Abstract
This study investigated the activity of lenalidomide in patients with relapsed/refractory chronic lymphocytic leukemia (CLL). Lenalidomide was given at 10 mg daily with dose escalation up to 25 mg daily. Three patients (7%) achieved a complete response (CR), one a nodular partial remission, and 10 patients a partial remission (PR), for an overall response (OR) rate of 32%. Treatment with lenalidomide was associated with an OR rate of 31% in patients with 11q or 17p deletion, of 24% in patients with unmutated VH, and of 25% in patients with fludarabine-refractory disease. The most common toxicity was myelosuppression, and the median daily dose of lenalidomide tolerated was 10 mg. Plasma levels of angiogenic factors, inflammatory cytokines, and cytokine receptors were measured at baseline, day 7, and day 28. There was a dramatic increase in median interleukin (IL)-6, IL-10, IL-2, and tumor necrosis factor receptor-1 levels on day 7, whereas no changes were observed in median vascular endothelial growth factor levels (20 patients studied). According to our experience, lenalidomide given as a continuous treatment has antitumor activity in heavily pretreated patients with CLL. This trial is registered at http://www.clinicaltrials.gov as no. NCT00267059.
Introduction
Whereas prolonged remissions are obtained in most patients affected by chronic lymphocytic leukemia (CLL) receiving chemoimmunotherapy combinations as frontline therapy,1 the results obtained with current treatment strategies in relapsed/refractory patients are less satisfactory. Salvage therapy with the monoclonal antibody alemtuzumab in patients who have failed treatment with fludarabine is associated with responses in one-third of the patients, with the majority of responses being partial remissions.2 Furthermore, patients with fludarabine refractory disease or bulky lymphadenopathy have a dismal prognosis, with a median survival of 9 months.3,4 Therefore, there is a need for new agents with activity in this setting
Lenalidomide is a derivative of thalidomide and belongs to a class of drugs known as immunomodulating drugs (IMiDs).5 Lenalidomide has shown clinical activity in multiple myeloma and in myelodysplastic syndrome.6,7 The mechanism of action of lenalidomide is not known and may be different depending on the disease. In myelodysplastic syndrome 5q− type, there is evidence of a direct inhibitory effect of lenalidomide on 5q− erythroid progenitors and of up-regulation of the tumor suppressor gene SPARC and the polytropic gene actinin.8 In multiple myeloma, lenalidomide increases the apoptotic rate of the neoplastic plasma cells, inhibits cell adhesion, and induces changes in the bone marrow (BM) microenvironment.9
With respect to the parent drug, thalidomide, lenalidomide is a more potent inhibitor of tumor necrosis factor-alpha (TNF-α) and has a weaker antiangiogenic effect. Lenalidomide also stimulates T-cell proliferation and activates natural killer (NK) cells.10 Owing to its ability to inhibit TNF-α and potentially disrupt the interaction between neoplastic cells and the bone marrow (BM) microenvironment, we decided to evaluate the activity of lenalidomide in patients with CLL. This approach was based on our earlier observation that CLL patients with high plasma levels of TNF-α have an inferior clinical outcome.11 This report summarizes our results obtained with continuous treatment with low-dose lenalidomide in patients with relapsed or refractory CLL.
Methods
Study design
From December 2005 to February 2007, 44 patients with relapsed/refractory CLL were enrolled in this study. All patients provided informed consent according to institutional guidelines and in accordance with the Declaration of Helsinki. This study was approved by the University of Texas M. D. Anderson Cancer Center Institutional Review Board. In this study, patients were required to have received at least one prior purine analog–based chemotherapy, have adequate performance status (Zubrod performance status ≤ 3), serum creatinine level less than 176.8 μM (2 mg/dL), and total bilirubin level less than 34.2 μM (2 mg/dL). No requirements were set for absolute neutrophil count or platelet count.
