Abstract
Graft-versus-host disease (GVHD) remains a major cause of morbidity and mortality in allogeneic stem cell transplantation. Effector memory T cells (TEM) do not cause GVHD but engraft and mount immune responses, including graft-versus-tumor effects. One potential explanation for the inability of TEM to cause GVHD is that TEM lack CD62L and CCR7, which are instrumental in directing naive T cells (TN) to lymph nodes (LN) and Peyer patches (PP), putative sites of GVHD initiation. Thus TEM should be relatively excluded from LN and PP, possibly explaining their inability to cause GVHD. We tested this hypothesis using T cells deficient in CD62L or CCR7, transplant recipients lacking PNAd ligands for CD62L, and recipients without LN and PP or LN, PP, and spleen. Surprisingly, CD62L and CCR7 were not required for TN-mediated GVHD. Moreover, in multiple strain pairings, GVHD developed in recipients that lacked LN and PP. Mild GVHD could even be induced in mice lacking all major secondary lymphoid tissues (SLT). Conversely, enforced constitutive expression of CD62L on TEM did not endow them with the ability to cause GVHD. Taken together, these data argue against the hypothesis that TEM fail to induce GVHD because of inefficient trafficking to LN and PP.
Introduction
The application of allogeneic stem cell transplantation is limited by graft-versus-host disease (GVHD). GVHD can be prevented by purging T cells from the graft; however, donor T cells are important for facilitating stem cell engraftment, providing immediate posttransplantation immunity, and eliminating residual leukemic cells.1 A key challenge is to balance the positive and negative effects of donor T cells to optimize outcome.
We showed that purified CD4+ TN (CD62L+CD44−CD25−) initiated severe GVHD, whereas effector memory T cells (TEM) (CD62L−CD44+CD25−) did not but mounted a strong recall responses to a vaccination antigen.2 Chen and colleagues3 demonstrated that total TEM (CD4 and CD8 cells) did not induce GVHD but if primed in vivo against leukemia, these cells induced graft-versus-leukemia responses. These studies and others2-5 suggested that transplanting memory T cells may provide some of the benefits of donor T cells without the risk of GVHD.
However, the reason for reduced GVHD induction by TEM is unclear. One fundamental difference between TN and TEM is their trafficking patterns. Naive T cells (TN) primarily circulate through the blood, spleen, and lymph nodes (LN) and are excluded from nonlymphoid tissues. In contrast, TEM generally bypass LN but can enter parenchymal tissues as well as spleen.6 These circulation patterns are largely determined by the expression of CD62L, CCR7, and CD44. TN express CD62L and CCR7, whereas TEM express neither but up-regulate CD44, which promotes entry to inflamed nonlymphoid tissues.7,8 CD62L binds to peripheral lymph node addressin (PNAd) ligands on high endothelial venules, is essential for T cell entry into peripheral lymph nodes (pLN), and promotes but is not required for T cell migration to mesenteric LN (mLN) and Peyer patches (PP).9,10 CCR7 is essential for optimal homing of TN to all LN and to a lesser extent governs entry to PP.11,12
In GVHD, donor TN and TEM encounter host antigens regardless of where they traffic. Secondary lymphoid organs, including LN and PP, contain high concentrations of “professional” antigen-presenting cells that display host antigens and are required to activate donor T cells.13-15 Therefore, it is reasonable to hypothesize that donor T cells will be effectively primed to recipient antigens in LN and PP. Thus, the inability of TEM to induce GVHD could be because they lack CD62L and CCR7 and, relative to TN, they are restricted from LN and PP. A corollary of this idea is that TN must be primed in LN and PP to induce GVHD.
In support of this hypothesis, in GVHD models, donor T cells homed to secondary lymphoid organs within 24 hours after infusion but did not infiltrate target organs until days later.16,17 Moreover, when TN and TEM were transplanted separately, TN accumulated in LN and PP, whereas TEM did not.16 Also consistent with an important role for LN priming is that interrupting the engagement of LN homing receptors CD62L and α4β7 on donor T cells can ameliorate GVHD. Incubating donor T cells with antibodies against CD62L and CD49d (α4 integrin) before their infusion reduced homing of T cells to recipient LN by half and significantly delayed or prevented GVHD18,19 and GVHD mediated by CD62L−/−β7−/− donor T cells was significantly delayed.20 However, in the latter studies, GVHD was not completely prevented, suggesting that LN priming of donor T cells may not be essential for GVHD. Thus, data conflict regarding the hypothesis that TEM do not cause GVHD because they do not traffic to LN.
Here we use several approaches to test the hypothesis that TEM do not induce GVHD because they are restricted from LN and PP and the related hypothesis that TN must access these SLT to cause GVHD. Contrary to our expectations, blocking homing of TN to LN—thereby rendering them more like TEM—had little impact on GVHD, whereas TEM genetically modified to improve access to LN still failed to induce GVHD. Overall, these results suggest that differential ability to traffic to LN/PP plays a limited role in explaining why TN cause GVHD and TEM do not.
Methods
Mice
BALB/c, C57BL/6 (B6), 129/Sv, and CD45.1 (B6) mice were from the National Cancer Institute (Frederick, MD). B6.C mice (B6-H2d congenics) were from The Jackson Laboratory (Bar Harbor, ME). B6bm12 and IAb−/− (B6 major histocompatibility complex [MHC] II-deficient) were from Taconic Farms (Hudson, NY). BALB.B mice were from Harlan (Indianapolis, IN). CD62L−/− (B6)9 and GST2/3−/− (B6)21 mice were gifts from Tom Tedder (Duke University Medical Center, Durham, NC) and Steve Rosen (University of California-San Francisco), respectively. CCR7−/− (BALB/c)11 were a gift from Martin Lipp and Uta Hopken (Max-Delbrück-Center for Molecular Medicine, Berlin, Germany). Alymphoplasia (B6) (aly/aly)22 and heterozygous littermates (aly/+) were obtained from Fadi Lakkis (University of Pittsburgh School of Medicine, PA). Mice transgenic for CD62L with a mutated protease site (LΔP) and control transgenic mice with a normal protease site were described.23,24 All mice were bred at Yale University or at the University of Minnesota under specific pathogen-free conditions. Recipients were 8 to 12 weeks at the time of initial transplantation.
Splenectomy
Mice were anesthetized with ketamine (100 mg/kg)/xylazine (10 mg/kg) solution via intraperitoneal injection and received 0.3 mg/kg meloxicam intraperitoneally before surgery for pain relief. Splenectomies were performed as described previously.25 Postoperative treatment included ibuprofen in the drinking water (30 mg/kg/day) for 3 days after surgery and antibiotic (trimethoprim/sulfamethoxazole)-supplemented water for 7 days after surgery. Mice were allowed to recover for 3 to 5 weeks before undergoing bone marrow transplantation (BMT).
