Abstract
To study prognostic factors of progression/relapse, data concerning 225 children enrolled between 1987 and 1997 in Berlin-Frankfurt-Münster, Société Française d'Oncologie Pédiatrique and United Kingdom Children's Cancer Study Group prospective studies for the treatment of anaplastic large cell lymphoma (ALCL) were merged. Median follow-up was 9.3 years. Five-year overall survival and event-free survival of the whole population was 81% (95% confidence interval, 76%-86%) and 69% (63%-74%), respectively. B symptoms, mediastinal involvement, skin lesions, visceral involvement, St Jude stage 3-4, Ann Arbor stage 3-4, and elevated lactate dehydrogenase increased the risk of progression/relapse in the univariate analysis. In the multivariate analysis, 3 factors remained significant: mediastinal involvement (relative risk [RR] = 2.1 [1.2-3.5]), visceral involvement defined as lung, liver, or spleen involvement (RR = 2.1 [1.3-3.6]), and skin lesions (RR = 1.9 [1.1-3.2]). Five-year progression-free survival (PFS) of the 81 patients with none of these risk factors was 89% [82%-96%], contrasting with a 5-year PFS of 61% [53%-69%] in the 144 patients with at least 1 risk factor (RR = 4.4 [2.2-8.9; P < .001). In conclusion, 3 factors associated with an increased risk of failure in childhood ALCL have been defined: mediastinal involvement, visceral involvement, and skin lesions.
Introduction
Anaplastic large cell lymphoma (ALCL) is a rare disease in children accounting for 10% to 15% of all childhood non-Hodgkin lymphomas.1 Diagnostic criteria are now well established. ALCL is characterized by the proliferation of large pleiomorphic cells of a T/null phenotype that tend to invade the lymph node sinuses and express the CD30 antigen. In children, most ALCLs exhibit a t(2;5) translocation, which can be detected by anaplastic lymphoma kinase (ALK) antibodies.2,3 The existence of B-cell ALCL (B-ALCL) and Hodgkin-like ALCL has been a matter of debate for years, but these lymphomas were excluded from ALCL in the recent classifications (Revised European-American Lymphoma [REAL] and World Health Organization classifications).4,5
There is no consensus regarding a standard treatment for ALCL. Some teams use short-pulse chemotherapy, which is similar to the treatment used for B-cell lymphoma.6-9 Others treat patients with more prolonged semicontinuous leukemia-type chemotherapy.10,11 Finally, in other groups and especially in adults, ALCL patients receive repeated-pulse chemotherapy according to protocols designed for all large-cell lymphomas, regardless of the immunophenotype, including large B-cell lymphoma and ALCL.12-14
In published pediatric series,6-9,11,15-20 the analysis of prognosis factors has been limited to date because of the small numbers of patients.
To identify patients at high risk of failure, a prognostic study was undertaken by the European Intergroup for Childhood Non-Hodgkin Lymphoma on the population of children enrolled in the prospective studies conducted by the Berlin-Frankfurt-Münster (BFM) group (Germany, Austria, and Switzerland) and French (Société Française d'Oncologie Pédiatrique [SFOP]) and British (United Kingdom Children's Cancer Study Group [UKCCSG]) groups, all of whom received a short intensive chemotherapy regime. A first analysis was performed in 1998.21 After publication of the results of the national studies,6-9 the Intergroup database was updated. We report here the results of the analysis performed on this large population of childhood ALCL with a very long follow-up.
Methods
The trial was approved by the French Society of Pediatric Oncology for French patients, by the Gesellshaft für Pädiatrische Onkologie und Hämatologie board for German patients, by the UKCCSG for United Kingdom patients, and by the local review boards at the participating institutions. Informed consent was obtained in accordance with the Declaration of Helsinki.
Patients
Overall, 225 patients were included: 88 BFM patients enrolled in the BFM86 and BFM90 studies between 1986 and 1995, 82 French patients enrolled in the SFOP HM89 and HM91 protocols between 1988 and 1997, and 55 British patients included in the UKCCSG 9001, 9002, and 9003 studies between 1990 and 1997.
