We describe the use of pretargeted radioimmunotherapy (PRIT) using an anti–murine CD45 antibody-streptavidin (SA) conjugate followed by radiobiotin to deliver radiation selectively to murine hematolymphoid tissues, which may potentially augment the efficacy and decrease the toxicity of radioimmunotherapy for disseminated murine leukemia. Biodistribution and therapeutic results demonstrated high target organ to nontarget organ ratios of radioactivity and significant long-term survival in leukemic mice using PRIT. These data suggest that anti-CD45 PRIT using an anti–CD45-SA conjugate in a syngeneic murine model of disseminated leukemia may be more effective and less toxic than directly labeled monoclonal antibodies.
Introduction
Acute myeloid leukemia (AML) currently kills the majority of afflicted patients despite treatment with combination chemotherapy and allogeneic hematopoietic cell transplantation (HCT).1 Although allogeneic HCT for relapsed leukemia patients may offer the best, and sometimes the only chance for cure, the procedure too often fails to eradicate the patient's malignancy or is associated with fatal toxicities. High-dose chemoradiotherapy with allogeneic HCT in high-risk patients with advanced AML produces a 5-year survival rate of only 20% to 30% because of both treatment-related mortality and leukemic relapse.2 As part of the transplant procedure, patients are typically treated with an intensive preparative regimen both to ensure allogeneic engraftment and to eradicate as much malignancy as possible. Attempts to develop improved preparative regimens with increased antitumor effects and less toxicity have met with limited success because the conditioning regimens are composed of relatively nonspecific agents, such as total body radiation (TBI) or high-dose alkylating agents
Prior studies have shown that increasing the level of TBI before HCT significantly diminishes the relapse rate but increases treatment-related toxicity, nullifying the advantage of the lower relapse rate.3,–5 Myeloablative radioimmunotherapy (RIT) is an emerging approach to increase the specific radiation dose delivered to malignant cells without increasing extramedullary toxicities, and has produced promising results in AML and lymphoma trials.6,,,,,,,,–15 Our group and others have shown that it is feasible to use directly labeled antibodies (Ab) to target radiation to hematopoietic tissues with greater absorbed radiation doses delivered to the bone marrow and spleen compared with the liver, lungs, and kidneys. An 131I-anti–CD45 Ab can deliver at least 2- to 3-fold more radiation to bone marrow, spleen, and sites of leukemia than to any normal organ, and that 131I-anti-CD45 Ab can be safely combined with high-dose cyclophosphamide (CY) and 12 Gy TBI.8 At the maximum tolerated dose (10.5 Gy to liver), this supplemental radiation delivers an average additional dose of 24 Gy to bone marrow and 50 Gy to spleen.
Despite the promise of this approach, the efficacy of RIT is currently limited by nonspecific delivery of radiation to normal tissues because of the long circulating half-life of radiolabeled Abs in the bloodstream. We and others have conducted pilot studies in other malignancies (eg, lymphoma, adult T-cell leukemia/lymphoma, and solid tumors) demonstrating convincingly that multistep pretargeting RIT methods for delivering radiation are superior to conventional RIT.16,,,,,,,,,,–27 In preliminary studies, we have shown that pretargeted RIT (PRIT) using BC8, an antihuman (h)CD45 Ab, conjugated with streptavidin (SA) to treat human leukemia xenografts in mice significantly augments the efficacy of RIT and decreases the toxicity of therapy compared with a conventional, directly labeled anti-CD45 radioimmunoconjugate (J.M.P. et al, manuscript in preparation). Although pilot mouse xenograft experiments with anti-hCD45 PRIT are very encouraging, their interpretation is confounded by unavoidable differences between murine tumor xenograft systems and naturally occurring tumors. In xenograft systems, human target antigens (including hCD45) are confined to tumor cells, whereas all other tissues are devoid of hCD45 and will not bind anti–hCD45 Ab. In contrast, in patients with cancer, hCD45 is present on many normal hematopoietic elements (eg, bone marrow, lymph node cells, Kupffer cells) as well as on AML cells and consequently toxicity profiles, particularly myeloid toxicities, in the human may not be reliably mimicked in xenograft systems. The classic xenograft systems are indeed more similar to chloromas than to typical disseminated leukemia. To overcome these limitations, we have now more rigorously tested anti–CD45 PRIT in a complementary, more relevant murine syngeneic myeloid leukemia system in which the target antigen is present on both leukemia cells as well as normal myeloid, lymphoid, and reticuloendothelial tissues.
Methods
Mice
Female SJL/J mice (H-2s), 6 to 8 weeks old, were purchased from Jackson Laboratories (Bar Harbor, ME). The animals were housed under protocols approved by the Fred Hutchinson Cancer Research Center (Seattle, WA) Institutional Animal Care and Use Committee.
