Abstract
This prospective study evaluated sexual function through 5 years after myeloablative allogeneic hematopoietic cell transplantation (HCT) for cancer to determine sexual function recovery and residual problems. Adults completed measures before HCT (N = 161), with survivors followed at 6 months and at 1, 2, 3, and 5 years. At 5 years case-matched controls also completed assessments. Analyses indicated that men and women differed in rates of being sexually active across time (P < .001) and in overall sexual function (P < .001). Both sexes declined in sexual activity rates and sexual function from before HCT to 6 months afterward (P ≤ .05). Activity rates recovered for men by 1 year (74%) and for women by 2 years (55%). Men improved from their 6-month nadir in sexual function by 2 years (P = .02), whereas women did not improve by 5 years (P = .17). Both male and female survivors were below controls in rates of sexual activity and sexual function at 5 years. Most women reported sexual problems (80% of survivors vs 61% of controls, P = .11); in contrast for men 46% of survivors versus 21% of controls (P = .05) reported problems. Thus, despite some recovery, sexual dysfunction remained a major problem for men and women after HCT. Aggressive efforts are needed to treat these deficits.
Introduction
Sexual dysfunction is widely noted in studies of quality of life after systemic cancer treatment, particularly after alkylating agents used in hematopoietic cell transplantation (HCT).1-5 Yet few studies have examined the nature of this dysfunction or followed survivors over time to determine whether function improves, stabilize, or declines with continued recovery. Although sexual problems also occur in a large percentage of the normal population, particularly among women,6,7 sexual function in cancer survivors after HCT has rarely been compared with that of case-matched controls.8,9
Numerous cross-sectional studies report sexual dysfunction after high-dose treatment and HCT.3,5,8-12 In a previous case-control cohort, we found that 10-year survivors had more sexual problems than did controls.8 Other investigations have focused on sexual function during the first year, but we are aware of none that describes prospective longitudinal outcomes beyond 1 year.3,4,9,13-16
This research provides results of a prospective evaluation of sexual function in survivors of myeloablative HCT through 5 years after treatment. Our goals included identifying the trajectory of recovery from the hypothesized 6-month nadir of function after HCT, as well as comparing survivor 5-year sexual function and residual sexual problems to case-matched controls. We selected 6 months as a point of reference because it is the hypothesized nadir in sexual function for all survivors among the time points assessed. In contrast, the pre-HCT time point represents a potentially highly variable point in the sexual function trajectories for transplant recipients. Treatment regimens before transplantation are not uniform. Depending on the extent of prior treatment, as well as many other factors, pre-HCT baseline may be a high point in function for some patients. For others it may represent a nadir, and for still others it may represent a midpoint from which either further recovery or decline is possible. Recent survivorship guidelines after HCT have recommended using the 6-month time point as a landmark from which to examine late effects.17 On the basis of previous research and this guideline, we hypothesized that (1) men would have higher rates of sexual activity and better sexual function over time compared with women; (2) men and women would decline in both rates of being sexually active and in quality and quantity of sexual function from before HCT to 6 months afterward; (3) between 6 months and 5 years men would recover in sexual function, whereas women would not recover3 ; and (4) 5 years after HCT, men and women would have lower sexual activity rates and poorer sexual function than would matched controls. Secondary goals of this research were to identify reasons that survivors do not return to sexual activity and to define long-term sexual problems, so that treatments can be designed for them.
Methods
Participants and procedures
The Institutional Review Board at the Fred Hutchinson Cancer Research Center approved all study procedures and forms. Written informed consent was obtained from all patients for the longitudinal study in accordance with the Declaration of Helsinki. All transplant recipients at the Hutchinson Center scheduled to receive myeloablative preparative regimens were screened and approached for consent within constraints of the study coordinator's ability to contact them before treatment started. Nonrelapsing survivors were followed from before HCT to 5 years afterward. These results are part of an investigation of recovery and long-term quality of life in adult survivors of HCT. Adult US residents (n = 236) preparing for a first HCT between March 1996 and May 1998 were invited to participate, 37 declined consent (16%); 199 were enrolled (Figure 1). Eligible participants were 22 years or older, receiving their first HC transplant for a malignancy, had sufficient English proficiency to complete assessments, did not have major psychiatric diagnoses as indicated by the medical team, and completed the baseline assessment before beginning HCT. No exclusions were made for sexual orientation or sexual partner status. Although allogeneic and autologous HC transplant recipients participated, the number of autologous recipients was small and diverse. Consequently, results are reported only for allogeneic recipients (n = 161).
