as HLA-G+ regulatory cells through membrane-bound HLA-G1 for a limited amount of time.
Trogocytosis of HLA-G might therefore explain how, within a heterogeneous environment, a few HLA-G–expressing cells can protect a comparatively larger number of HLA-G–negative cells from immune destruction. This is best illustrated in the case of NK cells which, on acquisition of HLA-G, change their functional behavior from that of killers set to lyse a tumor-cell target to that of regulatory cells that protect the targets they were supposed to kill. Trogocytosis of HLA-G1 also represents a new mechanism of regulatory cell generation: one that does not involve the maturation of long-lived regulatory cells but one that involves the direct and immediate conversion of effector cells into cells that acquire effective, but temporary, regulatory function in situ and so provide local emergency immune suppression (Figure 3). Such a mechanism of immune protection might occur in all contexts in which HLA-G is membrane-bound and be particularly useful in those where HLA-G–expressing cells are scarce. These contexts include pregnancy, where HLA-G expression is restricted to the trophoblasts at the maternal-fetal interface, transplantation where HLA-G expression is heterogeneous11 and/or restricted to few structures,56 or autoimmunity.12 In these cases, emergency immune suppression through trogocytosis of HLA-G might prove beneficial and cost-effective, but in other contexts such as cancer or viral infections, it might contribute to the generation of an immuno-privileged milieu particularly deleterious. This certainly emphasizes the significance of even a few HLA-G–expressing cells in the pathologic context.
Emergency immune suppression: a possible impact of HLA-G trogocytosis on immune responses. Within the microenvironment of a tumor, for example, in which some cells express HLA-G and some do not (A), the function of activated natural killer (NK) cells is directly blocked by the interaction of tumor cell HLA-G and NK-cell immunoglobulin-like transcript 2 (ILT2). This results in the immune escape of the HLA-G–positive tumor cell. (B) During this cell-to-cell contact, tumor cell-membrane patches containing HLA-G are acquired by activated NK cells within minutes (through a process known as trogocytosis). (C) This results in the generation of HLA-G–positive activated NK cells, which can inhibit other HLA-G–positive (cross-inhibition) or HLA-G–negative NK cells through HLA-G and ILT2 cross-linking (D). This suppressive function of HLA-G–positive NK cells results in the immune escape of HLA-G–negative tumor cells. (E) Immune suppression does not spread as NK cells do not translate HLA-G de novo and lose its surface expression quickly if not in the vicinity of HLA-G–positive tumor cells. Emergency immune suppression and in situ generation of regulatory cells, which is fast, local and temporary, might constitute an efficient mechanism of immune escape.
Emergency immune suppression: a possible impact of HLA-G trogocytosis on immune responses. Within the microenvironment of a tumor, for example, in which some cells express HLA-G and some do not (A), the function of activated natural killer (NK) cells is directly blocked by the interaction of tumor cell HLA-G and NK-cell immunoglobulin-like transcript 2 (ILT2). This results in the immune escape of the HLA-G–positive tumor cell. (B) During this cell-to-cell contact, tumor cell-membrane patches containing HLA-G are acquired by activated NK cells within minutes (through a process known as trogocytosis). (C) This results in the generation of HLA-G–positive activated NK cells, which can inhibit other HLA-G–positive (cross-inhibition) or HLA-G–negative NK cells through HLA-G and ILT2 cross-linking (D). This suppressive function of HLA-G–positive NK cells results in the immune escape of HLA-G–negative tumor cells. (E) Immune suppression does not spread as NK cells do not translate HLA-G de novo and lose its surface expression quickly if not in the vicinity of HLA-G–positive tumor cells. Emergency immune suppression and in situ generation of regulatory cells, which is fast, local and temporary, might constitute an efficient mechanism of immune escape.
Long-term relevance of HLA-G expression: the functions of HLA-G through regulatory cells
Initial studies showed that HLA-G, which is expressed by target cells, engages inhibitory receptors on effector cells, resulting in a transient block in their functions, and so acts as a shield against immune aggression.81 However, it is now clear that HLA-G–related regulatory cells exist (Table 2) and that some of these can have a long-lasting inhibitory effect on immune responses.