Treatment plan
Lenalidomide was given at the starting dose of 10 mg daily for 28 days. The dose was then increased by 5 mg every 28 days to a maximum of 25 mg daily, based on patient tolerability and response. The lenalidomide dose could be de-escalated in 5-mg decrements. Treatment was discontinued if disease progression or excessive toxicity was observed. A course of lenalidomide was defined as one month of treatment. Patients who had stable disease (SD) continued treatment until evidence of disease progression (PD). No routine antiviral, antibacterial, or Pneumocystis carinii pneumonia prophylaxis was used. Hematopoietic growth factors were used according to common practice guidelines.
Evaluation during study
Pretreatment evaluation consisted of history and physical examination; laboratory evaluation included complete blood counts, comprehensive metabolic profile, serum beta-2 microglobulin (β2M), bone marrow biopsy, and aspiration with immunophenotyping. Assessment of standard metaphase karyotype, fluorescence in situ hybridization (FISH) studies of common genomic abnormalities, immunoglobulin heavy chain variable gene (VH) mutational status, and assessment of ZAP-70 by immunohistochemistry were obtained at baseline. A complete hematologic profile was monitored weekly during the first month of therapy or until a stable dose of lenalidomide was tolerated. Blood counts were monitored monthly thereafter. Serum β2M and proportions of CD3+, CD4+, and CD8+ peripheral blood T lymphocytes were measured at baseline, 3 months, 6 months, and yearly thereafter. BM biopsy and aspiration were performed at 3 months, 6 months, and every 3 months thereafter in responding patients.
Response and toxicity criteria
Patients were evaluated for clinical response according to the National Cancer Institute Working Group (NCI-WG) criteria at 3 months, 6 months, and every 3 months thereafter.12 Patients who achieved a complete remission were assessed for residual disease by flow cytometry for the presence of a monoclonal B-cell population (negativity defined as less than 1% of lymphocytes that coexpressed CD5 and CD19 antigens with normalization of the κ/λ ratio) and/or polymerase chain reaction (PCR) to detect the IgVH gene of the malignant clone. Patients were monitored for toxicity according to the NCI common toxicity criteria (version 3.0; Bethesda, MD), and the dose of lenalidomide was reduced according to individual patient tolerability.
Plasma cytokines and soluble factors
To study the effect(s) of lenalidomide in CLL, plasma levels of angiogenic factors vascular endothelial growth factor (VEGF) and fibroblast growth factor-basic (FGF-basic), inflammatory cytokines (interleukin-6 [IL-6], IL-8, and IL-10, IFN-γ, IL-1β, IL-2, and TNF-α), and cytokine receptors (IL-2R and soluble TNF-receptor (sTNF-R1) were measured at baseline, day 7, and day 28 of treatment in 20 patients who agreed to additional blood collections for optional correlative studies.
Peripheral blood was drawn from all subjects; plasma was separated and stored frozen at −20°C for batch analysis at a later date. On the day of analysis, the frozen plasma samples were rapidly thawed and brought to room temperature and assayed for the presence of cytokines and soluble cytokine receptors using BioSource Multiplex Assays for Luminex (Biosource International, Camarillo, CA) and multiplex beads from R&D Laboratories (Minneapolis, MN), following the manufacturers' instructions and as previously described.13 Serial dilutions of known concentrations of recombinant cytokines were used to construct a standard curve for each of the analytes. The concentrations of cytokines in plasma samples were interpolated from their respective standard curves. The minimum detectable levels of analytes with the above-mentioned kits were as follows: IL-6, 3 pg/mL; IL-10, 5 pg/mL; IL-2R, 30 pg/mL; IL-8, less than 3 pg/mL; IFN-γ, less than 1 pg/mL; IL-1β, less than 1 pg/mL; IL-2, less than 6 pg/mL; TNF-α, less than 1 pg/mL; sTNF-R1, less than 15 pg/mL; VEGF, less than 10 pg/mL; and FGF-basic, less than 15 pg/mL. The interassay coefficient of variation was 4.6% to 12.6%.