Murine AML model
The radiation-induced murine SJL/J AML model used in this study has been previously described.28,–30 AML cells were maintained by serial transplantation of spleen mononuclear cells from leukemic mice into fresh SJL/J recipients. Mice injected intravenously with 105 leukemia cells routinely developed fatal disease in approximately 4 weeks. Weekly retro-orbital blood samples were used to evaluate for the presence of murine AML. Peripheral blood (75–100 μL) was incubated with appropriate amounts of CD45 fluorescein isothiocyanate, CD117 PE, and CD11b PerCP-Cy5.5 (all Abs from BD Biosciences Pharmingen, Franklin Lakes, NJ) at room temperature for 15 minutes in the dark, lysed with 1.5 mL of buffered NH4Cl containing 0.25% formaldehyde (Polysciences, Warrington, PA) for 15 minutes, followed by a single wash in phosphate-buffered saline. The entire cell suspension was acquired on an LSRII (BD Biosciences, San Jose, CA) where typically 200 000 to 300 000 events were obtained, and data were analyzed using using CellQuest Pro 5.2 software (San Jose, CA). The murine AML immunophenotype was CD45+, CD117 variable+, CD34 low+, and CD11b−.
Antibodies and production of DOTA-Ab and Ab-SA conjugates
The 30F11 hybridoma cell line expressing the rat IgG2b antimurine CD45 Ab was a gift from Irv Bernstein (Fred Hutchinson Cancer Research Center). Rat polyclonal IgG (MS163) was obtained from Biomeda (Foster City, CA) and used as nonspecific negative control. The 30F11 Ab was produced by injecting the hybridoma into pristane-primed mice to generate ascites. The 30F11 Ab was purified from ascitic fluid by protein G immunoabsorption column chromatography. DOTA-Ab and Ab-SA conjugates were produced as described previously.18
Radiolabeling
Abs were iodinated with Na125I or Na131I (PerkinElmer, Waltham, MA) by the chloramine T method as previously published.18 111In and 90Y (PerkinElmer) radiolabeling of intact Ab-SA conjugates for PRIT was performed as described.18 Radiochemical purity was typically greater than 99% as determined by HPLC for each construct, and labeling efficiencies were greater than 90%. DOTA-biotin was synthesized as described.18
Cytometric cell-binding analysis of 30F11 DOTA-Ab and Ab-SA conjugates
Indirect flow cytometry was used to assess binding of 30F11 DOTA-Ab and Ab-SA conjugates to peripheral blood mononuclear cells collected from leukemic female SJL/J mice using methods previously described.31 Briefly, SJL/J AML cells (0.5 × 106) were incubated with 10 μg/mL of primary Ab or molar equivalent for Ab-SA conjugate at 4°C for 30 minutes in phosphate-buffered saline supplemented with 2% fetal bovine serum. Cells were washed twice and incubated at 4°C for 30 minutes using 5 μg of secondary fluorescein isothiocyanate-labeled goat antirat IgG Ab (F-6258, Sigma-Aldrich, St Louis, MO). After 2 more washes, samples were preserved in 1% paraformaldehyde solution and analyzed using a FACScan II flow cytometer (BD Biosciences). Mean fluorescence intensity was determined using CellQuest Pro 5.2 software (San Jose, CA).
Blood clearance studies
To assess whole blood clearance, 0.67 or 2.67 nmol of 125I-labeled 30F11 Ab-SA was injected intravenously via the tail vein into groups of 5 female SJL/J mice who had received 105 SJL/J AML cells 23 days earlier. Serial blood samples were drawn from the retro-orbital venus plexus and radioactivity measured by gamma counting. The blood half-life (T1/2) was determined by fitting data for each mouse to a 2-compartmental nonlinear decay model with WinNonLin software (Pharsight, Mountain View, CA) and calculating the mean for the Ab-SA conjugate.
Biotinylated clearing agent
A synthetic biotinylated clearing agent (CA) containing 16 N-acetyl-galactosamine-residues per dendrimeric molecule was a gift from NeoRx (Seattle, WA) and was used for PRIT experiments to eliminate excess Ab-SA molecules from the circulation before the administration of radiolabeled biotin. The N-acetyl galactosamine residues have a high affinity for hepatic asialoglycoprotein receptors and thus facilitate the rapid hepatic clearance of residual Ab-SA conjugates from the bloodstream and their endocytosis into liver cells.21
Biodistribution studies
Female SJL/J mice were injected intravenously with 105 SJL/J AML cells either 2 or 23 days before anti-mCD45 Ab therapy, at a time when mAML was detectable in the peripheral blood of all mice by flow cytometry (as described above and shown in Figure S6, available on the Blood website; see the Supplemental Materials link at the top of the online article). Groups of 5 mice were then injected intravenously via the tail vein with either directly radioiodinated 30F11 DOTA-Ab (for conventional RIT studies) or unlabeled 30F11 Ab-SA conjugate (for PRIT studies). All leukemic mice were placed on a biotin-free diet (Harlan Teklad, Madison, WI) for 5 days before receiving either Ab construct. For conventional RIT Ab biodistribution studies, groups of 5 mice received 0.67 nmol radioiodinated 30F11 DOTA-Ab (100 μg; 50 μCi). For PRIT studies, groups of 5 mAML bearing SJL/J mice were injected with an equimolar amount (0.67 nmol) of unlabeled 30F11 anti-mCD45 Ab-SA conjugate (140 μg). Eight hours later, 5.8 nmol of CA was administered intravenously to half of the PRIT groups, followed by an intravenous injection of 1.2 nmol 111In-DOTA-biotin 10 hours after the delivery of the Ab-SA conjugate in PRIT mice. All mice were bled from the retro-orbital venous plexus, killed, and femoral marrow and organs (lung, liver, spleen, stomach, kidneys, small intestine, and colon) were harvested, weighed, and gamma counted for 125I or 111In activity. Mice in conventional RIT groups were killed 2, 4, 8, 24, 48, and 72 hours after 125I-DOTA-Ab injection, whereas mice in the PRIT groups were killed 24 hours after the 111In-DOTA-biotin injection. The percent-injected dose of 125I and 111In per gram (% ID/g) of blood, tumor and normal organs was calculated after correcting for radioactive decay using an aliquot of the injectate. Marrow-to-normal organ ratios of absorbed radioactivity were also calculated. Control groups were injected with 0.67 nmol of the nonbinding rat polyclonal IgG 125I-DOTA-Ab or Ab-SA conjugate followed by CA and 111In-DOTA-biotin.
