Receptor or nonreceptor tyrosine kinases (TKs) are known to play an important role in leukemogenesis. Here we studied the level of protein tyrosine phosphorylations in a series of fresh AML samples and evaluated the effect of TK inhibitors. Compared with normal hematopoietic progenitors, a high level of tyrosine phosphorylation was detected in most acute myeloid leukemia (AML) samples. The Src family kinases (SFKs) appeared constitutively activated in most cases, including in the CD34+CD38−CD123+ compartment as revealed by the level of phosphorylated tyrosine 416. Lyn was the major SFK family member expressed in an active form in AML cells where it was abnormally distributed throughout the plasma membrane and the cytosol as opposed to normal hematopoietic progenitors. The SFK inhibitor, PP2, strongly reduced the global level of tyrosine phosphorylations, inhibited cell proliferation, and induced apoptosis in patient samples without affecting normal granulomonocytic colony forming units. Moreover, silencing Lyn expression by small interfering RNA in primary AML cells strongly inhibited proliferation. Interestingly, a link between Lyn and the mTOR pathway was observed as PP2 and a Lyn knockdown both affected the phosphorylation of mTOR targets without inhibiting Akt phosphorylation. Lyn should be considered as a novel pharmacologic target for AML therapy.
Introduction
Acute myeloid leukemia (AML) is characterized by an accumulation of neoplastic myeloid precursors in the bone marrow and blood. The processes of neoplastic transformation are thought to arise at the hematopoietic stem cell or committed myeloid progenitor level, leading to the genesis of leukemic hematopoiesis sharing organizational similarities with its normal counterpart.1,2 Despite an apparent uniformity, AML cells are classified in different compartments. The less mature compartment includes a small fraction of leukemic stem cells (LSCs) that are mostly quiescent but capable of self-renewal or can be committed into proliferation-differentiation. Another compartment contains the more mature and highly cycling progenitors (CFU-L) that are incapable of self-renewal but largely committed to proliferation and limited differentiation. CFU-L gives rise to a large population of differentiated leukemic cells (blast cells), which are arrested at a given stage of granulomonocytic differentiation and represent the majority of leukemic cells in the blood and marrow of patients with AML. From a clinical viewpoint, AML is a heterogeneous disease with marked differences in response to therapy, occurrence of relapses, and overall survival according to prognostic factors, such as age and cytogenetic or molecular abnormalities.3
Many different genetic defects are thought to support the heterogeneity of this disease. Alterations in self-renewal, differentiation, and survival observed in AML cells are the consequence of 2 types of cooperating mutations. Mutations affecting transcription factors (class II mutations) lead to impaired differentiation, whereas mutations of receptor tyrosine kinases (RTKs), such as FLT3, c-KIT, or downstream effectors, such as Ras (class I mutations) enhance cell survival and proliferation.4 The molecular characterization of recurrent translocations and the development of murine models of the disease have established the common idea that these 2 types of mutations are sufficient to generate the leukemic phenotype. Activating mutations of RTKs have been identified in only 50% of all AMLs. Therefore, one can expect the involvement of other deregulated RTKs and/or nonreceptor protein tyrosine kinases (TKs) acting as key activators of intracellular signal-transduction pathways. Indeed, cell survival and proliferation pathways, including MAPK, phosphoinositide 3-kinase (PI3K)/Akt, mTOR, NF-κB or STATs are deregulated in most, if not all, AML cases.5,,,,,,,–13
Here, we investigated the profile of tyrosine phosphorylations in a large panel of fresh AML samples. We observed a high level of tyrosine phosphorylation in AML samples compared with normal hematopoietic progenitor cells (HPCs). Tyrosine kinase inhibitors, particularly the Src family kinase (SFK) inhibitor, PP2, strongly affected the level of global protein tyrosine phosphorylation. Moreover, PP2 induced the inhibition of cell proliferation and apoptosis in AML cell lines and patient samples without affecting colony-forming unit-granulocyte macrophage growth. SFKs appeared constitutively activated in leukemic cell lines, in AML samples as well as in the immature compartment of the leukemic clone (CD34+CD38−CD123+). Among SFKs, Lyn was consistently expressed at a high level and constitutively activated in leukemic cells. Accordingly, the specific down regulation of Lyn expression by small interfering RNA (siRNA) inhibited the clonogenic properties of AML cells. Moreover, PP2 and Lyn siRNA induced the inhibition of S6-ribosomal protein (RPS6) and 4E-BP1 phosphorylation, demonstrating a novel link between Lyn and the mTOR pathway in primary AML cells. These results indicate that Lyn is important for survival and proliferation of AML cells and represents a novel potential pharmacologic target for antileukemic drugs.
Methods
Cells and reagents
AML samples were obtained from patients diagnosed at the Department of Hematology, Toulouse University Medical Center (Toulouse, France), after informed consent was obtained in accordance with the Declaration of Helsinki. The institutional review board of the Hôpital Purpan (Toulouse, France) approved the study. AML cells and normal bone marrow CD34+ HPCs were isolated and processed as previously described.14 To assess the stimulation of the global phosphotyrosine profile, 5 × 105 CD34+ HPCs were incubated in Isocove modified Dulbecco medium (IMDM) 10% BIT (bovine serum albumin, insulin, transferrin) with or without granulocyte-macrophage colony-stimulating factor (GM-CSF, 10 ng/mL), stem cell factor (SCF, 100 ng/mL), and interleukin-3 (IL-3, 1 IU/mL) for 5, 15, 20 minutes or 4 hours, and then processed for Western blot analysis (see “Western blotting and immunoprecipitation”). The human leukemic cell lines KG1, U937, and UT7-EPO were purchased from American Type Culture Collection (Rockville, MD) and cultured in IMDM containing 20% fetal calf serum (FCS, KG1); RPMI 10% FCS (U937), and MEM 10% FCS and 1 UI/L erythropoietin (UT7-EPO). All AML cell lines were incubated in a humidified CO2 incubator (5% CO2, 37°C). Rapamycin and LY294002 were purchased from Sigma-Aldrich (St Louis, MO); PP2 [4-amino-5-(4-chlorophenyl)-7(t-butyl)pyrazolo[3,4-D]pyramidine], AG1296, and AG490 were purchased from Calbiochem (San Diego, CA). All these inhibitors were dissolved in DMSO, and stored at −20°C. Antibodies used included anti–phospho-Akt (Thr308 and Ser473), anti–phospho-Src (Y416), anti–phospho-p70S6K (Thr389), anti–phospho-4E-BP1 (Thr70), anti–phospho-RPS6 (Ser235/236), anti-p70S6K, anti–4E-BP1, anti–S6-ribosomal protein, anticaspase 3, horseradish peroxidase-conjugated secondary antibodies against mouse and rabbit immunoglobulins from Cell Signaling (Beverly, MA); anti–phospho Erk1/2 from Sigma-Aldrich; anti-Akt, anti-Fgr, anti-Fyn, anti-Hck, anti-Lck, anti-Lyn, anti-Src, and anti-PARP from Santa Cruz Biotechnology (Santa Cruz, CA); and anti-phosphotyrosine (mAb; clone 4G10) from Upstate Biotechnology (Charlottesville, VA). The antibody used to detect phosphorylated Src kinases recognizes the phospho site at position 416 in pp60c-src. The corresponding activation site detected by this antibody is 394 and 396 in Lck and Lyn, respectively.