Bone marrow transplantation
Transplantation was performed according to protocols approved the by Yale University or University of Minnesota IACUC. Recipients received total body irradiation as indicated from a 137Cs source (Yale) or x-irradiator (Minnesota). Three to 5 hours later, all recipients received T cell–depleted bone marrow (BM) with or without donor T cells via tail vein injection. Unfractionated spleen cells and spleen cells enriched for CD4+ or CD4+CD25− cells were transplanted to induce GVHD depending on the GVHD model. CD25+ cells were depleted in some experiments to reduce the GVHD-modulating effects of regulatory T cells.26-28 Transplanted T cell type and doses are indicated in the figure legends. For studies performed at Yale University, animals were fed moistened chow and water supplemented with trimethoprim/sulfamethoxazole for 2 weeks after BMT. For wild-type (WT) or MHC II−/−→aly/aly Spl− chimeras, splenectomized recipients received 2 doses of 500 cGy separated by 3 hours. Two hours later, mice received 8 × 106 CD45.1 (B6) or 10.5 × 106 MHC II−/−CD45.1 (B6) non-T cell–depleted BM cells. Mice were allowed to recover for 7 weeks, and then 3 mice of each group were killed and analyzed for full donor chimerism and antigen-presenting cell turnover by flow cytometry cells isolated from BM, liver, and intestine and tissue staining of skin and intestine. For BALB/c→C57BL/6 aly/+ or aly/aly transplants at the University of Minnesota, mice were given 800 cGy of total body irradiation on day −1 followed by pan-T cell–depleted BM (day 0) with or without supplemental BALB/c splenocytes, as described previously.28
Cell separations
BM cells were isolated and prepared as described previously.2 Remaining Thy1.2-positive cells were routinely less than 0.5% of BM cells as determined by flow cytometry.
CD4+CD25− cells were enriched from RBC-depleted splenocytes by negative selection using streptavidin-coated magnetic activated cell sorting (MACS) beads and an AutoMACS (Miltenyi Biotec, Auburn, CA) after incubating with blocking antibody (anti-FcR, clone 2.4G2), biotinylated anti-CD8 (TIB105) and biotinylated anti-CD25 (PC61). Resulting cells had less than 1% CD4+CD25+ or CD8+ cells as determined by flow cytometry.
CD4+ cells were positively selected using streptavidin-coated MACS beads and biotinylated anti-CD4 (GK1.5) on an AutoMACS. Resulting cells were more than 95% CD4+ as determined by flow cytometry. The type of CD4 purification used and whether CD25+ cells were depleted is stated in the text legend of each figure; for simplicity groups receiving any type of CD4+ cell are designated “CD4” on the graphs themselves.
TN and TEM CD4+CD25− subsets were isolated by staining positively selected CD4+ cells with streptavidin-Alexa 488 (Invitrogen, Carlsbad, CA), anti-CD45RB-phycoerythrin (PE) (C363.16A; BD Biosciences, San Jose, CA), CD25-PE/Cy7 (PC61; BD Biosciences) and CD44-APC (IM7; BD Biosciences). Cells were sorted into TN (CD45RBhighCD44−) and TEM (CD45RBlowCD44+) subsets using a FACSAria (BD Biosciences). We validated this approach by analysis of B6 splenocytes stained with anti-CD4, anti-CD25, anti-CD45RB, anti-CD44, and anti-CD62L. Back-gating showed the CD4+CD25−CD45RBhighCD44− TN population to be approximately 80% CD62L+ and the CD45RB−CD44+ TEM population to be approximately 90% CD62L−.
Antibodies and fusion proteins
Mel-14 protein was purified from serum-free tissue culture medium as described previously.29 LTβRIg and TNFRIg fusion proteins were kind gifts of Drs Jeff Browning and Evangelia Notidis (Biogen Idec, Cambridge, MA) and were used exactly as described previously.30 Rat IgG control protein was obtained from Sigma-Aldrich (St Louis, MO).
GVHD analysis
Recipients were weighed on day +1 or day −1 after BMT and every 3 to 4 days thereafter. Beginning on day +16, mice were monitored for clinical signs of GVHD: diarrhea, skin disease, and hunched posture. Mice that died of radiation toxicity early after transplantation (days 0-10) before the onset of GVHD were censored with the exception of experiments shown in Figure 3. Mice with evidence of severe GVHD (very hunched and/or lethargic) or having lost more than 20% of their original weight (after recovery from irradiation) or considered clinically moribund (University of Minnesota) were killed and scored as dead. The last weight recorded for killed animals remained in the dataset. Throughout this work, unless otherwise indicated, BM-only control mice among various types of recipients in the same experiment were indistinguishable and were thus pooled for the purposes of graphing and statistical analysis.
Pathologic analysis
Tissues were fixed in 10% phosphate-buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Slides were read by pathologists who were expert in liver (A.D.), gastrointestinal (A.D, A.P.-M., and D.J.), and skin disease (J.M.) without knowledge of experimental groups. Liver scoring criteria: portal inflammation (0-3), bile duct injury (0-3), central perivenulitis (0-3), and lobular necroinflammatory activity (0-4). Colon slides in Figure 3B were scored by D.J. Scoring criteria: inflammation (0-3), apoptosis (0-3), and crypt loss (0-3). Colon slides in Figure 3D were scored by A.P.M. using published criteria.31 Colon slides in Figures 4B and 5D were scored by A.D. Scoring criteria: inflammation (0-3), apoptosis (0-4), neutrophilic abscesses (0-3), crypt loss (0-3), and ulceration (0-3).2
Statistical methods
Significance for differences in weight loss was calculated by an unpaired t test. P values for survival curves were calculated by log rank test. P values for histology comparisons were calculated by Mann-Whitney or Fisher exact test (Figure 3F, BM controls vs CD4→aly/aly Spl− only; and Figure 3G, BM vs CD4→[MHC II−/−→aly/aly Spl−] and BM vs CD4→[WT→aly/aly Spl−] only).
Results
Functional CD62L on donor T cells was not required for GVHD
To test whether CD62L-mediated homing to recipient peripheral LN was required for GVHD, we transplanted into BALB/c recipient mice CD4+CD25− T cells from B6 wild-type (WT) or CD62L−/− donors9 (Figure 1A). Recipients of WT T cells were also treated on days −1, +2, and +5 with control rat IgG or an effective dose32 of blocking antibody to CD62L (Mel-1433 ), which markedly reduces T-cell migration to peripheral LNs in vivo.33 Mel-14 treatment resulted in a significant but slight delay in the kinetics of GVHD death compared with control treatment, although the final mortalities were identical (Figure 1A).