Bone marrow microvascular density
Angiogenesis was quantified by measuring the microvessel density (MVD) in BM biopsy sections that had involvement with B-CLL, regardless of the degree or pattern of involvement, at 3 and/or 6 months after treatment. Blood vessel formation in formalin-fixed, paraffin-embedded BM sections was highlighted by immunohistochemistry using antibodies to CD34 (MY10; BD Biosciences, San Jose, CA) by methods previously described.14 A modification of the criteria used by Kini et al to count microvessels was used.15 Microvessel counts were performed by one observer (E.J.S.) at 400× (40× objective, 10× ocular) magnification using an ocular microscopic grid with an area of 0.0625 mm2 to define the area assessed and to determine the MVD. Numbers of microvessels in the pretreatment and one posttreatment sample were counted in 5 grid areas involved by CLL, and an average number of microvessels was obtained and expressed as average number of microvessels/mm2 or MVD.
Statistical design
The statistical design of Thall, Simon, Estey for efficacy and safety monitoring (Thall and Simon16 and Thall et al17 ) was applied to ensure that the overall response (OR) rate was 20% or higher and that grade 3 to 4 toxicity was 30% or less. A maximum of 45 patients was established to ensure that a posterior 95% interval for any probability Pr(OR) or PR(toxicity) has a width at most of 0.30. The distribution of time to progression and survival were evaluated by the Kaplan-Meier method. The Kaplan-Meier curves for the categoric variables were generated for disease-free survival and overall survival.
Plasma cytokine levels of patients with SD or PD (SD + PD, N = 12) and complete remission (CR) or partial remission (PR; CR + PR, N = 8) were compared over 3 time points using the Friedman test. If statistically significant (P < .05), Wilcoxon signed ranked test was performed to determine differences between 2 time points.
Results
Patient characteristics
All patients were evaluable for response and toxicity. Their characteristics are listed in Table 1. The median age was 64 years (range: 49-86 years). The median number of prior treatments was 5 (range: 1-15). Median serum β2-M level was 4.3 g/mL (3.64 μmol/L), range: 1.6 g/mL-10.1 g/mL (1.35-8.55 μmol/L); 45% of the patients had advanced Rai stage (III and IV disease). Twenty-seven percent of patients were refractory to prior fludarabine treatment (defined as failure to achieve a PR or CR following fludarabine-based treatment, disease progression during treatment with fludarabine or within 6 months from the last dose); 39% had lymph nodes larger than 5 cm indicating bulky disease. Furthermore, 59% of patients carried an unfavorable genomic abnormality such as 11q− and/or 17q−; 88% of the 33 patients with known VH mutational analysis carried unmutated VH. ZAP-70, as assessed by immunohistochemistry, was available for 21 patients, with 17 of them having evidence of expression.
Clinical responses
Three patients (7%) achieved CR, 1 patient achieved a nodular partial remission and 10 patients (25%) achieved a partial remission (PR) for an overall response rate of 32% (Table 2). Time to best response was 6 months in 11 patients and 9 months in 3 patients. Eleven patients (25%) had stabilization of their disease or disease improvement without fulfilling the criteria for PR and they were able to continue on treatment past the third month. Resolution of bone marrow involvement was observed in 26% of the patients, reduction of the lymphocyte count greater than 50% was observed in 46% with normalization in 14% of the patients, and improvement of greater than 50% in organomegaly and lymphadenopathy was observed in 47% and 41% of the patients, respectively. Seventeen patients (39%) experienced disease progression during treatment (10 patients progressed prior to completing the third month of treatment and the remaining 7 progressed after 3 [3 patients], 4 [1 patient], 5 [1 patient], 6 [1 patient], and 8 months [1 patient]).
Two early deaths due to infections (5%) occurred within the first 30 days of treatment: one was an 86-year-old patient who developed pneumonia on day 11, another was a 76-year-old patient who died of disseminated mucormycosis on day 22 of treatment.
When residual disease was measured by flow cytometry in the 3 patients who achieved a CR, no monoclonal B-cell population was detected in 2 patients, and in the third patient, only 1% of the cells were monoclonal B cells. Molecular evidence of residual disease was detected in both CR patients in whom PCR testing was performed.