Inclusion criteria in these studies were newly diagnosed systemic ALCL and age less than 18 years. Exclusion criteria were previous treatment, severe immunodeficiency, and ALCL occurring as a second malignancy. In the SFOP studies, patients with central nervous system (CNS) disease were not included.
The diagnosis of ALCL was based on established morphologic and immunohistochemical criteria defined in the updated Kiel classification22 and in the revised REAL classification of lymphoma.5 Slides of 216 of 225 patients (96%) were reviewed for the purpose of each study by the national reference pathologists. Immunohistochemistry was performed on paraffin sections with a panel of monoclonal antibodies directed against CD20, CD79a, CD3, CD45RO, CD43, CD68, CD15, and CD30. During most of the accrual period, the ALK monoclonal antibody was not commonly available, but it was tested retrospectively on slides of 143/225 patients (80 in France, 51 in BFM, and 12 in the United Kingdom). Patients with B-ALCL (n = 10) and Hodgkin-like (n = 5) ALCL were excluded from the present analysis because these proliferations are no longer included among ALCL in the most recent classifications.
Staging procedures have been described previously for each study.6-9 All patients underwent a physical examination, a full blood count, bone marrow aspirate smears (and a bone marrow biopsy for patients included in the SFOP studies), and cerebro-spinal fluid (CSF) cytology. Imaging that varied by country and period of treatment included plain x-rays, ultrasonography, computed tomography, magnetic resonance imaging, and/or skeletal scintigraphy. Patients were staged according to the St Jude and Ann Arbor staging systems.23,24
The treatment schedules have already been described6-9 and are summarized in Table 1. BFM patients were treated according to the BFM86 protocol before 1990 (18 patients), either branch B–standard-risk group (SRG; 2 patients with stage 1 or 2 completely resected disease), branch B-RG (13 patients with stage 2 unresected and stage 3 disease), or branch B-IV (3 patients with stage 4 and/or multifocal bone disease), and then according to the BFM90 protocol, either branch K1 (7 patients with stage 1 or 2 completely resected disease), branch K2 (49 patients with stage 2 unresected and stage 3 disease), or branch K3 (14 patients with stage 4 and/or multifocal bone disease). In the SFOP group, patients were treated with the HM89 protocol until 1990 (18 patients), and with the HM91 protocol afterward (64 patients), whatever the disease extent. In the UKCCSG group, 5 patients were included in the 9001 protocol designed for stage 1 disease, 47 in the 9002 protocol for stage 2, stage 3 and non-CNS stage 4, and 3 in the 9003 protocol for patients with CNS disease.
Data
A common database was constructed in 1998, merging data collected previously in each prospective study group. Follow-up data were updated in April 2006.
Statistics
The distribution of initial characteristics in the different countries was compared using χ2 tests or exact tests when appropriate. The associations between initial characteristics were described using odds ratios (ORs) with 95% confidence intervals (95% CIs).
The main end point to evaluate prognostic factors was the progression-free survival (PFS), defined as the interval from the first day of chemotherapy and progression or relapse. Patients whose disease progressed while on treatment, before achieving a complete remission (CR), were considered an initial treatment failure, with the date of failure at day 1 of chemotherapy because it would have been difficult to define a date of progression in such cases. Patients who neither progressed nor relapsed were considered free of failure at the date of the last follow-up visit. Follow-up data were censored at the date of death for patients whose death was attributable to treatment toxicity. The secondary end point was the overall survival (OS), defined as the interval from the first day of chemotherapy to the time of death, whatever the cause.
The event-free survival (EFS), which takes into account progression and relapse but also secondary malignancy and toxic death, was used to compare results of study groups. It was defined as the interval from the first day of chemotherapy to the time of the first event (progression, relapse, secondary malignancy, or death, whatever the cause) or to the date of the last follow-up visit for those who were still in first CR.