Radioimmunotherapy studies
The therapeutic efficacy of pretargeted 30F11 anti-mCD45 Ab-SA chemical conjugate was evaluated using various doses of 90Y-DOTA-biotin in groups of 5 leukemic SJL/J mice. Mice received 105 SJL/J AML cells followed 2 days later with administration of 0.67 nmol of anti-mCD45 Ab-SA conjugate. A single dose of 1.2 nmol (1 μg) DOTA-biotin labeled with 100, 200, or 300 μCi 90Y-DOTA-biotin was administered 8 hours after delivery of the Ab-SA conjugate. Untreated mice were used as a negative control. Mice were assessed daily for weight changes and general appearance. White blood counts and immunophenotypic evidence of murine AML were monitored by retro-orbital bleeding every 7 days. Mice were killed if they became moribund from progressive leukemia or if weight loss exceeded 10% of total body weight.
Results
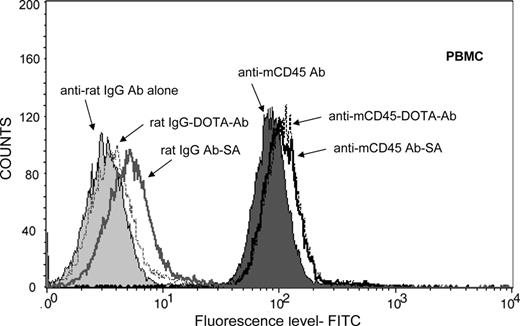
We used a PRIT approach using a Ab-SA conjugate directed against murine (m)CD45, administered sequentially with a dendrimeric N-acetylgalactosamine-containing CA followed by radiolabeled-DOTA-biotin.32 The anti-mCD45 DOTA-Ab and Ab-SA conjugates were tested for binding to leukemic SJL/J mouse peripheral blood mononuclear cells and splenocytes by flow cytometry (Figure 1). These studies demonstrated that the DOTA-Ab and Ab-SA conjugates displayed similar binding patterns on peripheral blood mononuclear cells and spleen cells. The specificity of tumor targeting by the anit-mCD45 Ab conjugates in these experiments was demonstrated by use of a nonspecific control Ab. Blood clearance studies were conducted in female BALB/c athymic mice to assess the pharmacokinetics of the anti-mCD45 Ab. In these studies 0.67 and 2.67 nmol (100 and 400 μg, respectively) of 125I-labeled anti-mCD45 Ab were injected via the tail vein and blood samples were serially drawn and counted for radioactivity. A blood clearance half-life (t1/2β) of 13.5 hours was observed after injection of 0.67 nmol, compared with 15.5 hours for the Ab administered at a dose of 2.67 nmol. Area-under-curve (AUC) calculations demonstrated fast clearance of the anti-mCD45 Ab at both doses (AUC 702 pmol/mL per hour vs 7245 pmol/mL per hour, respectively).
Cell binding studies. Flow cytometric analysis showing the mean fluorescence index for anti-mCD45 Ab, DOTA-Ab, and Ab-SA conjugates with peripheral blood mononuclear cells (PBMCs) from leukemic SJL/J mice. Included in these studies were the nonbinding control conjugates, including rat IgG Ab, rat IgG-DOTA-Ab, and rat IgG Ab-SA.
Cell binding studies. Flow cytometric analysis showing the mean fluorescence index for anti-mCD45 Ab, DOTA-Ab, and Ab-SA conjugates with peripheral blood mononuclear cells (PBMCs) from leukemic SJL/J mice. Included in these studies were the nonbinding control conjugates, including rat IgG Ab, rat IgG-DOTA-Ab, and rat IgG Ab-SA.