Western blotting and immunoprecipitation
Western blotting was performed as previously described.14 For immunoprecipitation, 107 fresh cells were lysed for 30 minutes at 4°C in 800 μL of a lysis buffer containing 20 mM Tris/HCl, pH 7,4, 150 mM NaCl, 1 mM EDTA, 0,8% Triton X-100, 4 mM NaF, 1 mM orthovanadate, and a protease cocktail inhibitor. After centrifugation, the supernatants were incubated for 1 hour at 4°C with 3 μg of Lyn antibody, followed by the addition for 1 hour of Protein A-Sepharose. After 3 washes with the same buffer without Triton X-100, the pellets were lysed in a Laemmli's buffer, loaded onto SDS-PAGE, and immunostained with the appropriate antibody. Lyn and p-Src antibodies were biotinylated using the BiotinTag Micro Biotinylation Kit (Sigma-Aldrich) and detected using a horseradish peroxidase coupled to streptavidin (Pierce, Rockford, IL).
Small interfering RNA experiments
Fresh AML cells, KG1 and U937 were transfected using the Amaxa nucleofection technology (Amaxa, Köln, Germany). Briefly, cells were resuspended in Amaxa solution kit R (KG1) and V (fresh samples and U937) following the Amaxa guidelines for cell lines. Leukemic cells (2 × 106) in 100 μL of solution kit V or R were mixed with 3 μg siRNA smartpool Lyn or with 3 μg siRNA control both for cell lines and primary AML samples (Dharmacon, Lafayette, CO) and immediately nucleofected with an Amaxa Nucleofector apparatus (program V-01 for both cell lines, program U-15 for primary AML cells). Cells were then immediately transferred into wells containing 37°C prewarmed culture medium in 6-well plates. After transfection, cells were cultured from 24 to 72 hours before analyzing by Western blot.
Flow cytometric analysis
Fresh leukemic cells and KG1 (2 × 105) were incubated for 1 hour with or without PP2, then fixed and permeabilized with intraprep kit (Beckman Coulter, Villepinte, France) according to the manufacturer's recommendations. Cells were then incubated for 1 hour at 4°C in the dark with antiphospho-Src (Y416), rabbit nonrelevant IgG (Santa Cruz) or no antibody. Samples were then washed twice with phosphate-buffered saline (PBS) and pellets were incubated for 30 minutes in the dark with the secondary goat antirabbit FITC antibody (BD Biosciences, San Jose, CA). All samples were analyzed by Epics XL (Beckman Coulter), and results were expressed as the ratio between mean fluorescence intensity of the stained sample and mean fluorescence intensity of the control. For several experiments on AML samples, a double immunostaining procedure combining Pe-Cy5 conjugated anti-CD34 (Beckman Coulter) surface staining and antiphospho-Src (Y416) intracellular staining was also conducted with the same results. Flow cytometric detection of the immature compartment of the leukemic clone (CD34+CD38−CD123+ cells) has been described elsewhere.15
Apoptosis
Fresh AML cells (2 × 106) were incubated in 96-well flat-bottomed plates with or without 10 μM PP2. After incubation, cells were washed twice with cold PBS (without Ca2+ and Mg2+), followed by addition of 5 μL propidium iodide and 5 μL annexin V-FITC. Cells were also incubated for 15 minutes at room temperature in the dark. The percentage of apoptotic cells was assessed by flow cytometry using an Epics XL (Beckman Coulter).
Clonogenic assays
Sensitivity of leukemic progenitors to PP2.
The percentage of leukemic cells after Ficoll separation ranged between 60% and 100% (median, 92%). AML cells were adjusted to a final concentration of 105 cells/mL and grown in H4230 Stem Cell Technologies methyl cellulose medium (Stem Cell Technologies, Vancouver, BC) supplemented with 10% 5637-conditioned medium as a stimulant (5637 is a bladder carcinoma cell line secreting GM-CSF, G-CSF, IL1beta, M-CSF, and SCF)16 and increasing concentrations of PP2 (0, 0.5, 1, 2, 5, 10, and 20 μM). The cells were then plated in 35-mm Petri dishes in duplicate and incubated for 7 days in a humidified CO2 incubator (5% CO2, 37°C). Leukemic colonies (> 20 cells) and clusters (> 5 cells) were scored under an inverted microscope at day 7 and were uniformly named colony forming unit-leukemia (CFU-L). In each case, the viability and the leukemic nature of CFU-L were confirmed by a blue trypan exclusion assay and by morphologic analysis after Giemsa staining. We also performed karyotypic analysis in some samples and, as expected, in each case, colony-forming cells displayed identical cytogenetic abnormalities to the primary leukemic cells.
Colony-forming assays of human bone marrow CD34+ cells.
Fresh CD34+ human bone marrow cells were washed twice in IMDM medium with 10% FCS and resuspended at a concentration of 7 × 103/mL in Stem Cell Technologies' methyl cellulose complete media: H4230 supplemented with 10% 5637-conditioned medium (CFU-GM growth), H4435 (CFU-M growth), and H4535 (BFU-E growth). PP2 was added at a concentration of 0, 2, 5, and 10 μM. The cells were then plated in 35-mm Petri dishes and incubated in a humidified CO2 incubator (5% CO2, 37°C) for 14 days. Colonies (> 50 cells) were then scored under an inverted microscope.
Confocal microscopy
AML primary cells, KG1, and U937 (2 × 105) were plated on poly-L-lysine precoated slides, fixed with 3.7% (w/v) paraformaldehyde for 30 minutes, washed twice with PBS, and then permeabilized by 0.2% (v/v) Triton X-100 in bovine serum albumin (BSA)-PBS (w/v) for 5 minutes. Slides were incubated for 30 minutes with 3% BSA-PBS for aspecific binding and then incubated for 30 minutes again with primary antibodies diluted in 3% BSA-PBS (1 of 500 for Lyn and 1 of 100 for p-Src Y416). After 3 washes in 3% BSA-PBS, secondary antibodies (antimouse FITC antibody and antirabbit Alexa Fluor 594) were added for 30 minutes. After several washes in PBS, the coverslips were mounted with Moewiol, and all the preparations were analyzed by confocal laser scanning using a Zeiss LSM510 equipped with a 63× objective.
Results
The level of protein tyrosine phosphorylation is high in AML and sensitive to SFK inhibition
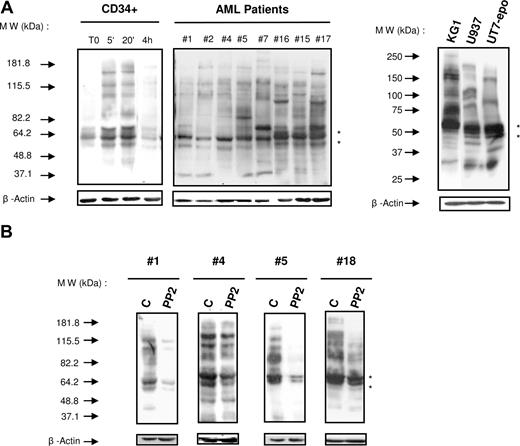
Abnormal activation of tyrosine kinases and of the signaling pathways they control is thought to play a critical role in leukemogenesis. To assess the role of tyrosine kinases in AML cells, we first examined the level of tyrosine phosphorylations in normal HPCs, AML samples (n = 38, Table 1), and leukemic cell lines (KG1, U937, UT7-Epo) by immunoblotting with an antiphosphotyrosine antibody. In normal quiescent CD34+ HPCs, the level of tyrosine phosphorylation was low but increased on stimulation with a cocktail of cytokines (SCF, IL3, GM-CSF). These phosphorylations increased rapidly and returned to the basal level after 4 hours of stimulation. Although somewhat heterogeneous, the tyrosine phosphorylation levels were consistently high in AML samples (Figure 1A). It is noteworthy that the tyrosine phosphorylation profiles appeared more homogeneous when assessed according to their cytogenetic or molecular background (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Strikingly, we observed a strong signal of tyrosine phosphorylation of proteins of approximately 55 to 60 kDa. This molecular weight may correspond to SFKs, which are known to be expressed in AML cells.17 Moreover, the SFK inhibitor PP2 (10 μM, 1 hour) dramatically reduced the degree of tyrosine phosphorylation in most samples tested (15 of 20). Samples variations or kinetic differences likely explain the degree of responses observed in Figure 1B. Altogether, these results suggest that Src kinases play an important role in AML.
