Abstract
Functional characterization of signaling pathways that critically control mantle cell lymphoma (MCL) cell growth and survival is relevant to designing new therapies for this lymphoma. We herein demonstrate that the constitutive activation of Akt correlates with the expression of the phosphorylated, inactive form of PTEN. Phosphatidyl-inositol-3 kinase (PI3-K)/Akt or mammalian target of rapamycin (mTOR) inhibition decreased the growth of both primary MCL cultures and established cell lines and antagonizes the growth-promoting activity of CD40 triggering and IL-4. These effects are mediated by nuclear accumulation of the p27Kip1 inhibitor induced by down-regulation of the p45Skp2 and Cks1 proteins, which target p27Kip1 for degradation. Moreover, Akt inhibition down-regulated cyclin D1 by promoting its proteasome-dependent degradation driven by GSK-3. Intriguingly, mTOR inhibition affected cyclin D1 proteolysis only in MCL cells in which GSK-3 is under the direct control of mTOR, suggesting that different MCL subsets could be differently responsive to mTOR inhibition. Finally, PI3-K/Akt inhibitors, but not rapamycin, induced variable levels of caspase-dependent apoptosis and reduced telomerase activity. These results indicate that Akt and mTOR activation have distinct functional relevance in MCL and suggest that targeting Akt may result in more effective therapeutic effects compared with mTOR inhibition.
Introduction
Mantle cell lymphoma (MCL) is a distinct B-cell non-Hodgkin lymphoma whose normal counterpart is likely represented by pre–germinal center, naive B cells that populates the mantle zone of lymph nodes.1 MCL is characterized by advanced stage at presentation, frequent extranodal localization, and aggressive clinical behavior, with poor response to conventional therapeutic regimens and a very unfavorable prognosis, even when the disease is treated with high-dose therapy and autologous bone marrow transplantation.1 More than 95% of MCLs show the t(11;14) (q13;q32) translocation, which results in a juxtaposition of the CCND1 gene locus to the immunoglobulin heavy chain promoter and the subsequent cyclin D1 overexpression,1,2 leading to the deregulation of the cyclin D/Rb pathway. Cyclin D1 deregulation, however, is not sufficient for lymphomagenesis; cooperation with microenvironmental stimuli, such as IL-4, IL-10, and CD40 activation, as well as additional genetic changes, are probably required to induce and sustain the transformed phenotype of mantle cells. Indeed, defects involving inhibitors of G1 cell-cycle progression, such as p53, p27Kip1, p16INK4a, and p15INK4, may also occur in MCL.3,4 More recently, gene-expression profiling and proteomic studies demonstrated that MCL cells carry a profound deregulation of multiple genes and pathways that are involved in the control of cell growth and survival.5,6 Despite these advances, however, only limited information is available on the mechanisms responsible for the constitutive activation of critical signaling pathways and their functional significance. Elucidation of these issues is of particular relevance to identify and exploit tumor-specific molecular signatures for new treatment strategies
Among the signaling pathways that may be deregulated in MCL cells, the phosphatidyl-inositol-3 kinase (PI3-K)/Akt pathway has recently attracted great interest as a possible therapeutic target. The PI3-K pathway is activated by a wide range of tyrosine kinase growth factor receptors and is the major activator of Akt, a serine/threonine protein kinase that modulates the function of a variety of downstream substrates involved in the regulation of cell-cycle progression, differentiation, transcription, translation, cell survival, and angiogenesis. Activation of PI3-K generates the membrane lipid phosphatidyl-inositol-trisphosphate (PIP3), which favors the recruitment of Akt to the plasma membrane, where the Akt kinase is activated upon phosphorylation by 3-phosphoinositide–dependent protein kinase-1 (PDK-1). Akt activation is counteracted by PTEN, a lipid phosphatase that dephosphorylates PIP3, whose expression is lost in a variety of tumor cells.7 Moreover, recent evidence also indicates that PTEN can be inactivated by phosphorylation of serine 380/threonine 382/383 on its carboxy-terminal regulatory domain.8-10 Nevertheless, the role of phosphorylated PTEN in human tumors has been poorly investigated so far.
Notably, the PI3-K/Akt pathway controls the expression of cell-cycle regulatory proteins, such as p27Kip1 and cyclin D1, that are deregulated in MCL. In particular, threonine 157 phosphorylation of p27Kip1 by Akt delocalizes the protein in the cytoplasm, thus preventing its inhibitory functions.11 Moreover, Akt may also regulate the levels of cyclin D1 through the inhibition of GSK-3β, a kinase that negatively regulates cyclin D1 expression at both the transcriptional and posttranscriptional levels.12 Activation of Akt promotes cell survival by directly phosphorylating key apoptotic regulators, including BAD, the apoptosis signal-regulating kinase 1, and members of the FoxO family of transcription factors. Furthermore, the Akt kinase enhances telomerase activity through the direct phosphorylation of the hTERT protein,13 the catalytic component of the telomerase complex.
One of the important downstream targets of Akt is the mammalian target of rapamycin (mTOR), a serine-threonine protein kinase which regulates cell growth and proliferation by integrating signals from growth factors, hormones, nutrients, and energy status. Similar to its yeast counterpart, mTOR resides in 2 distinct protein complexes, TORC1 and TORC2.14 TORC1 is sensitive to rapamycin and is responsible for the phosphorylation of the translational regulators p70S6 kinase and 4E-BPs, which results in enhanced protein synthesis.15 In particular, TORC1 controls the translation of proteins that critically regulate cell-cycle progression, including cyclin D1. TORC2 is rapamycin insensitive and is involved in actin cytoskeleton reorganization.14
Akt is overexpressed and/or constitutively activated in several tumor histotypes, often conferring resistance to chemo- and radiotherapy.16-18 Recent gene profiling studies have demonstrated that several members of the PI3-K/Akt pathway are up-regulated in MCL, and evidence has also been provided suggesting that Akt- and mTOR-dependent signaling may be constitutively active in MCL.6,19,20 Nevertheless, only limited information is currently available on the causes and functional consequences of Akt and mTOR activation in MCL cells.
The present study was aimed at further characterizing the biologic significance of the constitutive activation of the PI3-K/Akt and mTOR/p70S6K pathways in MCL, with the final goal to substantiate the rationale to exploit these signaling cascades for therapeutic purposes in the MCL setting.
Methods
Patient samples
A total of 5 patients with MCL (4 with leukemic-phase disease) were identified on the basis of morphologic, immunophenotypic, and molecular criteria according to World Health Organization (WHO) lymphoma classification. Of these, 4 patients (all with leukemic-phase disease) showed typical morphology, whereas the nodal patient had blastic/blastoid morphology (Table 1). All patients had been off treatment for more than 30 days at the time of the sample procurement. Approval was obtained from the CRO-IRCCS Institutional Review Board for these studies, and informed consent was obtained in accordance with the Declaration of Helsinki. In all cases in the leukemic phase, the percentage of circulating CD5+/CD19+ MCL cells was always greater than 80%. Mononuclear cells were isolated by density gradient centrifugation from peripheral blood samples or from unicellular suspension obtained from mechanically minced lymph nodes. After washing, cells were resuspended and cryopreserved in 10% DMSO until further study. Before use, the cells were thawed, washed, and resuspended in RPMI 1640 medium (Invitrogen, Carlsbad, CA) containing 10% fetal calf serum (FCS) and antibiotics. Non-B cells were removed using magnetic immunobeads (B-cell Isolation Kit; Miltenyi Biotech, Calderara di Reno, Italy). In all cases, more than 95% of purified cells were cyclin D1+, as shown by fluorescence-activated cell sorter (FACS) analysis. Lower but still detectable cyclin D1 expression levels were present after LY294002 treatment.
Cell lines
The 3 MCL cell lines included in this study are Granta 519, Jeko-1, and SP-53.21 Granta 519 cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 20 mM l-glutamine, and maintained in a humidified 5% CO2 incubator at 37°C. For Jeko-1 and SP-53, DMEM was substituted with RPMI 1640.