The median daily dose of lenalidomide tolerated by the patients in this study was 10 mg, and only 3 patients (7%) were able to tolerate the dose of 25 mg daily for at least 1 month. Myelosuppression was the most frequent reason for treatment interruption and dose reduction. Responses were seen at all dose levels as summarized in Table 3.
Disease progression and survival
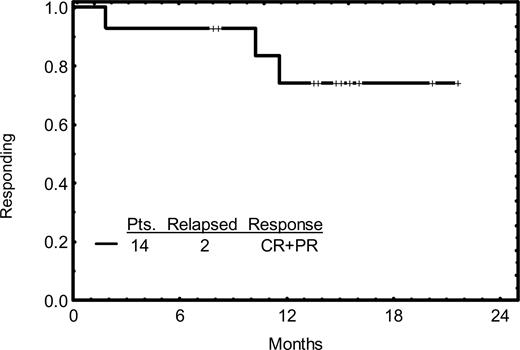
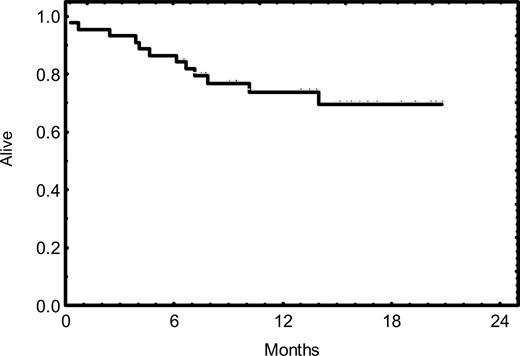
The response duration is illustrated in Figure 1; none of the 3 patients who achieved CR showed evidence of progression thus far. Two of the 11 patients who achieved PR have progressed. The overall survival rate is illustrated in Figure 2; 32 patients (73%) are alive with a median follow-up time of 14 months.
Kaplan-Meier survival curve. Thirty-two (73%) of 44 patients are alive with a median follow-up time of 14 months.
Kaplan-Meier survival curve. Thirty-two (73%) of 44 patients are alive with a median follow-up time of 14 months.
Response as per prognostic factor
In a prospective analysis of prognostic factors, we found that the overall response rate was 31% in patients having unfavorable genomic abnormalities (deletion 11q23 and/or 17p), 24% in patients with unmutated VH, and 25% in patients with fludarabine refractory disease (Table 4).
Treatment toxicity
A total of 333 courses of lenalidomide were administered to the 44 patients at the time of this report. The most common toxicity (Table 5) was myelosuppression. Neutropenia, grade III to IV was observed in 41% of the courses and grade I to II in 11%. Thrombocytopenia, grade III to IV was observed in 16% of the courses and grade I to II in 15%. Anemia was less common: anemia grade III to IV was observed in 3% of the courses, and grade I to II in 19%. Nonhematologic toxicity consisted of grade I to II fatigue, observed in 22% of the courses and grade III to IV in only 1%. Diarrhea and rash were commonly reported; diarrhea was grade III to IV in 2% and grade I to II in 13% of the courses, and rash was limited to grade I to II and observed in 13% of the courses. One patient developed an uncomplicated deep venous thrombosis of the right popliteal vein while receiving concomitant erythropoietin.
Tumor flare reaction
Tumor flare reaction is a toxicity described with lenalidomide18 and also known to occur with the parent drug thalidomide.19 In our patients, the tumor flare reaction was characterized by the development of painful and swollen lymphadenopathies occasionally associated with fever and/or bone pain. Grade 3 tumor flare reaction requiring narcotics was reported in 2% of the courses and grade I to II tumor flare was reported in 10% of the courses. The incidence of tumor flare reaction of any grade was higher (9 of 17, 53%) in patients with lymph nodes larger than 5 cm than in the remaining patients (4 of 27, 15%). The occurrence of a tumor flare reaction did not predict for a higher response rate (OR, 38%; and 34% in patients with and without a tumor flare reaction). Tumor flare was managed with a short 6-day course of oral methylprednisolone (Medrol Dosepak). Tumor flare reoccurred to a lesser extent at the time of dose escalation in a subset of patients. None of our patients experienced tumor lysis syndrome.