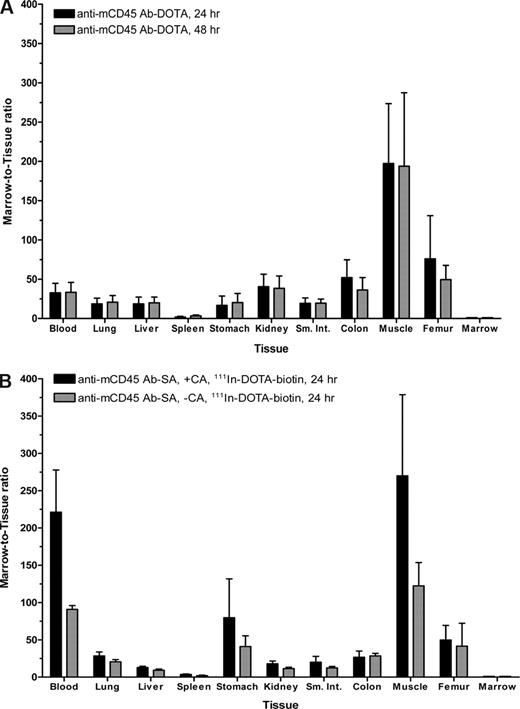
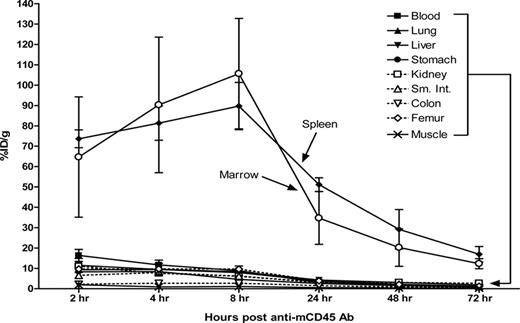
The biodistribution of radiolabeled anti-mCD45 Ab (30F11) was evaluated using conventional one-step RIT in SJL/J mice that received 105 myeloid leukemia cells via the tail vein 23 days before receiving directly labeled anti-mCD45 Ab. We first performed flow cytometric analyses and obtained tissues from target organs to confirm the presence of myeloid leukemia blasts in target organs 23 days after delivery of syngeneic leukemia cells. The flow cytometry data and histologic sections from the key target organs depict significant involvement of leukemia at this time point (Figure S6,S7). At 2, 4, 8, 24, 48, and 72 hours after injection, groups of 5 mice were killed, and their organs were harvested, washed, weighed, and assayed for 125I activity. Specific localization of Ab in bone marrow and spleen was documented in 3 separate experiments (Figure 2). At all time points, control animals injected with nonspecific 125I-labeled rat polyclonal antimurine IgG exhibited negligible tumor uptake of the radiolabel demonstrating the specificity of targeting in these experiments. Specific uptake of 125I-anti-mCD45 Ab within target tissues reached a maximum value at 8 hours with 105.6 plus or minus 27.1% (mean and SD) injected dose of absorbed radionuclide/gram (% ID/g) delivered to marrow and 89.7 plus or minus 11.6% ID/g to the spleen. The targeted concentrations of radioactivity remained at a relatively high levels in the bone marrow and spleen using the directly labeled anti-mCD45 Ab with more than 30% ID/g present in these target tissues 24 hours after administration of the radiolabeled Ab and 10% to 30% ID/g 48 hours after injection. The amount of absorbed radioactivity remained relatively high in the blood and normal organs, however, with 8.9 plus or minus 2.3% ID/g remaining in the blood and 6.1 plus or minus 0.7% ID/g in the kidneys after 8 hours (Figure 2). The marrow-to-normal organ ratios of radioactivity using the directly labeled anti-mCD45 Ab ranged from more than 65:1 (blood) to approximately 162:1 (muscle) after 24 hours (Figure 3A).
Biodistributions of radioactivity in blood, marrow, spleen, and normal organs of leukemic SJL/J mice injected with 125I-anti-mCD45 Ab. Leukemic mice were injected via the tail vein with 0.67 nmol of conventional trace-labeled 125I-anti-mCD45 Ab at time = 0 hours and killed 2, 4, 8, 24, 48, or 72 hours later. The radioactivity in blood, marrow, and spleen was quantified by gamma counting, corrected for decay and expressed as percent ID/g of tissue. Error bars are SD.
Biodistributions of radioactivity in blood, marrow, spleen, and normal organs of leukemic SJL/J mice injected with 125I-anti-mCD45 Ab. Leukemic mice were injected via the tail vein with 0.67 nmol of conventional trace-labeled 125I-anti-mCD45 Ab at time = 0 hours and killed 2, 4, 8, 24, 48, or 72 hours later. The radioactivity in blood, marrow, and spleen was quantified by gamma counting, corrected for decay and expressed as percent ID/g of tissue. Error bars are SD.
Marrow-to-normal organ ratios for conventional RIT with (A) 125I-anti-mCD45 DOTA-Ab and (B) PRIT with 111In-DOTA-biotin using an anti-mCD45 Ab-SA conjugate. Leukemic SJL/J mice were treated as described in Figures 2 and 4. Marrow-to-normal organ ratios of radioactivity using either (A) directly labeled 125I-DOTA-Ab or (B) pretargeted 111In-DOTA-biotin, delivered with or without CA at 8 hours, are shown 24 and/or 48 hours after injection of radioactivity. Error bars are SD.