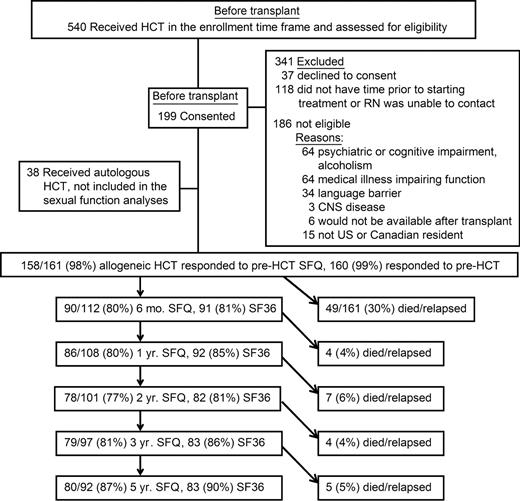
Flow diagram of patients through the study. Note that the 5-year relapse-free survival rate overall is 57% (92 of 161). The overall completion rate on SFQ for relapse-free survivors was 84%. HCT indicates hematopoietic cell transplantation; and SFQ, Sexual Function Questionnaire.
Flow diagram of patients through the study. Note that the 5-year relapse-free survival rate overall is 57% (92 of 161). The overall completion rate on SFQ for relapse-free survivors was 84%. HCT indicates hematopoietic cell transplantation; and SFQ, Sexual Function Questionnaire.
Pretransplantation assessment was conducted in the ambulatory clinic. Repeated self-report forms were mailed to nonrelapsed survivors after 6 months and after 1, 2, 3, and 5 years. Nonresponding participants were contacted by phone to facilitate return of assessments. All participants were followed until 5 years, request to withdraw, relapse, or death. At 5 years, surviving participants were asked to nominate a case-matched control. If possible the control was a biologic sibling of the same sex and within 5 years of the survivor's age. If no such person existed, survivors nominated a friend of the same sex, known since before transplantation, within 5 years of the survivor's age, and of the same ethnicity, race, and education (<4-year college degree versus at least a college degree). Because the larger study required face-to-face neuropsychological testing,18 controls needed to be seen by a traveling neuropsychometrist. When nominated controls were not accessible, we recruited community volunteers through posters at community sites in Seattle, also case-matched to survivors on sex, age, ethnicity, race, and education.
Measures
Medical records provided diagnosis, treatment regimen, type of stem-cell donor, chronic graft-versus-host disease (GVHD) status, and dates of recurrent malignancy or death. Chronic GVHD was scored by physician specialists at peak event as clinical, subclinical, abnormal, normal, or unevaluable. We summarized these codings as clinical, not clinical, or unevaluable.
Standard self-report questions asked about demographics, menopausal status, current medications, health function, and sexual function. Patient-reported medical outcomes have established reliability in various medical and nonmedical population, including HCT.19,20 Because survivors were not routinely seen at the transplantation center after 1 year, chronic GVHD severity was self-rated by survivors in follow-up assessments as “none,” “mild,” “moderate,” or “severe.” The 37-item Sexual Function Questionnaire (SFQ) has well-defined reliability and validity with HCT and normative samples.9 It assessed 9 domains (interest, desire, arousal, orgasm, satisfaction, activity, relationship, masturbation, and problems) established through principal components analysis with factor loadings all above 0.50 plus a mean sexual function score, with internal consistency ranging from α = 0.81 to 0.94. Male and female versions match in content and scoring with the exception of differing sexual problem content. Frequency of sexual thoughts and behaviors were reported, ranging from “not at all” to “more than once a day.” Sexual responses, satisfaction, and problems were rated for intensity (“not at all” to “extremely”) or frequency of occurrence during sexual activity (“not at all” to “always”). The 2 primary outcomes were sexual activity reported as “yes” or “no” to “have you been sexually active in the past month (alone or with a partner)” and mean score for quality and quantity of sexual function across items. If not sexually active, respondents were given a list of possible reasons and told to mark as many as apply. The Short Form 36 Health Survey (SF36)21 measured 8 dimensions of health function, with t scores for summary mental and physical components.22 The SF36 correlated at r greater than 0.50 with medical comorbidities in HCT survivors and controls8 and is provided to define and compare functional status of cohorts.
