Abstract
The role of platelets in hemostasis is to produce a plug to arrest bleeding. During thrombocytopenia, spontaneous bleeding is seen in some patients but not in others; the reason for this is unknown. Here, we subjected thrombocytopenic mice to models of dermatitis, stroke, and lung inflammation. The mice showed massive hemorrhage that was limited to the area of inflammation and was not observed in uninflamed thrombocytopenic mice. Endotoxin-induced lung inflammation during thrombocytopenia triggered substantial intra-alveolar hemorrhage leading to profound anemia and respiratory distress. By imaging the cutaneous Arthus reaction through a skin window, we observed in real time the loss of vascular integrity and the kinetics of skin hemorrhage in thrombocytopenic mice. Bleeding—observed mostly from venules—occurred as early as 20 minutes after challenge, pointing to a continuous need for platelets to maintain vascular integrity in inflamed microcirculation. Inflammatory hemorrhage was not seen in genetically engineered mice lacking major platelet adhesion receptors or their activators (αIIbβ3, glycoprotein Ibα [GPIbα], GPVI, and calcium and diacylglycerol-regulated guanine nucleotide exchange factor I [CalDAG-GEFI]), thus indicating that firm platelet adhesion was not necessary for their supporting role. While platelets were previously shown to promote endothelial activation and recruitment of inflammatory cells, they also appear indispensable to maintain vascular integrity in inflamed tissue. Based on our observations, we propose that inflammation may cause life-threatening hemorrhage during thrombocytopenia.
Introduction
Inflammation and hemostasis are tightly intertwined. In particular, this is becoming very evident in platelet biology. While the classical role of platelets is to mediate hemostatic plug formation, it has been demonstrated that platelets also play an important role in inflammation. For example, previous studies show that platelets promote inflammatory responses in atherosclerosis, in hepatitis, and after cerebral ischemia.1-4 Furthermore, early in inflammation prothrombotic functions of platelets are reduced,5 and activated platelets are capable of up-regulating inflammatory molecules on the endothelium.6,7 Recently, our group showed that in angiogenesis—which is strongly linked to inflammation8 —platelets play an important role in preventing hemorrhage of sprouting vessels.9 That platelets support vascular integrity during injury is well established. Early studies also demonstrated a supportive role for platelets during organ perfusion with buffers10,11 and growth-promoting effects on endothelial cultures.12 Whether platelets have a supportive role in inflamed microcirculation still remains experimentally unexplored.
In humans, profound thrombocytopenia is found, for example, in patients suffering from idiopathic thrombocytopenic purpura. Interestingly, in the absence of injury, some patients bleed while others do not show spontaneous bleeding despite equally low platelet counts.13 Thus, thrombocytopenia alone cannot explain this phenomenon and other, yet to be defined contributing factors are required to induce bleeding in thrombocytopenic patients, as suggested in a recent review.14 As thrombocytopenia may lead to life-threatening bleedings, it is important to further understand the cofactors leading to hemorrhage.
In the present study, we investigate the effects of inflammation on vascular integrity during thrombocytopenia. We challenged mice in 4 different inflammatory models and observed the affected blood vessels over time in the presence or absence of platelets. We show that thrombocytopenia rapidly induces massive bleeding in inflamed skin, brain, and lung. Thus inflammation is likely a key inducer of bleeding in thrombocytopenia.
Methods
Animals
Six- to 8-week-old C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice deficient in β3-integrin,15 Fcγ receptor (lacking glycoprotein VI [GPVI]),16 calcium and diacylglycerol-regulated guanine nucleotide exchange factor I (CalDAG-GEFI),17 von Willebrand factor (VWF),18 and P-selectin,19 and IL4Rα/GPIbα transgenic mice20 were bred and housed in our animal facility. Experimental procedures were approved by the Animal Care and Use Committee of the Immune Disease Institute.
Reagents and antibodies
Croton-oil, hemoglobin, Evans blue, Drabkin reagent, BSA, LPS, rabbit anti-BSA antibody, and rabbit IgG antibody were purchased from Sigma-Aldrich (St Louis, MO). Depleting anti-GPIbα, IgG-control, and anti-αIIbβ3 were from emfret Analytics (Wuerzburg, Germany) and anti-hIL4R antibody was from R&D Systems (Minneapolis, MN).
Platelet count and depletion
Mice were injected intravenously with a commercially available anti-GPIbα antibody21 and IL4Rα/GPIbα-tg mice with an anti-hIL4R antibody (2 μg/g body weight) for platelet depletion. For platelet counts, mice were bled from the retro-orbital plexus under isoflurane anesthesia (IsoFlo; Abbott Laboratories, Abbott Park, IL). Blood was collected into a heparin-containing tube. Platelets in whole blood were stained with FITC-labeled anti-αIIbβ3 antibody for 20 minutes at room temperature and analyzed immediately on a FACScalibur (Becton Dickinson, Rockville, MD). For quantification, fluorescent beads (Spherotec, Libertyville, IL) at defined concentrations were added.
Irritant contact dermatitis
Croton oil (10 μL of 10% solution) in acetone was applied topically to the shaved dorsal skin of platelet-depleted and control mice. Olive oil (10%) in acetone was used as a control. Mice were killed after 4 hours and the dorsal skins were harvested by careful dissection.