Characteristics of AML patients
| Patient no. . | Age, yr . | FAB . | WBC, × 109/L . | Cytogenetic . | FLT3 . | |
|---|---|---|---|---|---|---|
| ITD . | D835 . | |||||
| 1 | 75 | 5 | 46.5 | 46 XX | 0 | 0 |
| 2 | 51 | 1 | 1.1 | 46 XX | 0 | 0 |
| 3 | 38 | 2 | 1.8 | Tetrasomy 8 | 0 | 0 |
| 4 | 45 | 4 | 216 | 46 XY | 1 | 0 |
| 5 | 67 | t-AML | 7.6 | 46 XY | 0 | 0 |
| 6 | 59 | 4 | 162 | 46 XX | 0 | ND |
| 7 | 43 | 4Eo | 148 | 46 XY, inv (16) | 0 | 0 |
| 8 | 52 | 5 | 142.5 | 46 XY, t(3;6) | 1 | 0 |
| 9 | 53 | 1 | 225 | 46 XX | 0 | ND |
| 10 | 43 | 2 | 28 | 46 XY, t(8;21) | 0 | 0 |
| 11 | 53 | 4 | 54.5 | 46XX | 1 | 0 |
| 12 | 59 | 1 | 125 | Penta8; +(10); +(13) | 1 | ND |
| 13 | 46 | 1 | 33.3 | 46 XX | 1 | 0 |
| 14 | 52 | 1 | 167 | 46 XX | 1 | 0 |
| 15 | 53 | 4Eo | 98.6 | 46 XX, inv(16) | 0 | 0 |
| 16 | 40 | 5 | 107.7 | 46 XY | 1 | 0 |
| 17 | 64 | 4 | 91 | 46 XY, t(2;21) | 0 | ND |
| 18 | 60 | 2 | 70 | 46 XX | 0 | 0 |
| 19 | 59 | 4 | 20 | 46 XX | 0 | 0 |
| 20 | 72 | 2 | 18.9 | Complex | 0 | ND |
| 21 | 41 | 4Eo | 57.8 | 46 XY, t(4;16) | 0 | 0 |
| 22 | 54 | 2 | 35.7 | 47 XX, +(8) | 0 | ND |
| 23 | 35 | 2 | 3.3 | 46 XY, t(8;21) | 0 | 0 |
| 24 | 41 | 2 | 21 | 46 XY, t(8;21) | 0 | 0 |
| 25 | 56 | 4Eo | 23 | 46 XX, inv (16) | 0 | 0 |
| 26 | 15 | 2 | 11.2 | 46 XY, t(8;21) | 0 | ND |
| 27 | 54 | 2 | 11 | 46 XY, t(8;21) | 0 | 0 |
| 28 | 27 | 3 | 18.9 | 46 XX, t(15;17) | 1 | ND |
| 29 | 73 | 3 | 1.3 | 46 XY, t(15;17) | 0 | ND |
| 30 | 29 | 2 | 33.5 | 46 XX, t(8;21) | 0 | 0 |
| 31 | 44 | 2 | 211.8 | 46 XY, t(8;21) | 0 | 0 |
| 32 | 63 | 3 | 13.3 | 46 XX, t(15;17) | 0 | 1 |
| 33 | 45 | 3 | 10 | 46 XY, t(15;17) | 1 | ND |
| 34 | 42 | 3 | 42.1 | 46 XY,t(15;17) | 1 | 0 |
| 35 | 45 | t-AML | 39.6 | 45 XX, inv(3), del(5) | 0 | 0 |
| 36 | 78 | 2 | 98.6 | 46 XX, inv(3) | 1 | 0 |
| 37 | 62 | 2 | 142.5 | 46 XX, t(3;6), 7q− | 1 | 0 |
| 38 | 49 | 2 | 3.1 | Complex | 1 | ND |
| 39 | 66 | 2 | 106 | Complex | 0 | ND |
| 40 | 48 | 2 | 4.4 | Complex | ND | ND |
| 41 | 74 | 1 | 88 | 46 XY, t(8;22),5q−,20q− | ND | ND |
| 42 | 81 | 2 | 50 | 46 XY | 0 | 1 |
| 43 | 79 | t-AML | 188 | 47 XY, +(8) | 0 | 0 |
| 44 | 61 | 2 | 39.5 | 46 XY | 1 | 0 |
| 45 | 80 | 2 | 38.5 | 47 XY, +(8) | 0 | ND |
| 46 | 77 | 1 | 195 | 46 XX | 0 | 0 |
| 47 | 70 | 1 | 14 | Complex | 0 | ND |
| 48 | 63 | t-AML | 41 | Complex | 0 | 0 |
| 49 | 33 | 2 | 60.4 | 46 X−Y+X, t(8;21) | 1 | 0 |
| 50 | 76 | 4 | 136 | 47 XX, +(11) | 0 | 1 |
| 51 | 73 | 2 | 13.3 | 46 XX | 0 | ND |
| 52 | 68 | t-AML | 82.7 | 45 XY, −(7) | 0 | ND |
| 53 | 79 | 2 | 1.4 | Complex | 0 | 0 |
| 54 | 69 | 2 | 10.6 | 46 XY | ND | ND |
| 55 | 70 | t-AML | 19 | 46 XY, (7)q− | 0 | ND |
| 56 | 56 | 1 | 26.9 | ND | 1 | ND |
| 57 | 53 | 1 | 27.9 | 46 XY | 0 | 0 |
| 58 | 19 | 5 | 15.2 | 5q−, 17+, −20, t(5;17) | 0 | ND |
| 59 | 66 | 2 | 5,8 | Complex | 0 | ND |
| 60 | 60 | 2 | 41 | 46 XY | 0 | ND |
| 61 | 76 | 1 | 6.5 | 46 XX | ND | ND |
| 62 | 68 | 2 | 1.2 | Complex; 5q−;17p− | 0 | ND |
| Patient no. . | Age, yr . | FAB . | WBC, × 109/L . | Cytogenetic . | FLT3 . | |
|---|---|---|---|---|---|---|
| ITD . | D835 . | |||||
| 1 | 75 | 5 | 46.5 | 46 XX | 0 | 0 |
| 2 | 51 | 1 | 1.1 | 46 XX | 0 | 0 |
| 3 | 38 | 2 | 1.8 | Tetrasomy 8 | 0 | 0 |
| 4 | 45 | 4 | 216 | 46 XY | 1 | 0 |
| 5 | 67 | t-AML | 7.6 | 46 XY | 0 | 0 |
| 6 | 59 | 4 | 162 | 46 XX | 0 | ND |
| 7 | 43 | 4Eo | 148 | 46 XY, inv (16) | 0 | 0 |
| 8 | 52 | 5 | 142.5 | 46 XY, t(3;6) | 1 | 0 |
| 9 | 53 | 1 | 225 | 46 XX | 0 | ND |
| 10 | 43 | 2 | 28 | 46 XY, t(8;21) | 0 | 0 |
| 11 | 53 | 4 | 54.5 | 46XX | 1 | 0 |
| 12 | 59 | 1 | 125 | Penta8; +(10); +(13) | 1 | ND |
| 13 | 46 | 1 | 33.3 | 46 XX | 1 | 0 |
| 14 | 52 | 1 | 167 | 46 XX | 1 | 0 |
| 15 | 53 | 4Eo | 98.6 | 46 XX, inv(16) | 0 | 0 |
| 16 | 40 | 5 | 107.7 | 46 XY | 1 | 0 |
| 17 | 64 | 4 | 91 | 46 XY, t(2;21) | 0 | ND |
| 18 | 60 | 2 | 70 | 46 XX | 0 | 0 |
| 19 | 59 | 4 | 20 | 46 XX | 0 | 0 |
| 20 | 72 | 2 | 18.9 | Complex | 0 | ND |
| 21 | 41 | 4Eo | 57.8 | 46 XY, t(4;16) | 0 | 0 |
| 22 | 54 | 2 | 35.7 | 47 XX, +(8) | 0 | ND |
| 23 | 35 | 2 | 3.3 | 46 XY, t(8;21) | 0 | 0 |
| 24 | 41 | 2 | 21 | 46 XY, t(8;21) | 0 | 0 |
| 25 | 56 | 4Eo | 23 | 46 XX, inv (16) | 0 | 0 |
| 26 | 15 | 2 | 11.2 | 46 XY, t(8;21) | 0 | ND |
| 27 | 54 | 2 | 11 | 46 XY, t(8;21) | 0 | 0 |
| 28 | 27 | 3 | 18.9 | 46 XX, t(15;17) | 1 | ND |