Infectious complications (grade 3 or 4)
The most common infection was pneumonia, complicating 3% of the courses. Pneumocystis carinii pneumonia was documented in 2 patients. Fever of unknown origin (FUO) was observed in 2% of the courses. Two patients developed diarrhea of infectious etiology, cryptosporidium in one case and rotavirus infection in another. One patient developed disseminated mucormycosis (Table 5).
Changes in peripheral T cells
Data on blood T cells were available at baseline, month 3, and month 6 in 19 patients. As illustrated in Figure 3, the number of circulating lymphocytes decreased with treatment, whereas the mean number of CD3+ T cells remained stable: before treatment, 880/μL; 3 months, 930/μL; and 6 months, 1020/μL.
Total lymphocyte and T-cell counts. Total lymphocyte and T-cell counts in peripheral blood in 22 patients at baseline, month 3, and month 6 of treatment. The boxes represent the interquartile range, which extended from the 25th percentile to the 75th percentile, and the line across the box indicates the mean; error bars represent 95% confidence intervals.
Total lymphocyte and T-cell counts. Total lymphocyte and T-cell counts in peripheral blood in 22 patients at baseline, month 3, and month 6 of treatment. The boxes represent the interquartile range, which extended from the 25th percentile to the 75th percentile, and the line across the box indicates the mean; error bars represent 95% confidence intervals.
Changes in plasma cytokine levels
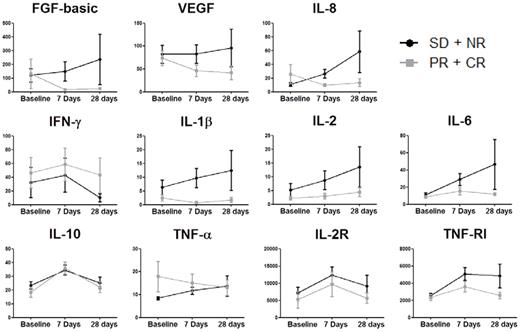
Plasma samples were available from 12 patients with NR (no response) + SD and 8 patients with CR + PR. For CR + PR patients, there were statistically significant changes in plasma levels of FGF-basic (P = .050), IL-10 (P = .050), and IL-2R (P = .018) over time. For NR + SD patients, the statistically significant changes in plasma cytokines over time were noted for IL-6 (P = .018), IL-10 (P = .018), IL-2R (P = .005), and TNF-R1 (P = .005; Figure 4). There were no statistically significant changes in the levels of plasma VEGF in either group of patients. There was a dramatic increase in median IL-6, IL-10, IL-2R, and tumor necrosis factor receptor-1 (TNF-R1) levels in both patient groups at day 7, and the levels dropped substantially at day 28. In contrast, the median level of FGF-basic declined for the CR + PR group at day 7, whereas the SD + NR group remained at the same median level. Furthermore, as seen in Figure 5, cross-sectional analysis revealed that the median levels of FGF-basic (P = .001) and IL-6 (P = .035) were significantly higher for the SD + NR group at day 7 compared with the CR + PR group.
Changes in circulating angiogenic and inflammatory factors during therapy with lenalidomide. Peripheral blood was collected from patients receiving lenalidomide therapy at baseline and after 7 days and 28 days of treatment. Plasma samples from patients with stable disease or no response (SD + NR, gray lines) and partial response or complete response (PR + CR, black lines) were analyzed for the angiogenic factors FGF-basic, VEGF, and IL-8; the inflammatory cytokines IFN-γ, IL-1β, IL-2, IL-6, IL-8, IL-10, and TNF-α; and the soluble cytokine receptors IL-2R and TNF-RI. Longitudinal analysis of SD + NR shows significant changes over time in IL-6, IL-10, IL-2R, and TNF-RI. PR + CR show significant changes over time in FGF-basic, IL-10, and IL-2R. The change in VEGF concentrations was not statistically significant for either group. Plasma concentrations are reported in mean (± SEM) picogram per milliliter.