Proliferation assays, cell-cycle analysis, and apoptosis detection
Cell proliferation was evaluated by 2 different methods. For 3H-thymidine uptake, the protocol used was previously described.22 For carboxyfluorescein diacetate succinimidyl ester (CFDA-SE; Molecular Probes, Eugene, OR) labeling,23 purified cells were stained with a predetermined optimal concentration of the dye (0.5 μM for 10 minutes at room temperature under continuous shaking), washed 3 times with complete medium, and seeded at 106 cells/mL. After culture, cells were stained with either propidium iodide (PI; to monitor cell viability) or with anti–cyclin D1 antibody (Upstate Biotechnology, Lake Placid, NY) as previously described.22 All flow cytometric analyses were performed on a EPICS ALTRA flow cytometer (Beckman Coulter, Milan, Italy), and cell division was analyzed using the FlowJo 4.3 proliferation module (TreeStar, Ashland, OR). Data were reported as “division index,” defined as the average number of division that a cell present in the starting population has undergone. Results of CFDA-SE experiments are reported as means plus or minus SE and a paired, 2-tailed Student t test was used to compare assay results. The influence of PI3-K/Akt inhibitors on cell-cycle parameters was investigated by flow cytometry after PI staining as previously described.22 Apoptosis was evaluated by fluorescence analysis after PI staining, nick translation labeling of DNA strand breaks (TUNEL), and annexin V labeling as previously reported.24,25
Antibodies and reagents
Phospho-Akt (Ser473), phospho-p70S6K (T389), phospho-S6rp (Ser235/236), phospho–GSK-3α/β (S21/9), phospho–4E-BP1 (T37/46), phospho-PTEN (Ser380), phospho-PTEN (Ser380/Thr382/Thr383), Akt, p70S6K, PTEN, caspase-8, caspase-9, caspase-10, and PARP antibodies were from Cell Signaling Technology (Danvers, MA); caspase-3 antibody was from Biosource (Camarillo, CA); β-tubulin and retinoblastoma protein were from BD Pharmingen (San Diego, CA); p27Kip1 was from BD Transduction Laboratories (Erembodegem, Belgium); and cyclin D1 (DCS-6), cyclin D3 (C-16), p45Skp2, and Cks1 was from Santa Cruz Biotechnology (Santa Cruz, CA). LY294002, wortmannin, and SH-5 were purchased from Alexis Biochemicals (Lausen, Switzerland); calf intestinal alkaline phosphatase (CIAP) was from Roche (Indianapolis, IN); Z-VAD was from Calbiochem (San Diego, CA); and SB216763, rapamycin, LLnL, and cycloheximide were from Sigma-Aldrich (St Louis, MO).
Extract preparation and Western analysis
Whole-cell lysates and nuclear/cytoplasmic extracts were prepared as previously described.22 For caspase analysis, cells were resuspended in CHAPS cell extract buffer (Cell Signaling Technology), frozen and thawed 3 times, and centrifuged at 16 000g. Proteins were fractionated using SDS-PAGE and transferred onto nitrocellulose membranes. Immunoblotting was performed using the enhanced chemiluminescence detection system (GE Healthcare, Little Chalfont, United Kingdom).
Immunohistochemistry
Formalin-fixed, paraffin-embedded lymph-nodes of 13 patients with MCL (age range, 41-78 years; 7 men and 6 women; 12 classic and 1 blastoid variant) and reactive tonsils from 3 pediatric patients were stained with antibodies against phospho-PTEN (1:200; Cell Signaling Technology), and phospho-Akt (1:100, Cell Signaling Technology) using an automatized immunostainer (Bond BioVision, Newcastle, United Kingdom) and Novolink detection kit (Novocastra, Newcastle, United Kingdom). Slides were counterstained with haematoxylin and evaluated independently by 2 investigators (C.D., M.P.). For phosphatase treatment, samples were incubated for 45 minutes at 37°C with CIAP (1000 U/mL in Tris-buffered saline [TBS]) and then washed 3 times before staining.
Telomerase activity
Telomerase activity was evaluated by using a telomeric repeat amplification (TRAP)–based quantitative assay, as previously reported.26 The amount of telomerase activity in each sample was expressed as the ratio between the counts per minute (cpm) relative to the telomerase ladder (cpm TL) and the standard control (cpm ITAS). The cpmTL/cpm ITAS ratios provided a more accurate quantification of telomerase levels and a wider range of linear relationship than those obtained by comparing sample values against an external standard control.27
Results
Proliferation of MCL cells is driven by the PI3-K/Akt and mTOR pathways
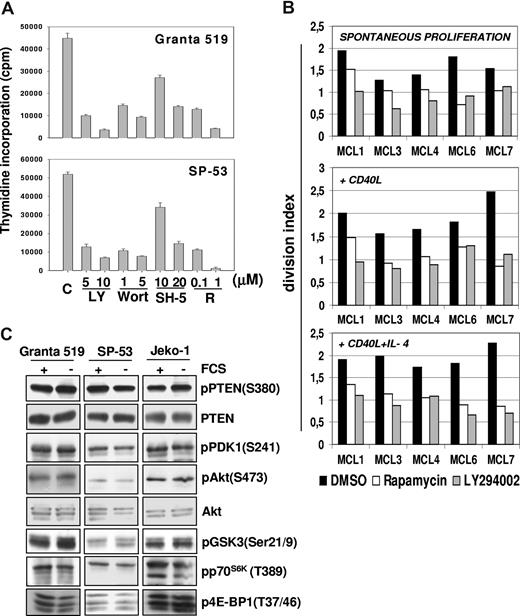
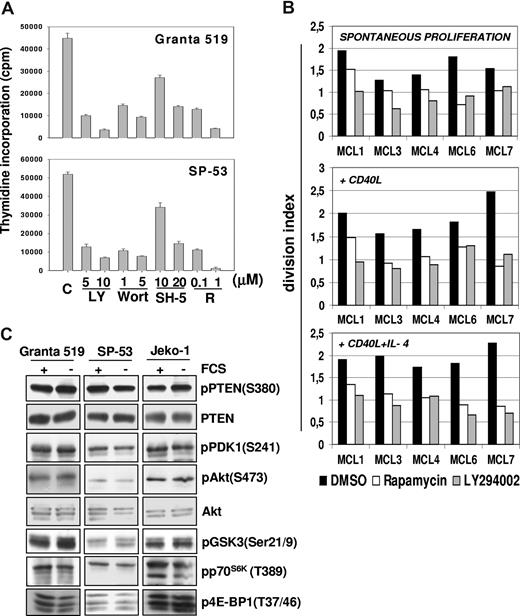
The effects of pharmacologic inhibition of PI3-K, Akt, and mTOR on MCL cell proliferation were initially investigated in Granta 519 and SP-53 MCL lines. As shown in Figure 1A, LY294002, wortmannin, and rapamycin reduced by about 80% the 3H-thymidine incorporation in both cell lines. The specific Akt inhibitor SH-5 was also active, with a more pronounced effect when administered at 20 μM compared with lower doses (Figure 1A). The effects of LY294002 and rapamycin were also verified in 5 primary MCL cultures using the CFDA-SE assay combined with intracellular cyclin D1 staining to avoid possible interference by residual normal B cells. Treatment for 3 days with 10 μM LY294002 significantly decreased the division index of cyclin D1+ cells of all 5 primary MCL cultures (mean ± SE, 1.59 ± 0.12 vs 0.89 ± 0.09; P = .01). Similar findings were obtained with rapamycin (0.1 μM; mean ± SE, 1.59 ± 0.12 vs 1.07 ± 0.12; P = .02; Figure 1B). These results indicate that the PI3-K/Akt and mTOR pathways have a relevant role in promoting MCL cell growth.