Statistical analysis
Sample descriptions and graphic displays included data on all responding participants and controls at each time point. Longitudinal analyses were conducted on the subset of 112 (70%) of 161 6-month relapse-free survivors (RFSs).17 Pretransplantation demographics, sexual activity, and mean sexual function were compared for 6-month RFSs versus non-RFS patients using t tests, chi-square tests, and Cochran-Armitage tests for trend. The same methods compared pretransplantation demographics.
Longitudinal regression models were fitted with the use of generalized estimating equations (GEEs) with independence correlation and robust standard errors. Confidence intervals (CIs) were set at 95%. GEEs with independence working correlation protect against regression model bias related to time-varying covariates23 and attrition because of death.24 Responses from 6 months to 5 years were modeled as categorical to avoid imposing a predetermined structure (ie, linear or quadratic) for decline and recovery. Interaction terms were fitted to compare sexual activity rates and function separately by sex, based on known differences of alkylating agents on male and female gonadal systems.25,26 Comparisons that involved sexual activity and function before and after HCT were based on Wald tests. Comparisons with matched controls were conducted by paired t tests and McNemar test for matched pairs, separately for men and women. Reasons for sexual inactivity and rates of problems were summarized descriptively. Statistical analyses were conducted using SAS/STAT 9.1 (SAS Institute, Cary, NC), and R 2.0.1 (R Project, http://www.r-project.org).
Results
Cohort characteristics
The SFQ response rate was 84%, averaged across time versus 87% on the SF36 (Figure 1). All 161 participants completed the SFQ at one or more time points. Six-month RFSs did not differ from those who died or relapsed by 6 months (non-RFSs) in age, sex, ethnicity, education level, income, marital status, or whether the donor was related or unrelated. RFSs had a higher rate of chronic-phase chronic myeloid leukemia and a lower rate of relapsed or persistent malignancy before transplantation (P = .003, Cochran-Armitage trend test), as expected. On the SF36, mental summary scores did not differ between RFS and non-RFS groups (P = .75), but non-RFSs had lower physical summary scores (P = .003). Analyzed separately for men and women, RFSs and non-RFSs did not differ in pretransplantation rates of sexual activity (Figure 2) or mean sexual function score (Figure 3), except that non-RFS men had marginally lower mean scores for sexual function (P = .06).
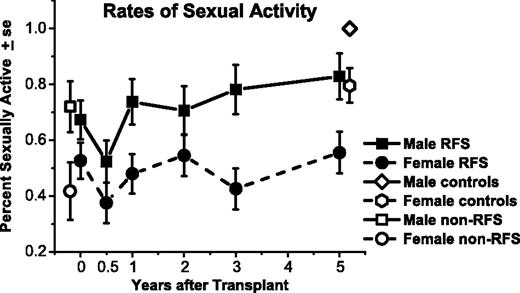
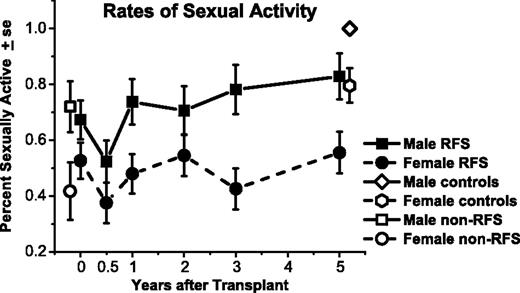
Percentage of males and females who were sexually active, among responding relapse-free-survivors (RFSs) from before transplantation (N = 109) to 5 years after transplantation (n = 80). Rates are also graphed for non-RFS patients before transplantation (n = 49) and controls at 5 years (n = 77). Both men and women declined in rates of being sexually active from before transplantation to 6 months afterward (P = .05). On average, men improved by 1 yea compared with 6-month levels (P = .02); women improved by 2 years (P = .03), but both remained below their respective controls at 5 years.