| 29 | 73 | 3 | 1.3 | 46 XY, t(15;17) | 0 | ND |
| 30 | 29 | 2 | 33.5 | 46 XX, t(8;21) | 0 | 0 |
| 31 | 44 | 2 | 211.8 | 46 XY, t(8;21) | 0 | 0 |
| 32 | 63 | 3 | 13.3 | 46 XX, t(15;17) | 0 | 1 |
| 33 | 45 | 3 | 10 | 46 XY, t(15;17) | 1 | ND |
| 34 | 42 | 3 | 42.1 | 46 XY,t(15;17) | 1 | 0 |
| 35 | 45 | t-AML | 39.6 | 45 XX, inv(3), del(5) | 0 | 0 |
| 36 | 78 | 2 | 98.6 | 46 XX, inv(3) | 1 | 0 |
| 37 | 62 | 2 | 142.5 | 46 XX, t(3;6), 7q− | 1 | 0 |
| 38 | 49 | 2 | 3.1 | Complex | 1 | ND |
| 39 | 66 | 2 | 106 | Complex | 0 | ND |
| 40 | 48 | 2 | 4.4 | Complex | ND | ND |
| 41 | 74 | 1 | 88 | 46 XY, t(8;22),5q−,20q− | ND | ND |
| 42 | 81 | 2 | 50 | 46 XY | 0 | 1 |
| 43 | 79 | t-AML | 188 | 47 XY, +(8) | 0 | 0 |
| 44 | 61 | 2 | 39.5 | 46 XY | 1 | 0 |
| 45 | 80 | 2 | 38.5 | 47 XY, +(8) | 0 | ND |
| 46 | 77 | 1 | 195 | 46 XX | 0 | 0 |
| 47 | 70 | 1 | 14 | Complex | 0 | ND |
| 48 | 63 | t-AML | 41 | Complex | 0 | 0 |
| 49 | 33 | 2 | 60.4 | 46 X−Y+X, t(8;21) | 1 | 0 |
| 50 | 76 | 4 | 136 | 47 XX, +(11) | 0 | 1 |
| 51 | 73 | 2 | 13.3 | 46 XX | 0 | ND |
| 52 | 68 | t-AML | 82.7 | 45 XY, −(7) | 0 | ND |
| 53 | 79 | 2 | 1.4 | Complex | 0 | 0 |
| 54 | 69 | 2 | 10.6 | 46 XY | ND | ND |
| 55 | 70 | t-AML | 19 | 46 XY, (7)q− | 0 | ND |
| 56 | 56 | 1 | 26.9 | ND | 1 | ND |
| 57 | 53 | 1 | 27.9 | 46 XY | 0 | 0 |
| 58 | 19 | 5 | 15.2 | 5q−, 17+, −20, t(5;17) | 0 | ND |
| 59 | 66 | 2 | 5,8 | Complex | 0 | ND |
| 60 | 60 | 2 | 41 | 46 XY | 0 | ND |
| 61 | 76 | 1 | 6.5 | 46 XX | ND | ND |
| 62 | 68 | 2 | 1.2 | Complex; 5q−;17p− | 0 | ND |
FAB indicates French-American-British classification; WBC, white blood cell count; FLT3-ITD, FLT3-internal tandem duplication; FLT3-D835, FLT3 mutation in the activation loop; t-AML, transformed-AML; and ND, not done.
Analysis of the global protein tyrosine phosphorylation in normal HPCs and AML cells. (A) The level of protein tyrosine phosphorylation in normal unstimulated and stimulated (GM-CSF, SCF, and IL-3 for 5 and 20 minutes or for 4 hours) CD34+ HPCs, fresh AML samples, and AML cell lines was studied under similar conditions by Western blot with the antiphosphotyrosine antibody 4G10. The membrane was stripped and reprobed for β-actin as a loading control (lower panel) and for Lyn (black stars). Results shown are representative of experiments performed with normal CD34+ HPCs from 3 healthy donors and 38 AML samples (patient nos. 1, 2, 4–22, 24–28, 30, 31, 33–37, 56–60). (B) A similar analysis was performed on fresh AML cells cultured alone or in the presence of PP2 (10 μM) for 1 hour. Results shown are representative of experiments performed with 20 AML samples (patients 1–20). Black stars indicate the position of Lyn.
Analysis of the global protein tyrosine phosphorylation in normal HPCs and AML cells. (A) The level of protein tyrosine phosphorylation in normal unstimulated and stimulated (GM-CSF, SCF, and IL-3 for 5 and 20 minutes or for 4 hours) CD34+ HPCs, fresh AML samples, and AML cell lines was studied under similar conditions by Western blot with the antiphosphotyrosine antibody 4G10. The membrane was stripped and reprobed for β-actin as a loading control (lower panel) and for Lyn (black stars). Results shown are representative of experiments performed with normal CD34+ HPCs from 3 healthy donors and 38 AML samples (patient nos. 1, 2, 4–22, 24–28, 30, 31, 33–37, 56–60). (B) A similar analysis was performed on fresh AML cells cultured alone or in the presence of PP2 (10 μM) for 1 hour. Results shown are representative of experiments performed with 20 AML samples (patients 1–20). Black stars indicate the position of Lyn.
SFKs are in an active form both in leukemic bulk and in immature CD34+CD38−CD123+ leukemic cells
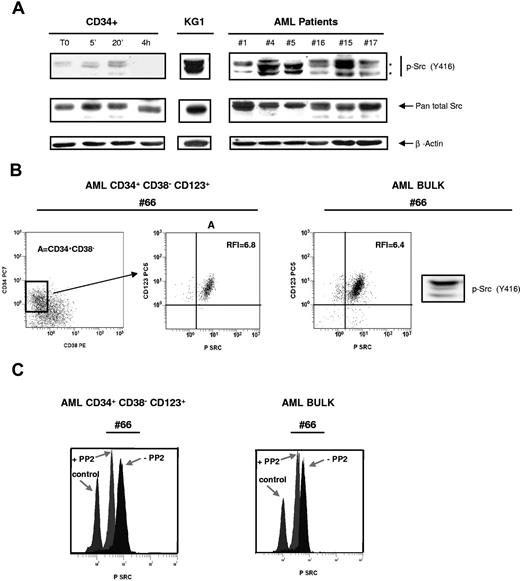
The phosphospecific antibody recognizing the active form of SFK members was used to monitor the activation state of these kinases by immunoblotting and flow cytometry. The level of activation of SFKs was assessed in normal CD34+, in the KG1 cell line, and in a series of 53 AML samples (Figure 2A). Normal CD34+ cells displayed a low level of activated SFKs, but this level increased after 5 and 20 minutes of cytokine stimulation. Contrasting with normal cells, SFKs were found in an active form in all AML samples tested as well as in KG1 cells.