The HLA-G–related regulatory cells
| Name . | Generation . | Phenotype . | HLA-G induction . | HLA-G expression . | Mechanism of action . | Reference . |
|---|---|---|---|---|---|---|
| Regulatory T cells | ||||||
| HLA-G–induced Treg | Periphery | None known/CD3+CD4low and CD3+CD8low | APC-HLA-G1+ or HLA-G5 | No | Unknown/soluble factors including IL-10 | 82/55, 57 |
| HLA-G+ Tregs | Thymus | CD3+HLA-G1+ | No | HLA-G1 and HLA-G5 | Soluble factors including HLA-G | 83 |
| HLA-G1acq+ T cells | Periphery | CD3+HLA-G1+ | Trogocytic HLA-G1 acquisition | HLA-G1 | HLA-G1 | 38 |
| CD4+CD25+CTLA4+/CD8+CD28− | Periphery | CD4+CD25+/CD8+CD28- | HLA-G–modified DC | No | —/possibly IL-10 | 40 |
| Tolerogenic APC | ||||||
| HLA-G1+ APC | Periphery | HLA-G1+ | No | HLA-G1 | HLA-G1 | 82 |
| HLA-G–modified DC | Periphery | HLA-DRlow, CD80low, CD86low | HLA-G tetramers | No | — | 40 |
| Suppressive NK cells: HLA-Gacq+ NK cells | Periphery | CD16+HLA-G1+ | Trogocytic HLA-G1 acquisition | HLA-G1 | HLA-G1 | 39 |
| Name . | Generation . | Phenotype . | HLA-G induction . | HLA-G expression . | Mechanism of action . | Reference . |
|---|---|---|---|---|---|---|
| Regulatory T cells | ||||||
| HLA-G–induced Treg | Periphery | None known/CD3+CD4low and CD3+CD8low | APC-HLA-G1+ or HLA-G5 | No | Unknown/soluble factors including IL-10 | 82/55, 57 |
| HLA-G+ Tregs | Thymus | CD3+HLA-G1+ | No | HLA-G1 and HLA-G5 | Soluble factors including HLA-G | 83 |
| HLA-G1acq+ T cells | Periphery | CD3+HLA-G1+ | Trogocytic HLA-G1 acquisition | HLA-G1 | HLA-G1 | 38 |
| CD4+CD25+CTLA4+/CD8+CD28− | Periphery | CD4+CD25+/CD8+CD28- | HLA-G–modified DC | No | —/possibly IL-10 | 40 |
| Tolerogenic APC | ||||||
| HLA-G1+ APC | Periphery | HLA-G1+ | No | HLA-G1 | HLA-G1 | 82 |
| HLA-G–modified DC | Periphery | HLA-DRlow, CD80low, CD86low | HLA-G tetramers | No | — | 40 |
| Suppressive NK cells: HLA-Gacq+ NK cells | Periphery | CD16+HLA-G1+ | Trogocytic HLA-G1 acquisition | HLA-G1 | HLA-G1 | 39 |
— indicates not applicable.
HLA-G–induced regulatory T cells were first observed after stimulation of T cells with HLA-G1–expressing APCs.82 These regulatory T cells arise during an antigenic stimulation, do not respond to stimulation, and can block the alloreactivity of autologous T cells in vitro. Such regulatory T cells can be generated by membrane-bound HLA-G138,82 or soluble HLA-G5,57 and were detected in vivo after transplantation.55,57 Phenotypically, HLA-G–induced regulatory T cells are distinct from other regulatory T cells and do not express the transcription factor forkhead box P3 (FOXP3) that is characteristic of some regulatory T-cell subsets. The only phenotypical characteristic that seems to characterize HLA-G–induced regulatory T cells is their reduced expression of CD4 and CD8, although all HLA-G–induced regulatory T cells might not present this phenotype. HLA-G–induced CD3+CD4low and CD3+CD8low regulatory T cells act through soluble molecules, including interleukin-10 (IL-10).55 Physiologic relevance for these regulatory T-cell subsets was established in transplant recipients for whom allograft acceptance correlated with high HLA-G plasma levels, high IL-10 levels, and an over-representation of circulating CD3+CD4low and CD3+CD8low T cells.55
Naturally occurring HLA-G+ regulatory T cells83 constitute a different subset to the HLA-G–induced regulatory T cells and express normal levels of CD4 and CD8. HLA-G+CD4+ or HLA-G+CD8+ T cells exist in the peripheral blood of healthy persons as a discrete subset and seem to be generated in the thymus. HLA-G+ regulatory T cells are phenotypically distinct from other regulatory T cells, being characterized by the expression of cell-surface HLA-G1 and secreted HLA-G5, and by a lack of CD25 and FOXP3 expression. HLA-G+ regulatory T cells are hypoproliferative, are hyporesponsive to stimulation, and have a cytokine expression profile that differs from those of other regulatory T cells, with no increased expression of the immunosuppressive cytokines IL-10 or transforming growth factor-β and reduced expression of IFN-γ. Despite this, CD4+HLA-G+ and CD8+HLA-G+ regulatory T cells mediate their suppressive function through HLA-G and soluble factors, possibly HLA-G5 and/or sHLA-G1. HLA-G+ regulatory T cells are a small subset in the peripheral blood of healthy donors, but their numbers increase at sites of inflammation, such as in the central nervous system of patients with neuroinflammatory disorders and in muscle tissue in patients with idiopathic myositis.83
HLA-G–induced regulatory T cells and HLA-G+ regulatory T cells are 2 different regulatory cell subsets. Yet, they have in common that in fine they rely on HLA-G to exist as regulatory cells, even if the exact contribution of HLA-G to HLA-G–dependent regulatory T-cell generation (HLA-G–induced regulatory T cells) and/or function (HLA-G+ regulatory T cells) remains unclear. More importantly, the existence of HLA-G–related regulatory T cells highlights a role for HLA-G in long-term immunomodulatory mechanisms and increases its in vivo relevance.