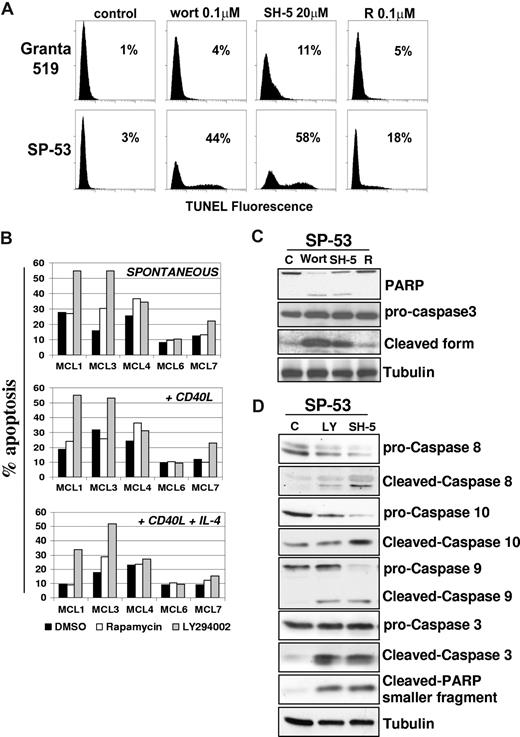
Constitutive activation of P13-K/Akt and mTOR pathways promotes MCL cells proliferation. (A) Inhibition of PI3-K/Akt and mTOR pathways strongly reduces the proliferation of MCL cell lines. Granta 519 and SP-53 cells were cultured at 105/mL in DMEM and RPMI, respectively, supplemented with 10% FCS for 24 hours with either solvent alone (“C”), LY294002 (“LY”) at 5 or 10 μM, wortmannin (“Wort”) at 1 or 5 μM, SH-5 at 10 or 20 μM, or rapamycin (“R”) at 0.1 or 1 μM. DNA synthesis was assessed by 3H-thymidine incorporation after 6 hours. Results were expressed as mean (± SD) counts per minute of quadruplicate wells. (B) Antiproliferative effects of PI3-K and TORC1 inhibition in primary MCL cultures. Effects of PI3-K or TORC-1 inhibition on the division index of 5 primary MCL cultures were evaluated by CFDA-SE labeling coupled with cyclin D1 staining. The results are relative to a 3-day culture period. Globally, cultures treated with LY294002 (10 μM) or rapamycin (0.1 μM) showed a significantly lower division index compared with controls. Effects of LY294002 (10 μM) and rapamycin (0.1 μM) were also investigated in the same primary MCL cultures stimulated by rhsCD40-L alone or in combination with IL-4, showing that PI3-K or TORC-1 inhibition also significantly decreased the division index in the presence of these stimuli. (C) The PI3K/Akt and mTOR/p70S6K pathways are constitutively activated in MCL cell lines. Granta 519, SP-53, and Jeko-1 cells were cultured in medium with 10% FCS or medium with 0.5% bovine serum albumin (BSA) for 24 hours. Total-cell lysates, corresponding to 100 μg of proteins, were subjected to immunoblotting analysis using phosphospecific antibodies.
Constitutive activation of P13-K/Akt and mTOR pathways promotes MCL cells proliferation. (A) Inhibition of PI3-K/Akt and mTOR pathways strongly reduces the proliferation of MCL cell lines. Granta 519 and SP-53 cells were cultured at 105/mL in DMEM and RPMI, respectively, supplemented with 10% FCS for 24 hours with either solvent alone (“C”), LY294002 (“LY”) at 5 or 10 μM, wortmannin (“Wort”) at 1 or 5 μM, SH-5 at 10 or 20 μM, or rapamycin (“R”) at 0.1 or 1 μM. DNA synthesis was assessed by 3H-thymidine incorporation after 6 hours. Results were expressed as mean (± SD) counts per minute of quadruplicate wells. (B) Antiproliferative effects of PI3-K and TORC1 inhibition in primary MCL cultures. Effects of PI3-K or TORC-1 inhibition on the division index of 5 primary MCL cultures were evaluated by CFDA-SE labeling coupled with cyclin D1 staining. The results are relative to a 3-day culture period. Globally, cultures treated with LY294002 (10 μM) or rapamycin (0.1 μM) showed a significantly lower division index compared with controls. Effects of LY294002 (10 μM) and rapamycin (0.1 μM) were also investigated in the same primary MCL cultures stimulated by rhsCD40-L alone or in combination with IL-4, showing that PI3-K or TORC-1 inhibition also significantly decreased the division index in the presence of these stimuli. (C) The PI3K/Akt and mTOR/p70S6K pathways are constitutively activated in MCL cell lines. Granta 519, SP-53, and Jeko-1 cells were cultured in medium with 10% FCS or medium with 0.5% bovine serum albumin (BSA) for 24 hours. Total-cell lysates, corresponding to 100 μg of proteins, were subjected to immunoblotting analysis using phosphospecific antibodies.
PI3-K/Akt and mTOR blockade inhibits the growth-promoting activity exerted by CD40 activation and IL-4 costimulation
The division index of primary MCL cultures was increased by exposure for 3 days to rhsCD40-L alone (1 μg/mL; mean ± SE, 1.9 ± 0.16) or its combination with IL-4 (1000 U/mL; mean ± SE, 1.94 ± 0.09), which is consistent with previous findings.22 Notably, LY294002 significantly inhibited the growth of MCL cells stimulated with either rhsCD40-L alone (mean ± SE, 1.9 ± 0.16 vs 1.09 ± 0.08; P = .03) or rhsCD40-L plus IL-4 (mean ± SE, 1.94 ± 0.09 vs 0.88 ± 0.09; P = .002; Figure 1B). Rapamycin induced similar effects in primary MCL cells cultured with rhsCD40-L alone (mean ± SE, 1.9 ± 0.16 vs 1.11 ± 0.11; P = .01) or rhsCD40-L plus IL-4 (mean ± SE, 1.94 ± 0.09 vs 1.05 ± 0.08; P = .003; Figure 1B).
The PI3-K/Akt pathway is constitutively activated in MCL cells
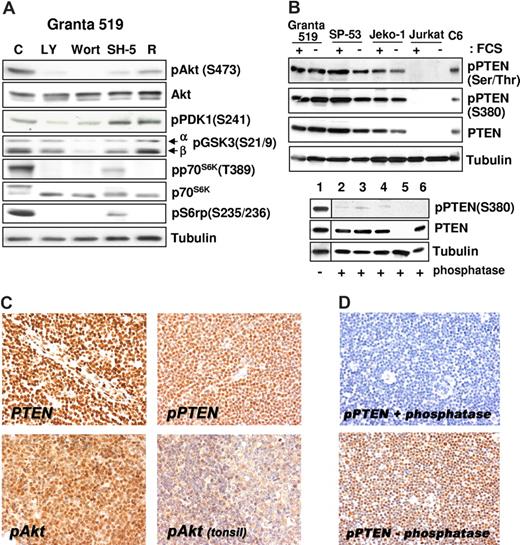
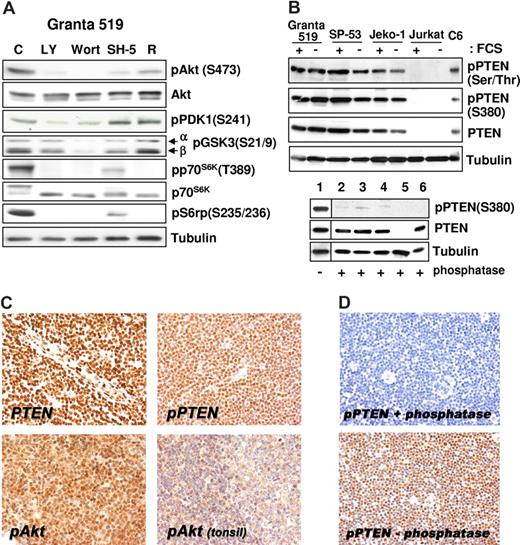
All MCL cell lines investigated expressed Akt phosphorylated on Ser473 independent of the presence of serum (Figure 1C), and the specificity of PI3-K downstream activation was confirmed by inhibition of the phosphorylation after treatment with LY294002 and wortmannin (Figure 2A). The aberrant activation of Akt in MCL cells was not related to loss of PTEN phosphatase, as Granta 519, SP-53, and Jeko-1 cells expressed PTEN (Figures 1C,2B). Notably, all 3 MCL cell lines expressed the functionally inactive PTEN as shown by Ser380 and Thr382/383 PTEN phosphorylation analysis both in the presence and in absence of serum (Figures 1C,2B). Specificity of the antibody to phospho-PTEN was confirmed by phosphatase treatment (Figure 2B).