Marrow-to-normal organ ratios for conventional RIT with (A) 125I-anti-mCD45 DOTA-Ab and (B) PRIT with 111In-DOTA-biotin using an anti-mCD45 Ab-SA conjugate. Leukemic SJL/J mice were treated as described in Figures 2 and 4. Marrow-to-normal organ ratios of radioactivity using either (A) directly labeled 125I-DOTA-Ab or (B) pretargeted 111In-DOTA-biotin, delivered with or without CA at 8 hours, are shown 24 and/or 48 hours after injection of radioactivity. Error bars are SD.
Despite the relatively rapid clearance of the Ab from the blood, the Ab may still be cleared from the circulation too slowly for optimal RIT without the use of a PRIT approach. Consequently, in 3 separate experiments, a single 5.6 nmol (50 μg) dose of a biotinylated polymeric, N-acetyl-galactosamine CA was given via intraperitoneal injection to SJL/J mice 8 hours after 125I-anti-mCD45 Ab-SA conjugate administration. The results demonstrated that the CA rapidly removed more than 80% of circulating labeled Ab-SA conjugate from the blood within 1 hour, with a drop in whole blood concentration to less than 1% ID/g (Figure S8).
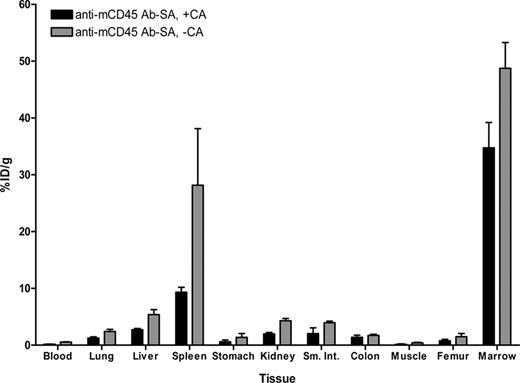
Biodistribution experiments using the PRIT strategy examined the effects of eliminating nonspecific radiation exposure from blood-borne radiolabeled Ab by delivering the unlabeled anti-mCD45 Ab-SA conjugate, either in the presence or absence of CA, in a syngeneic murine leukemia system. Biodistribution studies were performed using SJL/J mice inoculated via the tail vein with 105 leukemic cells 23 days before receiving 0.67 nmol of unlabeled anti-mCD45 Ab-SA conjugate. Eight hours after intravenous injection of the Ab-SA conjugate, 5.8 nmol of CA was delivered to half the mice to explore its efficacy. All mice received 1.2 nmol 111In-DOTA-biotin 10 hours after Ab-SA conjugate administration, and groups of 5 mice each were killed 24 and 48 hours later. Pretargeted anti-mCD45 Ab-SA conjugate resulted in favorable biodistributions of radioactivity at each time point assessed (Figure 4). The uptake of radioactivity in bone marrow and spleen of mice treated with pretargeted anti-mCD45 Ab-SA conjugate, followed the CA at 8 hours, reached a maximum of 34.8 plus or minus 4.5 and 9.3 plus or minus 0.4% ID/g, respectively, 24 hours after administration of 111In-DOTA-biotin. By contrast, mice that received pretargeted anti-mCD45 Ab-SA conjugate, without use of the CA, reached a maximum tumor uptake of radioactivity of 48.7 plus or minus 4.5 and 28.2 plus or minus 10.0% ID/g in the marrow and spleen after 24 hours, respectively, suggesting that the CA may remove a fraction of pretargeted Ab-SA conjugate from target CD45+ tissues (Figure 4). Despite decreasing the concentration of radioactivity in the bone marrow and spleen, however, the pretargeted marrow-to-normal organ ratios were superior with the use of the CA compared with the ratios seen without use of the CA, primarily because of the CA's efficient reduction of circulating radionuclide in the blood and in the normal organs (Figure 3B). After 24 hours, the marrow-to-blood ratio exceeded 220:1 using pretargeted anti-mCD45 Ab-SA conjugate followed 8 hours later by the CA, and was approximately 90:1 without using the CA. Nevertheless, it is important to recognize that the pretargeting approach, even without use of the CA, generated superior marrow-to-normal organ ratios of radioactivity with the anti-mCD45 Ab-SA conjugate compared with results with conventional radiolabeling (compare Figure 3A,B). The specificity of tumor targeting by anti-mCD45 Ab-SA conjugate in these experiments was demonstrated by control groups treated with negative control polyclonal rat antimurine IgG-SA, treated with and without CA, and 111In-DOTA-biotin. These control mice did not exhibit any appreciable radiobiotin uptake into tumors at any time point (eg, < 1% ID/g at 24 hours; data not shown).