How may HLA-G–related regulatory cells be useful in terms of diagnostic or therapeutic efforts is an important question. First, identifying such cells in situ may serve as a marker of bad (eg, cancer) or good (eg, transplantation) prognosis. Second, manipulating these regulatory cells for the purpose of immunotherapy may prove to be useful. In cancer, for instance, finding ways of overcoming the suppressive activity of these HLA-G–related regulatory T cells, or of selectively depleting these cells locally in tumors or draining lymph nodes, may represent an important therapeutic strategy aimed at inducing effective tumor-specific immune responses capable of controlling or eradicating tumors.84,85 An approach in which these cells are depleted or inactivated as part of a vaccination regimen might prove useful as well, not only for increasing the immunogenicity of the vaccine but also to ensure that these regulatory cells, which may favor tumor growth, are not promoted. Similarly, blocking Treg trafficking, differentiation, and/or function and reducing the susceptibility to suppression may constitute additional therapeutic strategies. In other contexts, such as transplantation, boost regulatory cell function or numbers might be equally useful. However, a greater understanding of the nature and role of these HLA-G–related regulatory cells in pathology is needed to address these points. One of the first questions to investigate would certainly be to understand the differences between naturally occurring HLA-G+ and HLA-G–induced regulatory T cells. Given their very different generation, phenotype, and mechanism of action, it seems unlikely that they mediate the same functions in the same contexts. In particular, whether or not either of these subsets require antigen specificity to act is crucial to the issue of whether or not either of these 2 regulatory subsets can be used to specifically alter responses against given antigens.
In conclusion, HLA-G is an important natural tolerogenic molecule in the context of fetal-maternal tolerance, which is being increasingly studied in pathologic contexts in adults. As such, research on HLA-G and its uses as a diagnostic, prognostic, and possibly therapeutic tool is gaining interest, as shown by the number of publications on HLA-G, which grew steadily from fewer than 10 in 1989 to more than 300 in 2007. Basic research on HLA-G continues to reveal surprising complexity. Indeed, HLA-G is no longer considered to be just a monomorphic, classic HLA class I–like molecule that acts as a shield against immune aggression, but is also regarded as a molecule whose expression regulation is complex, whose structures are multiple and yet to be fully identified, and whose functions span much further than direct protection against immune destruction, to long-term and local tolerance mechanisms. This complexity may be related to the fact that HLA-G is involved in pregnancy, the clearest demonstration that true tolerance is commonly achieved. HLA-G may therefore have been evolutionarily selected to be as reliable and adaptive as possible, targeting most immune-cell subsets, most steps of immune responses, and possibly more. Yet, within this apparent complexity, trends emerge that will lead to a better understanding of HLA-G biology and to a better use of its tolerogenic properties. Already, monoclonal antibodies are no longer a problem. sHLA-G titration tests are commercially available and widely used, with statistical significance on large cohorts of patients established. Obviously, not all studies lead in the same direction, and many disagree with the significance of HLA-G in one context or another. Yet this is not negative: HLA-G is no panacea and does not have to be significantly correlated with everything to be useful and important in some given situations. Thus, energy is devoted (1) to use HLA-G in those contexts where it is already significant, (2) to understand why it is not significant in other contexts, and (3) to find new contexts in which it is. What the currently obtained data imply is that HLA-G should not be overlooked in a pathologic situation where the immune system inexplicably misbehaves because HLA-G could provide an answer and possibly a solution. The rapidly increasing knowledge of HLA-G's true complexity also underlines a paucity of tools to detect, discriminate, study, or act on HLA-G's various structures, which might have led to an underestimation of HLA-G's relevance. Yet these are minor setbacks because along with the evidence of the problem was provided the solution: functional structures of HLA-G need to be specifically detected, and data revisited. Increasingly complex, HLA-G? Yes, but not increasingly chaotic because the field of HLA-G research benefits from a true asset: focus. Indeed, there might be disagreements over such technical subtlety, or such particular result, but there are few arguments over what HLA-G is, can do, and should be used for: HLA-G is at minima a tolerogenic molecule that should be used to monitor and cure. This is not bad for a molecule that barely existed 10 years ago.
Authorship
Contribution: E.D.C., B.F., N.R.-F., and P.M. contributed significantly to the design and writing of this review; J.L. designed and wrote this review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joel LeMaoult, CEA, I2BM, Service de Recherches en Hemato-Immunologie, Hôpital St Louis, 1 Av Claude Vellefaux, F-75475 Paris, France; e-mail: Joel.LeMaoult@cea.fr.