Impairing CD62L on donor T cells has minimal impact on GVHD. Survival curves are shown. (A) On day 0, BALB/c recipients received 800 cGy and 107 BM cells from WT B6 donors with or without 3 × 105 CD4+CD25− cells from WT B6 or CD62L−/− (B6) donors. Mice that received WT CD4+CD25− cells were injected on days −1 (intravenous), +2 (intraperitoneal) and +5 (intraperitoneal) with 250 μg of Mel-14 or control Rat IgG. *P < .05 for CD4 + Mel-14 versus CD4 + Rat IgG. P = .3334 for WT CD4 + Rat IgG versus CD62L−/− CD4. (B) BALB/c recipients were splenectomized (Spl−) or left intact (Spl intact) 3 weeks before BMT. On day 0, recipients received 800 cGy and 107 BM cells from WT B6 donors with or without 3 × 105 CD4+ cells from WT B6 or CD62L−/− (B6) donors. *P < .05 for Spl intact versus Spl− recipients of CD4 T cells. (C) On day 0, BALB.B recipients received 850 cGy and 107 BM cells from WT B6 donors with or without 3 × 106 total T cells from WT B6 or CD62L−/− (B6) donors. NS indicates not significant for recipients of WT versus CD62L−/− T cells.
Impairing CD62L on donor T cells has minimal impact on GVHD. Survival curves are shown. (A) On day 0, BALB/c recipients received 800 cGy and 107 BM cells from WT B6 donors with or without 3 × 105 CD4+CD25− cells from WT B6 or CD62L−/− (B6) donors. Mice that received WT CD4+CD25− cells were injected on days −1 (intravenous), +2 (intraperitoneal) and +5 (intraperitoneal) with 250 μg of Mel-14 or control Rat IgG. *P < .05 for CD4 + Mel-14 versus CD4 + Rat IgG. P = .3334 for WT CD4 + Rat IgG versus CD62L−/− CD4. (B) BALB/c recipients were splenectomized (Spl−) or left intact (Spl intact) 3 weeks before BMT. On day 0, recipients received 800 cGy and 107 BM cells from WT B6 donors with or without 3 × 105 CD4+ cells from WT B6 or CD62L−/− (B6) donors. *P < .05 for Spl intact versus Spl− recipients of CD4 T cells. (C) On day 0, BALB.B recipients received 850 cGy and 107 BM cells from WT B6 donors with or without 3 × 106 total T cells from WT B6 or CD62L−/− (B6) donors. NS indicates not significant for recipients of WT versus CD62L−/− T cells.
CD62L−/− CD4+CD25− (Figure 1A) or CD62L−/−CD4+ T cells (Figure 1B) caused GVHD-mediated mortality similar to that induced by WT T cells (Figure 1A,B, P > .3), confirming recently published results.20 GVHD is maximal with both CD4 and CD8 cells in the MHC-matched B6→BALB.B model,34 and total CD62L−/− T cells caused GVHD-related mortality indistinguishable from that of WT T cells (Figure 1C). Taken together, these experiments indicate that TN need not express CD62L to cause GVHD. Although Mel-14 treatment slightly reduced GVHD, given the results with CD62L-deficient T cells, this may be attributable to depletion of antibody-coated T cells and/or a partial blockade of neutrophil entry to inflamed sites.35,36
To test whether CD62L−/− T cells were primed in the spleen, we transferred B6 CD62L−/− CD4+ T cells into splenectomized BALB/c recipients (Figure 1B). Unexpectedly, GVHD mortality was paradoxically accelerated in splenectomized recipients of CD62L−/− CD4+ T cells compared with spleen-intact recipients (Figure 1B). Thus, splenic priming was not required, and presumably donor T cells can be primed in other locations less dependent on CD62L for T-cell entry, such as mLN and PP.
Impairing CD62L ligands on recipient LN did not ameliorate GVHD
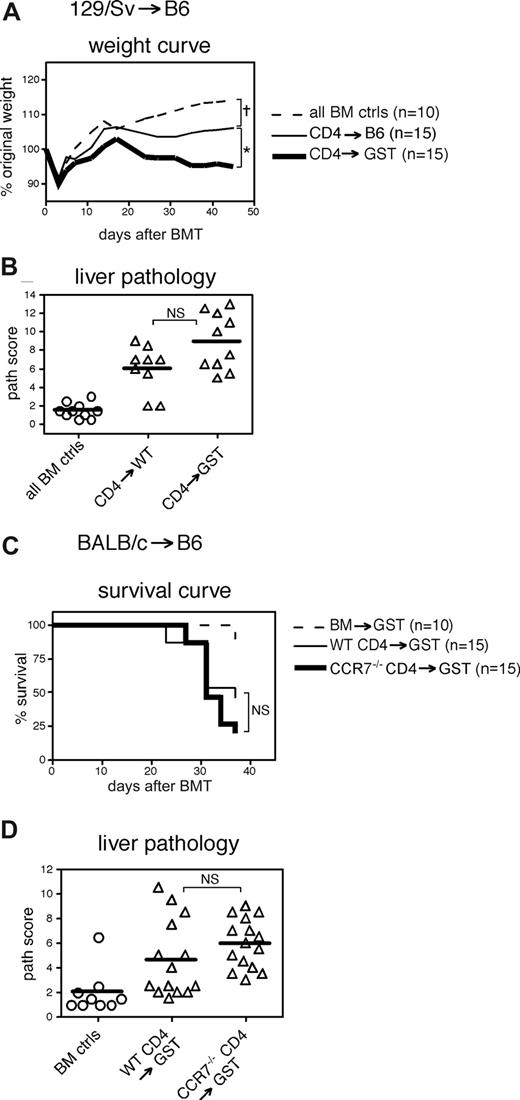
We also tested GVHD induction by WT T cells in recipients that lack glucosyl transferases 2 and 3 (GST2/3−/−), which are necessary to generate ligands for CD62L on HEV.21 There is greatly reduced homing of T cells to pLN and a substantial reduction in homing to mLN in these mice.21 We used GST2/3−/− mice as recipients in the MHC-matched 129/Sv→B6 nonlethal GVHD model, which typically demonstrates moderate weight loss and significant histopathology in the liver. Similar to the results with CD62L−/− T cells, GST2/3−/− recipients of WT CD4+CD25− T cells developed GVHD as severe, if not more so, as that in WT recipients (Figure 2A,B).