Constitutive activating phosphorylation of SFKs in leukemic bulk and in immature CD34+CD38−CD123+ leukemic cells. (A) Normal CD34+ HPCs unstimulated or stimulated with GM-CSF, SCF and IL-3, KG1, and 53 AML samples (patient nos. 1, 2, 4–22, 24–28, 30, 31, 33–37, 40–44, 47–53, 56–63) were analyzed by Western blot using the phosphospecific antibody recognizing the activated form of SFK members (anti-pSrc Y416). The membranes were stripped and reprobed with the pan total Src antibody and a β-actin antibody as loading control. Data shown are representative of 3 independent experiments. Black stars indicate the position of Lyn. (B) Flow cytometric analysis of a sample from patient 66 using the 4-color protocol as described in “Flow cytometric analysis.” The left panel shows the CD34 versus CD38 dot plot (CD34+CD38− blast cell population is represented in gate A). Middle panel shows the CD123+ and p-Src Y416 expression in gate A. The right panel shows a sample of the leukemic bulk from patient 66 analyzed both by flow cytometry and by Western blot using the anti-pSrc Y416 antibody. (C) Patient cells were cultured for 1 hour alone or in the presence of PP2 (10 μM), then processed using 4-color immunostaining. The effect of PP2 on SFK phosphorylation was analyzed in the CD34+CD38−CD123+ blast subcompartment (patient nos. 64–68) and in the leukemic bulk (patient nos. 20, 42–44, 47–50, 52–53, 61–63). Results shown are representative of the different patients analyzed.
Constitutive activating phosphorylation of SFKs in leukemic bulk and in immature CD34+CD38−CD123+ leukemic cells. (A) Normal CD34+ HPCs unstimulated or stimulated with GM-CSF, SCF and IL-3, KG1, and 53 AML samples (patient nos. 1, 2, 4–22, 24–28, 30, 31, 33–37, 40–44, 47–53, 56–63) were analyzed by Western blot using the phosphospecific antibody recognizing the activated form of SFK members (anti-pSrc Y416). The membranes were stripped and reprobed with the pan total Src antibody and a β-actin antibody as loading control. Data shown are representative of 3 independent experiments. Black stars indicate the position of Lyn. (B) Flow cytometric analysis of a sample from patient 66 using the 4-color protocol as described in “Flow cytometric analysis.” The left panel shows the CD34 versus CD38 dot plot (CD34+CD38− blast cell population is represented in gate A). Middle panel shows the CD123+ and p-Src Y416 expression in gate A. The right panel shows a sample of the leukemic bulk from patient 66 analyzed both by flow cytometry and by Western blot using the anti-pSrc Y416 antibody. (C) Patient cells were cultured for 1 hour alone or in the presence of PP2 (10 μM), then processed using 4-color immunostaining. The effect of PP2 on SFK phosphorylation was analyzed in the CD34+CD38−CD123+ blast subcompartment (patient nos. 64–68) and in the leukemic bulk (patient nos. 20, 42–44, 47–50, 52–53, 61–63). Results shown are representative of the different patients analyzed.
Flow cytometry allows the detection of the immature compartment of the leukemic clone which expresses the CD34+CD38−CD123+ phenotype.15 This subcompartment is thought to be enriched in leukemic stem cells.7,18,19 Interestingly, CD34+CD38−CD123+ leukemic cells displayed a high level of SFK phosphorylation that was inhibited by PP2 (Figure 2B,C). Indeed, the ratio between the mean fluorescence intensity of the stained cells and the mean fluorescence intensity of the isotypic control was 1.5 plus or minus 0.3, 4.1 plus or minus 2.3, and 4.6 plus or minus 2.5 in normal CD34+ HPCs, leukemic bulk, and CD34+CD38−CD123+ cells (mean of 5 AML patients tested), respectively. After 1 hour of incubation with 10 μM PP2, this ratio decreased in the leukemic bulk and CD34+CD38−CD123+ cells (Figure 2C) and in KG1 cells (not shown). Thus, SFKs are activated in the leukemic bulk as well as in early leukemic progenitors but not in their normal counterpart.
Lyn is highly expressed and constitutively phosphorylated in AML cells
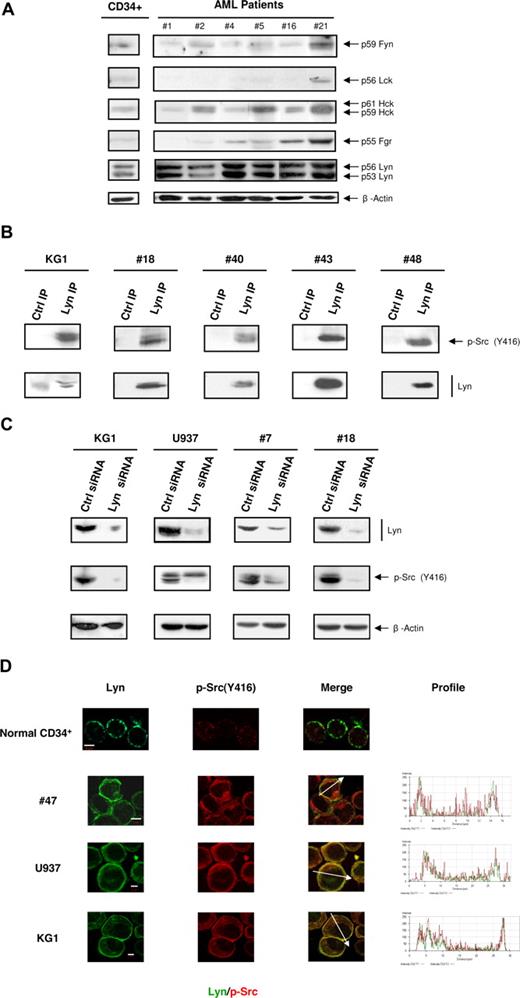
To identify the members of SFKs implicated in AML, we analyzed the level of expression of the various members of the family. In a large panel of fresh samples (n = 33), we found that Lyn, Hck, and Fgr were detected in 100%, 78%, and 66% of cases, respectively. Conversely, Fyn was weakly detected in most AML samples and Lck was hardly or not detectable in the majority of cases (Figure 3A, Figure S2A). It is noteworthy that the major bands detected by the antibody recognizing the active form of SFKs in KG1 and in patient samples shown in Figure 2A matched the 2 Lyn kinase isoforms (p53 and p56). When the nitrocellulose membrane shown in Figure 1A was stripped and reprobed for Lyn, the signal obtained matched the major band of 50/60 kDa observed with the antiphosphotyrosine antibody (Figure 1A). Depletion of Lyn from the lysate of U937 and KG1 cells strongly decreased the 50/60 kDa phosphotyrosine band, indicating that Lyn accounts for the major part of this signal, although other phosphoproteins of similar molecular weight are also likely present in this mass range (Figure S2B). Moreover, as shown by the immunoprecipitation of Lyn and immunoblotting with the antibody recognizing the active form of SFKs, Lyn was phosphorylated on the surrogate marker of its active form in KG1 cells as well as in fresh AML samples (Figure 3B). Accordingly, specific knockdown of Lyn expression using siRNA induced a dramatic reduction of the activated SFKs signal in KG1 cells, U937, and fresh AML cells (Figure 3C). Furthermore, an in vitro kinase assay based on the autophosphorylation of immunoprecipitated Lyn has revealed a highly active kinase in leukemic cells compared with normal cells (Figure S2C).
