Abstract
Children with Down syndrome (DS) have a markedly increased risk of acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). To identify chromosomal changes cooperating with +21 that may provide information on the pathogenesis of these leukemias, we analyzed 215 DS-ALLs and 189 DS-AMLs. Unlike previous smaller series, a significant proportion of DS-ALLs had the typical B-cell precursor ALL abnormalities high hyperdiploidy (HeH; 11%) and t(12;21)(p13;q22) (10%). The HeH DS-ALLs were characterized by gains of the same chromosomes as non–DS-HeH, suggesting the same etiology/pathogenesis. In addition, specific genetic subtypes of DS-ALL were suggested by the significant overrepresentation of cases with +X, t(8;14)(q11;q32), and del(9p). Unlike DS-ALL, the common translocations associated with non–DS-AML were rare in DS-AML, which instead were characterized by the frequent presence of dup(1q), del(6q), del(7p), dup(7q), +8, +11, del(16q), and +21. This series of DS leukemias—the largest to date—reveals that DS-ALL is a heterogeneous disorder that comprises both t(12;21) and HeH as well as DS-related abnormalities. Furthermore, this analysis confirms that DS-AML is a distinct entity, originating through other genetic pathways than do non–DS-AMLs, and suggests that unbalanced changes such as dup(1q), +8, and +21 are involved in the leukemogenic process.
Introduction
Since the late 1950s, children with Down syndrome (DS) have been known to have a significantly increased risk of developing acute leukemia–acute lymphoblastic leukemia (ALL) as well as acute myeloid leukemia (AML), with the subtype acute megakaryoblastic leukemia (AMKL) being particularly common1,2 ; the relative risks of ALL/AML and AMKL in DS patients have been estimated to be approximately 20 and 400 to 500, respectively.3-5 This, together with the fact that acquired gain of chromosome 21 occurs in approximately 20% and 5% of cytogenetically abnormal ALL and AML, respectively,6 clearly suggests that +21 is strongly associated with leukemogenesis. Since +21 in individuals with DS is present both in the leukemic as well as in the nonleukemic cells, its leukemogenic impact may be either cell autonomous, having a direct oncogenic effect, or non–cell autonomous, affecting the microenvironment. In the first situation, the DS-ALL/AMLs would have unique genetic features, whereas in the second case, one would expect a general increase of typical genetic subtypes of pediatric leukemias.7 Thus, the types and frequencies of acquired chromosome changes occurring in addition to the constitutional +21 in DS-ALL and DS-AML may provide important clues to the pathogenesis of acute leukemia in children with DS.
Although 1% to 3% of children with ALL and 2% to 15% of children with AML have DS (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), relatively little is known about the cytogenetic patterns in DS-associated leukemias, at least compared with the wealth of information on chromosomal and molecular genetic changes in non–DS-ALL/AML. The reasons for this are probably manifold. For example, most cytogenetic publications on DS-related leukemias have been single case reports or relatively small patient series, precluding detailed and quantitative analyses of the types and frequencies of chromosomal aberrations; only 2 previous studies have included more than 100 DS patients (Table S1). However, despite these limitations, several studies have shown that the karyotypic patterns of DS-ALL/AML in general seem to be different from the ones observed in acute leukemias in children without DS.
It has, for example, been reported that the ALL-associated translocations and abnormality patterns t(1;19)(q23;p13), t(4;11)(q21;q23), t(9;22)(q34;q11), hypodiploidy, and high hyperdiploidy (> 50 chromosomes; HeH) are less common in DS-ALL than in non–DS-ALL,8-15 whereas the t(8;14)(q11;q32) is overrepresented.16-21 Interestingly, the t(12;21)(p13;q22), which previously was considered to be rare in DS-ALL,12,13,22-24 has recently been suggested to be as common in DS-ALL as in non–DS-ALL.25
Regarding myeloid leukemias, it is quite clear that DS-AML is clinically and genetically unique. Most patients have acute megakaryoblastic leukemia (AMKL) that is highly responsive to chemotherapy and that has been shown to be characterized by acquired GATA1 mutations.26,27 These mutations arise in utero and are responsible for the congenital transient leukemia present in up to 10% of DS newborns.28 However, additional—at present unknown—genetic changes are needed for overt AMKL. Consistent with the distinct genetic nature of DS-AML, several studies have reported that they seldom harbor the characteristic AML-related aberrations inv(3)(q21q26), t(8;21)(q22;q22), MLL rearrangements, t(15;17)(q22;q21), and inv(16)(p13q22); furthermore, the t(1;22)(p13;q13), which encodes the chimeric RBM15/MKL1 transcript and that is characteristic for infant AMKL, is rarely found in DS-AMKL.4,10,15,29-39 On the other hand, gains of chromosomes 8, 19, 21, and 22 have been suggested to be more common in DS-AMKL than in non–DS-AML.4,10,29,34,38,40-47
To review in detail the types and frequencies of chromosomal aberrations and to identify novel genetic abnormalities in DS-ALL/AML, a collaborative study was initiated, collecting data on a total of 404 cytogenetically investigated cases, the largest series of such leukemias to date.