Akt constitutive activation correlates with the expression of phosphorylated/inactive form of PTEN. (A) The PI3-K/Akt cascade in MCL cell lines. Granta 519 cells were cultured in serum-free medium for 6 hours with solvent alone (“C”), 50 μM LY294002 (“LY”), 1 μM wortmannin (“Wort”), 20 μM SH-5, or 0.1 μM rapamycin (“R”). Total-cell lysates corresponding to 100 μg of proteins were analyzed by immunoblotting for the indicated proteins. Comparable results were also obtained in SP-53 and Jeko-1 cells (not shown). (B) MCL cell lines express the phosphorylated, inactive form of PTEN. All 3 cell lines investigated were cultured in medium with 10% FCS or medium with 0.5% BSA for 24 hours. Total-cell lysates, corresponding to 100 μg of proteins, were subjected to immunoblotting analysis using specific antibodies to the phosphorylated form of PTEN on Ser380 and Thr382/383. To check the antibody specificity after Western transfer, the membrane (bottom panel) was treated with CIAP for 3 hours at 37°C. Lane 1 was cut before CIAP treatment and used as positive control for the phosphospecific antibody. The samples are Granta 519 (lanes 1 and 2), SP-53 (lane 3), Jeko-1 (lane 4), Jurkat (lane 5), and C6 (lane 6). The Jurkat cell line was used as negative control for PTEN protein expression,10 and the C6 cell line was included as positive control. (C) Immunohistochemistry (magnification, ×400). PTEN protein expression is retained in all MCL cells (top left panel), which also expresses its phosphorylated form (top right panel). Most MCL cells show intense phospho-Akt nuclear signal (bottom left panel), whereas weak nuclear and cytoplasmic staining is evident in reactive mantle and germinal center lymphocytes (top right panel). (D) Original magnification, ×200. Staining with phospho-PTEN (Thr380) of samples treated (top) and untreated (bottom) with calf intestinal alkaline phosphatase for 45 minutes at 37°C. Images were acquired with a Nikon E80i microscope (Nikon, Tokyo, Japan) equipped with a Plan APO 20×/.075 objective and Nikon DS camera. ACT-1 software was used for image processing.
Akt constitutive activation correlates with the expression of phosphorylated/inactive form of PTEN. (A) The PI3-K/Akt cascade in MCL cell lines. Granta 519 cells were cultured in serum-free medium for 6 hours with solvent alone (“C”), 50 μM LY294002 (“LY”), 1 μM wortmannin (“Wort”), 20 μM SH-5, or 0.1 μM rapamycin (“R”). Total-cell lysates corresponding to 100 μg of proteins were analyzed by immunoblotting for the indicated proteins. Comparable results were also obtained in SP-53 and Jeko-1 cells (not shown). (B) MCL cell lines express the phosphorylated, inactive form of PTEN. All 3 cell lines investigated were cultured in medium with 10% FCS or medium with 0.5% BSA for 24 hours. Total-cell lysates, corresponding to 100 μg of proteins, were subjected to immunoblotting analysis using specific antibodies to the phosphorylated form of PTEN on Ser380 and Thr382/383. To check the antibody specificity after Western transfer, the membrane (bottom panel) was treated with CIAP for 3 hours at 37°C. Lane 1 was cut before CIAP treatment and used as positive control for the phosphospecific antibody. The samples are Granta 519 (lanes 1 and 2), SP-53 (lane 3), Jeko-1 (lane 4), Jurkat (lane 5), and C6 (lane 6). The Jurkat cell line was used as negative control for PTEN protein expression,10 and the C6 cell line was included as positive control. (C) Immunohistochemistry (magnification, ×400). PTEN protein expression is retained in all MCL cells (top left panel), which also expresses its phosphorylated form (top right panel). Most MCL cells show intense phospho-Akt nuclear signal (bottom left panel), whereas weak nuclear and cytoplasmic staining is evident in reactive mantle and germinal center lymphocytes (top right panel). (D) Original magnification, ×200. Staining with phospho-PTEN (Thr380) of samples treated (top) and untreated (bottom) with calf intestinal alkaline phosphatase for 45 minutes at 37°C. Images were acquired with a Nikon E80i microscope (Nikon, Tokyo, Japan) equipped with a Plan APO 20×/.075 objective and Nikon DS camera. ACT-1 software was used for image processing.
To confirm the constitutive Akt activation in MCL cells, phosphorylation of its downstream substrates was investigated. Immunoblotting analysis showed the presence of GSK-3–, p70S6K-, and 4E-BP1–phosphorylated forms on Ser21/9, Thr389, and Thr37/46, respectively (Figures 1C,2A).
Moreover, treatment with LY294002, wortmannin, or SH-5 markedly decreased the levels of phospho–GSK-3 (Ser21/9), phospho-p70S6K (Thr389), and phospho-S6rp (Ser235/236), demonstrating that the activation of these kinases is dependent on Akt activity (Figure 2A). Notably, p70S6K and S6rp phosphorylation was also prevented by rapamycin, confirming an mTOR-constitutive, Akt-downstream activation in these cell lines. Moreover, rapamycin also induced a slight decrease in the levels of pAkt in Granta 519 (Figure 2A), an effect observed also in other cellular systems after prolonged (more than 8 hours) exposure to this inhibitor,28,29 whereas rapamycin did not affect Akt phosphorylation in Jeko-1 and SP-53 cells, even after 72 hours (not shown). Treatment with okadaic acid abolished rapamycin-induced inhibition of phospho-Akt (not shown), indicating that this effect is probably due to the removal of mTOR-dependent restrain of the PP2A phosphatase, a regulator of Akt phosphorylation.30
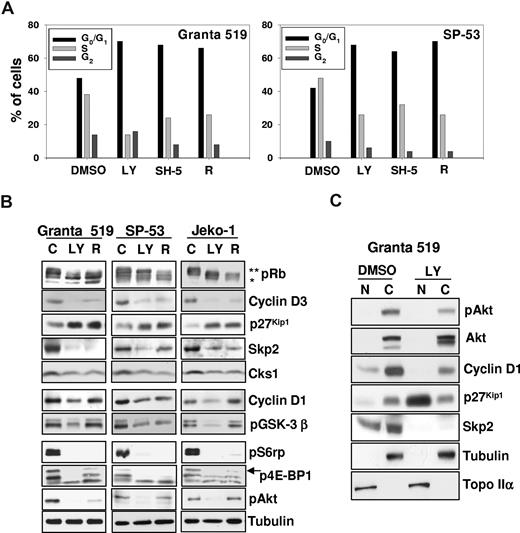
On the other hand, GSK-3β phosphorylation was unaffected by rapamycin in Granta 519 and Jeko-1 cell lines (Figure 3B), indicating that the GSK-3β kinase is not dependent on mTOR activation in these cells. Conversely, SP-53 cells showed a marked down-regulation of phospho–GSK-3β (Ser9) when treated with rapamycin (Figure 3B), suggesting that activation of GSK-3β in these cells is both mTOR- and Akt-dependent.31
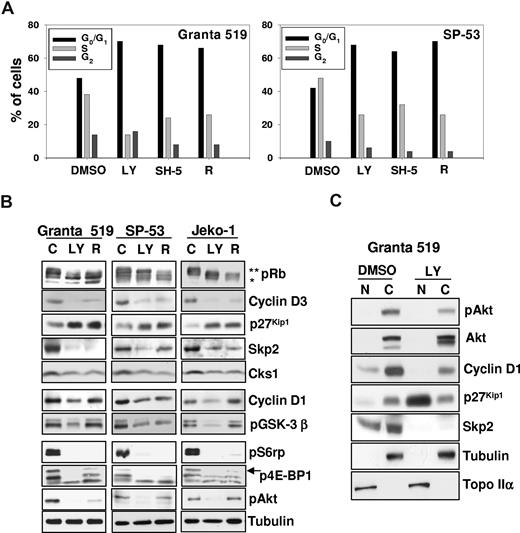
Inhibition of the PI3-K/Akt and mTOR/p70S6K signaling pathways induces G0/G1 cell-cycle arrest, up-regulates p27Kip1 inhibitor, and down-regulates Skp2 and cyclin D expression. (A) Granta 519 and SP-53 cells were cultured in presence of solvent alone (“C”), 50 μM LY294002 (“LY”), 20 μM SH-5, or 0.1 μM rapamycin (“R”) for 24 hours. Cell cycle was analyzed by FACS after DNA staining with PI in cells permeabilized with 0.01% NP40. (B) Granta 519, SP-53, and Jeko-1 cells were treated with solvent alone, 50 μM LY294002 (LY), or 0.1 μM rapamycin (R) for 24 hours. Total-cell lysates corresponding to 100 μg of proteins were analyzed by immunoblotting for the indicated proteins. Anti-pRb Ab recognizes the hyperphosphorylated (**) and hypophosphorylated (*) Rb proteins. (C) Granta 519 cells were treated with solvent alone (DMSO) or 50 μM LY294002 (LY) for 24 hours, and 100 μg of nuclear (N) and cytoplasmic (C) fractions were analyzed by immunoblotting for the indicated proteins. Localization of nucleus-specific protein topoisomerase IIα (Topo IIα) and cytoplasm-specific tubulin was carried out to confirm the purity of the nuclear and cytoplasmic fractions.
Inhibition of the PI3-K/Akt and mTOR/p70S6K signaling pathways induces G0/G1 cell-cycle arrest, up-regulates p27Kip1 inhibitor, and down-regulates Skp2 and cyclin D expression. (A) Granta 519 and SP-53 cells were cultured in presence of solvent alone (“C”), 50 μM LY294002 (“LY”), 20 μM SH-5, or 0.1 μM rapamycin (“R”) for 24 hours. Cell cycle was analyzed by FACS after DNA staining with PI in cells permeabilized with 0.01% NP40. (B) Granta 519, SP-53, and Jeko-1 cells were treated with solvent alone, 50 μM LY294002 (LY), or 0.1 μM rapamycin (R) for 24 hours. Total-cell lysates corresponding to 100 μg of proteins were analyzed by immunoblotting for the indicated proteins. Anti-pRb Ab recognizes the hyperphosphorylated (**) and hypophosphorylated (*) Rb proteins. (C) Granta 519 cells were treated with solvent alone (DMSO) or 50 μM LY294002 (LY) for 24 hours, and 100 μg of nuclear (N) and cytoplasmic (C) fractions were analyzed by immunoblotting for the indicated proteins. Localization of nucleus-specific protein topoisomerase IIα (Topo IIα) and cytoplasm-specific tubulin was carried out to confirm the purity of the nuclear and cytoplasmic fractions.
Immunohistochemical detection of phosphorylated Akt and PTEN in MCL biopsies
All 13 patients with MCL were evaluated and scored positive for both markers tested, displaying immunoreactivity in a variable proportion of neoplastic cells. Moderate to strong nuclear pAkt staining was observed in 40% to 80% of neoplastic cells. All patients with MCL analyzed demonstrated preserved PTEN nuclear reactivity (Figure 2C), and phospho–S380-PTEN was also homogenously expressed (Figure 2D). Specificity of the antibody to phospho-PTEN was confirmed by phosphatase treatment (Figure 2D). Notably, serial consecutive sections stained with pAkt and pPTEN antibodies showed diffuse and strong immunohistochemical signal in the same cells. Faint nuclear pAKT staining was detected in few lymphocytes in the mantle zone in reactive tonsils (Figure 2C). No staining was detected when the primary antibody was omitted.
PI3-K/Akt and TORC1 inhibition induces MCL cells accumulation in G0/G1, cyclin D down-regulation, and increased p27Kip1 expression levels
PI3-K, Akt, or TORC1 inhibitors induced a significant increase in G0/G1 MCL cells at the expense of those in S phase (Figure 3A). Consistently, LY294002 and rapamycin markedly decreased the levels of hyperphosphorylated, active pRb (Figure 3B). PI3-K and TORC1 inhibition induced a down-regulation of cyclin D3 expression in all cell lines tested (Figure 3B), suggesting that cyclin D3 may be an important target of both Akt and mTOR. LY294002 also markedly down-regulated cyclin D1 expression in all MCL cell lines analyzed, whereas rapamycin affected cyclin D1 levels in SP-53 cells only. Moreover, PI3-K/Akt or TORC1 inhibition resulted in a significant up-regulation of the p27Kip1 protein in Granta 519, SP-53, and Jeko-1 cells (Figure 3B). While untreated MCL cells lines carried p27Kip1 almost exclusively in the cytoplasm, PI3-K/Akt inhibition resulted in a nuclear accumulation of p27Kip1 (Figure 3C), consistent with the constitutive Akt activation. To better define the mechanisms underlying p27Kip1 up-regulation induced by PI3-K/Akt or TORC1 inhibition, we investigated the effects of LY294002, SH-5, and rapamycin on the expression of components of the SCFSkp2 ubiquitin ligase complex. PI3-K/Akt or TORC1 inhibition induced a down-regulation of the substrate-targeting p45Skp2 subunit and its cofactor Cks1 in all 3 MCL cell lines (Figure 3B). These findings indicate that in MCL cells, the PI3-K/Akt and TORC1 pathways control the expression of p45Skp2 and Cks1, 2 SCFSkp2 complex components that are required for proteasome-dependent p27Kip1 degradation.
PI3-K/Akt inhibition promotes GSK-3β–dependent degradation of cyclin D1 by the proteasome
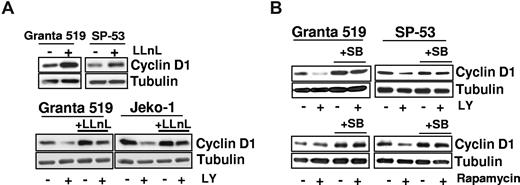
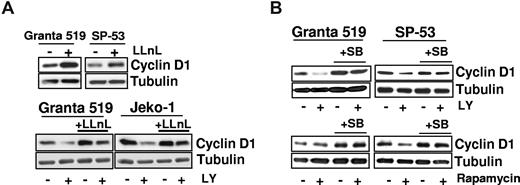
The down-regulation of cyclin D1 induced by PI3-K/Akt inhibition was associated with a reduced GSK-3β phosphorylation. Given the inherent overexpression of cyclin D1 and considering that GSK-3β may regulate cyclin D1 expression, we verified whether the effects of PI3-K/Akt inhibition on cyclin D1 protein levels involved posttranscriptional mechanisms. Incubation of MCL cells with the proteasome inhibitor LLnL induced an up-regulation of cyclin D1 protein levels (Figure 4A), confirming that cyclin D1 is mainly regulated by proteasome-mediated degradation in MCL cells. Notably, preincubation with LLnL prevented LY294002-induced cyclin D1 down-regulation, demonstrating that PI3-K/Akt inhibition down-regulates cyclin D1 by promoting its proteasome-dependent degradation (Figure 4A). To confirm the LY294002 effects on cyclin D1 protein stability, Granta 519 cells were pretreated with LY294002 and then exposed to cycloheximide. In several experiments, LY294002 decreased cyclin D1 half-life, whereas pretreatment with the GSK-3β–specific inhibitor SB216763 increased it (not shown). Considering that cyclin D1 degradation may be regulated by GSK-3β, we analyzed the role of this kinase in mediating LY294002-induced cyclin D1 down-regulation. While incubation with SB216763 alone slightly augmented cyclin D1 protein levels, SB216763 and LY294002 cotreatment abolished the cyclin D1 down-regulation induced by PI3-K/Akt inhibition (Figure 4B). These results indicate that LY294002-induced cyclin D1 down-regulation was mediated by the GSK-3β kinase.
Down-regulation of cyclin D1 is the result of enhanced proteasomal degradation and is a GSK-3–dependent event. (A) Granta 519 and SP-53 cells were cultured in presence of the proteasome inhibitor LLnL 100 μM for 7 hours (top), and Granta 519 and Jeko-1 cells were grown for 24 hours in the presence of 25 μM LY294002, 1 μM LLnL, and their combination (bottom). Equal amounts of total-cell lysates were analyzed by immunoblotting for cyclin D1 protein. (B) Analysis of cyclin D1 protein expression in lysates from Granta 519 and SP-53 treated with 30 μM SB216763 (a GSK-3–specific inhibitor) alone and in combination with LY294002 (25 μM) or rapamycin (0.1 μM) for 30 hours. Tubulin shows equal loading of protein for each lane.
Down-regulation of cyclin D1 is the result of enhanced proteasomal degradation and is a GSK-3–dependent event. (A) Granta 519 and SP-53 cells were cultured in presence of the proteasome inhibitor LLnL 100 μM for 7 hours (top), and Granta 519 and Jeko-1 cells were grown for 24 hours in the presence of 25 μM LY294002, 1 μM LLnL, and their combination (bottom). Equal amounts of total-cell lysates were analyzed by immunoblotting for cyclin D1 protein. (B) Analysis of cyclin D1 protein expression in lysates from Granta 519 and SP-53 treated with 30 μM SB216763 (a GSK-3–specific inhibitor) alone and in combination with LY294002 (25 μM) or rapamycin (0.1 μM) for 30 hours. Tubulin shows equal loading of protein for each lane.
TORC1 inhibition down-regulates cyclin D1 only in MCL cells in which mTOR controls GSK-3β
Unlike PI3-K and Akt inhibitors, rapamycin down-regulates cyclin D1 only in SP-53 cells (Figures 3B,4B). Notably, this effect was correlated with inhibition of GSK-3β phosphorylation since the activation of this kinase was unaffected by rapamycin in Granta 519 and Jeko-1 cells, in which TORC1 inhibition failed to down-regulate cyclin D1 (Figure 3B). Conversely, SP-53 cells showed a marked down-regulation of phospho–GSK-3β (Ser 9) when treated with rapamycin (Figure 3B), suggesting that GSK-3β activation in these cells is both mTOR- and Akt-dependent.31 As for PI3-K/Akt inhibition, SB216763 and rapamycin cotreatment of SP-53 cells abolished the cyclin D1 down-regulation induced by TORC1 inhibition (Figure 4B). These findings support a role of the GSK-3β kinase in mediating rapamycin-induced cyclin D1 down-regulation.
Inhibition of PI3-K/Akt induces caspase-mediated apoptosis in MCL cells
To assess whether the PI3-K/Akt and TORC1 pathways also regulate MCL cell survival, the effects of LY294002 were investigated in B cell–enriched primary cultures from 5 patients with MCL. Identification of apoptotic cells by double gating on low scatter/PI+ events showed that LY294002 markedly enhanced basal MCL cell apoptosis in MCL1, MCL3, and MCL7 cultures, whereas only a slight increase in the number of apoptotic cells was detected in the MCL4 culture (Figure 5B). Primary cells from the MCL6 patient were completely resistant to the proapoptotic effect of LY294002. Conversely, rapamycin induced only limited apoptotic responses in 2 (MCL3, MCL4) of the 5 primary MCL cultures investigated (Figure 5B). Moreover, rhsCD40-L did not significantly affect the extent of apoptosis induced by LY294002 in the 4 responsive MCL cultures, whereas in the presence of rhsCD40-L and IL-4, the proapoptotic effect of LY294002 was decreased but still evident in the MCL1, MCL4, and MCL7 cultures (Figure 5B).
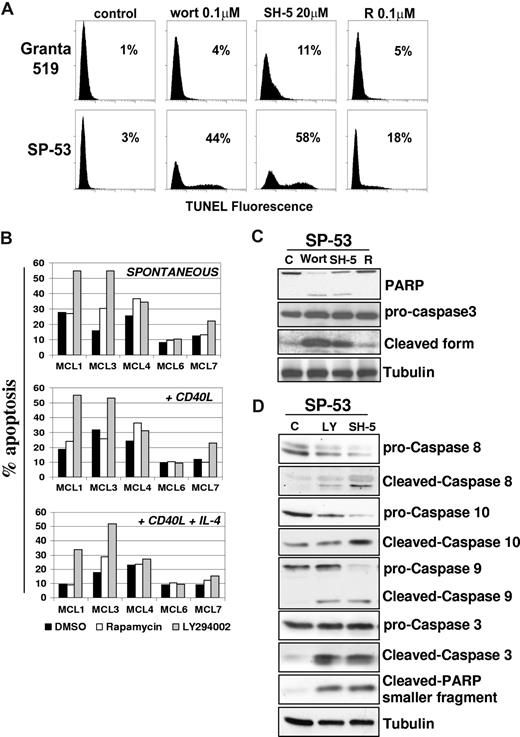
Inhibition of the PI3-K/Akt but not mTOR pathway induces apoptosis in MCL. (A) To assess whether the PI3-K/Akt and/or mTOR signalings regulate MCL cell survival, we investigated the effects of wortmannin, SH-5, and rapamycin in Granta 519 and SP-53 cells. Cells were treated with 0.1 μM wortmannin (Wort), 20 μM SH-5, or 0.1 μM rapamycin (R) for 24 hours, and apoptosis was quantified by TUNEL assay. Percentages of apoptotic cells are indicated. Comparable results were obtained in SP-53 and Jeko-1 cells, and a representative experiment with SP-53 is shown. (B) Effects of LY294002 and rapamycin on cell survival of 5 patients with primary MCL. B cell–enriched cultures were treated with LY294002 (10 μM) or rapamycin (0.1 μM) for 72 hours, and apoptosis was quantified by CFDA-SE/PI labeling. Effects of LY294002 and rapamycin were also investigated in the same primary MCL cultures stimulated by rhsCD40-L alone or in combination with IL-4. (C,D) Analysis of caspase activation by immunoblotting in SP-53 cells. LY294002-, wortmannin-, and SH-5–induced apoptosis in SP-53 cells were associated with activation of caspase-9, -8, and -10 and cleavage of caspase-3 and PARP. Tubulin shows equal loading of protein for each lane.
Inhibition of the PI3-K/Akt but not mTOR pathway induces apoptosis in MCL. (A) To assess whether the PI3-K/Akt and/or mTOR signalings regulate MCL cell survival, we investigated the effects of wortmannin, SH-5, and rapamycin in Granta 519 and SP-53 cells. Cells were treated with 0.1 μM wortmannin (Wort), 20 μM SH-5, or 0.1 μM rapamycin (R) for 24 hours, and apoptosis was quantified by TUNEL assay. Percentages of apoptotic cells are indicated. Comparable results were obtained in SP-53 and Jeko-1 cells, and a representative experiment with SP-53 is shown. (B) Effects of LY294002 and rapamycin on cell survival of 5 patients with primary MCL. B cell–enriched cultures were treated with LY294002 (10 μM) or rapamycin (0.1 μM) for 72 hours, and apoptosis was quantified by CFDA-SE/PI labeling. Effects of LY294002 and rapamycin were also investigated in the same primary MCL cultures stimulated by rhsCD40-L alone or in combination with IL-4. (C,D) Analysis of caspase activation by immunoblotting in SP-53 cells. LY294002-, wortmannin-, and SH-5–induced apoptosis in SP-53 cells were associated with activation of caspase-9, -8, and -10 and cleavage of caspase-3 and PARP. Tubulin shows equal loading of protein for each lane.
Treatment of SP-53 cells with LY294002, wortmannin, or SH-5 resulted in an increased number of apoptotic cells, from 25% to 60%, as evaluated by TUNEL assay. Similar results were obtained in the Jeko-1 cell line (not shown). Conversely, rapamycin had only a moderate effect on SP-53 cell survival (less than 20% of apoptotic cells), and it failed to induce apoptosis in Jeko-1 cells, even after 72 hours of treatment (Figure 5A). Experiments carried out with annexin V/PI gave similar results (not shown). These findings suggest that the Akt kinase is probably one of the main mediators of survival signals in MCL cells. Intriguingly, PI3-K/Akt inhibitors induced only limited levels of apoptosis in Granta 519 cells (less than 15%). This finding may be due to the fact that, unlike SP-53 and Jeko-1, Granta 519 is an Epstein-Barr virus (EBV)–positive cell line, which expresses viral proteins, such as LMP-1, that are known to have antiapoptotic properties (not shown). On these grounds, limited to the responsiveness to apoptotic stimuli, Granta 519 cells cannot be fully representative of MCL cells, since these malignancies are usually EBV−.
Treatment with wortmannin or SH-5 showed the appearance of cleaved forms of PARP and caspase-3, confirming the induction of apoptosis (Figure 5C). Moreover, caspase-8, -9, and -10 were all activated in SP-53 cells treated with LY294002 or SH-5 (Figure 5D), indicating an involvement of both the intrinsic and the extrinsic apoptotic pathways. Similar results were obtained in Jeko-1 cells (not shown). To rule out the possibility that cyclin D1 down-regulation induced by PI3-K/Akt inhibition is the consequence of apoptosis, the effects of LY294002 were also analyzed in the presence of the apoptosis inhibitor Z-VAD. While Z-VAD inhibited caspase-3 and PARP activation, cyclin D1 down-regulation induced by LY294002 occurred independently of MCL cell apoptosis and was associated with GSK-3β inhibition (not shown).
PI3-K/Akt regulates telomerase activity in MCL cells
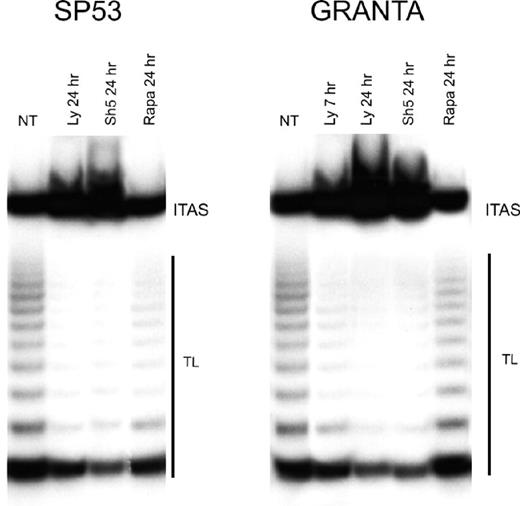
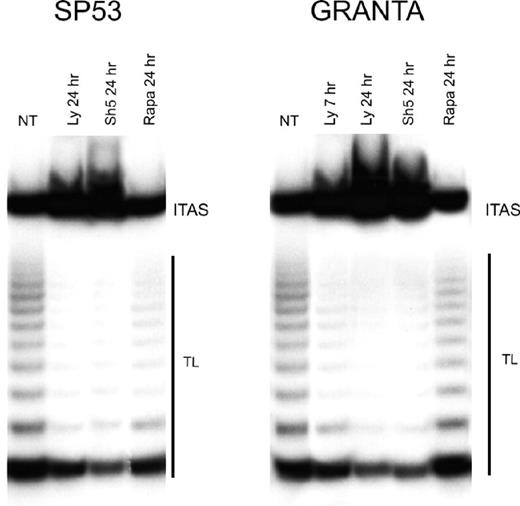
The Akt kinase enhances telomerase activity through the phosphorylation of hTERT protein,13 and inhibitors of the PI3-K/Akt cascade down-regulate telomerase activity in several tumor cell lines.32,33 As shown in Figure 6, both LY294002 and SH-5 greatly reduced telomerase activity. As soon as after 7 hours of treatment with LY294002, Granta 519 cells showed a reduction of more than 50% of telomerase activity compared with untreated cells; after 24 hours, both Granta 519 and SP-53 cells treated either with LY294002 or SH-5 showed a reduction of about 90% in telomerase activity. By contrast, rapamycin had no effect on telomerase activity on both cell lines.
Inhibition of PI3-K/Akt but not mTOR pathway decreases telomerase activity in MCL. Granta 519 and SP-53 cells were treated with the PI3-K/Akt inhibitors, 50 μM LY294002 (Ly), 20 μM SH-5, and 0.1 μM rapamycin (Rapa), for 7 and/or 24 hours and then analyzed for telomerase activity by TRAP assay. TL indicates telomerase ladder; ITAS, internal telomerase assay standard.
Inhibition of PI3-K/Akt but not mTOR pathway decreases telomerase activity in MCL. Granta 519 and SP-53 cells were treated with the PI3-K/Akt inhibitors, 50 μM LY294002 (Ly), 20 μM SH-5, and 0.1 μM rapamycin (Rapa), for 7 and/or 24 hours and then analyzed for telomerase activity by TRAP assay. TL indicates telomerase ladder; ITAS, internal telomerase assay standard.
Discussion
Improvement of our ability to control highly malignant cancers such as MCL depends not only on the identification of crucial signaling pathways inherently activated in tumor cells, but also on the definition of how the different kinases work and interact with each other to convey signals promoting cell growth and survival. In the present study, we demonstrate that PI3-K/Akt– and TORC1-dependent pathways are aberrantly activated in MCL cells, as shown by the detection of constitutive phosphorylation of different downstream substrates, including Akt, GSK-3β, p70S6K, S6rp, and 4E-BP1. Notably, activation of Akt and mTOR was also detected in short-term cultures and tumor biopsies derived from patients with MCL, demonstrating that these kinases are inherently activated also in vivo. These findings confirm and extend those recently reported by others34,35 and allow the inclusion of MCL among the lymphoid malignancies characterized by inherent PI3-K/Akt and TORC1 activation.36,37 Our finding that Akt is constitutively activated in our series that mainly includes typical MCL apparently contrasts with those of a recent study in which Akt activation was mainly detected in blastoid variants and in only one-third of patients with typical histology.34 This may be due to the different sensitivity of the method used (immunoblotting vs immunohistochemistry) and Akt probably being activated in all MCL, although blastoid variants may show more pronounced levels of activation, which is consistent with their aggressive behavior.
Unlike what observed in other systems,16 the inherent PI3-K/Akt activation characterizing MCL cells is independent on serum factors, which is probably due to transformation-related events. While mutations activating PI3KCA were not detected in MCL, loss of PTEN expression was found in fewer than 30% of patients with MCL with activated Akt, suggesting that PTEN inactivation by genetic and/or epigenetic changes may contribute to Akt activation in a fraction of patients.34 Here, we provide new evidence indicating that PTEN is constitutively phosphorylated, and therefore probably inactivated, in both MCL cell lines and in tumor biopsies. Notably, PTEN phosphorylation correlated with Akt activation, providing thus a possible additional mechanism leading to aberrant Akt activation in MCL. These findings are similar to those recently reported in Hodgkin lymphoma and acute myeloid leukemia, in which Akt activation was not associated with loss of PTEN expression, but with its inactivating phosporylation,38,39 suggesting that this mechanism likely occurs in a broad spectrum of haematologic malignancies.
Several lines of evidence indicate that microenvironmental stimuli may cooperate with inherent genetic alterations, leading to enhanced cell-cycle progression of MCL cells. In particular, CD40 activation is sufficient to promote proliferation of primary MCL, supporting the possible pathogenic role of CD40 stimulation in vivo.40 Here, we show that inhibition of PI3-K/Akt or TORC1 not only blocks basal cell proliferation, but also antagonizes the growth-promoting activity of CD40 triggering and IL-4 in primary MCL cultures. On these grounds, therapeutic approaches targeting PI3-K/Akt or mTOR may directly inhibit MCL growth and simultaneously counteract microenvironmental signals able to promote the entry of resting MCL cells into cell cycle.
Consistent with the notion that the PI3-K/Akt and TORC1 critically regulate G1/S cell-cycle progression, inhibition of these pathways resulted in G0/G1 accumulation of MCL cells together with down-regulation of D type cyclins and/or up-regulation of p27Kip1. Interestingly, cyclin D3 and p27Kip1 constitute targets shared by PI3-K/Akt and TORC1, since inhibition of both these pathways modulated expression levels of these proteins in all MCL cell lines. Cyclin D3, however, is often down-regulated in MCL in vivo,41,42 suggesting that targeting cyclin D3 probably results in limited effects in these patients. The up-regulation of p27Kip1 may be at least in part due to direct transcriptional activation of the CDKN1B gene by FoxO transcription factors that are negatively regulated by Akt.43 It should be considered, however, that we and others have shown that, in MCL cells, the amount of the p27Kip1 protein is mainly regulated at the posttranscriptional level by the ubiquitin-proteasome machinery. Akt was recently shown to directly phosphorylate p27Kip1 on Thr157, thereby favoring the mislocalization of the protein in the cytoplasm and its proteasome-mediated degradation.11,44 Indeed, p27Kip1 phosphorylated on Thr157 was recently detected in MCL cells showing constitutive Akt activation.34 Here, we demonstrate that the PI3-K/Akt and TORC1 pathways control the expression of 2 members of the SCFSkp2 E3 ligase complex, which is involved in recognizing and targeting p27Kip1 for ubiquitination.45-47 In fact, PI3-K/Akt or TORC1 inhibition results in a marked down-regulation of p45Skp2, a protein that directly binds to phosphorylated p27Kip1 and promotes its ubiquitination and degradation.45 Moreover, PI3-K/Akt and TORC1 inhibition also down-regulates Cks1, another component of the SCFSkp2 complex essential for p27Kip1 ubiquitination.46 Notably, p45Skp2 overexpression was associated with constitutive PTEN phosphorylation in acute myelogenous leukemias.48 These findings, together with those presented herein, reinforce the notion that aberrations in the PTEN/Akt/p45Skp2 pathway may have a relevant role in the pathogenesis of several hematologic malignancies, including MCL.
Intriguingly, the effects of PI3-K/Akt and TORC1 inhibition on cyclin D1 expression differed among the MCL lines analyzed. In fact, PI3-K/Akt inhibition resulted in a rapid decrease of cyclin D1 levels in all cell lines investigated. Here, we provide direct evidence indicating that in our MCL cell lines, cyclin D1 levels are mainly regulated by the proteasome, and that PI3-K/Akt inhibition down-regulates cyclin D1 by promoting its proteasome-dependent degradation. This effect is mediated by reactivation of the GSK-3β kinase following PI3-K/Akt inhibition. By contrast, rapamycin down-regulated cyclin D1 in SP-53 cells but not in Granta 519 and Jeko-1 cell lines, despite the induction of similar antiproliferative effects. These results indicate that cyclin D1 down-regulation is not the only contributor to the induction of cell-cycle arrest by TORC inhibitors, a phenomenon that is likely mediated also by cyclin D3 and p27Kip1 deregulation. Available data on the effects of rapamycin on cyclin D1 expression in MCL are conflicting.19,35 Here, we demonstrate that TORC1 inhibition results in cyclin D1 down-regulation only in MCL cells in which the GSK-3β kinase is under the direct control of mTOR, such as in SP-53 cells. Conversely, MCL cell lines resistant to rapamycin-induced inhibition of GSK-3β phosphorylation do not down-regulate cyclin D1 following TORC1 inhibition. This is consistent with the effects of rapamycin in breast cancer cell lines, which showed an increase in the GSK-3β kinase activity and, consequently, enhanced cyclin D1 proteolysis.31 These findings, taken together, further support the functional relevance of the GSK-3β kinase in regulating cyclin D1 expression and could help reconcile the discrepant results obtained so far with rapamycin in different MCL cell lines.19,35
In keeping with the notion that the PI3-K/Akt pathway critically regulates cell survival, we found that inhibition of this cascade may induce MCL cell apoptosis. This response involved both the intrinsic and the extrinsic apoptotic pathways, as shown by the concomitant activation of caspase-8, -9, and -10. Apoptosis induced by PI3-K or Akt inhibition was so far only demonstrated in established MCL cell lines,34,35 useful in vitro models which however cannot be fully representative of the properties of MCL cells in vivo. Indeed, our analysis of primary MCL cultures disclosed markedly heterogeneous apoptotic responses following PI3-K inhibition. Notably, this treatment induced comparable levels of growth inhibition in the same MCL cultures, indicating a dissociation between growth-inhibitory and proapoptotic effects. It is worth mentioning, however, that in responsive MCL cultures, PI3-K inhibition also induced significant levels of apoptosis in the presence of costimulatory signals, such as CD40 activation and IL-4, further strengthening the potential therapeutic effectiveness of targeting this pathway. On the other hand, while TORC1 inhibition resulted in MCL cell accumulation in G0/G1, rapamycin induced no or only limited apoptotic responses in either established cell lines or primary MCL cultures.
Enhanced telomerase activity characterizes aggressive lymphomas, including MCL, suggesting that telomerase could constitute a potentially relevant therapeutic target for these malignancies. Telomerase activity can be regulated by transcriptional modulation of the hTERT gene and by phosphorylation of the hTERT protein operated by different kinases, including Akt.49 Notably, rapamycin was recently shown to suppress hTERT mRNA expression in gynecologic malignancies independent of cell-cycle arrest.50 Our findings are consistent with the possibility that the sustained telomerase activity detected in MCL is at least in part due to the underlying constitutive activation of Akt, whereas TORC1 is probably not involved.
In conclusion, the results of the present study add further support to the potential therapeutic relevance of targeting PI3-K/Akt and TORC1 in MCL. Nevertheless, our findings suggest that the Akt kinase may constitute a more effective target in MCL compared with TORC1, since Akt inhibition induces significant apoptotic responses coupled with down-regulation of telomerase activity. On the other hand, the variable extent of apoptosis showed by primary MCL cultures after PI3-K/Akt inhibition confirms the clinical observation that MCL is a heterogeneous entity, an issue that deserves further investigation to achieve a better control of this aggressive lymphoma.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Italian Association for Cancer Research (AIRC) to R.D. and A.D.R. J.D.C. is a fellowship recipient of AIRC.
Authorship
Contribution: J.D.C. and P.Z. performed experiments, interpreted data, and drafted the manuscript; L.T., M.G., A.P., S.B., and S.R. performed research and analyzed data; M.S. and U.T. performed patient provision; M.P. and C.D. performed immunohistochemistry and interpreted data; A.D.R. interpreted data and contributed to the drafting of the manuscript; and R.D. designed research, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Riccardo Dolcetti, Cancer Bio-Immunotherapy Unit, Department of Medical Oncology, CRO–National Cancer Institute, Via F. Gallini 2, 33081 Aviano (PN), Italy; e-mail: rdolcetti@cro.it.