Reverse passive Arthus reaction in the skin
Shaved mice were anesthetized with inhalation of isoflurane and the reverse passive Arthus reaction (rpA) reaction was elicited by intravenous injection of BSA (75 μg protein/g mouse in PBS) immediately followed by intradermal injection of anti-BSA antibody (5 μg/μL in PBS) with a 30-G needle. Nonimmunizing rabbit IgG was injected as a control. Platelets were depleted 5 minutes later to avoid bleeding from needle injury. Mice were killed after 4 hours and skins harvested. For quantification studies of the inflammatory area, 100 μL Evans blue (1%) was administered with the BSA injection. The diameter of leaked Evans blue spot was measured.
Hemoglobin tissue analysis
Punch biopsies were taken from treated and control mice, weighed, and blended in 500 μL Drabkin reagent. The homogenate was spun at 16 000g for 10 minutes and the supernatant taken for analysis. The OD was measured at 530 nm (reference wavelength set at 630 nm) on a plate reader. A standard curve was generated by diluting pure hemoglobin in Drabkin reagent. Samples were measured in triplicates.
In vivo imaging of Arthus reaction
Dorsal skinfold chamber and surgical preparation was performed as described.22 Mice were sedated, hair was removed from the back of the animals, and 2 symmetric titanium frames were positioned to sandwich the extended double layer of skin of the dorsal skinfold. One layer of skin was completely removed in a circular area of 15 mm in diameter, and the remaining layers, consisting of the epidermis, subcutaneous tissue, and striated skin muscle, were covered with a 12-mm glass coverslip that was incorporated into one of the frames. The animals tolerated the dorsal skinfold chambers well and showed no signs of discomfort. The rpA was elicited as described in “Reverse passive Arthus reaction in the skin.” During in vivo microscopy, mice were sedated with 100 mg/kg ketamine and 10 mg/kg xylazine. Light microscopy imaging was performed on an upright microscope (Axioplan; Zeiss, Oberkochen, Germany) with a 2.5× magnification objective and a digital camera (AxioCam HSc) attached to it. Data acquisition was done with the time-lapse function in the software from the same manufacturer (Axiovision 4.6.3). Photographs were taken with a digital camera (D 70; Nikon, Tokyo, Japan). To visualize platelets, washed platelets from IL4Rα/GPIbα-tg mice were labeled with calcein-AM and transfused as described previously.23 Endogenous platelets were depleted after initiation of the Arthus reaction. The inflamed microcirculation was observed over 4 hours with a 10× magnification objective, and images were acquired with the equipment described.
Platelet transfusion
Washed platelets were prepared from IL4Rα/GPIbα-tg mice as described.23 Various amounts of platelets were transfused into WT recipient mice. Endogenous platelets were depleted and blood was drawn for platelet count. IL4Rα/GPIbα-tg platelets were stained with Alexa 647–labeled anti-IL4R antibody to differentiate between transfused and host platelets. Platelet counts were expressed in relation to initial platelet count. Bleeding was quantified by pixel area of hemorrhage.
Stroke model
Stroke was induced as described.24 In brief, we induced transient focal cerebral ischemia by 90 minutes occlusion of the right middle cerebral artery with a 7.0 siliconized filament in male mice. Mice were anesthetized with isoflurane in a mixture of 30% oxygen. Body temperature was maintained at 37°C (± 1.0°C) using a heating pad. We used laser Doppler flowmetry in all mice to confirm induction of ischemia and reperfusion. Platelets were depleted 3 hours after induction of ischemia.
Lung inflammation model
Groups of mice were sedated and inoculated intranasally with 10 μg Pseudomonas aeruginosa LPS or PBS vehicle, as described.25 Ten minutes after intranasal application, platelets were depleted. Bronchoalveolar lavage (BAL) was performed by canulating the trachea with an 18-gauge angiocath and lungs were lavaged 5 times with 0.8 mL cold sterile PBS. Red blood cells (RBCs) were measured in pooled BAL from each mouse using an AcT2diff cell analyzer (Beckman Coulter, Fullerton, CA). The same analyzer was used to measure hemoglobin concentration in whole blood for anemia studies.
Statistical analysis
Data are presented as mean plus or minus SEM and were analyzed by unpaired, 2-tailed Student t test. P values less than .05 were regarded as statistically significant.
Results
Induction of profound thrombocytopenia
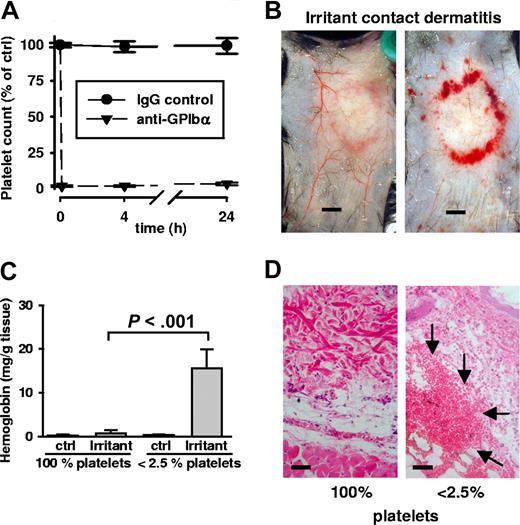
To study the role of platelets in inflammation, we induced thrombocytopenia in mice by injection of an anti-GPIbα antibody.21 Resulting platelet counts were 25 (± 0.1) × 109/L (< 2.5%) compared with 1150 (± 231) × 109/L (100%) during the whole experiment. Injection of control IgG did not alter platelet count (Figure 1A). The mice with low and normal platelet counts were then submitted to various inflammatory models.