Changes in circulating angiogenic and inflammatory factors during therapy with lenalidomide. Peripheral blood was collected from patients receiving lenalidomide therapy at baseline and after 7 days and 28 days of treatment. Plasma samples from patients with stable disease or no response (SD + NR, gray lines) and partial response or complete response (PR + CR, black lines) were analyzed for the angiogenic factors FGF-basic, VEGF, and IL-8; the inflammatory cytokines IFN-γ, IL-1β, IL-2, IL-6, IL-8, IL-10, and TNF-α; and the soluble cytokine receptors IL-2R and TNF-RI. Longitudinal analysis of SD + NR shows significant changes over time in IL-6, IL-10, IL-2R, and TNF-RI. PR + CR show significant changes over time in FGF-basic, IL-10, and IL-2R. The change in VEGF concentrations was not statistically significant for either group. Plasma concentrations are reported in mean (± SEM) picogram per milliliter.
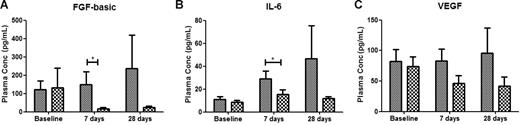
Responders to therapy with lenalidomide have significantly different plasma concentrations of angiogenic and inflammatory cytokines. Cross-sectional analysis of plasma angiogenic and inflammatory factors shows that patients who responded to lenalidomide therapy (PR + CR,  ) have significantly lower plasma concentrations (mean ± SEM) of the angiogenic factor FGF-basic (A) and the inflammatory cytokine IL-6 (B) than patients who failed to respond to treatment (SD + NR; ▩). Plasma samples were taken at baseline and following 7 days and 28 days of lenalidomide therapy. Although a trend suggesting decreasing VEGF concentrations in responders was noted, no statistical difference in VEGF plasma concentration was observed between the SD + NR and the PR + CR groups (C). *P < .05 (A); P = .018 (B).
) have significantly lower plasma concentrations (mean ± SEM) of the angiogenic factor FGF-basic (A) and the inflammatory cytokine IL-6 (B) than patients who failed to respond to treatment (SD + NR; ▩). Plasma samples were taken at baseline and following 7 days and 28 days of lenalidomide therapy. Although a trend suggesting decreasing VEGF concentrations in responders was noted, no statistical difference in VEGF plasma concentration was observed between the SD + NR and the PR + CR groups (C). *P < .05 (A); P = .018 (B).
Responders to therapy with lenalidomide have significantly different plasma concentrations of angiogenic and inflammatory cytokines. Cross-sectional analysis of plasma angiogenic and inflammatory factors shows that patients who responded to lenalidomide therapy (PR + CR,  ) have significantly lower plasma concentrations (mean ± SEM) of the angiogenic factor FGF-basic (A) and the inflammatory cytokine IL-6 (B) than patients who failed to respond to treatment (SD + NR; ▩). Plasma samples were taken at baseline and following 7 days and 28 days of lenalidomide therapy. Although a trend suggesting decreasing VEGF concentrations in responders was noted, no statistical difference in VEGF plasma concentration was observed between the SD + NR and the PR + CR groups (C). *P < .05 (A); P = .018 (B).
) have significantly lower plasma concentrations (mean ± SEM) of the angiogenic factor FGF-basic (A) and the inflammatory cytokine IL-6 (B) than patients who failed to respond to treatment (SD + NR; ▩). Plasma samples were taken at baseline and following 7 days and 28 days of lenalidomide therapy. Although a trend suggesting decreasing VEGF concentrations in responders was noted, no statistical difference in VEGF plasma concentration was observed between the SD + NR and the PR + CR groups (C). *P < .05 (A); P = .018 (B).
Effect on bone marrow microvessel density
For 22 patients, the pretreatment BM biopsy specimens as well as the 3-month and/or 6-month posttreatment BM biopsy specimens were available for MVD assessment. For the 22 pretreatment BM specimens, the median MVD was 52.8 vessels/mm2 (range: 16-156.8 microvessels/mm2). Nineteen BM biopsy specimens were available at 3 months after treatment; the median MVD was 76.8 vessels/mm2 (range: 6.4-160 microvessels/mm2). Thirteen BM biopsy specimens were available at 6 months after treatment; the median MVD was 60.8 vessels/mm2 (range: 38.4-108.8 microvessels/mm2). There was no significant difference between MVD prior to lenalidomide treatment and after treatment (P = .697 from Wilcoxon rank test). Similarly, there was no significant difference in MVD changes between nonresponders (9 patients) and responders (13 patients; P = .300 from Wilcoxon rank test).