Percentage of males and females who were sexually active, among responding relapse-free-survivors (RFSs) from before transplantation (N = 109) to 5 years after transplantation (n = 80). Rates are also graphed for non-RFS patients before transplantation (n = 49) and controls at 5 years (n = 77). Both men and women declined in rates of being sexually active from before transplantation to 6 months afterward (P = .05). On average, men improved by 1 yea compared with 6-month levels (P = .02); women improved by 2 years (P = .03), but both remained below their respective controls at 5 years.
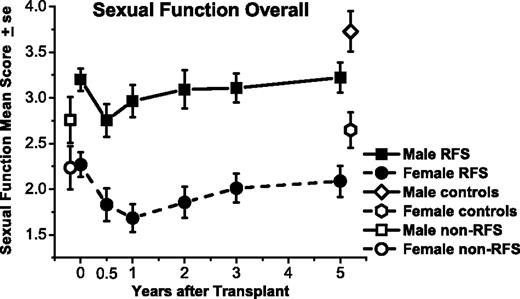
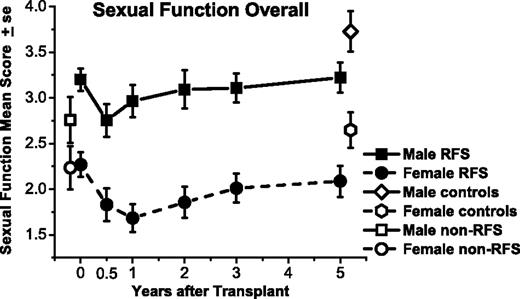
Sexual function means for responding male and female relapse-free survivors (RFSs) from before transplantation (N = 109) to 5 years after transplantation (n = 80), measured on the same scale, although with different problem items. Means are also graphed for non-RFS patients before transplantation (n = 49) and controls at 5 years (n = 77). Both men and women declined in average sexual function from before transplantation to 6 months afterward (P < .01). Women did not improve from 6-month post-transplantation levels by 5 years (P = .17) and remained below matched controls (P = .03). Men improved by 2 years (P = .02) but remained below their respective controls at 5 years (P = .01).
Sexual function means for responding male and female relapse-free survivors (RFSs) from before transplantation (N = 109) to 5 years after transplantation (n = 80), measured on the same scale, although with different problem items. Means are also graphed for non-RFS patients before transplantation (n = 49) and controls at 5 years (n = 77). Both men and women declined in average sexual function from before transplantation to 6 months afterward (P < .01). Women did not improve from 6-month post-transplantation levels by 5 years (P = .17) and remained below matched controls (P = .03). Men improved by 2 years (P = .02) but remained below their respective controls at 5 years (P = .01).
In the RFS sample (Table 1), men and women did not differ by age at transplantation, ethnicity, education, income, marital status, or receipt of total body irradiation. RFS men and women differed in likelihood of persistent or relapsed disease (P = .04), with men more likely to have relapsed and to have poorer SF36 physical component scores before transplantation (P = .007), whereas the sexes did not differ on mental component scores (P = .09). Relapsed or persistent disease was associated with poorer physical component scores (P = .001). In rate of post-transplantation chronic GVHD, the RFS cohort and the 5-year survivors were similar, with 69% (n = 77) and 70% (n = 54) having clinical chronic GVHD, respectively. Three percent were unevaluable for chronic GVHD in the RFS cohort. Among 5-year survivors 65% (n = 50) had been diagnosed at some point with clinical chronic GVHD and 1% (n = 1) was unevaluable. By 5 years, survivors reported the following rates of current chronic GVHD: 1.5% severe, 4.5% moderate, 28% mild, and 66% none currently. Thirteen percent continued to take an oral immunosuppressant medication. Standard procedure at discharge from the transplantation center is to place all women on hormone therapy unless medically contraindicated. At 6 months 83% of female survivors reported taking prescribed hormones. At 1 year the rate remained fairly stable at 78% but declined to 57% by 5 years. None of the men indicated that they were taking testosterone therapy at the 5-year assessment. Nor did they indicate use of sildenafil or similar erectile function agents, although we did not specifically ask about this use.