Impairing CD62L ligands on recipient LN has minimal impact on GVHD. (A,B) On day 0, WT B6 or GST2/GST3−/− (B6) (GST) recipients received 1000 cGy and 7 × 106 BM cells with or without 4 × 106 CD4+CD25− cells from 129/Sv donors. Mice were killed on days 38 and 41 to collect tissues for histopathologic analyses. (A) Weight curve. †P < .05 for BM controls versus CD4→WT or CD4→GST for all days 24 and later; *P < .05 for CD4→WT versus CD4→GST for all days 14 and later. (B) Liver histopathology. NS indicates not significant for CD4→WT versus CD4→GST; P < .001 for BM controls versus CD4→WT or CD4→GST. (C,D) On day 0, GST recipients received 1000 cGy and 1.3 × 107 BM cells from BALB/c donors with or without 3 × 105 CD4+ cells from BALB/c or CCR7−/− (BALB/c) donors. (C) Survival curve. NS: not significant for WT CD4→GST versus CCR7−/− CD4→GST; P < .05 for BM controls versus WT CD4→GST or CCR7−/− CD4→GST. (D) Liver histopathology. NS, not significant for WT CD4→GST versus CCR7−/− CD4→GST; P < .01 for BM controls versus CD4→WT or CD4→GST.
Impairing CD62L ligands on recipient LN has minimal impact on GVHD. (A,B) On day 0, WT B6 or GST2/GST3−/− (B6) (GST) recipients received 1000 cGy and 7 × 106 BM cells with or without 4 × 106 CD4+CD25− cells from 129/Sv donors. Mice were killed on days 38 and 41 to collect tissues for histopathologic analyses. (A) Weight curve. †P < .05 for BM controls versus CD4→WT or CD4→GST for all days 24 and later; *P < .05 for CD4→WT versus CD4→GST for all days 14 and later. (B) Liver histopathology. NS indicates not significant for CD4→WT versus CD4→GST; P < .001 for BM controls versus CD4→WT or CD4→GST. (C,D) On day 0, GST recipients received 1000 cGy and 1.3 × 107 BM cells from BALB/c donors with or without 3 × 105 CD4+ cells from BALB/c or CCR7−/− (BALB/c) donors. (C) Survival curve. NS: not significant for WT CD4→GST versus CCR7−/− CD4→GST; P < .05 for BM controls versus WT CD4→GST or CCR7−/− CD4→GST. (D) Liver histopathology. NS, not significant for WT CD4→GST versus CCR7−/− CD4→GST; P < .01 for BM controls versus CD4→WT or CD4→GST.
Although the prior experiments with CD62L−/− T cells and GST2/3−/− recipients excluded an essential role for the engagement of CD62L on donor T cells with PNAd ligands in the host, TN also use CCR7 to enter LN and PP,11,12 and residual homing to LN in GST2/3−/− mice is probably mediated by CCR7.21 To impair both CD62L- and CCR7-mediated T-cell homing, we transplanted into GST2/3−/− recipients CCR7−/− donor CD4 T cells in the BALB/c→B6 strain pairing. At day 37 after transplantation, there was equivalent death and liver pathology in GST2/3−/− recipients of either T-cell type (Figure 2C,D). Therefore reducing LN homing mediated by both CD62L and CCR7 did not significantly ameliorate GVHD.
LN-, PP-, and/or spleen-deficient recipients developed GVHD
That CD62L−/− T cells induced GVHD in splenectomized recipients raised the possibility that even without CD62L or CCR7, donor T cells could gain sufficient access to LN and PP to cause GVHD. Although trafficking studies that used large numbers of transferred cells have clearly shown important roles for these molecules in LN entry one or a few hours after transfer, this has been less well studied in the context of GVHD; studies have visualized T cells only from day 2 and beyond.16,17,20 In our experience, it was technically difficult to track the smaller numbers of cells typically used in a GVHD experiment at very early time points (day 1 and earlier) that reflect direct homing potential. It was not possible to sort enough TEM cells to clearly visualize them after transplantation (B.E.A., unpublished observations, January 2007).
We therefore took a more definitive approach by using recipient mice that lack LN and PP altogether. aly/aly mice are devoid of most lymphoid tissue,22 although they retain nasal-associated lymphoid tissue (NALT) and cryptopatches.22,37-39 aly/+ (with WT phenotype) or aly/aly mice were splenectomized or left intact to create the following types of recipients: WT (aly/+); LN/PP present, no spleen (aly/+Spl−); no LN/PP, spleen present (aly/aly); and no LN/PP or spleen (aly/aly Spl−). We initially used these recipients in the B6bm12→B6 GVHD model along with minimally lethal transplantation conditions so that histologic GVHD could be more readily and comparably assessed. As expected, aly/+ recipients of CD4+CD25− cells had significant weight loss compared with BM controls. Most importantly, GVHD occurred in the absence of LN/PP or LN/PP and spleen, because aly/aly and aly/aly Spl− recipients of CD4+CD25− cells lost weight, albeit significantly less than aly/+ recipients. aly/aly Spl− recipients had even less weight loss compared with aly/aly recipients, suggesting in this context that lack of spleen led to amelioration of disease. Unexpectedly, aly/+ Spl− T-cell recipients developed accelerated and severe GVHD (Figure 3A). Thus, in this model, spleen or LN/PP alone are sufficient for moderate to severe clinical GVHD to develop. Conversely, despite the absence of nearly all organized SLT, even aly/aly Spl− recipients developed significant weight loss compared with BM controls, although weight loss was reduced compared with the other T-cell recipient groups.