Discussion
Continuous treatment with low-dose oral lenalidomide resulted in an overall response rate of 32% in a population of heavily pretreated patients with CLL. Furthermore, considering the high proportion of patients carrying unfavorable prognostic factors, such as poor prognostic genomic abnormalities and unmutated immunoglobulin heavy chain gene, the response rate observed in these subgroups (OR: 31% and 24%, respectively) indicates activity of lenalidomide in patients unlikely to respond to standard approaches. Chanan-Khan et al reported clinical responses with a higher dose of lenalidomide, 25 mg a day, given in an intermittent fashion to previously treated patients with CLL. The OR rate in this study was 47%, with a CR rate of 9%.18 Myelosuppression was the major toxicity of lenalidomide in our study and the Chanan-Khan et al study18 ; severe neutropenia was observed in 70% of the patients in the study of Chanan-Khan et al18 and in 41% of the courses in our study. Interestingly, myelosuppression in our experience was also seen during the later phases of treatment in responding patients, suggesting a direct effect of lenalidomide on hematopoietic progenitors. The infection rate was significant, but not surprising considering the profound immunosuppressed status of these patients and also considering that no antibiotic prophylaxis was administered and many patients had long-standing neutropenia that preceded treatment with lenalidomide. Our low-dose continuous lenalidomide regimen was associated with a modest rate of tumor flare reaction (12%), lower than the 58% incidence reported with the higher dose regimen by Chanan-Khan et al.18 None of our patients experienced clinically relevant tumor lysis syndrome, despite having large tumor volumes. Only one case of uncomplicated deep vein thrombosis was reported, suggesting that this complication may be more common when lenalidomide is used in association with steroids and in patients with plasma cell dyscrasias. Our impression is that patients require a prolonged treatment to reach their response, as we observed further improvement of the quality of responses after 9 months of treatment. We believe that the most active dose of lenalidomide for the treatment of CLL has not been defined yet. In our experience, both 10 and 15 mg daily appear active in the setting of relapsed CLL. The question of whether continuous exposure is superior to the 3 weeks on/1 week off schedule reported by Chanan-Khan et al18 cannot be answered by this relatively small phase 2 trial experience and will require further investigation. Our decision to investigate a continuous schedule of lenalidomide was based on the potential advantages of inhibiting the production of prosurvival cytokines and the supportive effect of stroma on the malignant cells for an extensive period of time. We also hypothesized that a sustained exposure to this agent would not allow the CLL cells to recuperate from these inhibitory effects. We observed that in the majority of our patients, treatment with lenalidomide was not associated with lymphopenia, as often seen with other agents such as purine analogues and monoclonal antibodies, but with a stable or increased number of T cells, suggesting that immunomodulation with this agent may be of pivotal relevance in CLL. Thalidomide and IMiDs in general have been associated with immunologic activation in patients with multiple myeloma.9 We measured plasma levels of sIL-2R, IL-2, IFN-γ, and TNF-α to identify any evidence of immune activation in patients with CLL receiving lenalidomide. Despite the small number of patients in this study, transient immune activation occurred in both CR + PR and NR + SD patients. Specifically, increases in sIL-2R levels on day 7 in both NR + SD (P = .005) and CR + PR (P = .018) patients and return to baseline levels by day 28 are indicative of transient immune activation that had abated by day 28. Similarly, by day 7 there were significant increases in the plasma levels of IL-6 (P = .018) and IL-10 (P = .018) in NR + SD patients and IL-10 in CR + PR patients, suggesting that immune activation was present. Our observations of increases in the level of sIL-2R and immune activation are consistent with an earlier report of malignant melanoma patients receiving lenalidomide in doses of up to 25 mg daily for 28 days.20