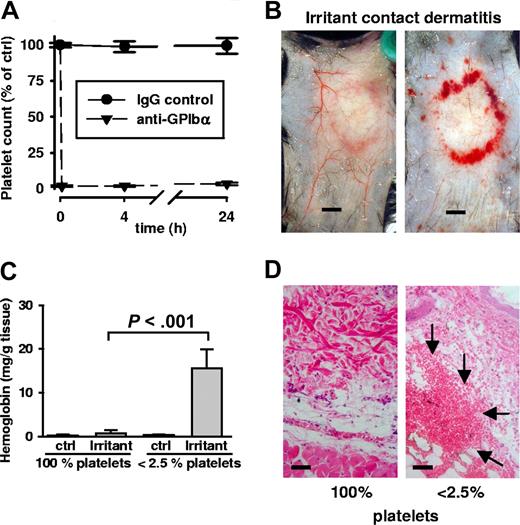
Bleeding in irritant contact dermatitis in thrombocytopenic mice. (A) Thrombocytopenia was achieved by administration of a rat antimouse GPIbα antibody. Platelet counts were determined at indicated time points and displayed in relation to the baseline level (100%). Platelet depletion occurred within minutes (first data point = 0.1 hours) and platelet counts remained < 2.5% for or over 24 hours). Control IgG antibody injection did not alter platelet counts. (B) WT mice were subjected to irritant contact dermatitis (ICD) in the presence and absence of platelets. Erythema and edema were seen in both groups, while massive bleeding into the skin at the inflammatory ring was seen only in thrombocytopenic mice (right). Mice were killed 4 hours after stimulation. Bar = 5 mm. (C) Hemoglobin content in biopsies of the ICD-treated skin. Control skin has barely detectable levels of hemoglobin that were significantly elevated in the inflamed skin of thrombocytopenic mice (P < .001; n = 5). Error bars represent SEM. (D) Skin sections stained with hematoxylin and eosin show massive RBC accumulation (arrows) in the tissue only of ICD-challenged thrombocytopenic animals. Bar = 40 μm.
Bleeding in irritant contact dermatitis in thrombocytopenic mice. (A) Thrombocytopenia was achieved by administration of a rat antimouse GPIbα antibody. Platelet counts were determined at indicated time points and displayed in relation to the baseline level (100%). Platelet depletion occurred within minutes (first data point = 0.1 hours) and platelet counts remained < 2.5% for or over 24 hours). Control IgG antibody injection did not alter platelet counts. (B) WT mice were subjected to irritant contact dermatitis (ICD) in the presence and absence of platelets. Erythema and edema were seen in both groups, while massive bleeding into the skin at the inflammatory ring was seen only in thrombocytopenic mice (right). Mice were killed 4 hours after stimulation. Bar = 5 mm. (C) Hemoglobin content in biopsies of the ICD-treated skin. Control skin has barely detectable levels of hemoglobin that were significantly elevated in the inflamed skin of thrombocytopenic mice (P < .001; n = 5). Error bars represent SEM. (D) Skin sections stained with hematoxylin and eosin show massive RBC accumulation (arrows) in the tissue only of ICD-challenged thrombocytopenic animals. Bar = 40 μm.
Irritant contact dermatitis
As a challenge, we epicutaneously applied 10% croton oil to the backs of experimental mice. Animals were killed 4 hours after stimulation, and the skin was removed and examined. Surprisingly, there was extensive bleeding in the area of inflammation in the thrombocytopenic animals (Figure 1B). No such bleeding was observed in control mice with normal platelet counts. The control mice showed typical inflammatory signs of erythema and edema—but only thrombocytopenic mice invariably revealed massive red blood cell (RBC) loss to the skin. Bleeding was quantified by determining hemoglobin (Hb) content in the tissue. While a minor increase of Hb content was observed in control mice, it was in the platelet-depleted animals that tissue hemoglobin levels increased significantly (Figure 1C). Skin sections stained with hematoxylin and eosin (H&E) confirmed the massive presence of red blood cells in the tissue of thrombocytopenic mice (Figure 1D). Bleeding upon inflammation in thrombocytopenic mice revealed a crucial protective role for platelets during acute dermatitis.