LN, PP (aly/aly) and/or spleen-deficient mice develop GVHD. (A,B) aly/+ or aly/aly recipients were splenectomized (Spl−) or left intact 3 to 5 weeks before BMT. On day 0, recipients received 1000 cGy and 8-10 × 106 BM cells with or without 106 CD4+CD25− cells from B6bm12 donors. All CD4→aly/+ Spl− animals died by day 10 or were killed in a premorbid state for pathologic examination. Shown are combined data from 2 experiments. (A) Weight curve. †P < .01 for BM controls versus all CD4 groups for all days 6 and later; *P < .01 for CD4→aly/+ versus CD4→aly/aly or CD4→aly/aly Spl− for all days 10 and later, @P < .05 for CD4→aly/aly versus CD4→aly/aly Spl− for all days 17 and later. (B) Skin and colon histopathology. †P < .05 for BM controls versus all CD4 groups; other P values are indicated on the graphs. (C,D) On day 0, aly/+ or aly/aly recipients received 800 cGy and 107 T cell–depleted BM cells with or without 5 × 105 spleen cells from BALB/c donors. (C) Survival curve. (D) Colon histopathology at day 9 after BMT. †P < .05 for BM controls versus Spl→aly/+ or versus Spl→aly/aly. (E,F) aly/+ or aly/aly recipients were splenectomized (Spl−) or left intact 3-5 weeks before BMT. On day 0, recipients received 1000 cGy and 107 BM cells with or without 5 × 106 CD4+CD25− cells from 129/Sv donors. Shown are combined data from 2 experiments. (E) Incidence of clinical skin disease. †P < .05 for BM controls versus CD4→aly/aly or CD4→aly/aly Spl−. (F) Skin histopathology. †P < .0001 for BM controls versus CD4→aly/aly. (G) aly/aly recipients were splenectomized, allowed to rest for 3 to 5 weeks, and then transplanted with WT B6 BM (WT→aly/aly Spl−) or MHC II−/− (B6) BM (MHC II−/−→aly/aly Spl−). Eight weeks later, these mice received 2 doses of 450 cGy (separated by 3 hours) and 107 BM cells with or without 106 CD4+CD25− cells from B6bm12 donors. On day 27 after transplantation, mice were killed, and colon tissue was collected for histologic analysis. Colon histopathology; note that BM control groups are shown separately. NS indicates not significant for BM versus CD4→(MHC II−/−→aly/aly Spl−); P = .0005 for CD4→(WT→aly/aly Spl−) versus CD4→(MHC II−/−→aly/aly Spl−); P<.001 for BM versus CD4→(WT→aly/aly Spl−).
LN, PP (aly/aly) and/or spleen-deficient mice develop GVHD. (A,B) aly/+ or aly/aly recipients were splenectomized (Spl−) or left intact 3 to 5 weeks before BMT. On day 0, recipients received 1000 cGy and 8-10 × 106 BM cells with or without 106 CD4+CD25− cells from B6bm12 donors. All CD4→aly/+ Spl− animals died by day 10 or were killed in a premorbid state for pathologic examination. Shown are combined data from 2 experiments. (A) Weight curve. †P < .01 for BM controls versus all CD4 groups for all days 6 and later; *P < .01 for CD4→aly/+ versus CD4→aly/aly or CD4→aly/aly Spl− for all days 10 and later, @P < .05 for CD4→aly/aly versus CD4→aly/aly Spl− for all days 17 and later. (B) Skin and colon histopathology. †P < .05 for BM controls versus all CD4 groups; other P values are indicated on the graphs. (C,D) On day 0, aly/+ or aly/aly recipients received 800 cGy and 107 T cell–depleted BM cells with or without 5 × 105 spleen cells from BALB/c donors. (C) Survival curve. (D) Colon histopathology at day 9 after BMT. †P < .05 for BM controls versus Spl→aly/+ or versus Spl→aly/aly. (E,F) aly/+ or aly/aly recipients were splenectomized (Spl−) or left intact 3-5 weeks before BMT. On day 0, recipients received 1000 cGy and 107 BM cells with or without 5 × 106 CD4+CD25− cells from 129/Sv donors. Shown are combined data from 2 experiments. (E) Incidence of clinical skin disease. †P < .05 for BM controls versus CD4→aly/aly or CD4→aly/aly Spl−. (F) Skin histopathology. †P < .0001 for BM controls versus CD4→aly/aly. (G) aly/aly recipients were splenectomized, allowed to rest for 3 to 5 weeks, and then transplanted with WT B6 BM (WT→aly/aly Spl−) or MHC II−/− (B6) BM (MHC II−/−→aly/aly Spl−). Eight weeks later, these mice received 2 doses of 450 cGy (separated by 3 hours) and 107 BM cells with or without 106 CD4+CD25− cells from B6bm12 donors. On day 27 after transplantation, mice were killed, and colon tissue was collected for histologic analysis. Colon histopathology; note that BM control groups are shown separately. NS indicates not significant for BM versus CD4→(MHC II−/−→aly/aly Spl−); P = .0005 for CD4→(WT→aly/aly Spl−) versus CD4→(MHC II−/−→aly/aly Spl−); P<.001 for BM versus CD4→(WT→aly/aly Spl−).
T-cell infiltration and target tissue damage occurred in the majority of T-cell recipients compared with BM controls, regardless of aly genotype and spleen presence (Figure 3B). The severity of histologic GVHD paralleled the weight loss data. In both the colon and skin, aly/+ mice had the highest average pathology scores, followed by aly/aly and then aly/aly Spl− recipients. It is difficult to make comparisons with aly/+ Spl− T-cell recipients because these organs were harvested between days 8 and 10 after BMT compared with day 23 for the other recipients. We could not evaluate the liver for GVHD because aly/aly mice develop spontaneous hepatic inflammation that is difficult to distinguish from GVHD.40 Taken together, these data show that GVHD can develop in the complete absence of LN and PP, as well as in recipients without LN, PP, and spleen. However, that GVHD is markedly reduced in aly/aly Spl− recipients relative to mice that have either LN, PP, or spleen indicates that these are important sites for donor T-cell priming that are redundant among themselves.
We extended the investigation of the roles of LN/PP to a second MHC-mismatched strain pairing, BALB/c→B6. B6 aly/+ recipients of BALB/c spleen cells died of rapidly lethal GVHD, whereas the majority of aly/aly recipients survived for the duration of the experiment (Figure 3C). Although in this strain pairing the absence of LN/PP was protective, aly/aly spleen cell recipients still had clear histologic evidence of colonic GVHD, even at day 9 after transplant (Figure 3D). Therefore, although the clinical disease was reduced in the absence of LN/PP, donor T cells could still be primed.
Because most human allogeneic stem cell transplants are MHC-matched, we investigated requirements for CD4 T-cell priming in the MHC-matched 129/Sv→B6 model. This is generally a nonlethal model with little clinical skin disease (B.E.A. and W.D.S., unpublished observations, March 2006). We were therefore surprised that severe clinical skin disease developed in nearly all aly/aly (19/22) and a quarter of the aly/aly Spl− (5/20) CD4 cell recipients (Figure 3E). In contrast, 0/35 BM control mice, 1/23 aly/+ CD4 cell recipients, and 1/19 aly/+ Spl− CD4 cell recipients had clinical skin disease. Pathologic analysis of skin sections confirmed that aly/aly T cell recipients had typical cutaneous GVHD (Figure 3F). Although several mice in the aly/aly Spl− cohort had very high skin pathology scores, statistical significance (by Mann-Whitney test) was not reached compared with the BM control group, most likely because of fluctuation of scores in the mildly affected range. A Fisher exact test on the number of unaffected (score < 5) and affected (score > 5) mice demonstrated a significant difference (P < .01) between the groups. Thus, CD4 cells need not be primed in LN, PP, or spleen to induce cutaneous GVHD—even in MHC-matched allogeneic BMT. Furthermore, in the absence of LN/PP, priming of TN donor T cells in the spleen and elsewhere may generate T cells that preferentially induce skin GVHD.