Biodistributions of 111In-DOTA-biotin in blood, marrow, spleen, and normal organs after pretargeting with anti-mCD45 Ab-SA conjugate. Leukemic SJL/J mice were injected via the tail vein with 0.67 nmol of anti-mCD45Ab-SA conjugate, administered either alone or 8 hours later with 5.8 nmol of CA, followed by 1.2 nmol of 111In-labeled DOTA-biotin 10 hours after delivery of the Ab-SA conjugate in all mice. Mice were killed 24 hours later, and blood, marrow, spleen, and normal organs were harvested, weighed, and analyzed for levels of radioactivity by gamma counting to determine percent ID/g. Error bars are SD.
Biodistributions of 111In-DOTA-biotin in blood, marrow, spleen, and normal organs after pretargeting with anti-mCD45 Ab-SA conjugate. Leukemic SJL/J mice were injected via the tail vein with 0.67 nmol of anti-mCD45Ab-SA conjugate, administered either alone or 8 hours later with 5.8 nmol of CA, followed by 1.2 nmol of 111In-labeled DOTA-biotin 10 hours after delivery of the Ab-SA conjugate in all mice. Mice were killed 24 hours later, and blood, marrow, spleen, and normal organs were harvested, weighed, and analyzed for levels of radioactivity by gamma counting to determine percent ID/g. Error bars are SD.
Based on encouraging results from the biodistribution experiments, we conducted PRIT studies to evaluate the efficacy of targeting the CD45 antigen in the murine syngeneic leukemia model. To ensure that targeting CD45+ tissues 2 days after delivery of therapeutic radiobiotin would be appropriate for assessing therapeutic response, we performed a biodistribution experiment in mice that received pretargeted antimurine CD45 Ab-SA conjugate 2 days after delivery of AML cells. The results from this day 2 biodistribution study confirmed that the anti-mCD45 Ab-SA conjugate will adequately localize to target organs with a maximum tumor uptake of radioactivity seen in the marrow and spleen after 8 hours of 99.1 plus or minus 23.4 and 52.5 plus or minus 9.8% ID/g, respectively (Figure 5A). The therapeutic efficacy of pretargeted 90Y-DOTA-biotin was assessed after administration of the anti-mCD45 Ab-SA conjugate in SJL/J mice. Treatment consisted of a single dose of pretargeted 90Y-DOTA-biotin delivered 2 days after tail vein injection of 105 myeloid leukemia cells. Treatment groups of 5 animals each received 100, 200, 300, or 800 μCi 90Y-DOTA-biotin 8 hours after administration of anti-mCD45 Ab-SA conjugate. All control mice that were either untreated or received polyclonal IgG Ab-SA conjugate and 90Y-DOTA-biotin developed disseminated myeloid leukemia as demonstrated by flow cytometric evidence of myeloid blasts in the peripheral blood by day 23. Furthermore, all control mice developed rapidly escalating blood leukocyte counts by day 30 and required euthanasia by day 35 due to progressive myeloid leukemia (Figure 5B). Animals treated with PRIT using anti-mCD45 Ab-SA conjugate followed by 100 or 200 μCi of 90Y-DOTA-biotin exhibited prolonged survival but eventually died of leukemia progression. Death was required after treatment of all 5 mice in the 100 μCi group by day 56 and by day 62 in mice receiving 200 μCi 90Y-DOTA-biotin. By comparison, 4 of 5 mice treated with pretargeted anti-mCD45 Ab-SA conjugate followed by 300 μCi 90Y-DOTA-biotin survived leukemia free for the duration of the experiment (> 100 days), whereas the fifth mouse was killed on day 78.
Biodistribution and cumulative survival of leukemic mice treated with anti-mCD45 Ab-SA. (A) Biodistribution of radioactivity in blood, marrow, spleen, and normal organs of leukemic SJL/J mice injected with 125I-anti-mCD45 Ab-SA on day 2 post-AML inoculation. Leukemic mice were injected via the tail vein with 0.67 nmol of conventional trace-labeled 125I-anti-mCD45 Ab-SA or 125I-IgG Ab-SA at time = 0 hours and killed 8 hours later. The radioactivity in blood, marrow, and spleen was quantified by gamma counting, corrected for decay, and expressed as percent ID/g of tissue. Error bars are SD. (B) Analysis of cumulative survival of SJL/J mice inoculated with myeloid leukemia treated with PRIT using anti-mCD45 Ab-SA conjugate. Groups of 5 mice harboring murine leukemia were treated as described in the legend to Figure 4 and analyzed for survival as a function of time. Treatment groups included mice treated with 0.67 nmol of anti-mCD45 Ab-SA or control IgG-SA conjugate, mice that did not receive any Ab-SA conjugate, or control untreated mice, followed 8 hours later by 100, 200, or 300 μCi of 90Y-DOTA-biotin.