The association between lenalidomide therapy and immune activation is further exemplified by changes in the levels of TNF-α and its soluble receptor TNF-R1. Based on our observation that increased TNF-α plasma levels are associated with an inferior outcome in patients with CLL and the potency of lenalidomide as a TNF-α inhibitor, we expected to see a decline in TNF-α levels during treatment. We, therefore followed TNF-α and TNF-R1 plasma levels. Since our assay measured free TNF-α and not TNF-α bound to its receptor, we hypothesized that TNF-R1 would be a better indicator of TNF-α levels. We observed that treatment with lenalidomide did not induce any statistically significant changes in TNF-α levels, and, in terms of TNF-R1 levels, we observed a sharp increase in its levels on day 7 that was followed by a decline to basal level at later time points, and we attributed this fluctuation to an immunologic flare. These data are at variance with an earlier study that showed an increase in serum TNF-α levels of patients with malignant melanoma receiving lenalidomide at a dose of 25 mg daily.20 Finally, the significant increases in the levels of IL-6 and sTNF-R1 in NR + SD patients may be due to the down-regulation of TNF-α by increasing levels of IL-6, which also induced increased levels of sTNF-R1 in NR + SD patients on day 7.21,22
Next, we assessed plasma levels of proangiogenic factors in both patient groups as lenalidomide has been known to reduce angiogenesis.5 The antiangiogenic mechanism of the thalidomide is not well known,23 and that of its analog lenalidomide has been demonstrated in in vitro systems.24 However, these findings have been difficult to confirm in studies with multiple myeloma patients.25 Even though the antiangiogenic effect of thalidomide is thought to be mediated through the inhibition of VEGF and bFGF biologic activity,26 studies with lenalidomide have not consistently demonstrated a reduction in VEGF and bFGF.20 Our data show that while VEGF levels remained unchanged in CR + PR as well as NR + SD patients, bFGF levels decreased by day 7 in CR + PR patients (P = .05). Moreover, the lack of a significant reduction in neovascularization seen in the bone marrow biopsies suggests that the antiangiogenic effect may not play a major role in this setting. Both of these observations are not inconsistent with other studies with these agents and may reflect a lack of sensitivity of these assays and their limitation to detect relevant changes in angiogenic activity.27 Despite these results, we detected significant changes in the level of the proangiogenic factor IL-8 between NR + SD and CR + PR (P = .018) patients on day 7 after therapy with lenalidomide. An earlier study reported increases in the levels of circulating IL-8 following therapy with a daily dose of 25 mg lenalidomide.20 In the current study, we report that NR + SD patients had significantly higher levels of plasma IL-8 than those of CR + PR patients on day 7 after therapy (P = .018).
In conclusion, according to our experience, lenalidomide has shown antitumor activity in patients who have failed numerous prior treatments and carry unfavorable prognostic factors.
Correlative studies suggest that this agent may have a unique mechanism of action in CLL and may be working through an immunostimulatory effect. The exact mechanisms of action in CLL need to be further explored in larger studies and may be more informative in a less heavily pretreated population. Work continues in evaluating the most effective dose and schedule of this agent in CLL and further assessing its activity as initial therapy and in combination with other agents.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mary L. Browning, BSN, and Susan C. Smith, MS, for data collection.
Authorship
Contribution: A.F. designed and performed research, analyzed data, and wrote the paper; B.-N.L., H.G., E.N.C., and C.L. performed correlative studies; E.J.S. performed MVD measurements; S.M.O., W.G.W., Z.E., and S.F. performed research; S.W. designed statistical analysis; J.M.R. designed and performed research, and analyzed correlative studies; M.J.K. designed and performed research.
Conflict-of-interest disclosure: A.F. is a member of the Celgene Corporation Advisory Board. The remaining authors declare no competing financial interests.
Correspondence: Alessandra Ferrajoli, Department of Leukemia/Unit 428, The University of Texas, M. D. Anderson Cancer Center, PO Box 301402, Houston, TX 77230-1402; e-mail: aferrajo@mdanderson.org.