Case-matched controls included 36% siblings, 40% friends, and 23% community volunteers. The types of controls did not differ in demographic characteristics, SF36 physical or mental scores, or SFQ scores, except that volunteers were less likely to have annual income more than $75 000 (P = .005). Five-year survivor and control cohorts were comparable (Table 1), with the exception that most female survivors (93%) were postmenopausal versus 50% of female controls (P < .001), and 57% of female survivors were taking hormone therapy versus 25% of female controls (P < .01). In addition, on the SF36 the control cohort had higher physical component t scores on the SF36 than did 5-year survivors (P < .01).
Sexual function changes
Results for the longitudinal GEE analyses are displayed graphically in Figures 2 and 3, with key comparisons in Table 2. Men had higher rates of sexual activity than did women across time for RFSs (P = .003), for non-RFSs before HCT (P = .03), and for controls (P = .006).
Odds of women being sexually active were less likely after 6 months, when only 38% were active than before HCT [Figure 2; odds ratio (OR), 1.9; 95% CI, 1.0-3.4; P = .05]. By 2 years after HCT, with 55% active, women recovered to rates higher than at 6 months (OR, 2.0; 95% CI, 1.1-3.7; P = .03) and comparable with before HCT (OR, 1.1; 95% CI, 0.6-1.9; P = .80). The 5-year rate of 56% continued higher than at 6 months afterward (OR, 2.1; 95% CI, 1.1-4.0; P = .03), not different from before HCT (OR, 1.1; 95% CI, 0.6-2.1; P = .71), and lower than matched controls (P = .02).
Men were also less likely to be sexually active at 6 months afterward (52% active) compared with before HCT (67% active; OR, 1.9; 95% CI, 1.0-3.5; P = .05). By 1 year, the 74% active rate for men had improved from their 6-month nadir (OR, 2.5; 95% CI, 1.1-5.7; P = .02) and was not different from before HCT (OR, 1.4; 95% CI, 0.7-2.6; P = .36), with 83% active after 5 years. Nonetheless, male 5-year survivors were less likely to be sexually active than were matched controls (P = .04).
Quality and quantity of sexual function (SFQ mean) was 0.4 points lower after 6 months than before HCT for women (95% CI, −0.7 to −0.2; P = .003; Figure 3; Table 2). Average sexual functioning at 5 years did not differ significantly from either 6 months after HCT (difference, 0.3; 95% CI, −0.1 to 0.6; P = .17) or before HCT (difference, −0.2; 95% CI, −0.5 to 0.2; P = .31). After 5 years female survivors' scores averaged 0.5 points lower than matched female controls (95% CI, 0.1-0.9; P = .03).
Men also declined in quality and quantity of sexual function between before HCT and 6 months afterward (difference, −0.4; 95% CI, −0.7 to −0.2; P = .001). By 2 years, male SFQ mean scores improved compared with 6 months afterward (difference, 0.3; 95% CI, 0.0-0.6; P = .02). After 5 years mean scores remained improved from 6 months and were similar to before HCT (95% CI, −0.3 to 0.4; P = .90). However, at 5 years scores of male survivors were an average of 0.51 points lower than scores of matched controls (95% CI, 0.12-0.89; P = .01).
Reasons for sexual inactivity and types of sexual problems
For female controls and survivors across time, the most prevalent reason given for absence of sexual activity was lack of a partner (Table 3). For male survivors, reasons for sexual inactivity varied (Table 3). Nearly 20% of women at both 6 months and 5 years were inactive in part because of lack of interest or libido, suggesting that this problem did not improve over time. In contrast, for men, lack of interest or libido as a reason for inactivity declined from 14% to 6% between 6 months and 5 years.
Respondents who were not attempting sexual activity had difficulty identifying their sexual problems. Consequently, sexual problems at 5 years are described for those who were sexually active (Table 4). Nearly half (46%) of male survivors reported at least one sexual problem at least half the time versus 21% of male controls (P = .05). This compares with 80% of female survivors versus 61% of female controls reporting problems (P = .11).