Characterization of SFK members in AML cells. (A) The levels of Fyn, Lck, Hck, Fgr, and Lyn from normal CD34+ HPCs and AML samples were analyzed by Western blot using specific antibodies (patient nos. 1, 2, 4–7, 9, 10, 12–16, 18, 19, 21, 22, 24–28, 30, 31, 33–41). The level of β-actin was assessed as loading control (lower panel). Results shown are representative of the different patients analyzed. (B) Protein lysates from KG1 cells and from AML samples (patient nos. 18, 40, 43, 48) were immunoprecipitated using an anti-Lyn antibody. The immunoprecipitates (IP) were probed with anti-pSrc Y416. The membranes were then stripped and reprobed with a biotinylated anti-Lyn antibody revealed with streptavidin-HRP. Control immunoprecipitates were performed with protein A beads only. (C) KG1, U937, and fresh AML samples (patient nos. 7, 15, 18, 45) were cultured after electroporation with Lyn siRNA or control siRNA. The Western blot was performed 48 to 72 hours after electroporation. Results shown are representative of 4 experiments performed with KG1 cells and of the different patients analyzed. (D) Immunolocalization of Lyn and active forms of SFKs was performed by confocal microscopy using specific antibodies in normal CD34+ HPCs and AML samples. Results shown are representative of 3 (for HPC, KG1, and U937) and 8 AML samples (patient nos. 8, 16, 21, 30, 40, 43, 47, 48) independent experiments (original magnification, ×63). Bars represent 5 μm.
Characterization of SFK members in AML cells. (A) The levels of Fyn, Lck, Hck, Fgr, and Lyn from normal CD34+ HPCs and AML samples were analyzed by Western blot using specific antibodies (patient nos. 1, 2, 4–7, 9, 10, 12–16, 18, 19, 21, 22, 24–28, 30, 31, 33–41). The level of β-actin was assessed as loading control (lower panel). Results shown are representative of the different patients analyzed. (B) Protein lysates from KG1 cells and from AML samples (patient nos. 18, 40, 43, 48) were immunoprecipitated using an anti-Lyn antibody. The immunoprecipitates (IP) were probed with anti-pSrc Y416. The membranes were then stripped and reprobed with a biotinylated anti-Lyn antibody revealed with streptavidin-HRP. Control immunoprecipitates were performed with protein A beads only. (C) KG1, U937, and fresh AML samples (patient nos. 7, 15, 18, 45) were cultured after electroporation with Lyn siRNA or control siRNA. The Western blot was performed 48 to 72 hours after electroporation. Results shown are representative of 4 experiments performed with KG1 cells and of the different patients analyzed. (D) Immunolocalization of Lyn and active forms of SFKs was performed by confocal microscopy using specific antibodies in normal CD34+ HPCs and AML samples. Results shown are representative of 3 (for HPC, KG1, and U937) and 8 AML samples (patient nos. 8, 16, 21, 30, 40, 43, 47, 48) independent experiments (original magnification, ×63). Bars represent 5 μm.
Lyn distribution was analyzed in normal CD34+ HPCs, KG1 cells, U937, and AML samples by confocal microscopy. The distribution of Lyn in normal CD34+ HPCs was restricted to distinct points at the plasma membrane, and the signal obtained with the antibody recognizing the active form of SFKs was very weak (Figure 3D). In contrast, Lyn appeared to be distributed over the entire plasma membrane and also in the cytoplasm where it colocalized with the antibody recognizing the active form of SFKs staining (Figure 3D).
Antileukemic activity of PP2 and Lyn siRNA
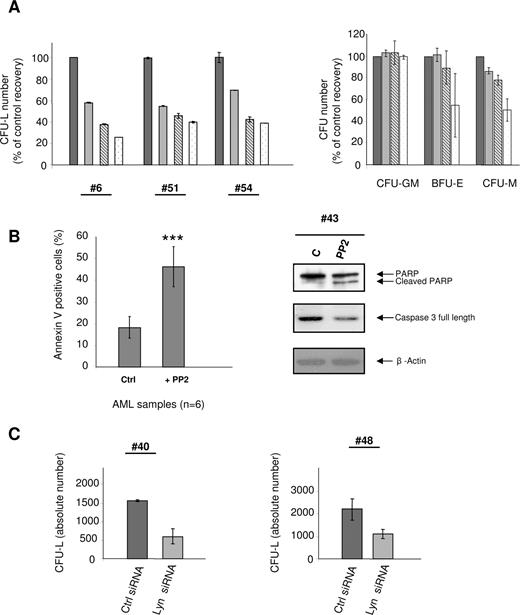
To further characterize the role of SFKs in AML cells, we analyzed the effect of the SFK inhibitor PP2 on survival and proliferation of KG1 cells and AML samples. PP2 inhibited KG1 cell growth in a time- and dose-dependent manner both in clonogenic assays and in liquid culture, and this effect was mainly the result of a cell cycle arrest in G1 (Figure S3). Because fresh AML cells usually hardly proliferate in liquid culture, we used a clonogenic assay in methylcellulose conditioned with hematopoietic growth factors to study the effect of PP2 on samples from 8 AML patients. PP2 inhibited the capacity of AML cells to generate CFU-L, in a dose-dependent manner, with an IC50 less than 10 μM in most cases (5 of 8; Figure 4A left panel).
Activity of PP2 on normal HPCs and AML cell proliferation and survival. (A) Fresh AML cells (patient nos. 6, 51, 54) and normal HPC (right panel) were grown in clonogenic assays in the presence of increasing doses of PP2 (black bars, control; gray bars, 2 μM; hatched bars, 5 μM; white bars, 10 μM). Results are presented as percentage of control and are mean plus or minus SEM of duplicates. Results shown for normal CD34+ HPCs are mean plus or minus SEM of 2 independent experiments performed in duplicate. IC50 values were 4.8, 3.5, and 7.7 μM for patient nos. 6, 51, and 54, respectively. Five other patients (nos. 42, 47, 48, 50, 55) were also analyzed with IC50 values of 28.2, 9.5, 10.2, 14.5, and 0.5 μM, respectively. (B) AML samples (patient nos. 13, 42, 43, 44–46) were incubated with or without 10 μM PP2 for 24 hours, then processed for apoptosis studies using annexinV/PI staining and Western blot analysis using anti-PARP and anti-caspase 3 antibodies. Results are presented as mean percentages (± SEM) of annexin V-positive cells in treated vs untreated cells (***P < .001). (C) Fresh AML samples (patient nos. 40 and 48) were electroporated with Lyn siRNA or control siRNA, and their clonogenic properties were analyzed. CFU-Ls numbers were scored at day 7. Results are expressed as absolute number of leukemic colonies and are mean plus or minus SEM of duplicates.
Activity of PP2 on normal HPCs and AML cell proliferation and survival. (A) Fresh AML cells (patient nos. 6, 51, 54) and normal HPC (right panel) were grown in clonogenic assays in the presence of increasing doses of PP2 (black bars, control; gray bars, 2 μM; hatched bars, 5 μM; white bars, 10 μM). Results are presented as percentage of control and are mean plus or minus SEM of duplicates. Results shown for normal CD34+ HPCs are mean plus or minus SEM of 2 independent experiments performed in duplicate. IC50 values were 4.8, 3.5, and 7.7 μM for patient nos. 6, 51, and 54, respectively. Five other patients (nos. 42, 47, 48, 50, 55) were also analyzed with IC50 values of 28.2, 9.5, 10.2, 14.5, and 0.5 μM, respectively. (B) AML samples (patient nos. 13, 42, 43, 44–46) were incubated with or without 10 μM PP2 for 24 hours, then processed for apoptosis studies using annexinV/PI staining and Western blot analysis using anti-PARP and anti-caspase 3 antibodies. Results are presented as mean percentages (± SEM) of annexin V-positive cells in treated vs untreated cells (***P < .001). (C) Fresh AML samples (patient nos. 40 and 48) were electroporated with Lyn siRNA or control siRNA, and their clonogenic properties were analyzed. CFU-Ls numbers were scored at day 7. Results are expressed as absolute number of leukemic colonies and are mean plus or minus SEM of duplicates.