Immune complex–mediated inflammation
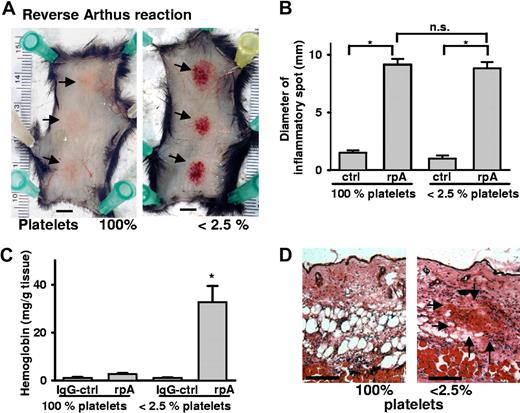
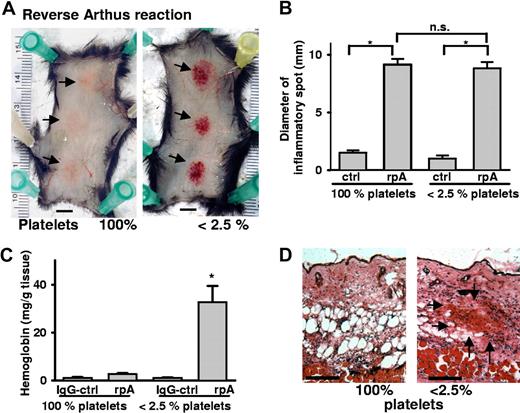
To confirm the hemostatic role of platelets during skin inflammation, we examined a different model of dermatitis, the reverse passive Arthus reaction (rpA). The rpA was elicited by intravenous administration of BSA followed by anti-BSA antibody injection into 3 sites of the dorsal skin. Local formation of immune complexes induced tissue swelling and erythema in the injected area of wild-type (WT) mice (Figure 2A). In thrombocytopenic mice, in addition, massive petechial bleeding occurred at the inflammatory site that was not seen in mice with normal platelet count or in thrombocytopenic mice injected with nonimmunizing rabbit IgG antibody. The rpA induced bleeding in 43 of 44 platelet-depleted mice, whereas none of 32 mice with normal platelet count showed macroscopic bleeding. The area of inflammation as determined by the diameter of Evans blue leakage was not statistically different between thrombocytopenic and nondepleted mice (Figure 2B). RBC infiltration assessed by Hb levels was significantly elevated in inflamed tissue of the thrombocytopenic mice, while only a minor elevation of Hb levels was seen in nondepleted animals (Figure 2C). H&E staining of inflamed skin sections confirmed massive accumulation of RBCs in platelet-depleted mice that did not even occur in control animals observed for 24 hours (Figure 2D).
Hemorrhage at sites of the reverse passive Arthus reaction in platelet-depleted mice. (A) Mice were subjected to rpA ( ) in the presence and absence of platelets and killed at 4 hours. Erythema was seen in control mice (left), while massive petechial bleeding was observed only in thrombocytopenic animals (right). Bar = 5 mm. (B) Evans blue leakage into the tissue was measured to assess the size of the inflammatory area. No differences were found between control and platelet-depleted animals. (C) Hemoglobin content in biopsies of the inflamed and control skin. Induction of the rpA, but not the IgG control, led to significantly elevated Hb levels in thrombocytopenic mice (*P < .001; n = 4). Error bars represent SEM. (D) Skin sections stained with H&E revealed accumulation of RBCs (
) in the presence and absence of platelets and killed at 4 hours. Erythema was seen in control mice (left), while massive petechial bleeding was observed only in thrombocytopenic animals (right). Bar = 5 mm. (B) Evans blue leakage into the tissue was measured to assess the size of the inflammatory area. No differences were found between control and platelet-depleted animals. (C) Hemoglobin content in biopsies of the inflamed and control skin. Induction of the rpA, but not the IgG control, led to significantly elevated Hb levels in thrombocytopenic mice (*P < .001; n = 4). Error bars represent SEM. (D) Skin sections stained with H&E revealed accumulation of RBCs ( ) in the inflamed skin of thrombocytopenic mice. Bar = 100 μm.
) in the inflamed skin of thrombocytopenic mice. Bar = 100 μm.
Hemorrhage at sites of the reverse passive Arthus reaction in platelet-depleted mice. (A) Mice were subjected to rpA ( ) in the presence and absence of platelets and killed at 4 hours. Erythema was seen in control mice (left), while massive petechial bleeding was observed only in thrombocytopenic animals (right). Bar = 5 mm. (B) Evans blue leakage into the tissue was measured to assess the size of the inflammatory area. No differences were found between control and platelet-depleted animals. (C) Hemoglobin content in biopsies of the inflamed and control skin. Induction of the rpA, but not the IgG control, led to significantly elevated Hb levels in thrombocytopenic mice (*P < .001; n = 4). Error bars represent SEM. (D) Skin sections stained with H&E revealed accumulation of RBCs (
) in the presence and absence of platelets and killed at 4 hours. Erythema was seen in control mice (left), while massive petechial bleeding was observed only in thrombocytopenic animals (right). Bar = 5 mm. (B) Evans blue leakage into the tissue was measured to assess the size of the inflammatory area. No differences were found between control and platelet-depleted animals. (C) Hemoglobin content in biopsies of the inflamed and control skin. Induction of the rpA, but not the IgG control, led to significantly elevated Hb levels in thrombocytopenic mice (*P < .001; n = 4). Error bars represent SEM. (D) Skin sections stained with H&E revealed accumulation of RBCs ( ) in the inflamed skin of thrombocytopenic mice. Bar = 100 μm.