Biodistribution and cumulative survival of leukemic mice treated with anti-mCD45 Ab-SA. (A) Biodistribution of radioactivity in blood, marrow, spleen, and normal organs of leukemic SJL/J mice injected with 125I-anti-mCD45 Ab-SA on day 2 post-AML inoculation. Leukemic mice were injected via the tail vein with 0.67 nmol of conventional trace-labeled 125I-anti-mCD45 Ab-SA or 125I-IgG Ab-SA at time = 0 hours and killed 8 hours later. The radioactivity in blood, marrow, and spleen was quantified by gamma counting, corrected for decay, and expressed as percent ID/g of tissue. Error bars are SD. (B) Analysis of cumulative survival of SJL/J mice inoculated with myeloid leukemia treated with PRIT using anti-mCD45 Ab-SA conjugate. Groups of 5 mice harboring murine leukemia were treated as described in the legend to Figure 4 and analyzed for survival as a function of time. Treatment groups included mice treated with 0.67 nmol of anti-mCD45 Ab-SA or control IgG-SA conjugate, mice that did not receive any Ab-SA conjugate, or control untreated mice, followed 8 hours later by 100, 200, or 300 μCi of 90Y-DOTA-biotin.
Mice treated with higher doses of pretargeted 90Y-DOTA-biotin (eg, 800 μCi) developed profound weight loss, melanotic stools, and required death by day 12 due to radiation toxicity. Necropsy revealed no evidence of leukemia, but extensive gastric and small intestinal ulcerations and perforations were seen, apparently because of cross-target irradiation of the upper gastrointestinal tract by high concentrations of 90Y in the adjacent spleen (J.M.P. et al, manuscript in preparation). We hypothesize that these complications might be alleviated by using isotopes emitting β particles with shorter path lengths than Y-90 (∼5 mm), such as Lu-177 (β path length ∼0.7 mm), thereby permitting further dose escalation.
Discussion
Chemotherapy and external beam radiation therapy regimens expose normal and neoplastic cells to identical doses of cytotoxic agents and depend on the enhanced sensitivity of rapidly dividing cancer cells to achieve preferential killing. Therapeutic efficacy should be markedly enhanced and toxicity diminished if tumoricidal agents could be selectively focused on malignant cells. Therefore, Abs directed against tumor-associated antigens and conjugated to toxins, drugs, or radionuclides afford an attractive treatment option. Radiolabeled Abs appear particularly attractive for hematologic malignancies because: (1) their surface antigens are well delineated, (2) multiple high-quality Abs are available, (3) the best results of clinical trials with unmodified Abs and with radiolabeled Abs have been observed with hematologic malignancies, and (4) hematologic malignancies are exquisitely sensitive to radiation therapy.
The feasibility and safety of using radiolabeled Abs to treat hematologic malignancies have been well documented; however, the efficacy of RIT has been limited by the protracted circulating half-life of conventional radiolabeled Abs, resulting in low attainable tumor-to-normal organ ratios of absorbed radiation. Several approaches to decrease radiation at nontumor sites have recently been evaluated, with a primary focus on methods to remove circulating labeled Ab from the bloodstream. One particularly attractive approach to improve the success of RIT uses a multistep, pretargeting method that has demonstrated marked improvements in the tumor-to-normal organ ratios of absorbed radiation achievable in models of human lymphoma.17,18,21,22,33 In this manuscript, we describe for the first time the application of PRIT in a murine syngeneic myeloid leukemia system where an AML-associated antigen is present on both leukemia cells as well as normal myeloid, lymphoid, and reticuloendothelial tissues. We report 5 significant findings. First, in a model of murine leukemia, we demonstrate that conventional and pretargeted RIT using antimurine CD45 Ab-SA conjugates permit selective delivery of high absorbed radiation doses to hematopoietic tissues with relative sparing of nonhematopoietic organs. Second, we show in the murine leukemia model that an N-acetyl-galactosamine-containing dendrimeric CA can efficiently reduce circulating Ab-SA conjugate from the blood and thus limit the amount of radionuclide delivered to the normal organs. Third, we show that all 3 components of the pretargeting system can be administered to leukemic mice with negligible toxicity. Fourth, we demonstrate superior marrow-to-normal organ ratios using the PRIT approach with the use of the CA, compared with the ratios seen without the use of the CA. Lastly, we document that delivery of therapeutic doses of 90Yttrium using an antimurine CD45 Ab-SA conjugate, but not a control Ab-SA conjugate or radiobiotin alone, can achieve durable remissions in mice bearing systemic CD45+ myeloid leukemia.
Many cell surface antigens have been targeted with Abs for treatment of leukemia. To date, anti-CD33 Abs have been the most widely tested, either as unmodified Ab or conjugated to drugs or radionuclides.11,34,,–37 Other investigators have pursued RIT for AML by targeting CD66, which is present on maturing hematopoietic cells but not on leukemic blasts. CD66 targeting has been used to deliver homogenous radiation to normal cells throughout marrow, relying on bystander irradiation of leukemic blasts to improve the outcome of conditioning before HCT.12,38,39 We have focused on the CD45 antigen as an attractive alternative target for RIT of AML. Most hematologic malignancies, including 85% to 90% of acute lymphoid and myeloid leukemias, express CD45; however, the CD45 antigen is not found on tissues of nonhematopoietic origin.40,41 Available data suggest that CD45 is not shed into the bloodstream, is not rapidly internalized,42 and is also expressed at a high surface density on the majority of leukemias and lymphomas.40 Although some have expressed concerns about targeting an antigen expressed as broadly as CD45, important advantages also must be recognized. “Lineage-specific” radiolabeled Abs, such as those targeting CD45, may be superior to “leukemia-specific” radiolabeled Abs for patients in remission or in relapsed patients with subclinical involvement of extramedullary tissues, such as lymph nodes, because in these settings isolated malignant cells are surrounded by normal hematopoietic cells. Because the radiation from a radionuclide attached to an Ab bound to the surface of a cell can be emitted in any direction within a geographic area defined by the path length of the radionuclide, the isolated malignant cell may receive a significantly greater absorbed dose if the surrounding normal cells are targeted as well. Indeed, the therapeutic benefit of the PRIT approach demonstrated in this report may have predominantly resulted from crossfire irradiation from targeted neighboring CD45-expressing nonmalignant cells and not solely from direct targeting of the tumor. This contention is supported by the relatively small disease burden in the peripheral blood (Figure S6) and the extensive uptake of radioactivity into marrow and spleen (Figure 5A) on day 2 of therapy. Therefore, translation of the PRIT technology to human trials will likely require stem cell rescue because of the high probability of myeloablation.
In clinical trials, our group has escalated conventional RIT using 131I-anti-CD45 radiolabeled Ab combined with chemotherapy with or without TBI, to myeloablative levels, and relied on either HLA-matched allogeneic HCT to reconstitute hematopoiesis.8,9,15 These studies have demonstrated safety and specificity of radiation targeting to hematopoietic tissues compared with critical normal organs, and suggest efficacy against minimal residual disease. Although this myeloablative approach is effective, the attendant toxicity is substantial and the dose intensity of antileukemic radiation is limited by the radiation doses delivered to lung and liver, partially because of the long-circulating half-life of radiolabeled Ab in the bloodstream. The PRIT technology tested in this study may allow for delivery of dose-intensified RIT that might achieve improved outcomes without these toxicities and that may be applicable to higher risk (relapsed) patients suffering from greater disease burdens. The results presented in this study support the use of the CA as part of a PRIT approach to remove excess circulating Ab-SA conjugate from the circulation and conjugate that may be nonspecifically localized to normal tissues. The CA provides improved target-to-nontarget ratios of absorbed radioactivity, albeit at the risk of removing a nonnegligible amount of pretargeted Ab-SA conjugate from the marrow and spleen as was seen in our results; CA led to an approximately 30% decrease in the % ID/g of radioactivity in the marrow and 67% reduction in the spleen compared with results seen using of the PRIT strategy without the CA (Figure 4). It is possible that the CA may not be necessary for future studies and that translation of the PRIT approach to the clinic may not require use of a CA because of the relatively rapid inherent clearance of the Ab-SA conjugate from the circulation, combined with the apparent CA-mediated removal of Ab-SA conjugate from target tissues.
In conclusion, the data presented here using a relevant preclinical leukemia model suggest that PRIT using an idealized radioimmunoconjugate, which rapidly distributes to sites of disease with a prolonged residence time but is also efficiently cleared from the circulation if not targeted to tumor cells, may further improve the therapeutic index (target-to-nontarget ratio) over that currently achievable with conventional RIT methodologies. The advantages afforded by an anti-CD45 PRIT strategy lay the foundation for logical progression to clinical trials translating the PRIT technology to human patients with CD45+ myeloid malignancies. Translation of this novel anti-CD45 PRIT strategy for targeted anticancer therapy, if successful, may allow the application of this treatment not only to patients with acute leukemias, but to patients with virtually any CD45+ hematologic malignancy, including chronic leukemias and non-Hodgkin lymphomas, as well as AML and myelodysplasia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grants RO1 CA76287 and K08 CA095448) and the Frederick Kullman and Penny E. Petersen Memorial Funds. O.W.P. is supported by an endowed Chair from James and Shirley Raisbeck. J.M.P. is supported by Career Development Awards from the Lymphoma Research Foundation and the Damon Runyon Cancer Foundation.
National Institutes of Health
Authorship
Contribution: J.M.P contributed to the conception, design, analysis, and interpretation of the research and wrote the manuscript; N.H. contributed to the design of the research and performed research and analyzed data; B.L.W. performed research and collected data; L.D. performed research and collected data; A.P. contributed to the conception and interpretation of research; Y.L. contributed to the conception and interpretation of research; D.K.H. contributed vital reagents; D.S.W. contributed vital reagents and contributed to the interpretation of data; A.K.G. contributed to the interpretation of data; D.G. contributed to the interpretation of data; F.R.A. contributed to the conception and interpretation of research and revised the manuscript; O.W.P. contributed to the conception, design, analysis, and interpretation of the research and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John Pagel, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, M/S D5-380, Seattle, WA 98109; e-mail: jpagel@fhcrc.org.
References
Author notes
J.M.P. and N.H. contributed equally to this study.