We then studied the effect of PP2 on the capacity of normal CD34+ HPCs to generate granulomonocytic (CFU-GM), erythroid (BFU-E), and monocytic (CFU-M) colonies. No significant change in the number of colony-forming CFU-GM was observed on PP2 treatment. The number of BFU-E and CFU-M was significantly decreased on treatment with the highest concentration of PP2 (Figure 4A right panel). Altogether, these results demonstrate that, in most cases, PP2 targets AML progenitors at concentrations less than 10 μM while having no effect on normal granulomonocytic progenitors.
As shown in Figure 4B, incubation of fresh AML samples with 10 μM PP2 for 24 hours significantly induced apoptosis as assessed by annexin V binding (46%, PP2 vs 18%, control), PARP cleavage, and decrease in full-length caspase 3.
As Lyn appeared to be a major SFK in AML, we analyzed the effect of a specific decrease of its expression in fresh AML samples using siRNA. Figure 4C shows that the knockdown of Lyn strongly reduced the clonogenic properties of AML cells.
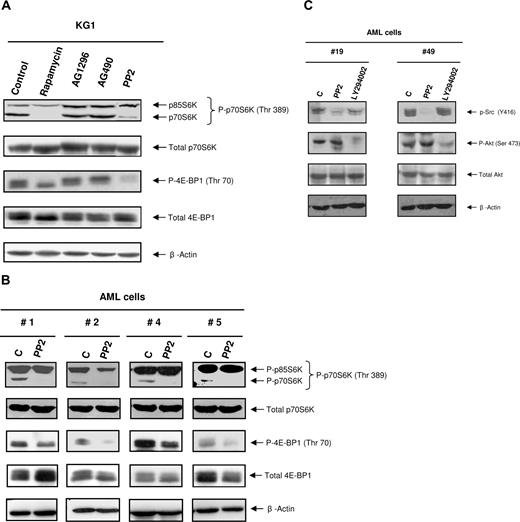
The SFK inhibitor PP2, but not AG1296 or AG490, inhibits the phosphorylation of mTOR targets in the KG1 cell line and AML samples
We have recently demonstrated that the mTOR/4E-BP1/p70S6K pathway is constitutively activated in AML cells but not in their normal counterpart.14 Rapamycin, a selective inhibitor of mTOR, inhibits the capacity of AML cells to generate CFU-L without affecting the growth of normal CD34+ HPCs. However, the mechanisms of activation of the mTOR/4E-BP1/p70S6K pathway in AML remain poorly understood. Because the effect of PP2 on both leukemic cells and normal HPCs mimicked rapamycin activity, we hypothesized a putative regulatory role of SFKs on mTOR activity in AML cells. We first treated KG1 cells, which display a high level of mTOR activation, with PP2 and other tyrosine kinase inhibitors, including AG1296 (targeting c-Kit, PDGFR, and FLT3) and AG490 (targeting JAK2). As expected, rapamycin inhibited the phosphorylation of mTOR targets. Interestingly, PP2 (10 μM) also inhibited the phosphorylation of p70S6K and 4E-BP1, whereas AG1296 and AG490 had no effect (Figure 5A). Moreover, a 1-hour treatment of fresh AML samples with PP2 consistently inhibited the phosphorylation of p70S6K and affected the phosphorylation of 4E-BP1 to different extent according to the patient samples, without affecting their expression level (Figure 5B). These results suggested that member(s) of the SFKs could play a role in regulating the mTOR pathway in AML cells. Consistent with the results obtained in the leukemic bulk, the high level of SFKs activation in CD34+CD38−CD123+ cells correlated with an elevated phosphorylation of RPS6. RPS6 phosphorylation was also inhibited by PP2 treatment in this immature sub-compartment (not shown). Interestingly, PP2 did not affect Akt phosphorylation, suggesting that SFKs are acting either downstream of this kinase in the mTOR pathway or in an alternative regulatory pathway. It is noteworthy that the PI 3-kinase inhibitor LY294002 had no significant effect on the SFKs Y416 phosphorylation, suggesting that these pathways are distinct (Figure 5C).
Inhibition of SFKs affects the phosphorylation of mTOR targets in AML cells. (A) KG1 cells were cultured for 1 hour alone (control) or in the presence of rapamycin (10 nM), AG1296 (25 μM), AG490 (20 μM), and PP2 (10 μM). Cells were lysed and analyzed by Western blot with the indicated antibodies. Results shown are representative of 3 independent experiments. (B) Fresh AML cells (patient nos. 1–20) were cultured for 1 hour alone or in the presence of PP2 (10 μM), lysed, and analyzed as in panel A. Results shown are representative of the different patients analyzed. (C) Fresh AML cells (patient nos. 7, 19, 21, 42, 43, 45, 49, 53, 59) were cultured alone or in the presence of PP2 (10 μM) or LY294002 (25 μM) for 4 hours, then processed for Western blot analysis.
Inhibition of SFKs affects the phosphorylation of mTOR targets in AML cells. (A) KG1 cells were cultured for 1 hour alone (control) or in the presence of rapamycin (10 nM), AG1296 (25 μM), AG490 (20 μM), and PP2 (10 μM). Cells were lysed and analyzed by Western blot with the indicated antibodies. Results shown are representative of 3 independent experiments. (B) Fresh AML cells (patient nos. 1–20) were cultured for 1 hour alone or in the presence of PP2 (10 μM), lysed, and analyzed as in panel A. Results shown are representative of the different patients analyzed. (C) Fresh AML cells (patient nos. 7, 19, 21, 42, 43, 45, 49, 53, 59) were cultured alone or in the presence of PP2 (10 μM) or LY294002 (25 μM) for 4 hours, then processed for Western blot analysis.
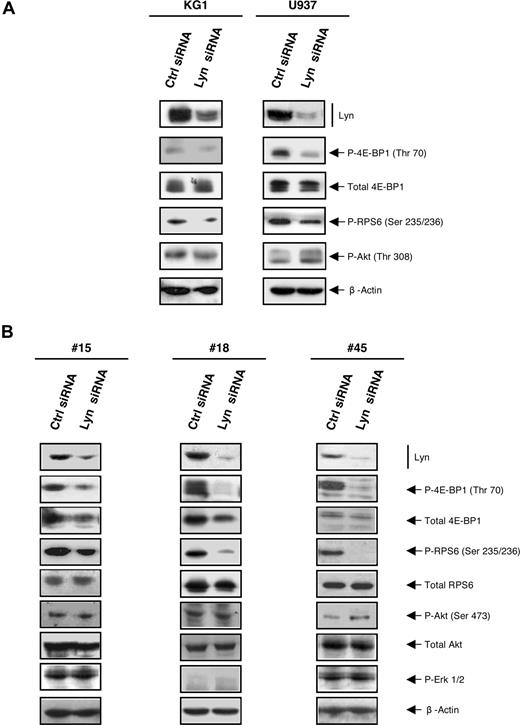
To investigate whether Lyn is involved upstream of mTOR, the level of phosphorylation of mTOR targets was assessed after silencing Lyn with siRNA in KG1 and U937 cell lines (Figure 6A) and in AML samples (Figure 6B). The reduction of Lyn expression induced a significant decrease in the level of phosphorylation of 4E-BP1 and RPS6 compared with control siRNA. Again, Lyn knockdown did not influence the phosphorylation of Akt both in cell lines and in fresh AML samples. As a control, the activation of another unrelated key signaling protein, Erk1/2, was not affected by Lyn knockdown.
Silencing Lyn inhibits the mTOR pathway independently of Akt in AML cells. (A) KG1 and U937 were cultured after electroporation with Lyn siRNA or control siRNA. After 72 hours, cells were processed for Western blot analysis using appropriate antibodies. Results shown are representative of 4 independent experiments. (B) Fresh AML samples (patient nos. 15, 18, 45) were cultured during 48 hours after electroporation with Lyn siRNA or control siRNA, lysed, and analyzed by Western blot using indicated antibodies.