Methods
Patients
The karyotypic features of 215 DS-ALLs and 189 DS-AMLs, diagnosed between 1992 and 2005, were reported to the International Berlin-Frankfurt-Münster (iBFM) cytogenetic register. The cases were collected from the following countries: Austria (n = 30), Czech Republic (n = 3), Germany (n = 105), Israel (n = 8), Italy (n = 6), the Netherlands (n = 38), the Nordic countries (Denmark, Finland, Iceland, Norway, and Sweden; n = 111), and the United Kingdom (n = 103). The iBFM register was created solely for this study and only includes DS-ALL and DS-AML from the participating countries (and not karyotypic data on ALL and AML in children without DS). Thus, to compare DS-leukemias and non–DS-leukemias, a control group was retrieved from the Mitelman Database of Chromosome Aberrations in Cancer,6 searching for cases without DS in the same age ranges as the corresponding DS-ALL (range, 0-20 years) and DS-AML (range, 0-12 years). The cases ascertained were registered as “unselected,” that is they were not reported because of specific or unusual karyotypic features, and all ALL had a B-lineage immunophenotype. The study was approved by the research ethics committees at the participating departments/universities and informed consent was obtained in accordance with the Declaration of Helsinki.
DS-ALL.
Of the 215 DS-ALLs, all except one were B-cell precursor (BCP) ALL; the immunophenotype of the remaining case was unknown. Thus, none of the characterized cases had T-cell ALL. The median age of the patients was 4.8 years (range, 0-20.2 years; Figure S1) and the sex ratio (male/female) was 1.2 (119/96).
DS-AML.
Among the 189 DS-AMLs, the frequencies of the various morphologic subgroups were as follows: 9 (4.8%) unspecified, 13 (6.9%) M0, 14 (7.4%) M1/M2, 2 (1.1%) M3, 5 (2.6%) M4, 4 (2.1%) M5, 6 (3.2%) M6, and 136 (72%) M7. The median age was 1.8 years (range, 0-11.9 years; Figure S1), with the AMKL patients having a median age of 1.7 years (range, 0-4.2 years), and the sex ratio (male/female) was 0.9 (89/100).
Cytogenetic investigations and karyotypic patterns ascertained
Chromosome banding analyses were performed using standard methods, and the definition and description of clonal abnormalities followed the recommendations of ISCN.48 During recent years, fluorescent in situ hybridization (FISH) and molecular genetic analyses have been increasingly applied to verify or characterize more precisely the chromosomal abnormalities found as well as to detect the specific changes t(12;21) and MLL rearrangements in ALL and t(8;21), MLL rearrangements, t(15;17), and inv(16) in AML.
The karyotypic patterns ascertained were (1) modal chromosome numbers; only the most basic clone and the lowest mode in cases with modal number spans were registered, (2) types (balanced and unbalanced) and numbers of chromosomal aberrations; cases with incomplete karyotypes were excluded in these analyses, (3) balanced rearrangements; the Mitelman Database of Chromosome Aberrations in Cancer6 was used to assess whether the aberrations had previously been reported, (4) chromosomal breakpoints, and (5) genomic imbalances, that is, whole or partial chromosome gains and losses (in relation to the nearest ploidy level). Identical breakpoints and imbalances were registered only once per case, and when a case harbored different imbalances involving the same chromosome, the largest imbalance was recorded.
Statistical analyses
The chi-square test, with Yates correction for continuity when applicable, was used to compare karyotypic complexity, modal chromosome number distribution, and frequencies of abnormalities between DS-ALL and non–DS-ALL and between DS-AML and non–DS-AML. P values less than .05 were considered significant.