Survival curves (PFS, EFS, and OS) were estimated using the Kaplan-Meier method, with Rothman's 95% CIs.25 Median follow-up was estimated using Schemper's method.26 Statistical differences in PFS, EFS, and OS were tested by the 2-tailed log-rank test. Relative risks (RRs) were estimated with their 95% CI using Cox's regression. Data were analyzed with the SAS software (version 8.2; SAS Software, Cary, NC).
The prognostic factor analysis of PFS and OS was stratified on the study group. The variables studied were sex, age (10 years or younger vs 11 years or older), immunophenotype (T/null), disease extent (mediastinal involvement, visceral involvement, skin lesions, bone lesions, bone marrow involvement, soft tissue mass, St Jude staging, Ann Arbor staging), and elevated lactate dehydrogenase (LDH; < 2× upper limit of normal [ULN] vs ≥ 2×ULN). The robustness of the final model was studied according to the study group.
Results
Initial characteristics
Age ranged from 10 months to 17 years (median 10.2 years). The population is described in Table 2. Among the 190 patients for whom the immunophenotype was available, 135 (71%) were positive for T-cell markers. This proportion was higher in the SFOP group than in the other groups. Positivity for ALK was found in 128 of 143 (90%) patients tested.
B symptoms were present in 54% of the patients. Lymph nodes were involved in 92% of patients, including mediastinal involvement in 36%. Extranodal disease was found in 68% of the cases, including visceral involvement in 43% and skin lesions in 26%. Bone marrow and CNS disease were rare.
B symptoms, bone marrow involvement, and elevated LDH were significantly more frequent in the SFOP group than in the other groups, whereas visceral involvement other than spleen, liver, and lung disease was rarer in the SFOP group. Other characteristics did not differ significantly between countries.
Numerous significant associations were observed among clinical characteristics: B symptoms were significantly associated with mediastinal involvement (OR = 2.3; P = .004), visceral involvement (OR = 4.2; P < .001), St Jude stage 3 or 4 (OR = 7.0; P < .001), and Ann Arbor stage 3 or 4 (OR = 3.3; P < .001). Mediastinal involvement was significantly linked to visceral involvement (OR = 3.6; P < .001) and bone marrow involvement (OR = 4.8; P = .002). Bone marrow involvement was also significantly associated with visceral involvement, especially lung disease (OR = 6.2; P < .001), and elevated LDH (OR = 5.8; P = .002). Visceral involvement was frequently multiple (eg, spleen plus liver involvement). In contrast, skin lesions and bone lesions were independent of other lesions. St Jude stage 3 or 4 was by definition linked to mediastinal and bone marrow involvement; it was also significantly associated with visceral involvement (OR = 77; P < .001) but appeared to be independent of skin lesions (OR = 1.2; P = .68). Ann Arbor stage 3 or 4 was by definition linked to visceral involvement but was not significantly associated with mediastinal involvement (OR = 1.75; P = .10).
Outcome
Overall, 197 patients (88%) achieved a CR with chemotherapy. Of the 28 who did not achieve a CR, 13 had a partial remission with residual disease at the end of treatment; 13 patients experienced early progression under treatment, and 2 died of treatment-related toxicity.
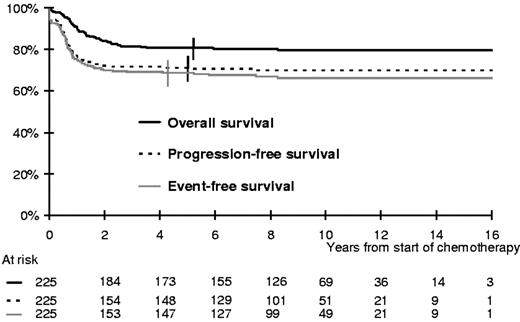
The median follow-up of the whole population is 9.3 years and the maximum 18 years; only 5 patients were lost to follow-up within 2 years of treatment onset. Overall, 50/197 patients who achieved a CR and 3/13 patients with residual disease subsequently relapsed or progressed at a median time of 8.1 months (range 3 months to 7.5 years) after the start of chemotherapy. Only 6 relapses occurred on treatment. Most of the relapses (31/53) were diagnosed within the first 6 months after the end of treatment. On the other hand, only 3 patients experienced a first relapse more than 3 years after the start of chemotherapy (maximum 7.5 years).