Silencing Lyn inhibits the mTOR pathway independently of Akt in AML cells. (A) KG1 and U937 were cultured after electroporation with Lyn siRNA or control siRNA. After 72 hours, cells were processed for Western blot analysis using appropriate antibodies. Results shown are representative of 4 independent experiments. (B) Fresh AML samples (patient nos. 15, 18, 45) were cultured during 48 hours after electroporation with Lyn siRNA or control siRNA, lysed, and analyzed by Western blot using indicated antibodies.
Discussion
In this study, we show that AML cell lines and freshly isolated cells from AML patients exhibit a high level of tyrosine phosphorylation compared with their normal counterpart. Strikingly, all AMLs displayed high levels of SFKs activation regardless of their cytogenetic or molecular background. SFK activation was also observed in the CD34+CD38−CD123+ compartment of the leukemic clone, indicating that SFKs are activated in the sub-compartment enriched in leukemic stem cells. The SFKs inhibitor PP2 efficiently inhibited cell proliferation and survival both at the leukemic bulk and clonogenic levels.
Among the SFKs, we show in a large series of AML cases that activated Lyn is consistently expressed at a high level. Two other members of the family, Hck and Fgr, are also strongly expressed in a significant proportion of AML patients. We focused our interest on Lyn because it appeared to be responsible for the main SFK activity in leukemic cells, as shown by the silencing experiments. Lyn was phosphorylated on the tyrosine residue classically used to monitor its activation state, although we cannot exclude that the phosphorylation of other sites also has an impact on its activity.20 However, an in vitro kinase assay based on the autophosphorylation of immunoprecipitated Lyn has revealed a highly active kinase in leukemic cells compared with normal cells. Consistent with our observations, previous reports indicated that Lyn was activated both in a small series of AML samples and in cell lines.17,21 Moreover, we show that in AML cells Lyn is distributed over the entire plasma membrane and in the cytoplasm possibly because of association with internal membranes as previously described in chronic lymphocytic leukemia cells.22 Conversely, Lyn is concentrated in several distinct points across the membrane in normal CD34+ HPCs, and the level of SFKs activation is low in these cells. In normal cells, Lyn is known to concentrate in lipid rafts where it is tightly regulated by the C-terminal Src kinase-binding protein (Cbp)–CSK complex, which phosphorylates tyr507, resulting in its inactivation.23,–25 Thus, mislocalized Lyn could escape the Cbp-CSK–mediated negative regulation in leukemic cells. Loss of the negative regulation of SFKs could also be explained by a possible defect in the activity of phosphatases, such as SHP1 and SHP2.26,–28 Other regulators of the level of expression of SFKs, such as the ubiquitine ligase Cbl or the suppressor of cytokine signaling proteins, may also contribute to the deregulation of SFKs in AML.29 Indeed, inactivating mutations of c-Cbl and Cbl-b have recently been shown in AML patient cells contributing to leukemogenesis through RTK activation.30,31
Interestingly, Lyn is considered as a negative regulator of normal myeloid hematopoiesis, except for terminal maturation of erythroblasts, and can have both positive and negative roles in B cells.25,32,,–35 Thus, according to the cell context, Lyn can have opposite effects on the regulation of signaling pathways. The high level of Lyn expression and its aberrant activation in leukemic cells possibly cause it to switch from being a negative to a positive regulator of cell proliferation, as recently proposed in Philadelphia chromosome–positive acute lymphoblastic leukemia.36,37 Consistent with this idea, PP2 has no significant effect on normal CFU-GM proliferation and differentiation, whereas it strongly blocks the clonogenic properties of AML cells in a dose-dependent manner. Importantly, it has been shown that specific down-regulation of Lyn had no effect on the survival of normal HPCs.37 In contrast, the specific knockdown of Lyn expression by siRNA significantly inhibited the clonogenic properties of fresh AML cells, comparable with the effect of the pan SFK inhibitor PP2. Similar results have been obtained in hematopoietic cell lines expressing mutated RTK such as FLT3.21,38 Overall, these results strongly suggest an important role for Lyn in AML biology.
One critical pathway overactivated in most AML cases is the mTOR/p70S6K/4E-BP1 pathway. In this study, we show that Lyn plays a role in the activation of this pathway. Indeed, the inhibition of SFKs by PP2 strongly affected the phosphorylation of p70S6K and 4E-BP1 both in cell lines and in fresh AML samples. Moreover, the treatment with Lyn siRNA decreased the phosphorylation of mTOR targets. One could hypothesize that Lyn regulates the PI3K/Akt pathway upstream of mTOR/p70S6K/4E-BP1 because it was previously shown that Lyn activates PI3K/Akt in response to G-CSF in nonleukemic hematopoietic cells.39,40 However, both PP2 and Lyn siRNA efficiently inhibited the phosphorylation of mTOR targets without affecting Akt phosphorylation. This result suggests that Akt signaling is not sufficient for mTOR activation in AML. In agreement, a recent report indicated that Lyn silencing in FLT3-ITD expressing 32D cells has no effect on Akt phosphorylation.38 It is also noteworthy that the selective inhibition of PI 3-kinase δ abolishes Akt activation in a large proportion of AML samples without affecting mTOR in most cases.12,41,42 Thus, alternative mechanisms other than the well-established PI3K/Akt pathway may link TKs, particularly Lyn, to mTOR in AML. In this respect, it is noteworthy that a recent report suggests that another nonreceptor tyrosine kinase, Syk, can activate the mTOR pathway independently of Akt in follicular lymphoma cells.43 How tyrosine kinases can regulate the mTOR pathway is still unknown. However, consensus tyrosine phosphorylation sites for SFKs (EEXIYGEIEA) are found in several upstream regulators of mTOR, including TSC1/2 and Rheb and in mTOR itself as shown through sequence analysis (NetPhos 2.0 Server), suggesting possible direct regulations. As in addition to growth factors or oncogenes mTOR integrates signals from nutrients and energy status, we cannot exclude a possible role for Lyn in regulating one of these mechanisms. Of note, Lyn did not appear to regulate the ERK/MAPK pathway, which is frequently activated in AML.5 This result is consistent with another study showing that Lyn down-regulation induced a reduction of STAT5 phosphorylation without affecting MAPK and Shc.38
Our study demonstrates that Lyn is highly expressed and activated in AML cells, including leukemic progenitors. Lyn controls cell survival and proliferation as demonstrated by the antileukemic activity of both SFK inhibitors and Lyn siRNA. Surprisingly, ablation of Lyn expression in primary cells uncovered a novel link between this kinase and the activation of the mTOR pathway. Altogether, these results support the use of SFK and Lyn inhibitors for new therapeutic approaches in AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sophie Allart and Fatima L'Faqihi from the Imaging and flow cytometry Core Facility of IFR 30, Monique Larroche and Nicole Lhermie for technical assistance, and Drs S. Manenti, C. Racaud-Sultan, and H. Tronchère for helpful discussions.
This work was supported by grants from La Ligue Nationale contre le Cancer, the Association pour la Recherche sur le Cancer (contract 3122), the Association Laurette Fugain, the Institut National du Cancer, and the Region Midi-Pyrénées.
Authorship
Contribution: C.D.S participated in designing, performing the research, and analyzed data; C.D. centralized the cytologic review and performed the research; V.B and N.P.-H performed the research, B.P and C.R. controlled, analyzed data, and wrote the paper. All authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christian Récher, Service d'Hématologie, CHU Purpan, place du Dr Baylac, 31059 Toulouse Cedex, France; e-mail: recher.c@chu-toulouse.fr.
References
Author notes
B.P. and C.R. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal