Acute promyelocytic leukemia (APL) is a distinct subtype of acute myeloid leukemia. Morphologically, it is identified as the M3 subtype of acute myeloid leukemia by the French-American-British classification and cytogenetically is characterized by a balanced reciprocal translocation between chromosomes 15 and 17, which results in the fusion between promyelocytic leukemia (PML) gene and retinoic acid receptor α (RARα). It seems that the disease is the most malignant form of acute leukemia with a severe bleeding tendency and a fatal course of only weeks. Chemotherapy (CT; daunorubicin, idarubicin and cytosine arabinoside) was the front-line treatment of APL with a complete remission (CR) rate of 75% to 80% in newly diagnosed patients. Despite all these progresses, the median duration of remission ranged from 11 to 25 months and only 35% to 45% of the patients could be cured by CT. Since the introduction of all-trans retinoic acid (ATRA) in the treatment and optimization of the ATRA-based regimens, the CR rate was raised up to 90% to 95% and 5-year disease free survival (DFS) to 74%. The use of arsenic trioxide (ATO) since early 1990s further improved the clinical outcome of refractory or relapsed as well as newly diagnosed APL. In this article, we review the history of introduction of ATRA and ATO into clinical use and the mechanistic studies in understanding this model of cancer targeted therapy.
A historical view of acute promyelocytic leukemia (APL)
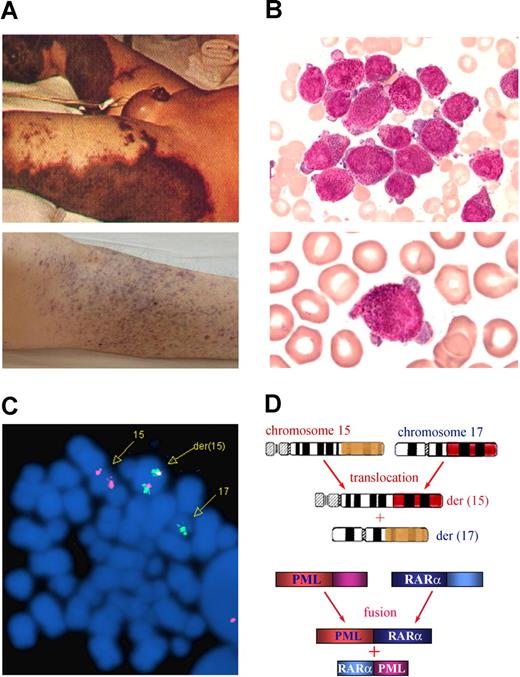
Acute promyelocytic leukemia (APL) is a distinct subtype of acute myeloid leukemia (AML; Figure 1). Morphologically, it is identified as AML-M3 by the French-American-British (FAB) classification. Cytogenetically, APL is characterized by a balanced reciprocal translocation between chromosomes 15 and 17, which results in the fusion between the promyelocytic leukemia (PML) gene and retinoic acid receptor α (RARα). Variant chromosomal translocations (eg, t(11;17), t(5;17)) can be detected in no more than 2% of APL patients. As a special entity, APL was first described in 1957 by a Swedish author, Hillestad,1 when he reported 3 patients characterized by “a very rapid fatal course of only a few weeks' duration,” with a white blood cell (WBC) picture dominated by promyelocytes and a severe bleeding tendency. He concluded that the disease “seems to be the most malignant form of acute leukemia.” More detailed features of APL were described by Bernard et al2 in 1959, and the severe hemorrhagic diathesis has been ascribed to disseminated intravascular coagulation (DIC) or hyperfibrinolysis.
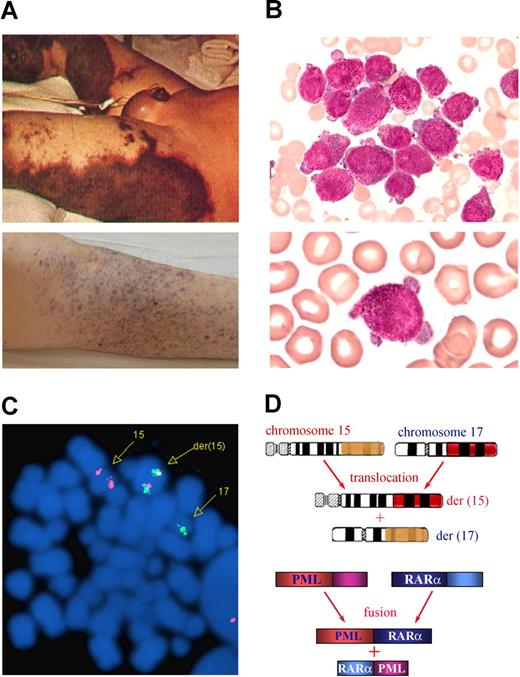
Clinical and molecular characteristics of APL. The 3 features of APL are (A) a severe bleeding tendency due to fibrinogenopenia and disseminated intravascular coagulation, (B) accumulation of abnormal promyelocytes in bone marrow (top panel) and peripheral blood (bottom panel), and chromosomal translocation t(15;17)(q22;q21) (C) with the resultant fusion transcripts between PML and RARα (D). (C) t(15;17) detected by fluorescence in situ hybridization using PML-RARα dual-color, dual-fusion translocation probes (Vysis, Downers Grove, IL). (D) Schematics representing the formation of 15;17 reciprocal chromosomal translocations (top panel) and fusion transcripts (bottom panel). Stains were analyzed using an Olympus BX51 research microscope equipped with a 100×/1.30 numeric aperture (NA) oil objective (Olympus, Tokyo, Japan). Images were processed using Adobe Photoshop CS (Adobe Systems, San Jose, CA). Original magnification, ×1000.
Clinical and molecular characteristics of APL. The 3 features of APL are (A) a severe bleeding tendency due to fibrinogenopenia and disseminated intravascular coagulation, (B) accumulation of abnormal promyelocytes in bone marrow (top panel) and peripheral blood (bottom panel), and chromosomal translocation t(15;17)(q22;q21) (C) with the resultant fusion transcripts between PML and RARα (D). (C) t(15;17) detected by fluorescence in situ hybridization using PML-RARα dual-color, dual-fusion translocation probes (Vysis, Downers Grove, IL). (D) Schematics representing the formation of 15;17 reciprocal chromosomal translocations (top panel) and fusion transcripts (bottom panel). Stains were analyzed using an Olympus BX51 research microscope equipped with a 100×/1.30 numeric aperture (NA) oil objective (Olympus, Tokyo, Japan). Images were processed using Adobe Photoshop CS (Adobe Systems, San Jose, CA). Original magnification, ×1000.
In 1973, Bernard et al3 demonstrated that APL leukemic cells were relatively sensitive to chemotherapy (CT: daunorubicin) that yielded a complete remission (CR) rate of 19 (55%) in 34 patients with APL. From then on, CT composed of an anthracycline (daunorubicin, idarubicin, or others) and cytosine arabinoside (Ara-C) was the frontline treatment of APL, and the CR rates could reach 75% to 80%4,5 in newly diagnosed patients. However, the frequently observed aggravation of bleeding syndrome by CT, leading to high early death rate, necessitated intensive platelet and fibrinogen support. Despite such progress, the median duration of remission ranged from 11 to 25 months and only 35% to 45% of the patients could be cured by CT alone as judged by the criterion of 5-year disease-free survival (5-year DFS).6,39 In 1985, the introduction of all-trans retinoic acid (ATRA) opened a new page in the history of APL treatment. Optimization of the ATRA-based regimens combining ATRA and CT has further raised the CR rate up to 90% to 95%, and a 6-year DFS up to 86% (± 10%) in low-risk patients in a report (Table 1). The application of arsenic trioxide (ATO) since the early 1990s further improved the clinical outcome of refractory or relapsed as well as newly diagnosed APL. A more profound reduction in PML-RARα transcript and longer survival in newly diagnosed APL were achieved when ATRA was combined with ATO compared with therapy with ATRA or ATO alone. Thus, the history of APL treatment can be subdivided into 4 periods: (1) pre-ATRA period: recognition of APL as a highly fatal disease entity and its response to CT (1957-1985) as discussed above; (2) introduction of ATRA in APL differentiation therapy and optimization of ATRA-based regimens (1985 to mid-1990s); (3) use of ATO in APL treatment (since mid-1990s); and (4) ATRA/ATO combination as a synergistic therapy and development of some new agents. In this article, we review the history of introduction of ATRA and ATO into clinical use and the mechanistic studies important in understanding this model of cancer-targeted therapy.
Introduction of ATRA as a differentiation therapy for APL: the first model of targeted therapy for cancer
In vitro studies
In the late 1970s, when studies on the treatment of acute leukemia were restarted in China after the chaos of the so-called cultural revolution, we faced a challenge in choosing a research orientation: to find new cytotoxic CT agents or to try other strategies? Until the mid-1970s, antileukemia therapy was mainly based on CT, aiming to inhibit the proliferation of malignant cells. However, it became well known that leukemic cells possess other biologic properties such as differentiation arrest, deregulation of programmed cell death (apoptosis), and the ability to disseminate. The fact that accumulation of abnormal promyelocytes within the bone marrow is characteristic of APL strongly suggested blockage of granulocytic differentiation. A question was then raised: could approaches other than killing, such as inducing cellular differentiation, be effective in the treatment of leukemia? Two factors inspired us to orient our research to differentiation therapy. First, the disease control model in China had been influenced by the Chinese ancient philosophy on the management of society, as illustrated by Confucius' famous saying: “If you use laws to direct the people, and punishments to control them, they will merely try to evade the laws, and will have no sense of shame. But if by virtue you guide them, and by the rites you control them, there will be a sense of shame and of right.” (Confucian Analects. Republished by Zhong-Hua-Shu-Ju, Beijing, 2005.) The translation of the essence of Confucius' philosophy into cancer therapy could be, if cancer cells are considered elements with “bad” social behavior in our body, “educating” rather than killing these elements might represent a much better solution. Second, in Western medicine, some evidence was emerging for cancer differentiation therapy. In 1961, Pierce and Verney7 observed differentiation of teratocarcinoma cells. Ten years later, Friend et al8 reported dimethyl sulfoxide–induced erythroid differentiation in murine virus–induced leukemia cells, while Schubert et al9 demonstrated differentiation of neuroblastoma cells.
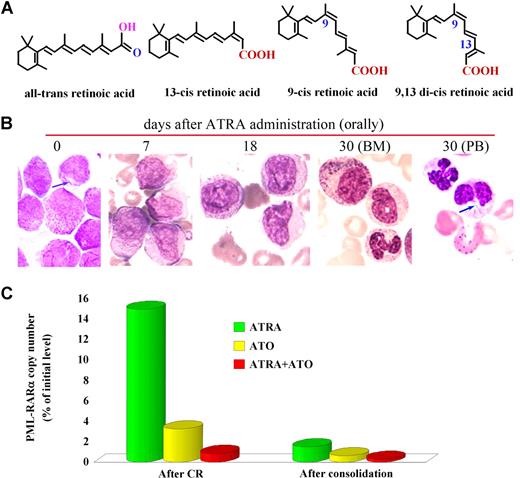
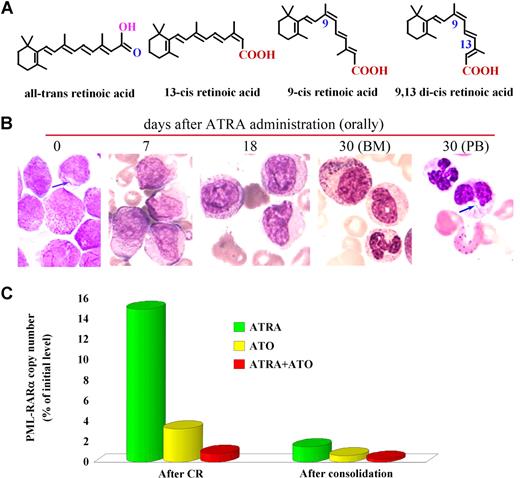
A major breakthrough was made by Sachs in 197810 when he discovered that leukemia cells could be triggered to undertake differentiation upon the action of certain agents. In the early 1980s, Breitman et al11,12 described a wide variety of compounds, including butyrate, dimethyl sulfoxide, and retinoic acid (RA), which were capable of inducing morphologic and functional maturation of HL-60 cells, a line with some features of promyelocytes. They also identified the specific response of APL specimens to RA. Then, the reports by Flynn et al13 and Nilsson14 on 2 isolated cases provided the first clues to the clinical effects of RA as a differentiation inducer for APL, since the use of 13-cis retinoic acid (13 cis-RA), an isomer of RA distinct from ATRA only in the orientation of the terminal COOH as shown in Figure 2A, induced clinical improvement or CR accompanied by maturation of promyelocytes. In 1986, Daenen et al15 used 13 cis-RA to treat an APL patient who went into CR with disappearance of signs of coagulopathy. Hence, our efforts at Shanghai Rui Jin Hospital affiliated to the Shanghai Second Medical University (SSMU, now the Shanghai Jiao Tong University School of Medicine) fit well into a field where Eastern philosophy meets Western biomedical science. When we started to screen for differentiation inducers for the treatment of leukemia in 1980, we were lucky that the isomer of RA available in Shanghai at that time was ATRA, just approved by the Shanghai Municipality for the treatment of skin diseases such as psoriasis and acne, and was later on shown to be superior to13 cis-RA in both in vitro and in vivo settings.16 We then demonstrated that ATRA strongly induces terminal differentiation of HL-60 and fresh APL cells. In 1985, during an informal meeting with Degos of the Institute of Hematology of Paris VII at Saint Louis Hospital, we discussed the feasibility of treating leukemia by inducing differentiation. The effects of ATRA discovered in Shanghai and that of low-dose Ara-C in Paris in inducing differentiation of leukemia cells were both appreciated. This meeting laid the foundation for a long-term cooperation between the Shanghai and Paris groups. At about the same time, SSMU sent researchers to Waxman's lab at Mount Sinai Medical Center in New York to conduct experiments on cancer differentiation. As such, the study of APL and ATRA marked the beginning of a long intercontinental journey.
ATRA in treating APL. (A) Isomers of retinoic acid. (B) ATRA induces terminal differentiation of abnormal promyelocytes in vivo. On day 30 of treatment, Auer bodies (arrow) are found in neutrophils circulating in the peripheral blood, indicating these cells are derived from leukemic promyelocytes. (C) ATRA treatment leads to elimination of PML-RARα–positive cells revealed by detection of minimal residual disease (MRD) using quantitative real-time RT-PCR for assessment of PML-RARα transcript. Effects of ATO and ATRA in combination with ATO are also shown. Stains were analyzed using an Olympus BX51 research microscope equipped with a 100×/1.3 NA oil objective, and images were processed using Adobe Photoshop CS.
ATRA in treating APL. (A) Isomers of retinoic acid. (B) ATRA induces terminal differentiation of abnormal promyelocytes in vivo. On day 30 of treatment, Auer bodies (arrow) are found in neutrophils circulating in the peripheral blood, indicating these cells are derived from leukemic promyelocytes. (C) ATRA treatment leads to elimination of PML-RARα–positive cells revealed by detection of minimal residual disease (MRD) using quantitative real-time RT-PCR for assessment of PML-RARα transcript. Effects of ATO and ATRA in combination with ATO are also shown. Stains were analyzed using an Olympus BX51 research microscope equipped with a 100×/1.3 NA oil objective, and images were processed using Adobe Photoshop CS.
Early results of ATRA alone as a remission induction treatment for APL
The first APL patient treated with ATRA was a 5-year-old girl who received medical care in Shanghai Children's Hospital in 1985. After anthracycline-based CT, she did not achieve remission and was in critical condition with high fever, skin and mucosal hemorrhage, and septicemia with a vaginal-rectal fistula resulting from a local infection. Her parents felt that their child's condition was hopeless and wanted to abandon treatment. We suggested to them that they consider ATRA for their child and finally they agreed to try it. ATRA was administered orally at a dose of 45 mg/m2 per day. After 1 week, the temperature fell to normal. Three weeks later, the girl miraculously went into CR and a postremission treatment composed of alternating ATRA/CT lasted for 1 year. Since then, she has been in remission and is now 26 years old in good health with a good career. Encouraged by the success of this pilot case, we extended the clinical trial. The first 6 APL patients (4 newly diagnosed and 2 refractory to CT) treated with ATRA all entered CR, accompanied by a gradual differentiation of leukemic promyelocytes in bone marrow and peripheral blood (Figure 2B).17 In 1988, the Shanghai Institute of Hematology (SIH) published in Blood18 the results of treatment of 24 APL patients (16 newly diagnosed and 8 refractory cases) given ATRA alone; of these, 23 cases achieved CR with differentiation of promyelocytes, while the single nonresponder also achieved CR by adding low-dose Ara-C. The efficacy of ATRA against APL was confirmed by other hematology/oncology centers worldwide.19,,,–23 Importantly, both the European APL 91 Group24 and the North American Intergroup25 demonstrated that, although the CR rates of APL patients treated with CT alone were not significantly different from those treated with ATRA, the long-term outcome of patients treated with ATRA was better than that of the CT group. In the former study, the 12-month event-free survivals (EFSs) in ATRA and CT groups were 79% (± 7%) and 50% (± 9%), respectively, whereas in the latter study, the 5-year DFSs were 69% and 29%, respectively, in the ATRA and CT groups.
Optimization of regimens by combining ATRA and CT for APL treatment
Even though a CR rate of approximately 85% can be achieved in APL with ATRA alone, continuous treatment of APL with ATRA will cause progressive resistance to the drug and reduction of its plasma concentration because of accelerated clearance, resulting in relapse usually within 3 to 6 months. Furthermore, the administration of ATRA is able to induce an elevation of white blood cell (WBC) count with fatal retinoic acid syndrome (RAS). These adverse effects instigated many investigators to further optimize ATRA-based regimens for better CR rate and survival time. In the early 1990s, a multicenter clinical study on 544 cases in China clearly showed the benefits of combining ATRA and CT as part of remission induction therapy.26 In addition, a large number of prospective randomized studies have been conducted since the early 1990s, particularly by the European APL Study Group,24,27 GIMEMA (Gruppo Italiano Malattie Ematologiche dell'Adulto),28 PETHEMA (Programa de Estudio y Tratamiento de las Hemopatías Malignas),29 the US North American Intergroup,25 and JALSG (Japan Adult Leukemia Study Group),30 that aimed to address the following issues: (1) Is ATRA combined with CT beneficial for yielding better outcome and reducing the incidence of RAS? (2) How should postremission treatment be conducted and how long should the continuation therapy be? (3) What could be the appropriate marker to evaluate the efficacy of APL therapy? The following general conclusions have been drawn from the above-mentioned studies.