Results
DS-ALL
Basic cytogenetic features.
Chromosome banding analyses were successful in 93% (201/215), of which 54% (109/201) harbored clonal aberrations. Fourteen cases were karyotypic failures; however, FISH and/or reverse-transcription–polymerase chain reaction (RT-PCR) revealed that 2 of these were BCR/ABL1 positive and that 7 harbored the ETV6/RUNX1 fusion. Furthermore, 12 of the cases with a seemingly normal karyotype were shown to be t(12;21) positive. Thus, taking the findings from targeted analyses into account, the frequencies of informative and aberrant cases increased to 98% (210/215) and 62% (130/210), respectively.
Modal chromosome numbers.
As seen in Table 1, the most common ploidy levels were pseudodiploidy and low hyperdiploidy (39% each). HeH was seen in 11% of the cases. None had fewer than 46 chromosomes.
Types and numbers of chromosomal aberrations.
Among the cytogenetically abnormal cases with complete karyotypes, 82% (79/96) harbored unbalanced changes only; the remaining cases had either both unbalanced and balanced aberrations (13/96; 14%) or only balanced abnormalities (4/96; 4.2%). The numbers of chromosomal changes varied between 0 and 19, with a median of 1 aberration per case (Figure S2). Sole anomalies were seen in 36 (33%) of the 109 karyotypically aberrant cases.
Balanced chromosomal abnormalities.
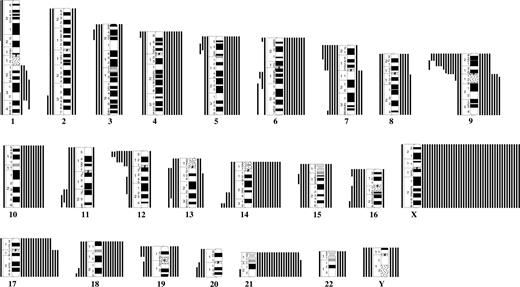
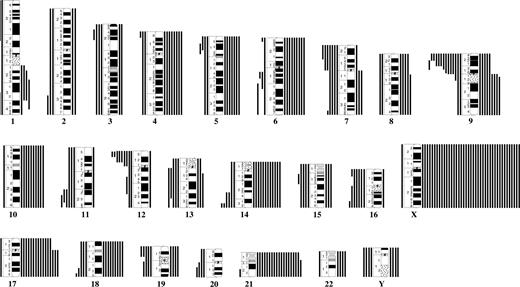
A total of 20 different translocations or inversions were identified (Table S2). The t(12;21) was found in 22 (10%) of 210 cases. The median age of the ETV6/RUNX1-postive ALL was 6.1 years. The second most common balanced aberration was t(8;14), seen in 2.8% (3/109). The only other recurrent translocation was t(9;22), which was detected by RT-PCR in 1.0% (2/210). The following breakpoints were recurrently involved: 1q32, 8q11, 9q34, 12p13, 12q13, 14q32, 20q13, 21q22, and 22q11 (Figure 1).
Breakpoint map of structural chromosomal abnormalities in 210 cytogenetically abnormal Down syndrome–acute lymphoblastic leukemias. Cases ascertained with targeted analyses for t(9;22) and t(12;21) are also included. ● and ○ represent unbalanced and balanced abnormalities, respectively.
Breakpoint map of structural chromosomal abnormalities in 210 cytogenetically abnormal Down syndrome–acute lymphoblastic leukemias. Cases ascertained with targeted analyses for t(9;22) and t(12;21) are also included. ● and ○ represent unbalanced and balanced abnormalities, respectively.
Genomic imbalances.
The most common gains were +X, +21, +17, +14, and +10; losses were rare and mainly involved chromosomes 16, Y, 7, and 15. The most frequent partial imbalances were del(9p) and del(12p) (Table 1; Figure 2). Common (≥ 3) breakpoints involved in unbalanced abnormalities were 1q32, 9p21-p22, 9q10, 11q23, 12p12-p13, 14q32, 17q10, and 19p13 (Figure 1). Among the 36 ALLs with sole anomalies, 8 (22%) had +X, 6 (17%) had del(9p), and 3 (8.3%) had del(12p); no other imbalance was recurrent as a single change.
Map of karyotypic imbalances in 109 cytogenetically abnormal Down syndrome–acute lymphoblastic leukemias. Gains are indicated to the right and losses to the left of each chromosome.
Map of karyotypic imbalances in 109 cytogenetically abnormal Down syndrome–acute lymphoblastic leukemias. Gains are indicated to the right and losses to the left of each chromosome.
Cytogenetic features in DS-ALL versus non–DS-ALL.
The numbers of anomalies and modal chromosome number distributions in DS-ALL differed significantly from those observed in non–DS-ALL (Table 1); the DS-ALL more frequently harbored 2 anomalies (P < .001) and were more often low hyperdiploid (P < .001). The most frequent additional chromosomes in the 11 cytogenetically informative HeH DS-ALLs were X (10/11), 4 (8/11), 17 (8/11), 21 (8/11), 10 (7/11), 14 (7/11), 18 (7/11), and 6 (5/11); these 8 chromosomes are also the most commonly gained in high hyperdiploid non–DS-ALL.6 Gain of the X chromosome, t(8;14), and del(9p) were significantly more common in the DS-ALL, whereas t(9;22), 11q23 rearrangements, and +21 were overrepresented in the non–DS-ALL (Table 1).
DS-AML
Basic cytogenetic features.
Chromosome banding analyses were successful in 99% (188/189), of which 72% (135/188) were karyotypically abnormal.
Modal chromosome numbers.
As seen in Table 2, the most common ploidy levels were low hyperdiploidy (45%) and pseudodiploidy (43%); none had less than 43 chromosomes.
Types and numbers of chromosomal aberrations.
Among the cytogenetically abnormal cases with complete karyotypes, 85% (113/133) harbored unbalanced changes only, 6.8% (9/133) had both unbalanced and balanced aberrations, and 8.3% (11/133) had only balanced abnormalities. The numbers of chromosomal changes varied between 0 and 9, with a median of 1 aberration per case (Figure S3). Sole anomalies were found in 57 (42%) of the 135 aberrant cases.
Balanced chromosomal abnormalities.
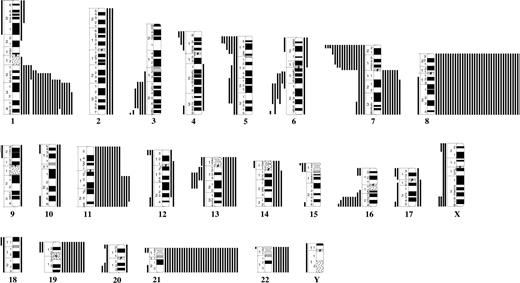
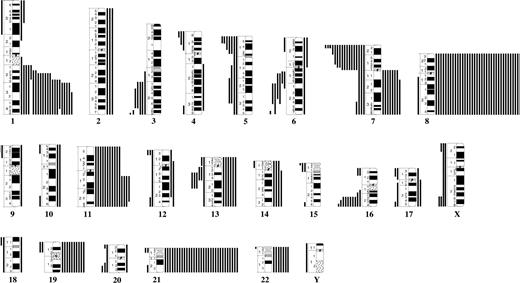
No recurrent translocations/inversions were identified, but 20 balanced changes were found in single cases (Table S3). The following breakpoints were recurrently involved: 3q25, 5p15, 11q13, and 17q21 (Figure 3).
Breakpoint map of structural chromosomal abnormalities in 135 cytogenetically abnormal Down syndrome–acute myeloid leukemias. ● and ○ represent unbalanced and balanced abnormalities, respectively.
Breakpoint map of structural chromosomal abnormalities in 135 cytogenetically abnormal Down syndrome–acute myeloid leukemias. ● and ○ represent unbalanced and balanced abnormalities, respectively.
Genomic imbalances.
The most common trisomies were +8, +21, +11, and +19; the only recurrent monosomies involved chromosomes X (females only), 5, and 7. The most frequent partial imbalances were dup(1q), del(7p), and del(16q) (Figure 4). Common (≥ 3) breakpoints involved in unbalanced abnormalities were 1p36, 1q21-q23, 1q25-q31, 3q21, 5p15, 5q31, 6q13, 6q21, 7p22, 7q10, 11q13, 13q14, and 16q22 (Figure 3). Among the 57 AML with single anomalies, 17 (30%) had +8, 3 (5.3%) had +21, and 2 (3.5%) each had i(7q) or del(16q); no other imbalance was recurrent as a single change. Three possibly recurrent unbalanced translocations were identified: der(4)t(1;4)(q21;p13)/der(4)t(1;4)(q23;p15), der(7)t(1;7)(q22;p22)/der(7)t(1;7)(q23;p22)/der(7)?t(1;7)(q31-32;p?22), and der(16)t(11;16)(q13;p13)/der(16)t(11;16)(q13;p12); all these occurred in AMKL.
Map of karyotypic imbalances in 135 cytogenetically abnormal Down syndrome–acute myeloid leukemias. Gains are indicated to the right and losses to the left of each chromosome.
Map of karyotypic imbalances in 135 cytogenetically abnormal Down syndrome–acute myeloid leukemias. Gains are indicated to the right and losses to the left of each chromosome.
Cytogenetic features in DS-AML versus non–DS-AML.
The numbers of anomalies and modal chromosome number distributions in DS-AML differed significantly from those observed in non–DS-AML (Table 2). The DS-AML less often had single anomalies (P < .001) and were more frequently low hyperdiploid (P < .001) than non–DS-AML. In DS-AML, dup(1q), del(6q), del(7p), dup(7q), +8, +11, del(16q), and +21 were more frequent, whereas loss of the Y chromosome, t(8;21), 11q23 rearrangements, and inv(16) were significantly more common in non–DS-AML (Table 2).
Discussion
The present series of 404 karyotypically investigated DS-associated acute leukemias, the largest to date, is admittedly not population based, considering the varied numbers of patients entered into the iBFM cytogenetic database from the participating countries. It is highly unlikely that this variation reflects geographic differences in incidence of DS or that children with DS have a higher risk of developing acute leukemias in certain countries. Instead, the most likely explanation is underreporting or exclusion of DS patients in some treatment studies. Despite this, we nevertheless believe that the cytogenetic patterns identified are representative, partly because of the size of the study and partly because the age distributions and sex ratios agree well with previous reports from population-based studies of DS-ALL and DS-AML.2
The previous findings of low frequencies of T-cell immunophenotypes (none found in the present series), hypodiploidy, HeH, t(1;19), t(9;22), and 11q23 translocations in DS-ALL8-15 were confirmed in this study. In fact, we showed that most of these genetic aberrations were statistically significantly less common in DS-ALL (Table 1). Although acquired +21 was, except for +X, the most frequent abnormality in DS-ALL (Figure 2), it was nevertheless significantly less frequent than in non–DS-ALL (Table 1). The reason for this is most likely the relatively low proportion of HeH DS-ALLs, a cytogenetic subgroup in which chromosome 21 is tetrasomic in the majority of the cases.50,51 However, it should be emphasized that a sizeable fraction (11%) of DS-ALL was HeH (Table 1) and that the most frequent supernumerary chromosomes, namely X, 4, 6, 10, 14, 17, 18, and 21, were the same as in HeH cases in patients without DS6 ; these cytogenetic similarities indicate that a constitutional +21 has no major impact on type of trisomies/tetrasomies occurring in HeH DS-ALL. Indirectly, this also suggests that the underlying mechanism behind high hyperdiploidy, shown previously to involve—most likely—one single, highly abnormal cell division,52-54 is the same irrespective of whether the patient has DS or not. If so, then the etiology of HeH ALL could also be similar in DS and non–DS patients.
In agreement with a previous study,25 the ETV6/RUNX1 fusion was found in a substantial proportion of the DS-ALLs (Table 1). Although the observed frequency (10%) is lower than the approximately 25% seen in most series of pediatric BCP ALL,49 it should be noted that the true prevalence of t(12;21) in the present patient cohort most likely is higher, considering that not all DS-ALL cases had been screened for this abnormality. Indeed, among the 34 BCP DS-ALLs in the Nordic countries and the 60 BCP DS-ALLs in the United Kingdom tested for ETV6/RUNX1, 6 (18%) and 9 (15%), respectively, have been positive for this gene chimera (data not shown). It is thus puzzling why some studies have reported much lower frequencies, that is 0% to 9%.12,13,22-24 It may be noteworthy that the median age of the t(12;21)-positive DS-ALL patients was 6.1 years, that is, clearly higher than the median age of 4.3 years for those without DS.55 It is today well established that the t(12;21) often, perhaps always, originates prenatally and that additional genetic changes are necessary for leukemic transformation.56 It would hence seem that such secondary hits occur later in patients with DS or, perhaps less likely, that ETV6/RUNX1 does not occur in utero in individuals with DS. Interestingly, the t(12;21) was the only ALL-associated translocation that may be equally frequent in DS and non–DS patients. This implies a complex pathogenesis of DS-ALL, with a constitutional +21 not only increasing the risk for the common t(12;21)-positive and HeH ALL but also for DS-ALL characterized by genetic changes less often seen in non–DS-ALL.
Given that the prevalence of ALL in children with DS is increased approximately 20-fold,5 the substantial proportion of common ALL subtypes, that is HeH and t(12;21), in the present series strongly indicates that constitutional +21 also increases the absolute risk for these genetic subgroups—if the increased risk applied only to other cytogenetic subsets, then the frequencies of HeH and t(12;21) would have been close to 1%. Interestingly, immune responses to infections have been suggested to be involved in the etiology of common childhood ALL.55,57 It is tempting to speculate that the well-known immune deficiency in children with DS plays a role in this context, something that would support the non–cell autonomous hypothesis.7
Three abnormalities—+X, t(8;14), and del(9p)—were significantly more common among the DS-ALLs (Table 1). That +X is frequent has been reported previously.8,10,15 A novel finding, however, was the high frequency (20%) of +X as the sole acquired anomaly. In contrast, among the unselected BCP non–DS-ALLs with single changes reported in the literature, only 1% has had +X as the sole change.6 Frequent loss of 9p, through deletions, unbalanced translocations, or i(9q), has previously been noted.8,58 Although the leukemogenic impact of 9p deletions often is considered to be due to loss of tumor suppressor genes, in particular CDKN2A,59 haploinsufficiency may also play a role, as recently shown for the PAX5 gene at 9p13 in pediatric ALL.60 Considering that del(9p) was the sole change in close to 20% of DS-ALLs with single abnormalities and hence likely of great importance in the pathogenetic process, it would seem fruitful to investigate further its molecular genetic consequences, focusing on the minimally deleted 9p22-pter segment (Figure 2). The overrepresentation of t(8;14) was expected because several previous studies have described an association between DS-ALL and this translocation.16-21 In fact, of the 46 t(8;14)-positive cases reported to date,6 11 (24%) have had DS. Akasaka et al61 recently showed that it results in dysregulation of the CCAAT/enhancer binding protein delta (CEBPD) gene at 8q11 through illegitimate recombination with IGH@ at 14q32. In their study, 4 additional IGH@ rearrangements were reported to deregulate other members of the CEBP gene family: inv(14)(q11q32) or t(14;14)(q11;q32) (CEBPE), t(14;19)(q32;q13) (CEBPA, CEBPG), and t(14;20)(q32;q13) (CEBPB). One of these—the inv(14)—has previously been reported in one DS-ALL.61 Interestingly, t(14;14) was found in a single case in the present compilation (Table S2). Thus, not only CEBPD deregulation but also aberrant expression of CEBPE may be a common feature of DS-ALL. Future global gene expression analyses should be able to clarify whether there are significant differences in expression patterns of CEBP gene family members between DS-ALL and non–DS-ALL.
Regarding AML, and in line with several previous studies,4,29,34,38,39 the present compilation clearly revealed that t(8;21), 11q23 translocations, and inv(16) are significantly less common in DS-AML than in non–DS-AML (Table 2). Furthermore, none of the 136 DS-AMKL harbored the t(1;22), verifying that this translocation is very rare indeed in this AML type.4,10,32,33,35,37 Thus, GATA1 mutations, characteristic for DS-AMKL, and RBM15/MKL1, typical for AMKL in infants without DS, are in all practice mutually exclusive.
It is today well established that the GATA1 mutations arise in utero and that they are necessary but insufficient for the development of overt DS-AMKL.26-28 The chromosomal aberrations in DS-AMKL could hence provide important clues to the genetic events associated with the transformation of the preleukemic GATA1-postive clone. Of particular interest in this context are the 8 genomic imbalances shown to be significantly more common in DS-AML, that is, dup(1q), del(6q), del(7p), dup(7q), +8, +11, del(16q), and +21 (Table 2).
Recurrent gains of 1q, through duplications or unbalanced translocations, have previously been reported in DS-AML.4,10,62 In these studies as well as in the present one, dup(1q) occurred mainly in DS-AMKL; only a handful cases have been other subtypes. It is in this context noteworthy that 3 possibly recurrent unbalanced translocations were identified in DS-AMKLs, namely der(4)t(1;4) der(7)t(1;7), and der(16)t(11;16); notably, 2 of these result in gain of 1q. The duplicated segment often comprised almost the entire 1q, making it difficult to delineate a minimally gained region; however, gain of 1q32-q42 was most common (Figure 4). Whether the functionally important outcome is duplication of one or several mutated genes or a general gene dosage effect remains to be elucidated. A relatively high frequency of del(6q) in DS-AML has previously been suggested only in one study.29 Deletions involving 6q are otherwise quite rare in AML, being associated mainly with lymphoid malignancies.63-65 As yet, the target gene(s) is unknown, and because no clear-cut minimally deleted segment was identified (Figure 4), it may prove difficult to identify the genes involved. The losses of 7p and gains of 7q often occurred together in DS-AML, as a consequence of i(7q). The fact that i(7q) occasionally was the sole chromosomal anomaly, as seen in 2 of the present cases and in 5 previously published cases,6 indicates that it plays an important role during the early stages of leukemogenesis. Recently, FISH mapping of i(7q) in acute leukemias revealed clustered breakpoints in 7p11.2, suggesting that genes in 7p11.2 may be rearranged.66 However, considering that all but one of the dup(7q) occurred as a result of i(7q) but that half of the del(7p) arose through other mechanisms—deletions or unbalanced translocations—it seems likely that loss of 7p, rather than gain of 7q, is the pathogenetically important outcome of the i(7q) in DS-AML. If so, the putative target gene may reside at 7p22 (Figure 4). The frequent occurrence of 16q loss in DS-AML was an unexpected finding that, to the best of our knowledge, has previously never been reported. As seen in Figure 4, the minimally deleted segment was 16q24, which hence may harbor genes of leukemogenic importance, not least considering that del(16q) was recurrent as a primary chromosomal abnormality.
The most common abnormality in DS-AML was trisomy 8, seen in close to 30% of the cytogenetically abnormal cases (Figure 4; Table 2). Not surprisingly, frequent gain of this chromosome has been found in most previous studies of DS-AML.4,10,15,29,38,40-43,45-47 Less well appreciated is the fact that +8 often is the sole acquired anomaly in DS-AML, being found in 30% of the cases with single anomalies. Thus, this abnormality is undoubtedly intimately involved in the leukemogenesis of DS-AML. However, its functional and pathogenetic consequences in DS-AMLs, as well as in non–DS-related malignancies, remain elusive.67 Other common trisomies in DS-AML included +11 and, particularly in AMKL, +21; the latter gain has also been emphasized in several previous studies.4,15,34,42,43,46 The fact that trisomies are quite characteristic for DS-AML suggests that DS patients may be more susceptible to nondisjunctional events during cell division, as suggested by Fong and Brodeur already in 1987.68
In conclusion, the present study reveals that DS-ALL is a genetically heterogeneous disorder that comprises both the well-known ALL-associated abnormalities HeH and t(12;21) as well as changes significantly more common in DS-ALL such as +X, del(9p), and CEBPD rearrangements. Taken together, this suggests a complex pathogenesis of ALL in children with DS. Furthermore, this analysis clearly confirms that DS-AML is a distinct entity, as also recognized in the present WHO classification,69 originating through other genetic pathways than do non–DS-AML, including GATA1 mutations and secondary, mainly cytogenetically, unbalanced changes such as dup(1q), +8, and +21.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Swedish Children's Cancer Foundation (E.F. and B.J.), Israeli Cancer Association (S.I. and B.S.), Israel Science Foundation (S.I. and B.S.), the Austrian Kinderkrebsforschung (O.A.H.), Parents' Foundation for Children with Cancer, Giessen (A.T.-S.), and Leukemia Research, United Kingdom (C.J.H.)
Authorship
Contribution: E.F. designed and performed the research, analyzed data, and wrote the paper. S.I., H.B.B., O.A.H., A.P., K.M., B.S., C.J.H., A.T.-S., and B.J. performed the research, analyzed data, and wrote the paper.
A complete list of the participants in the iBFM-SG study is provided in Document S1, available on the Blood website.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Erik Forestier, Pediatrics Unit, Department of Clinical Sciences, University of Umeå, SE-901 85 Umeå, Sweden; e-mail: erik.forestier@pediatri.umu.se.