) in the inflamed skin of thrombocytopenic mice. Bar = 100 μm.
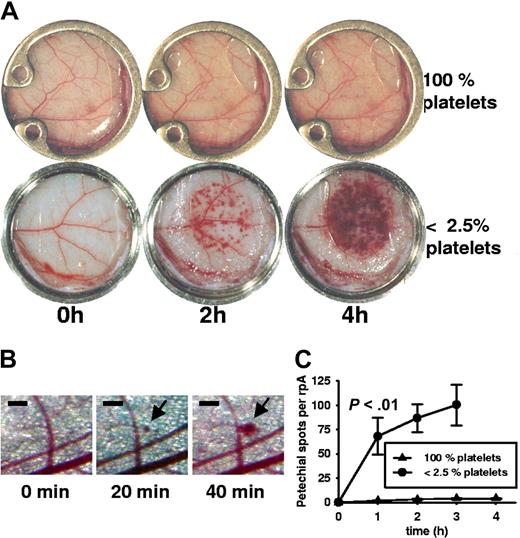
Kinetics of vessel damage and bleeding
To further understand the dynamics of inflammatory bleeding, we visualized the rpA by installing a dorsal skinfold chamber with a glass window for a real-time observation of the skin microcirculation.22 Installation of the skinfold chamber did not affect the development of rpA (Figure 3A). In platelet-depleted mice, the first microscopic appearance of petechiae was seen 20 minutes after stimulation (Figure 3B), and numerous petechiae were clearly visible after 1 hour of stimulation (microscopic petechial spots 68 ± 19 vs 2 ± 0.3 in nondepleted mice; n = 4; P < .01). During the first 2 hours, petechiae appeared and grew at the site of rpA induction with continuous RBC loss into the tissue so that eventually neighboring spots overlapped (Figure 3A). Microscopic observation showed that inflammatory bleeding occurred mainly from vessels with a diameter smaller than 100 μm, many of which could be identified as veins (Figure 3B; Video S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). After 4 hours, confluence of lesions impaired visual quantification. There was still active bleeding when the experiment was stopped after 6 hours. In nondepleted mice, only a few microscopic petechiae appeared over time (Figure 3A; Video S2). These experiments indicate that platelets secure integrity of the microcirculation of the skin during inflammatory challenge.
Kinetics of inflammatory bleeding in thrombocytopenic mice during reverse passive Arthus reaction. (A) Photographs of progressing rpA in the dorsal skinfold chamber. In the absence of platelets, petechial bleeding was clearly visible after 2 hours and increased with time. In nondepleted animals there were virtually no petechial spots. Window diameter was 12 mm. (B) Microscopic view of the progressing rpA in a thrombocytopenic mouse. Petechial bleeding was detected at 20 minutes, with further growth of the spot at 40 minutes. Bar = 200 μm. (C) Petechial spots visible to the eye (∼ 100 μm) were counted during rpA in thrombocytopenic and control animals. The difference in incidence of petechiae became statistically significant within 1 hour (P < .01; n = 4). At t = 4 hours, the petechiae became confluent, impairing quantification. Error bars represent SEM.
Kinetics of inflammatory bleeding in thrombocytopenic mice during reverse passive Arthus reaction. (A) Photographs of progressing rpA in the dorsal skinfold chamber. In the absence of platelets, petechial bleeding was clearly visible after 2 hours and increased with time. In nondepleted animals there were virtually no petechial spots. Window diameter was 12 mm. (B) Microscopic view of the progressing rpA in a thrombocytopenic mouse. Petechial bleeding was detected at 20 minutes, with further growth of the spot at 40 minutes. Bar = 200 μm. (C) Petechial spots visible to the eye (∼ 100 μm) were counted during rpA in thrombocytopenic and control animals. The difference in incidence of petechiae became statistically significant within 1 hour (P < .01; n = 4). At t = 4 hours, the petechiae became confluent, impairing quantification. Error bars represent SEM.
Bleeding in dermatitis is not observed in mice lacking major platelet adhesion receptors and can be prevented in thrombocytopenic mice by a minor platelet transfusion. To evaluate the role of the major platelet adhesion receptors in inflammatory bleeding, we tested mice deficient in the fibrinogen or VWF receptor (β3-integrin−/−; IL4Rα/GPIbα-tg) in the rpA model.15,20 In addition, mice deficient in up-regulation of all platelet integrins (CalDAG-GEFI−/−) or lacking P-selectin, VWF, and the collagen receptor GPVI (FcγR−/−) were challenged. As the rpA requires Fcγ receptor signaling, the irritant contact dermatitis model was studied in those mice.26 While no tissue bleeding was observed in the inflamed skin of knockout mice, platelet depletion invariably induced hemorrhage in all strains tested (Table 1). This indicated that the classical firm adhesion of platelets needed for plug formation may not be required for platelets to prevent skin bleeding in thrombocytopenia. To address this question visually, we used the dorsal skinfold chamber to observe platelet behavior. We could see rolling of fluorescently labeled platelets in the area of inflammation, but no platelet accumulation or apparent plug formation (data not shown). These findings further indicate that firm adhesion of platelets via classical adhesion receptors is not required to prevent inflammatory bleeding.