Overall, the 5-year PFS was 71% (95% CI; 65%-77%; Figure 1). Three patients developed a secondary malignancy. Overall, 45 patients died, 38 of disease progression (13 after initial failure and 25 after a relapse) and 7 of treatment complications (2 early toxic deaths and 3 treatment-related deaths in first CR, one attributable to a second malignancy, and one after a relapse). The 5-year EFS and OS rates were 69% (95% CI; 63%-74%) and 81% (95% CI, 76%-86%), respectively (Figure 1).
Prognostic factor study
As detailed in Table 3, B symptoms, a mediastinal mass, spleen, liver, or lung involvement, skin lesions, a high stage in the St Jude or Ann Arbor classifications, and elevated LDH significantly increased the risk of progression or relapse in the univariate analysis. Patients with bone lesions had a significantly better prognosis than patients without bone lesions. The immunophenotype, marrow involvement, a soft tissue mass, and visceral involvement other than spleen, liver, and lung involvement were not found to be associated with the risk of failure.
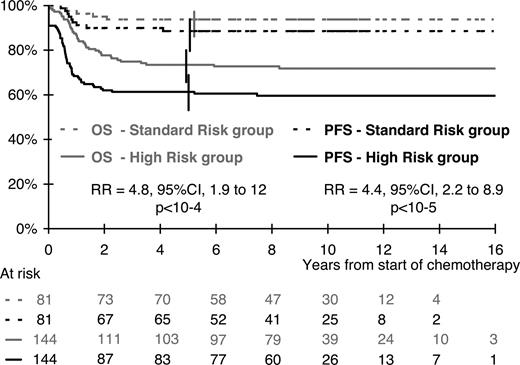
In the multivariate analysis, the risk of progression or relapse was correlated with visceral involvement (lung, liver, or spleen), skin lesions, and a mediastinal mass (Table 3). A high stage in the St Jude or Ann Arbor classification had no additional prognostic value when these 3 risk factors were taken into account. The risk of progression or relapse increased with the number of risk factors. Consequently, considering patients with none of these 3 risk factors as the reference group (n = 81), the risk was multiplied by 3.3, 5.3, and 10.2 for patients with only 1 risk factor (n = 85), 2 risk factors (n = 45), and all 3 risk factors (n = 14), respectively (overall comparison; P < .001). Overall, 64% of patients (144/225) had at least 1 risk factor, 63% in the BFM population, 61% in the SFOP population, and 71% in the UKCCSG population (P = .46). As illustrated in Figure 2, these high-risk patients had a risk of progression or relapse multiplied by 4.4 (95% CI, 2.2-8.9; P < .001) compared with the 81 standard risk group patients with no risk factor: 61% (95% CI; 53%-69%) and 89% (95% CI, 82%-96%), respectively, were free of disease failure at 5 years. This result did not vary significantly between countries: RR = 3.0 (95% CI, 0.9-10.4) in the BFM population, RR = 4.1 (95% CI, 1.6-10.8) in the SFOP population, and RR = 10.1 (95% CI, 1.3-76) in the UKCCSG population (test for heterogeneity; P = .21).
OS and PFS according to risk group. Standard risk group indicates no risk factor (ie, no mediastinal involvement and no lung, spleen, or liver involvement, and no skin lesion). High-risk group, at least 1 risk factor, mediastinal involvement, visceral involvement, or skin lesion.
OS and PFS according to risk group. Standard risk group indicates no risk factor (ie, no mediastinal involvement and no lung, spleen, or liver involvement, and no skin lesion). High-risk group, at least 1 risk factor, mediastinal involvement, visceral involvement, or skin lesion.
This definition of risk groups was also found to be relevant in terms of OS, with 73% (95% CI, 66%-80%) and 94% (95% CI, 89%-99%) of survivors at 5 years in the high-risk and in the standard risk groups, respectively (RR = 4.8; 95% CI, 1.9-12; P < .001).
Twelve patients with St Jude stage 1 or stage 2 completely resected disease were accrued to the BFM90-K1 or the UK9001 protocol, and received only short-duration and nonintensive chemotherapy; all of them are alive and free of disease more than 5 years after the start of treatment.
Discussion
The present study reports on a prognostic study performed on a large unselected group of children with ALCL included in several consecutive prospective studies conducted by 3 European study groups between 1987 and 1997. In the multivariate analysis, 3 factors were found to be significantly associated with a high risk of disease failure: mediastinal involvement, visceral involvement (defined as lung, liver, or spleen involvement) and skin lesions. These factors singled out 2 groups of patients: a poor prognosis group with at least 1 risk factor, accounting for 64% of the population with a 5-year PFS of 61%, and standard risk group, with a 5-year PFS of 89%.
This analysis is based on prospective recording of initial characteristics and long-term follow-up of patients included in several consecutive studies spanning a 10-year period. Because these studies were conducted a long time ago and updated recently, this was a unique opportunity to study long-term outcome and especially the risk of a late relapse. Furthermore, this collaborative study allowed us to assemble a large number of patients with this rare disease.
During this time period, diagnostic criteria for ALCL evolved. This led us to secondarily exclude patients with B-ALCL or Hodgkin-like ALCL, which are no longer considered ALCLs.4,5 Furthermore, significant progress has been achieved in the characterization of ALCL such as the identification of t (2;5)27 and variant translocations,28 the availability of the ALK antibody,29,30 and a better definition of ALCL histologic subtypes.2 These assessments were not commonly available during the accrual period and could not be studied in the prognostic factor analysis because of the high number of patients with missing data.
This study was conducted by merging preexisting databases and not as a prospective collaborative study. Thus, data collection and disease assessment were not identical across countries. This could explain some differences between study populations such as the higher percentage of tumors expressing T-cell markers in the French group, which was probably related to the greater number of T-directed antibodies tested by the French national reference pathologist. Similarly, the higher rate of bone marrow involvement in this group may have been attributable to more extensive exploration of the bone marrow with systematic bone marrow biopsies in addition to the bone marrow aspirations performed in all countries. These differences among the 3 countries might be more apparent than real. Yet there were some real differences in initial characteristics, such as a higher frequency of B symptoms and elevated LDH in the French group, which prohibits any conclusion regarding observed differences in outcome among the 3 study groups.
The aim of our study was to identify patients at a high risk of progression and relapse. We were able to identify several characteristics associated with a high risk of disease failure: mediastinal, visceral, and/or skin involvement. Mediastinal and visceral involvement have been identified previously in the SFOP6 and UKCCSG studies9 as predictive factors for a higher risk of failure, whereas none of these factors were identified as predictive of failure in the BFM90 study,8 in which only B symptoms were found to be associated with an increased risk of failure. The negative prognostic impact of skin involvement was described in the first series of the BFM group.7 Elevated LDH has also been associated previously with a higher risk of failure in the SFOP, study whereas its prognostic value was not evidenced in the BFM study. These discrepancies are probably attributable to the small number of patients in each study group and underline the importance of conducting a multivariate analysis in a large population of patients. The absence of significant heterogeneity among the 3 study groups in terms of the risk associated with the prognostic factors identified in the present study is in favor of greater applicability of these findings.
The St Jude24 and Ann Arbor staging systems23 currently used for lymphomas are not optimal for treatment decision-making in ALCL. In both classifications, more than two-thirds of the patients are classified as stages 3 and 4 based on the anatomic sites of the disease. It is difficult to apply these classification systems to ALCL patients because a high proportion have extranodal disease, which is not adequately taken into account in the St Jude classification and often arouses debate about the appropriate stage allocation in the Ann Arbor classification. Moreover, these staging systems are not adapted for the classification of patients with skin lesions. In our study, both staging systems were found to be associated with the risk of progression/relapse in the univariate analysis, but this prognostic value was no longer significant when other identified risk factors were taken into account in the multivariate analysis because of the relationships between high stages and the identified risk factors. Only a few patients had St Jude stages 1 and 2 resected disease. In the BFM and UKCCSG studies, they were treated with short-duration chemotherapy with very good results. This group of patients was not singled out in the present multivariate analysis because it was designed to identify patients at a high risk of failure. However, it is probably a subgroup of patients with a very low risk who could benefit from less intensive treatment.
Patients with bone lesions may also have a better outcome, although this factor did attain significance in the multivariate analysis. The good prognostic value of bone lesions in ALCL has already been reported by others.31
The International Prognostic Index,32 based on the LDH level, performance status, and Ann Arbor classification for patients younger than 60, is widely used for adult lymphomas. It has not yet been studied in children and could not be evaluated in our study because data on the performance status were not collected prospectively.
The outcome of the whole population is quite comparable with the results obtained by other groups with different regimens11-14,16,17,19,20,33 with a CR rate of 88% and a 5-year EFS of 71% (95% CI, 65%-77%). In this study, it is noteworthy that the risk of relapse was not higher in the small population of patients with residual disease at the end of treatment (3/13) than in the patients who attained a CR (50/197). However, this result should be interpreted with caution because of the absence of a radiologic review in this study and will have to be confirmed in a larger population of patients. Biopsy of residual mass and newer imaging methods such as positron emission tomography scan might help to assess which residual abnormalities are associated with a high risk of relapse.
This study was not designed to compare the efficacy of different treatment protocols because the different treatments were not randomly assigned and there may be systematic differences between the populations.
In all 3 study groups, most of the relapses occurred during the 6 months after the end of the treatment. The high risk of relapse at the end of chemotherapy led us to design a trial testing the interest of maintenance therapy after induction chemotherapy, which is the aim of the ongoing European Intergroup study ALCL99. This study will also allow us to validate the results of our prognostic factor study in patients assessed and treated homogeneously and will analyze the prognostic value of biologic characteristics recently available such as histologic subtypes, the immunophenotype, presence of the t (2;5) translocation and variants, and minimal disseminated disease.
In conclusion, this prognostic study performed on a large number of children with ALCL allowed us to define 2 groups of patients with very different outcomes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are indebted to all the children and parents who accepted to participate in the studies, to Lorna Saint-Ange for editing, to Ulrike Meyer and Rachel Hobson for data management, to Katelle Joly for statistics, to the pathologists who reviewed the slides, and to all the doctors who participated in the studies.
This work was supported by the Institut Gustave-Roussy, Cent pour Sang la Vie, France.
Authorship
Contribution: M.-C.L.D. performed research, analyzed data, and wrote the paper. A.R., M.Z., and D.W. designed research and collected data. G.D., I.O., and K.M. reviewed slides. L.B. designed research, collected and analyzed data, and wrote the paper. All coauthors have read and approved the submission of this manuscript.
The authors have written this paper on behalf of the Société Franc̨aise d'Oncologie Pédiatrique (SFOP) group (L.B.), the Berlin-Frankfurt-Münster (BFM) group (A.R.), and the United Kingdom Children's Cancer Study Group (UKCCSG; D.W.).
A complete list of the members of the European Intergroup for Childhood Non-Hodgkin Lymphoma is provided in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Marie-Cécile Le Deley, MD, PhD, Service de Biostatistique et d'Epidémiologie, Institut Gustave-Roussy, rue Camille Desmoulins, 94805 Villejuif, France; e-mail: le_deley@igr.fr.