aly/aly mice have defects in NFκ B signaling, which could have contributed to our findings, independent of the absence of LN/PP.22 Although one would have anticipated that host APCs defective in nuclear factor-κB signaling would induce blunted T cell responses, to confirm our findings in mice rendered LN/PP-deficient as a result of the aly/aly mutation, we created LN/PP-deficient mice by treating wild-type BALB/c pregnant mothers with both lymphotoxin β receptor immunogloblulin (LTβRIg) and tumor necrosis factor receptor immunoglobulin (TNFRIg), which prevents the development of LN and PP in pups.30 The absence of LN/PP was confirmed in littermates before transplantation and at the time of sacrifice after transplantation. Both WT and LN/PP-deficient (LTβRIg/TNFRIg) BALB/c recipients of B6.C CD4+CD25− cells developed similar GVHD as revealed by survival and histologic score (Figure 4A,B).
LN and PP deficient LTβRIg/TNFRIg-treated recipients develop GVHD. LN- and PP-deficient BALB/c recipients were created by treating pregnant mothers with LTβRIg and TNFRIg. On day 0, WT or LN/PP-deficient (LTβRIg/TNFRIg) recipients received 750 cGy and 9 × 106 BM cells with or without 1.3 × 106 CD4+CD25− cells from B6.C donors. (A) Survival curve. †P < .05 for BM controls versus CD4→WT or CD4→LTBRIg/TNFRIg. Two BM control mice were killed at day 17 because of extensive weight loss (> 30% of original weight), but there was no histologic evidence of GVHD in these animals. (B) Liver, colon, and skin histopathology. P < .05 for BM controls versus CD4→WT or CD4→LTβRIg/TNFRIg for all analyses; *P < .05 for CD4→WT versus CD4→LTβRIg/TNFRIg. NS indicates not significant for CD4→WT versus CD4→LTβRIg/TNFRIg.
LN and PP deficient LTβRIg/TNFRIg-treated recipients develop GVHD. LN- and PP-deficient BALB/c recipients were created by treating pregnant mothers with LTβRIg and TNFRIg. On day 0, WT or LN/PP-deficient (LTβRIg/TNFRIg) recipients received 750 cGy and 9 × 106 BM cells with or without 1.3 × 106 CD4+CD25− cells from B6.C donors. (A) Survival curve. †P < .05 for BM controls versus CD4→WT or CD4→LTBRIg/TNFRIg. Two BM control mice were killed at day 17 because of extensive weight loss (> 30% of original weight), but there was no histologic evidence of GVHD in these animals. (B) Liver, colon, and skin histopathology. P < .05 for BM controls versus CD4→WT or CD4→LTβRIg/TNFRIg for all analyses; *P < .05 for CD4→WT versus CD4→LTβRIg/TNFRIg. NS indicates not significant for CD4→WT versus CD4→LTβRIg/TNFRIg.
Thus in 4 different strain pairings, CD4+ TN did not require priming in LN and PP to induce GVHD. Interestingly, depending on the model studied, manifestations of GVHD were ameliorated, equivalent or more severe in the absence of recipient LN/PP, suggesting that the relative importance of LN/PP to the GVHD syndrome is model-dependent.
MHCII expression on hematopoietic cells was required for T-cell priming in aly/aly Spl− recipients
Occurrence of GVHD in aly/aly Spl− recipients suggested that parenchymal tissues alone could activate TN donor T cells when priming in SLT is prevented. To address this, we created B6/MHC II−/−→B6/aly/aly Spl− and control WT→aly/aly Spl− BM chimeric recipients in which host APCs were MHC class II–deficient or intact and used these mice as recipients in a second GVHD-inducing transplantation (B6bm12→B6 model). We observed histologic GVHD in the colons of CD4→(WT→aly/aly Spl−) recipients, which paralleled the disease seen in the CD4→aly/aly Spl− (nonchimeric) recipients shown in Figure 3B. However, we observed no histologic disease in the CD4→(MHC II−/−→aly/aly Spl−) (Figure 3G and data not shown). Thus, even in the absence of LN, PP, and spleen, hematopoietically derived APCs were still required to prime donor T cells.
Memory cells constitutively expressing CD62L did not cause GVHD
We addressed the hypothesis that TEM would induce GVHD if more efficiently recruited into LN by taking advantage of transgenic mice we created in which T cells constitutively express CD62L. These transgenic mice (LΔP) express CD62L driven by the CD2 promoter and have been back-crossed to B6 CD62L−/− mice. In addition, the metalloprotease site in CD62L was mutated such that CD62L cannot be shed. A control transgenic mouse with an intact metalloproteinase site constitutively expresses CD62L that can be shed after activation. Activated LΔP T cells enter pLN more efficiently than activated WT T cells, although somewhat less efficiently than unactivated, naive WT or LΔP T cells.23
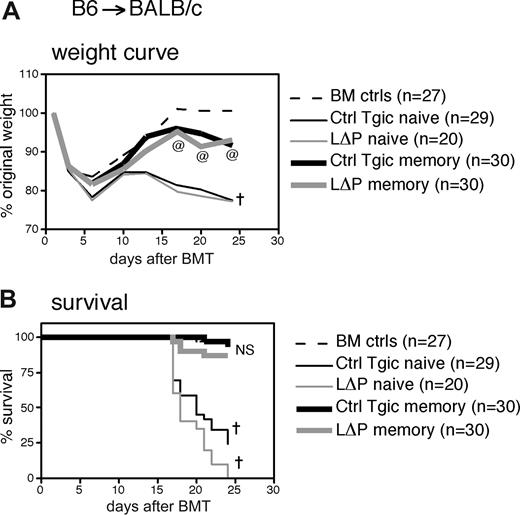
Because we could not use CD62L as a marker to sort LΔP TEM, CD4+CD25− cells from WT B6 or LΔP mice were sorted into TN and TEM subsets based on CD45RB and CD44 expression (Figure 5A and “Methods”) and transplanted separately into BALB/c recipients. Recipients of TN from either WT or LΔP donors developed severe GVHD as demonstrated by extensive weight loss, death and high histopathology scores (Figure 5B-D). Hence, CD62L down-regulation, which does not occur in LΔP mice, is not required for T cells to exit LN and infiltrate tissues during GVHD. In contrast, all recipients of TEM from either WT or LΔP donors survived and returned to approximately 100% of their original weight (Figure 5B,C). Weight gain and pathologic scores for recipients of LΔP TEM versus WT TEM were not statistically different, indicating that CD62L-expressing LΔP TEM did not have an enhanced ability to cause GVHD. Similar studies using control transgenic (instead of WT) donor T cells versus LΔP donor T cells also showed minimal GVHD caused by both transgenic TEM (Figure 6). Overall, these data demonstrate that constitutive CD62L expression on TEM does not endow them with the ability to cause severe GVHD similar to that caused by TN.
Memory cells constitutively expressing CD62L do not cause severe GVHD. (A) Sorting strategy. MACs-purified CD4+CD25− cells (left panel) were subsequently sorted into TN and TEM fractions based on CD45RB and CD44 expression according to the indicated gates; postsort analysis in rightmost panels. Sort of LΔP cells is shown; sorting parameters for WT and LΔP cells were identical. (B-D) On day 0, BALB/c recipients received 800 cGy and 107 BM cells from CD45.1 B6 donors with or without 3 × 105 naive or memory cells from WT B6 or LΔP (B6) donors. (B) Weight curve. †P < .05 for BM controls versus WT naive or LΔP naive for all days 10 and later; @P < .05 for BM controls versus LΔP memory for all days 20 and later. *P < .05 for BM controls versus WT memory at day 23 only. (C) Survival curve. †P < .01 for BM controls versus WT naive or versus LΔP naive. (D) Liver, colon, and skin histopathology. P < .05 for BM controls versus WT naive or LΔP naive for all analyses; NS indicates not significant for WT memory versus LΔP memory.
Memory cells constitutively expressing CD62L do not cause severe GVHD. (A) Sorting strategy. MACs-purified CD4+CD25− cells (left panel) were subsequently sorted into TN and TEM fractions based on CD45RB and CD44 expression according to the indicated gates; postsort analysis in rightmost panels. Sort of LΔP cells is shown; sorting parameters for WT and LΔP cells were identical. (B-D) On day 0, BALB/c recipients received 800 cGy and 107 BM cells from CD45.1 B6 donors with or without 3 × 105 naive or memory cells from WT B6 or LΔP (B6) donors. (B) Weight curve. †P < .05 for BM controls versus WT naive or LΔP naive for all days 10 and later; @P < .05 for BM controls versus LΔP memory for all days 20 and later. *P < .05 for BM controls versus WT memory at day 23 only. (C) Survival curve. †P < .01 for BM controls versus WT naive or versus LΔP naive. (D) Liver, colon, and skin histopathology. P < .05 for BM controls versus WT naive or LΔP naive for all analyses; NS indicates not significant for WT memory versus LΔP memory.
Memory cells constitutively expressing CD62L do not cause severe GVHD. On day 0, BALB/c recipients received 800 cGy and 107 BM cells from CD45.1 B6 donors with or without 3 × 105 naive or memory cells from control transgenic (metalloproteinase site intact, Ctrl Tgic) or LΔP donors. Shown are combined data from 3 experiments with sort-purified cells as described in the Figure 5 legend. (A) Weight curve. †P < .05 for BM controls versus Ctrl Tgic naive or LΔP naive for all days 6 and later; @P < .05 for BM controls versus Ctrl Tgic memory or LΔP memory for all days 17 and later. (B) Survival curve. NS indicates not significant for BM controls versus WT memory or LΔP memory and for WT memory versus LΔP memory; †P < .0001 for BM controls versus Ctrl Tgic naive or LΔP naive.
Memory cells constitutively expressing CD62L do not cause severe GVHD. On day 0, BALB/c recipients received 800 cGy and 107 BM cells from CD45.1 B6 donors with or without 3 × 105 naive or memory cells from control transgenic (metalloproteinase site intact, Ctrl Tgic) or LΔP donors. Shown are combined data from 3 experiments with sort-purified cells as described in the Figure 5 legend. (A) Weight curve. †P < .05 for BM controls versus Ctrl Tgic naive or LΔP naive for all days 6 and later; @P < .05 for BM controls versus Ctrl Tgic memory or LΔP memory for all days 17 and later. (B) Survival curve. NS indicates not significant for BM controls versus WT memory or LΔP memory and for WT memory versus LΔP memory; †P < .0001 for BM controls versus Ctrl Tgic naive or LΔP naive.
Discussion
Compared with TN, TEM are much less effective at causing GVHD.2-5 We tested 2 related hypotheses to explain why TN cause GVHD and TEM do not: (1) donor T cells need to be primed in recipient LN to cause GVHD, and (2) donor TEM cells lack the expression of LN-homing molecules, in particular CD62L and CCR7, which reduces their entry to and priming in recipient LN and dampens their ability to cause GVHD.
Our results argue against both of these hypotheses. With regard to the first, although CD62L and CCR7 are critically important for T-cell entry into LN, TN lacking CD62L or CCR7 caused severe GVHD. Furthermore, recipients with defective or completely absent LN still developed GVHD. Thus, restricting TN homing to sites accessible by TEM did not impair their ability to cause GVHD. With respect to the second hypothesis, we found that TEM with constitutive expression of CD62L still had very limited capacity to cause GVHD. These data formally exclude the hypothesis that priming in LN is required for GVHD induction and suggest that, at least as a consequence of reduced CD62L-expression, reduced LN homing by TEM does not by itself account for why TN cells cause GVHD but TEM do not.
We took multiple approaches to suppress TN cell entry into LN. CD62L-deficiency had no effect on GVHD, while inhibiting WT donor T cells with CD62L blocking antibody delayed GVHD only slightly. We further inhibited T-cell migration to LN by infusing CCR7−/− T cells into GST2/3−/− mice, resulting in simultaneous inhibition of both chemokine and CD62L-mediated T-cell entry, yet GVHD was essentially unaffected and in some cases even exacerbated (Figure 2). Given the lack of impact of these pathways on GVHD-induction by TN, we reason that the absence of CCR7 and CD62L on TEM in turn could not solely explain their inability to cause GVHD.
Dutt et al20 also found that CD62L−/− and WT T cells initiated comparable GVHD but concluded that the ability to traffic to mLN was nonetheless critical for GVHD, because recipients of CD62L−/−β7−/− T cells had improved survival and, in particular, little gut GVHD. Yet, in several of the models we tested, gut GVHD was evident in the absence of LN/PP. To reconcile these results, we first note that even in the recipients of CD62L−/−β7−/− T cells, weight loss and histologic changes were consistent with GVHD. In addition, the apparent difference in the conclusions from the 2 studies could be ascribed to the interpretation of the effect of β7 deficiency. We suggest that genetic deletion of β7 on donor T cells ameliorated GVHD by reducing the entry of activated T cells not only into mLN but also into intestinal tissue. Thus, if priming can occur alternatively in the spleen, as our data would indicate, the α4β7hi cells generated in mice lacking LN/PP could still enter target tissues, causing GVHD, as we observed, whereas β7-deficient cells would be impaired in ability to enter target tissues, thus leading to reduced GVHD. Indeed, α4β7hi but not a4β7low cells enter intestinal sites.41 This explanation is supported by the finding that, after transplantation, donor T cells did not highly up-regulate α4β7 until 5 rounds of cell division in recipient mLN,16 suggesting that high levels of this molecule were not necessary for SLT entry and priming. Consistent with this view, Petrovic et al42 found that α4β7-deficient T cells caused reduced intestinal but similar skin and thymic GVHD compared with WT cells.
Experiments exploiting genetic deficiency of CD62L, CCR7, and GST2/3 were limited by how well the particular gene deficiencies restrict T-cell trafficking as well as their potential to affect T-cell entry into target tissues. To eliminate T-cell priming in LN/PP completely, we induced GVHD in recipient animals lacking all LN/PP. In all strain pairs tested, recipients deficient in all LN/PP, but with an intact spleen, still developed GVHD. GVHD was ameliorated (but present) in 2 models, exacerbated in one model, and equivalent in a fourth. Taken together, these data suggest that priming in LN/PP is not required to induce GVHD but may be required for maximal GVHD in certain genetic mismatches or experimental conditions.
Somewhat surprisingly, we found that GVHD developed in splenectomized aly/aly recipients that lacked essentially all SLT, although the disease was reduced compared with intact controls. BM chimera experiments proved that BM-derived APCs were still required to initiate GVHD in the absence of spleen, LN, and PP. These experiments did not address where T cells were primed. Potential sites include the liver,43 bone marrow,44 NALT or cryptopatches, or other inducible gut- or lung-associated lymphoid tissue.45 Indeed, recent studies showed that CD8 responses to influenza infection do not require secondary lymphoid organs.45,46 Although GVHD initiation is not accompanied by the same infection-induced inflammatory signals, secondary lymphoid architecture may not be absolutely required when antigen is ubiquitous, as is the case with GVHD.
We unexpectedly observed that altering the trafficking and priming site of donor T cells affected the nature of GVHD. In 3 MHC-mismatched models, LN/PP-intact but splenectomized recipients developed more rapidly lethal GVHD than normal recipients. There are several nonexclusive explanations for this finding. The spleen may be a source of radiation-resistant recipient regulatory cells, which can suppress GVHD.47 If the spleen traps alloreactive cells,48,49 in its absence donor T cells may have enhanced ability to migrate to target organs and LN. Finally, T cells activated in the spleen versus LN may develop differently. For example, there may be altered cytokine skewing or expression of alternate homing receptors,6,50-52 which in some contexts could affect GVHD induction.
These results are relevant to a related article that was submitted and published while our manuscript was under review.53 In parallel with our studies, but using a single MHC-mismatched model of GVHD, Beilhack et al53 also used a variety of strategies to restrict trafficking and priming sites of GVHD-inducing lymphocytes. These researchers found that preventing priming in the LN/PP had no effect, from which they concluded that priming site did not alter GVHD. In contrast, we clearly found effects of absence of LN/PP alone on GVHD in 3 models—decreased GVHD in 2 and augmented skin GVHD in the third. Mice lacking spleen, leading therefore to priming in LN/PP and elsewhere, also had more severe and different GVHD. Thus, we conclude that priming site does affect the character of GVHD. The different conclusions could be attributable to different methods, as we documented GVHD by clinical assessment, weight loss, and quantitative pathologic assessment, whereas Beilhack et al53 relied on bioluminescence imaging and in some cases survival curves. Alternatively or in addition, these outcomes are model-dependent, and by studying more and different models, we uncovered the dependence of GVHD on priming site that was not found in the model used by Beilhack et al.53 These authors also concluded that there was no GVHD in mice lacking LN/PP (B6.LTa KO mice) that had also been splenectomized. However, we found, in both MHC-matched and MHC-mismatched models, that mice completely lacking SLT do get GVHD. Again, differences between our positive result and their negative result could be attributable to methods, model systems, or both.
Although our studies restricting the trafficking and priming venues of TN cells suggested that the reason TEM are unable to mediate GVHD is not the inability to traffic to particular sites, a complementary aspect of the work used the opposite approach—to restore homing capacity to TEM cells using our LΔP transgenic system in which CD62L cannot be down-regulated or shed.23 Activated LΔP cells can enter LN more efficiently than activated or memory WT cells. Therefore, LΔP memory cells should have better trafficking to recipient LN than WT memory cells. Nevertheless, LΔP TEM cells were no different from WT TEM in promoting minimal or no GVHD.
Considering our results in context,16,20,54-56 it is no longer reasonable to think that any one site, or even SLT per se, is required or critical for the induction of GVHD. However, because altering the priming site modulates disease quality or severity, we suggest that the priming site does qualitatively affect the nature of GVHD. This for example would account for the predominant effect on gut GVHD of α4β7 integrin expression and presumptive T-cell priming in mLN.20,42 By the same token, GVHD induction in the absence of all SLT, as well as the inability of constitutive CD62L expression by TEM to restore the ability to mediate GVHD, argues that trafficking differences alone do not account for the markedly greater GVHD-inducing potency of TN over TEM cells, as had been proposed.16,20 Rather, additional explanations for this clinically relevant phenomenon remain to be uncovered.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Cuiling Zhang, Catherine Matte-Martone, William Tan, and Srividhya Venkatesan for outstanding technical support. We thank Drs Steve Rosen, Thomas Tedder, Uta Hopken, Martin Lipp, Jeff Browning, Evangelia Notidis, and Fadi Lakkis for gifts of reagents. We thank Yale Animal Resources Center for expert animal care.
This work was supported by National Institutes of Health grants R01-HL066279 (M.J.S. and W.D.S.), T32-HL07974 (B.E.A.), R01-AI34495, and R37HL56067 (B.R.B.). W.D.S is supported by a Clinical Scholar Award from the Leukemia & Lymphoma Society.
National Institutes of Health
Authorship
Contribution: B.E.A. designed and executed experiments, analyzed data, and wrote the manuscript. A.A. contributed unique reagents and insights. P.A.T. and B.R.B designed and carried out experiments. J.M.M., D.J., A.J.D., and A.P.-M. interpreted and scored histopathologic sections. W.D.S. and M.J.S. designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no compet-ing financial interests.
Correspondence: Mark J. Shlomchik, Department of Laboratory Medicine, Box 208035, Yale University School of Medicine, New Haven, CT 06520-8035; e-mail: mark.shlomchik@yale.edu.
References
Author notes
*W.D.S. and M.J.S. contributed equally to this study.