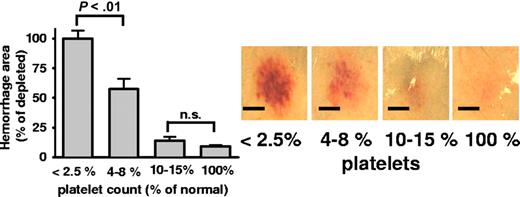
Similar to clinical findings in humans where minor increase of the platelet count prevents bleeding, we performed platelet transfusion studies in inflamed mice. Platelets of IL4Rα/GPIbα-tg mice do not carry the extracellular part of GPIbα recognized by the depleting antibody and therefore they are depletion resistant. After transfusing IL4Rα/GPIbα-tg platelets into WT mice, the endogenous platelets were selectively depleted, while transfused IL4Rα/GPIbα-tg platelets remained in the circulation as checked by flow cytometry (not shown). In transfused animals, the rpA reaction developed normally, but the inflammatory bleeding was not detected (Figure 4). Titrating down the numbers of transfused platelets, we observed that 10% to 15% of WT platelet count is sufficient to prevent inflammatory bleeding, while partial rescue was seen with platelet levels as low as 4% to 8% of WT (Figure 4).
Rescue of inflammatory hemorrhage by minor platelet transfusion. The rpA model was studied in thrombocytopenic mice receiving transfusion of depletion-resistant platelets from IL4Rα/GPIbα-tg mice. Bars indicate the area of hemorrhage in mice at various platelet counts. Photographs (right) show representative rpA reaction spots. Bar = 5 mm. Transfusion of platelets to 5% to 8% of normal platelet count significantly prevented bleeding (P < .01). At levels of 10% to 15% circulating platelets, the hemorrhagic area was similar to nondepleted control mice (P = .15; n = 5). Error bars represent SEM.
Rescue of inflammatory hemorrhage by minor platelet transfusion. The rpA model was studied in thrombocytopenic mice receiving transfusion of depletion-resistant platelets from IL4Rα/GPIbα-tg mice. Bars indicate the area of hemorrhage in mice at various platelet counts. Photographs (right) show representative rpA reaction spots. Bar = 5 mm. Transfusion of platelets to 5% to 8% of normal platelet count significantly prevented bleeding (P < .01). At levels of 10% to 15% circulating platelets, the hemorrhagic area was similar to nondepleted control mice (P = .15; n = 5). Error bars represent SEM.
Platelets prevent brain hemorrhage after stroke
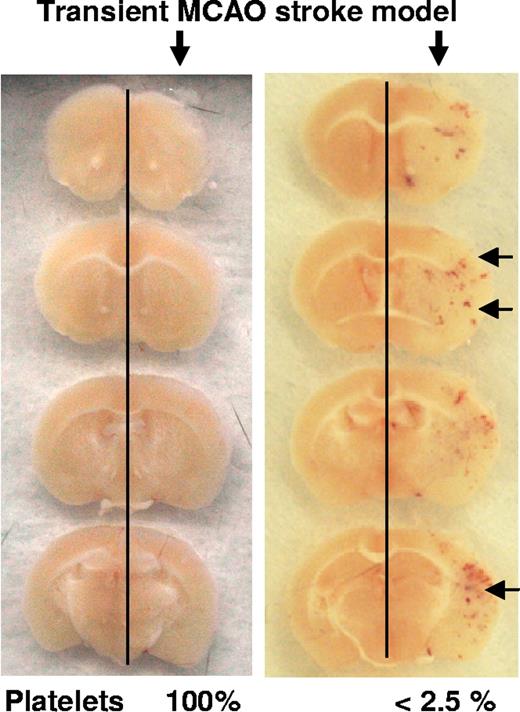
To examine whether inflammatory bleeding in thrombocytopenia is skin specific or a generalized phenomenon, we studied inflammation in 2 additional organs. Inflammation was induced in cerebral microcirculation by a transient occlusion of the middle cerebral artery. Twenty-four hours after ischemia/reperfusion, mice were killed and serial sections of the brain examined. Multiple hemorrhagic foci were seen around the infarction area in 4 of 4 thrombocytopenic mice that were not observed in mice with normal platelet count (9/9; Figure 5). Platelet depletion did not significantly affect the infarct size (not shown).
Inflammatory hemorrhage in stroke. Brain sections of mice challenged by the middle cerebral artery occlusion stroke model and analyzed 24 hours after reperfusion. The inflamed hemisphere (right) shows hemorrhagic spots in the thrombocytopenic mice ( ).
).
Inflammatory hemorrhage in stroke. Brain sections of mice challenged by the middle cerebral artery occlusion stroke model and analyzed 24 hours after reperfusion. The inflamed hemisphere (right) shows hemorrhagic spots in the thrombocytopenic mice ( ).
).