Discussion
This is the first prospective longitudinal study of sexual function in survivors for 5 years after HCT. It clearly documents that sexual dysfunction is a major problem that does not fully recover after cancer treatment and HCT for many male and female survivors. Men by and large recover from HCT-specific impairments, whereas women do not fully recover from the effect of HCT on sexuality. Consistent with studies of sexual function in the general population and among cancer survivors, men reported better sexual function than did women across cohorts and time. Sexual function declined as predicted for survivors from before HCT to 6 months afterward. Also as predicted, men recovered from 6-month lows to pre-HCT rates of being sexually active by 1 year, but they took until 2 years to improve in quality and quantity of sexual function, indicating that most recover from the effects of transplantation. However, male survivors continued to have lower sexual function than controls at 5 years, suggesting they did not fully recover from the effects of cancer itself or treatments received before HCT. Nearly half (45%) of the men at 5 years reported problems that disrupted sexual function.
Women improved compared with their 6-month activity nadir by 2 years and declined in rates of some problems, but they did not significantly progress in overall quality and quantity of sexual function between 6 months and 5 years. By 3 and 5 years their scores did not differ from either their 6-month nadir or pre-HCT levels. At 5 years, scores for both activity and function were lower than controls. The 0.5-point difference between survivors and controls in sexual function and the 0.4-point drop from before HCT to 6 months afterward correspond to roughly half a standard deviation for the sexual function measure. A difference of a third to half a standard deviation is accepted as clinically meaningful in quality-of-life measures.27,28 At least 40% of female survivors were not sexually active at each time point assessed, compared with 21% of female controls at the 5-year time point. These results are consistent with population-based reports that women in general have more sexual problems than men (80% of female survivors and 61% of female controls reported one or more sexual problems).7
There are several other important considerations and implications of these results. Although it would be inappropriate to statistically compare baseline rates of activity and function for HC transplant recipients with 5-year controls, it is notable that both men and women entered transplantation with sexual function descriptively below the controls who were assessed at an average of 5 years older than patients were at their pre-HCT baselines. These deficits that predate HCT document a need for evaluation and treatment of sexual difficulties in cancer survivors whether they are expected to continue on to HCT. We also note that the finding that men, on average, recover to pre-HCT levels of function does not rule out the likelihood that a minority of men have long-term sexual difficulties as a result of transplantation. Although it is beyond the aims of this paper to examine the complex interaction of risk factors related to sexual function outcomes, we expect that the longer the sexual deficits exist, the more intractable they will be to treat. As an additional point that is important for future studies and clinical care, we determined that cancer survivors will respond to sensitively and confidentially asked questions about their sexual function (84% response rate) at nearly the same rates they respond to requests for other health-related quality-of-life information (87% response rate).
Results are consistent with previous research, indicating that men and women decline in rates of sexual activity and satisfaction after HCT.3,9,10 Deficits have been reported relative to function before HCT and in comparison with either population norms or patients who receive chemotherapy without HCT.9,11-13 These findings apply across time after HCT and across ages at transplantation.3,10,11,14,16,29,30 Problem rates in the controls are consistent with normative studies reporting 25% to 63% of women in the general population and 10% to 52% of men report sexual problems.6,7
Sexual dysfunction in HCT survivors is presumably caused by systemic therapies, particularly the alkylating agents and total body irradiation used in myeloablative preparative regimens. These are known to permanently damage hypothalamic-pituitary-gonadal axis function.25,26 Follicle stimulating hormone is elevated in more than 90% of women and most men.25,26 Luteinizing hormone is elevated in most female survivors and normal in most men.25,26 Most women have primary ovarian failure with consequent low endogenous estrogen levels. Chronic GVHD may also contribute to vaginal introital stenosis and mucosal changes that contribute to dyspareunia, vaginal irritation, and increased sensitivity of genital tissues.31 Male sexual problems have been attributed to gonadal and cavernosal arterial insufficiency with resulting libido and erectile dysfunction.32,33 Most men recover Leydig cell function by 1 year.25,26 However, men with sexual problems have been noted to have testicular insufficiency with diminished libido or erectile dysfunction even when serum testosterone levels are within normal range.32-34 Thus, they may require dynamic testing of pituitary-gonadal function. Although biologic changes, and particularly hormones, may contribute to some of the observed sexual dysfunction, we recognize that neither hormone levels nor questionnaires can capture the full complexity of human sexual experience and satisfaction. Furthermore, although this study does not provide evidence for causal factors related to sexual dysfunction, it supports the importance of understanding these mechanisms along with recognizing the complexity of sexuality when designing interventions for these widespread problems.