Severe lung hemorrhage and anemia in inflamed lungs of thrombocytopenic mice
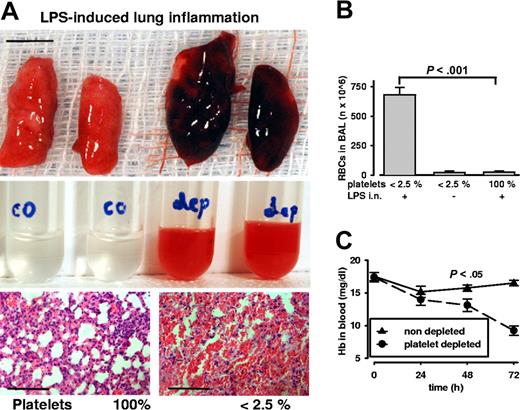
Pulmonary inflammation was induced by intranasal endotoxin application. After 24 hours of stimulation, we found massive pulmonary bleeding exclusively in platelet-depleted mice (Figure 6A). The bronchoalveolar lavage (BAL) revealed an opaque red fluid. Quantifying the cellular contents of BAL, we found high numbers of RBCs in inflamed thrombocytopenic animals. Importantly, during thrombocytopenia only inflammation led to pulmonary bleeding (Figure 6B): there were virtually no RBCs detectable in BAL of noninflamed platelet-depleted mice (inflamed 681 ± 103 vs noninflamed 21 ± 19 Mio RBCs; n = 4; P < .001). In thrombocytopenic mice, bleeding to the lung led to significantly reduced Hb levels in blood (Figure 6C). These mice also showed labored breathing and decreased activity that was not seen in LPS-stimulated mice with normal platelet count. Therefore, it appears that during inflammation platelets preserve physiologic organ function by maintaining the organ's vascular integrity.
Lung inflammation induces severe hemorrhage and anemia during thrombocytopenia. (A) Top: Postmortem analysis of lungs after intranasal LPS challenge. Massive pulmonary hemorrhage is observed in platelet-depleted lungs on the right. Bar = 5 mm. Middle: Bronchoalveolar lavage reveals red opaque fluid from depleted (dep) mice and not from control (co) mice with normal platelet count. Bottom: Lung sections stained with hematoxylin and eosin show massive RBC infiltration in airways and alveolar space of thrombocytopenic mice. Bar = 100 μm. (B) Significantly increased amounts of RBCs were found in the lavage of inflamed thrombocytopenic mice (P < .001; n = 5). Platelet depletion per se did not induce RBC loss into the lung. (C) Hemoglobin levels in whole blood of thrombocytopenic and control mice challenged in the lung inflammation model. Pulmonary hemorrhage is accompanied by a significant drop in hemoglobin levels in the thrombocytopenic animals. Error bars represent SEM.
Lung inflammation induces severe hemorrhage and anemia during thrombocytopenia. (A) Top: Postmortem analysis of lungs after intranasal LPS challenge. Massive pulmonary hemorrhage is observed in platelet-depleted lungs on the right. Bar = 5 mm. Middle: Bronchoalveolar lavage reveals red opaque fluid from depleted (dep) mice and not from control (co) mice with normal platelet count. Bottom: Lung sections stained with hematoxylin and eosin show massive RBC infiltration in airways and alveolar space of thrombocytopenic mice. Bar = 100 μm. (B) Significantly increased amounts of RBCs were found in the lavage of inflamed thrombocytopenic mice (P < .001; n = 5). Platelet depletion per se did not induce RBC loss into the lung. (C) Hemoglobin levels in whole blood of thrombocytopenic and control mice challenged in the lung inflammation model. Pulmonary hemorrhage is accompanied by a significant drop in hemoglobin levels in the thrombocytopenic animals. Error bars represent SEM.
Discussion
Here we show that, in the absence of platelets, there is massive bleeding in all inflamed organs examined: the skin, the brain, and the lung. This striking observation points to an important role for platelets in maintaining vascular integrity from the very onset of inflammation and throughout the inflammatory process. Thrombocytopenia alone did not cause bleeding in any of the experimental animals.
In the thrombocytopenic mice, organ bleeding was seen only upon inflammatory stimulation. This finding is in line with previous experimental data that thrombocytopenia per se is not sufficient to induce bleeding in mice.27 In human studies, 50% of 185 adults with immune thrombocytopenic purpura were diagnosed by routine blood tests without clinical signs of bleeding.13 This suggests that also in thrombocytopenic humans, additional factors are required to provoke bleeding. In another study, patients suffering from Wiskott-Aldrich syndrome (WAS) were subdivided into the WAS protein–negative and WAS protein–positive groups. While both groups showed similar levels of thrombocytopenia, the WAS protein–negative group showed significantly more bleeding events. Interestingly, in this subgroup the incidence of infections was significantly higher.28 In mice, petechiae have been reported in a complex model of idiopathic thrombocytopenic purpura that induces both thrombocytopenia and systemic inflammation.29 We here show clear experimental evidence that it is the inflammatory response that leads to hemorrhage in thrombocytopenia. In this context, it is noteworthy that bleeding in thrombocytopenic patients is first seen in the skin and mucosae. Perhaps this is due to ongoing subclinical local inflammatory processes in these organs that are constantly exposed to proinflammatory microbial and environmental irritants. As in humans, hemorrhage in thrombocytopenic mice can be rescued by platelet transfusion. For this, only a slight elevation in platelet count is required, pointing to a narrow threshold-level regulating hemorrhage (Figure 4).
Bleeding to the skin upon inflammatory challenge was not seen in mice with a single deficiency of any of the major platelet adhesion receptors (Table 1). While these animals are models for major bleeding disorders (Bernard-Soulier syndrome, Glanzmann thrombasthenia), their platelets are fully capable of preventing hemorrhage during inflammation. This indicates that the platelet role in inflammation is different from that needed during platelet plug formation. Although no single platelet adhesion receptor was absolutely required to prevent hemorrhage, it cannot be excluded that several types of receptors could support necessary adhesion, thus providing a backup mechanism. In contrast to previous findings in angiogenic vessels,9 VWF receptor–deficient platelets (IL4Rα/GPIbα-tg) rescued inflammatory bleeding (Figure 4), indicating that vascular instability induced by inflammation is different from that occurring during experimental angiogenesis. The importance of platelets for vascular integrity during inflammation is further underlined by the fact that P-selectin−/− mice with reportedly reduced inflammatory activity30 still showed massive tissue hemorrhage during thrombocytopenia (Table 1). While the role of platelet adhesion receptors was here excluded for the early phase of the Arthus reaction, it is likely that at later time points and in more stressful inflammatory models that lead to endothelial death and exposure of extracellular matrix the platelet adhesion receptors are required to prevent bleeding. This may explain why inhibition of GPIIbIIIa recently was shown to induce brain hemorrhage upon stroke.31 Observing fluorescently labeled platelets in the dorsal skinfold chamber, we did see rolling but no accumulation of platelets in the inflamed tissue (data not shown). This again suggests that platelet plug formation is not required to prevent hemorrhage during inflammation.
We speculate that platelet storage granules are important in the prevention of inflammatory hemorrhage and that platelets may act in a paracrine fashion. Indeed, platelets' storage granules contain an array of vasoactive molecules that regulate vascular junctions, permeability, and endothelial migration such as serotonin, sphingosine-1P, and angiopoietin-1 to name a few.32-38 Platelets could be required to deliver such agents to the site of inflammatory challenge and thereby secure vascular integrity as recently suggested for angiogenesis.39 From our current study, it appears that platelets were particularly needed to protect smaller venules surrounded by few smooth muscle cells and pericytes as these presented the main sites of inflammatory hemorrhage as visualized in the skin model (Figure 3; Video S1). All the tested inflammatory models critically depend on leukocyte activity, with neutrophils being the first cells to infiltrate the inflammatory spot.25,26,40-43 Whether leukocytes play a role in inflammatory hemorrhage by destabilizing vascular integrity still needs to be established.
Our latest studies show that inflammatory hemorrhage in thrombocytopenia is not restricted to skin microcirculation (Figures 5,6). This indicates that hemorrhage can occur at any inflamed site. While we currently do not know whether the inflammatory bleeding around the infarcted areas of the brain is accompanied by worsening of recovery from stroke, the pulmonary hemorrhage significantly affected lung function. Thrombocytopenic mice suffering from lung inflammation became anemic, indicating that such bleeding could also become life threatening (Figure 6C). These results suggest that inflammation in thrombocytopenic patients may have deleterious consequences inducing bleeding and thus affecting survival.
Our findings shed new light on platelet function during inflammation as we demonstrate a crucial role for platelets in preventing tissue-damaging hemorrhage or even severe blood loss from the inflamed organs. So while platelets have been known to promote recruitment of inflammatory cells and thus stimulate the inflammatory response, they also prevent the deleterious consequences of inflammation on the vasculature. Identification of the protective factor(s) provided by the platelets could be of major clinical importance.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Julia Kahn from Rakesh Jain's laboratory for teaching us to prepare the dorsal skinfold chamber, Jerry Ware and Richard Hynes for providing IL4Rα/GPIbα-tg and β3integrin-deficient mice, Lesley Cowan for assistance in preparing the paper, and Wolfgang Bergmeier for helpful discussions.
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (R37 HL41002, P01 HL066105 [D.D.W.], HL066548 [E.R.]); Fondation pour la Recherche Médicale (B.H.-T.-N.); grant 2006FI00092 from the Departament d'Universitats, Recerca i Societat de la Informació de la Generalitat de Catalunya i Fons Social Europeu (C.C.); and GO1360 from the Deutsche Forschungsgemeinschaft (T.G.). C.B. is a Parker B. Francis Fellow in Pulmonary Research.
National Institutes of Health
Authorship
Contribution: T.G., B.H.-T.-N., and D.D.W. designed research; T.G., B.H.-T.-N., C.C., C.B., B.-Q.Z., and S.M.C. performed research; T.G., B.H.-T.-N., C.B., E.R.-O., and D.D.W. analyzed data; T.G. and D.D.W. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Denisa D. Wagner, Immune Disease Institute, 800 Huntington Avenue, 02115 Boston, MA; e-mail: wagner@idi.harvard.edu.
References
Author notes
*T.G. and B.H.-T.-N. contributed equally to this study.