Treatment studies of sexual function after cancer or HCT are rare. Communication reluctance on the part of health professionals and patients is a recognized barrier to improved treatment.35 Prospective studies indicate that hormone therapy with oral estrogen ameliorates more serious decline in sexual function in women after HCT, but it does not eliminate problems.3,36 Androgen therapy, although promising in some studies, raises concerns about second cancers.37-39 Although vaginal lubricants, dilatators, or vibrators may be recommended to improve comfort,1 evidence-based studies are lacking.
For male HCT survivors, case series suggest that testosterone injections and sildenafil improve sexual performance for those with erectile dysfunction, low libido, and ejaculatory disorders in 2- to 24-month survivors.32 However, other data, including our own, indicate that most men recover testosterone and sexual function between 6 months and 2 years after HCT.40 Randomized controlled trials are needed to determine whether treatment improves the rate or pace of recovery. Rehabilitation studies with survivors of prostate cancer have found initial efficacy but difficulties with treatment participation and maintenance of gains.1,41
Strengths of this study include the prospective, long-term follow-up of a relatively large cohort, with controls at 5 years, low dropout rates, and detailed assessment of sexual function. Limitations of the study include that all participants were enrolled from one transplantation center, and sexual function before diagnosis was not known. Sample size was insufficient to examine problem-specific differences within groups beyond sex. Results are not generalizable to autologous HC transplant recipients or other cancer survivors. Another limitation is that matched controls were not randomly designated. Using siblings and friends as controls with similar biology, lifetime exposures, and social environment, but not HCT, is standard for survivorship research, including adults after HCT.42-44 Siblings control for biologic influence, and friends and siblings control for sociodemographic differences that may differ from general population rates. However, this method could be susceptible to bias from survivors with multiple control options nominating healthier siblings or friends. In addition, community volunteers could be sociodemographically different from other controls or survivors in ways we did not detect.
In conclusion, this research confirms that all men and women who receive a HC transplant for malignancies require discussion and, potentially, treatment for sexual dysfunction. Men on average recover from the acute effects of treatment on their sexual function, but they do not reach sexual function levels of age-matched controls. Nearly half of men and 80% of women will have long-term sexual problems. The presence of problems in many patients before HCT supports a need for similar sexual function outcome studies in survivors who do not receive a transplant and the importance of intervention planning for all survivors of hematologic malignancies. When designing treatments for these survivors, clinicians need to recognize that some problems may be longstanding and therefore potentially more difficult to treat. Communication between oncologists and other health care professionals and all of their patients is needed to normalize discussion of sexual concerns. This will facilitate detecting problems early after treatment. If strategies to improve sexual function are tried before avoidance behaviors or negative expectations become entrenched, this may improve long-term outcomes. Men may benefit from reassurance that erectile function and sexual desire should improve by 1 to 2 years after treatment when testosterone levels have normalized. However, men also need to know that methods such as testosterone replacement, erectile function medications, and other adaptive strategies can be considered if problems continue. For women, methods that focus on communication with their partners about changes in sensation, strategies for enhancing libido, routine use of vaginal lubricants if needed, and dilators or vibrators to assist with adapting to genital changes may help to maintain sexual responsiveness. Although evidence-based treatments have been minimally tested, the prevalence and extent of sexual problems mandates that survivors be made aware of potential changes and given resources to address these needs.
An Inside Blood analysis of this article appears at the front of this issue.
Presented in part at the annual meeting of American Psycho-Oncology Society (APOS), January 2006, and the annual meeting of The European Group for Blood Marrow Transplantation (EBMT), March 2006.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Tarah Helliwell, Sandy Lee, and Jo Ann Broeckel Elrod for their assistance with the study, and the dedicated transplant recipients, siblings, friends, and community members who have participated in this long-term study.
This work was supported by grants from the National Cancer Institute (CA63030, CA78990, and CA112631).
Authorship
Contribution: K.L.S. designed the research, directed research implementation, assisted with analyses, and wrote the paper; B.F.K. contributed to conceptualizing the manuscript, analyzed the data, and contributed to the writing; J.R.A. contributed to design of the research, performed the research, and assisted with writing the paper; J.E.S. and J.R.H. contributed to design of the research and writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karen Syrjala, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D5–220, Seattle, WA, 98109; e-mail: ksyrjala@fhcrc.org.