First, ATRA/CT in combination is superior to CT or ATRA alone, particularly with regard to reduction of relapse.27,31 CT usually includes one anthracycline (idarubicin [I], daunorubicin [D], mitoxantrone [M], or homoharringtonine [H]), and Ara-C and should be started early with ATRA or when WBC count exceeds 5 to 10 × 109/L. Incorporation of CT into remission induction also reduces the incidence of RAS.32 When RAS occurs, treatment with 10 mg dexamethasone intravenously, twice daily for 3 or more days, tremendously reduces mortality.6,32
Second, consolidation and maintenance therapies are necessary. The protocol recommended for consolidation is 3 monthly courses of anthracycline-based CT,24,28,–30 sometimes with high-dose Ara-C,33 while maintenance therapy consists mostly of 6-mercaptopurine (6-MP) and methotrexate (MTX) with ATRA for 15 days every 3 months, or anthracycline-based CT, 6-MP + MTX, and ATRA alternately, with a duration of usually 2 years.27,–29,38 As shown in Table 1, long-term outcome among large series of APL patients treated with optimized ATRA-based regimens yielded 70% 5-year EFS and 6-year DFS of 68% with as high as 86% in low risk objects. The best outcome was observed in patients who received ATRA during both induction and maintenance with a 5-year DFS of 74%.25
Third, detection of the PML-RARα fusion transcript is not only necessary for the diagnosis of APL, but also provides a valuable tool for detecting minimal residual disease (MRD), revealing early relapse after consolidation and guiding further treatment.34 Quantitative real-time reverse-transcription–polymerase chain reaction (RT-PCR) for analyzing PML-RARα (Figure 2C) is useful for the assessment of the prognosis of the disease.35,36
Mechanisms of action of ATRA in APL differentiation therapy
The striking clinical benefits of ATRA in treating APL gave rise to enthusiasm in clarifying the mechanisms of its action.
Dissecting APL leukemogenesis: transcriptional repression leads to abnormal promyelocyte accumulation.
In 1977, Rowley et al40 from the University of Chicago reported a consistent chromosomal translocation between chromosomes 15 and 17 in APL. t(15;17)(q22;q21) can be detected in more than 95% of APL patients. The breakpoints lie within the RARα locus on chromosome 17 and the PML locus on chromosome 15, resulting in a combination of the 2 genes as reported by de Thé et al,41 Kakizuka et al,42 and several other groups. The fact that the fusion transcript of PML-RARα could be detected in 100% of patients with t(15;17) while that of the reciprocal RARα-PML is absent in 10% to 20% of these cases suggests an essential role for PML-RARα in leukemogenesis. PML/RARα is able to form homodimers and sequesters RXR and/or PML proteins in a large protein complex. The homodimers repress the transcriptional expression of target genes essential for granulocytic differentiation through binding to a set of typical or variant retinoic acid response elements (RAREs) in the regulatory region of these genes and recruiting corepressor (CoR) proteins (such as Daxx and mSin3A/nuclear receptor corepressor [NcoR]/histone deacetylase [HDAC]) on both PML and RARα moieties. In addition, recent evidence suggests that PML-RARα is also capable of recruiting the methylating enzymes (DnmT1 and Dnmt3a), leading to the hypermethylation of the RA downstream gene promoter, resulting in transcriptional repression.43 Hence, the ultimate result of the t(15;17) as a genetic defect is an aberration of epigenetic control in terms of both aberrant histone modification and DNA methylation at critical gene chromatin domains. Transgenic mice experiments by Pandolfi's group and others showed that the PML-RARα fusion gene expressed in myeloid lineage is crucial for the pathogenesis of APL (He et al44 ), even though other genes such as FLT-345 and K-ras46 are required for a fully transformed phenotype. APL transgenic mice showed hematologic features mirroring the human APL, including sensitivity to ATRA treatment.44
Studies on t(11;17) and PLZF-RARa as well as other variant translocations and resultant fusion genes to further elucidate leukemogenesis in APL.
In 1991, the karyotype of a special case of APL drew the attention of Sai-Juan Chen at SIH.47 This case was relatively resistant to ATRA treatment. Cytogenetic analysis revealed a t(11;17)(q23;q21) and molecular cloning by our group in collaboration with Zelent and Waxman showed a fusion between RARα and the PLZF (for promyelocytic leukemia zinc finger) gene (Chen et al48 ). The PLZF-RARα fusion receptor behaves distinctly from PML-RARα since it recruits CoR with a tighter affinity and thereby leads to deeper transcriptional repression. A study on a group of APL patients with t(11;17)(q23;q21) by Licht et al49 established a new entity within APL with unique biologic features and poor prognosis. Afterward, other variant translocations were also reported, including t(5;17)(q35;q21), where RARα was fused with nucleophosmin (NPM); t(11;17)(q13;q21), in which a fusion gene nuclear matrix–associated (NuMA)–RARα was formed; and dup17(q11;q21), which generated a Stat5b-RARα fusion.50,51 A common feature of all fusion RA receptors in APL is that they are able to form homodimers with higher affinity for the CoR complex. Transgenic mouse models were reported for PLZF-RARα, NPM-RARα, and NuMa-RARα, and all these models resulted in leukemia. Interestingly, PLZF-RARα leukemic mice displayed partial resistance to ATRA at both cellular and organism levels.52
Mechanisms of action of ATRA.
The discovery of PML/RARa in APL pathogenesis pointed to a possible molecular mechanism underlying ATRA-specific therapy. Indeed, PML-RARα is a “druggable” target. It is generally accepted that a pharmacological concentration (10−6-10−7 M) of ATRA causes a configuration change of PML-RARα. As a result, the CoR complex dissociates from the receptor, whereas a coactivator complex composed of proteins with histone acetylase (HAT) activity is recruited, opening the chromatin structure and relieving transcriptional repression. This coregulator exchange model seems to get support from recent transcriptome and proteome analyses,53 with modulation of a large number of genes involved in the initiation/promotion of granulocytic differentiation, such as the up-regulation of granulopoiesis-associated transcription factors C/EBPs, cytokines/cytokine receptors, as well as their corresponding postreceptor signal transduction molecules. It is worth noting that another effect of ATRA in modulating PML-RARα is to induce its degradation. Although it was reported that ATRA could trigger caspase-mediated cleavage of the PML-RARα chimeric protein,54 further dissection of the pathways involved in PML-RARα catabolism led to the discovery of a ubiquitin/proteasome system (UPS)–mediated degradation of PML-RARα and RARα, which was dependent on the binding of SUG-1 in the AF-2 transactivation domain of RARα.55,56 Indeed, a number of components of the UPS necessary for the degradation of PML-RARα can be significantly enhanced upon ATRA. Moreover, in leukemic cells with PLZF-RARα, exposed to even 10−5 M of ATRA, the coregulator exchange is not sufficient, while the HDAC inhibitors TSA (trichostatin) or SAHA (suberoylanilide hydroxamic acid) cannot only reverse the transcriptional repression but also allow terminal differentiation of t(11;17) cells in combination with ATRA.
Use of ATO in the treatment of APL: taming an evil with a toxic agent
History of arsenic as a drug
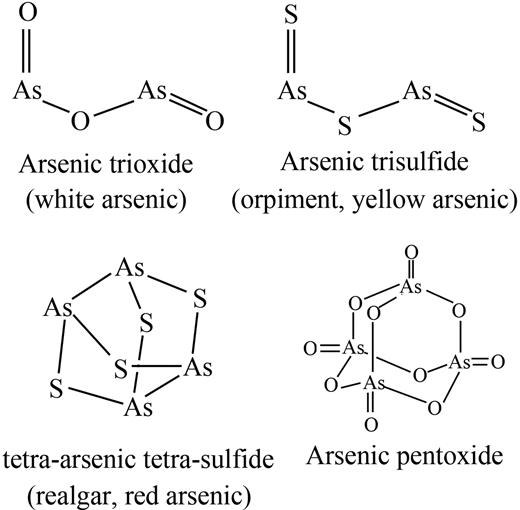
Arsenic is a common, naturally occurring substance that exists in organic and inorganic forms. There are 3 inorganic forms of arsenic: red arsenic (As4S4, also known as realgar); yellow arsenic (As2S3, also known as orpiment); and white arsenic or ATO (As2O3), which is made by burning realgar or orpiment (Figure 3). Although a well-known poison, arsenic is also one of the oldest drugs in both Western medicine and traditional Chinese medicine (TCM), since it was mentioned by Hippocrates (460-370 BC) for treatment of skin ulcer and by the Chinese Treaty NeiJing (263 BC) for treatment of malaria-associated periodic fever.57 In the late 18th and early 19th centuries, arsenic, in the form of Fowler solution (potassium bicarbonate–based solution of arsenic), was introduced in clinics to treat periodic fever, chronic myelogenous leukemia (CML), and many other diseases. However, it was discarded as a treatment in the early 20th century because of its toxicity and the advent of radiation and cytotoxic CT.
Arsenic in APL treatment
In TCM, arsenic is applied to only severe diseases with the principle of “taming an evil with a toxic agent.” In the early 1970s, a group from Harbin Medical University in northeastern China identified ATO as an active ingredient from an anticancer remedy and then used an arsenic compound to treat a variety of cancers.57 In 1992, Sun et al58 reported that, by administration (intravenous) of a crude solution of ATO composed of 1% ATO with a trace amount of mercury chloride, 21 of 32 APL patients entered CR with an impressive 30% survival rate after 10 years. In 1996 to 1997, groups from Harbin59 and SIH60,,–63 reported respective results using pure ATO in treating APL. In the Harbin series, CR rates of 73% and 52% were obtained in 30 newly diagnosed and 42 relapsed APL cases, respectively. From SIH, 15 APL patients at relapse after ATRA/CT received ATO at a dose of 0.16 mg/kg per day intravenously for 28 to 54 days. CR was achieved in 9 (90%) of 10 patients treated with ATO alone and in the remaining 5 treated by the combination of ATO and low-dose CT drugs or ATRA. During the treatment with ATO, there was no bone marrow depression and only limited side effects were encountered. These results were further confirmed by SIH in a larger group of 47 relapsed and 11 newly diagnosed APL cases63 with CR rates of 85.1% and 72.7%, respectively, and then by many groups worldwide.64,,,,–69 Furthermore, after CR is achieved by ATO alone, a molecular remission is obtainable in a relatively high proportion of the patients, from 72%66 to 91%67 in different multicenter studies, demonstrating that ATO is a highly effective drug for APL. Using ATO as a single agent, a relatively good long-term remission can be obtained in newly diagnosed patients, as evidenced by a 2-year DFS of 63.7% and a 3-year DFS of 87.2% in 2 recent studies.64,68
It is worth noting that another arsenic compound, As4S4, was also effective in the treatment of APL. Clinical use of As4S4 can be either in composite formulas as a standard practice of TCM or as a single agent. In 1995, Huang et al70 introduced orally used “composite Realgar-indigo naturalis tablets” for APL treatment, which contain realgar, indigo naturalis, Radix salviae miltiorrhizae, and Radix pseudostellariae. A CR rate of 98% was achieved in 60 APL patients. This result was recently confirmed by a multicenter study in China and a CR rate of 96.7% was achieved in a series of 78 cases.71 On the other hand, Lu et al72 reported in 2002 that by using pure As4S4, 103 (79.8%) of 129 APL patients achieved CR. There were 19 newly diagnosed APL cases in that series and all these cases obtained CR.
Mechanisms of action
Before the first controlled clinical trial of ATO in APL, SIH conducted a study on the cellular and molecular mechanisms of action of this ancient remedy. Interestingly, ATO exerts dose-dependent effects on APL cells.61 Under high concentration (1-2 × 10−6 M), ATO induces apoptosis, mainly through activating the mitochondria-mediated intrinsic apoptotic pathway. Under low concentrations (0.25-0.5 × 10−6 M) and with a longer treatment course, ATO tends to promote differentiation of APL cells. Since a range of ATO concentrations could exist in vivo as revealed by pharmacokinetic studies,62 we proposed that induction of both apoptosis and differentiation be a possible cellular mechanism in the clinical setting. This point of view was then supported by examination of bone marrow under ATO treatment in APL patients and in the PML-RARα/APL mouse model.73 The mechanism of proapoptotic activity of ATO was further scrutinized by many groups at the gene/protein levels, and a large body of information has been gathered, including histone H3 phosphoacetylation at CASPASE-10,74 the involvement of JNK signaling,75 anion exchanger 276 and GSTP1-1,77 up-regulation of a set of genes responsible for reactive oxygen species (ROS) production, intracellular oxidative DNA damage,78 suppression of human telomerase reverse transcriptase gene (hTERT), C17, and c-Myc genes through Sp1 oxidation,79 repression of NFκB activation,80 and down-regulation of Wt1 gene.81 Recently, a pathway composed of ATR, PML, Chk2, and p53 has been proposed to mediate ATO-induced apoptosis.82
The fact that ATO exerts selective therapeutic effects against APL but not against other subtypes of leukemia suggests a crucial link between its mechanism of action and PML-RARα. Indeed, we found that both PML-RARα and wild-type PML, but not wild-type RARα, were induced to be degraded in APL cells upon ATO in vitro and in vivo. This observation suggests that ATO might target the PML moiety in the fusion protein.60,61 Subsequent studies by several groups found that treatment of APL cells with ATO led to a significant degree of sumoylation of PML and PML-RARα. It was shown that sumoylation might take place at amino acids K65, K160, and K490, but only lysine 160 was important for the effect of ATO, since it mediated not only sumoylation but also subsequent recruitment of 11S proteasome, a process essential for the degradation of PML and PML-RARα proteins.73 When transcriptome/proteome platforms were used to analyze the effect of ATO and the data were compared with those of ATRA, we made an interesting observation: ATO could regulate a significant proportion of genes also modulated by ATRA, but the extent of modulation was much less than that by ATRA. In contrast, ATO induces a deeper change of proteome pattern, suggesting that protein modification, rather than gene expression modulation, could be the major molecular mechanism of ATO.53
ATRA/ATO combination as a synergistic therapy and development of some new agents for APL-targeted therapy
Combination of ATRA and ATO in taming APL
Rationale.
In 1998, at a meeting in Shanghai with Degos and Waxman, we discussed the possibility of using a triad of CT, ATRA, and ATO for newly diagnosed patients in an attempt to maximize the 5-year DFS in APL. Subsequently, in a PML/RARα mouse model and a human NB4 APL cell line–based ascites/leukemic mouse model, de Thé's group (Lallemand-Breitenbach et al84 ) and a group jointly led by Waxman and us (Jing et al83 ) showed that this combination could dramatically prolong the survival or even eradicate disease in animals. These results encouraged us to conduct a clinical trial using ATRA/ATO combination to treat newly diagnosed APL.
Marked clinical benefits.
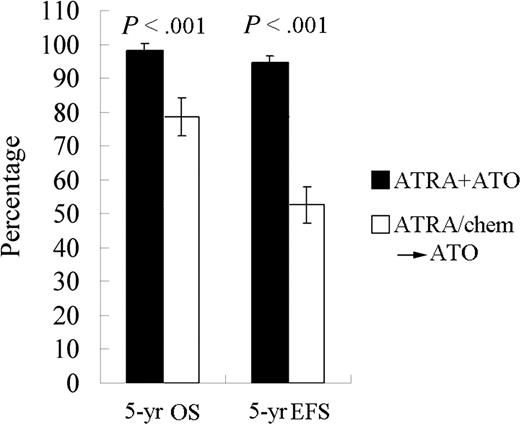
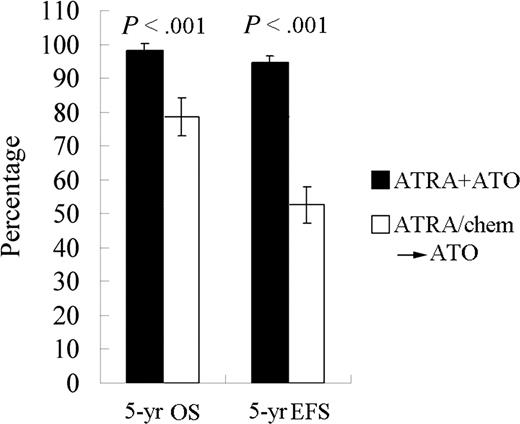
A randomized study with ATRA or ATO as a single agent or in combination for remission induction, followed by CT consolidation/continuation, was carried out at SIH beginning in April 2000 and the results were published in 2004.85 Sixty-one APL subjects were randomized into 3 groups treated with (1) ATRA, (2) ATO, or (3) the combination of the 2 drugs. The tumor burden was examined with real-time RT-PCR for the PML-RARα transcripts. Although CR rates in the 3 groups were similar (≥ 90%), the time to achieve CR was much shorter in the combination group than in the others (P < .05). The disease burden reflected by a fold change of PML-RARα transcripts at CR decreased more significantly in the combination therapy group compared with the monotherapy groups (P < .01; Figure 2C). This difference persisted after consolidation (P < .05). Importantly, all 20 cases in the combination group remained in CR, whereas 7 of 37 cases treated with monotherapy relapsed (P < .05) after a medium follow-up (MFU) of 18 months (range: 8-30 months). In 2006, we reported the results of 56 newly diagnosed APL patients treated with ATRA/ATO/CT since 2001 with an MFU of 48 months and compared the data with the conventional ATRA-ATO transition treatment group of 56 relatively well-matched cases treated by ATRA/CT and then ATO at relapse. The 4-year DFS and the 4-year overall survival (OS) rates in the study group were estimated at 94.2% (± 3.3%) and 98.1% (± 1.8%), respectively, compared with those of 45.6% (± 7.6%) and 83.4% (± 5.4%), respectively, in controls (P < .001 and P = .012, respectively).86 Our most recent data with an MFU of 60 months in these 2 groups showed a similar situation (Figure 4; Y. F. Liu, J. Hu, S. J. Chen, Z.C., unpublished data, June 2007). These results, together with some recent reports from other centers,87,88 clearly demonstrate superiority in treating APL simultaneously with ATRA and ATO.
The 5-year EFS and OS for APL patients treated with ATRA/ATO combination or each monotherapy (ATRA/chem→ATO).
The 5-year EFS and OS for APL patients treated with ATRA/ATO combination or each monotherapy (ATRA/chem→ATO).
Mechanisms of synergistic effect in combination therapy.
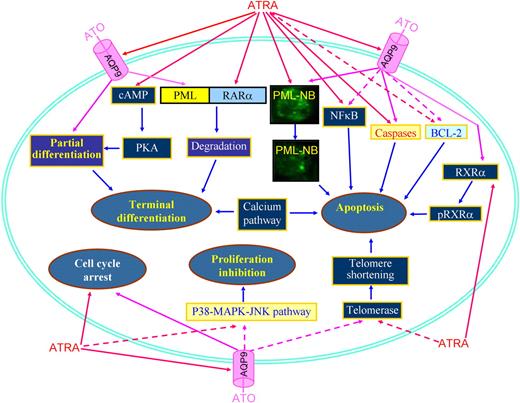
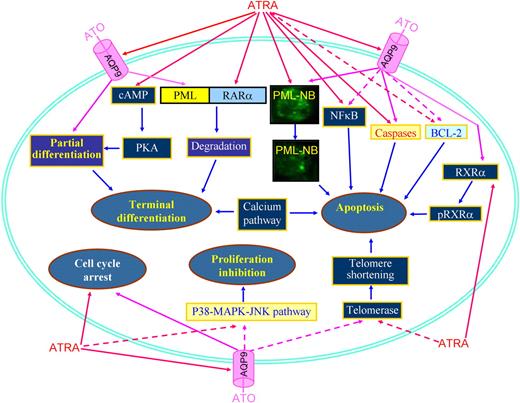
Applying an approach integrating cDNA microarray, proteomics, and methods of computational biology to study the effects on APL cells treated with ATRA and/or ATO, it was revealed that ATRA-induced differentiation involves essentially transcriptional remodeling, while the effects of ATO reside mainly at the proteome level, creating a molecular foundation for the synergistic/addictive effects between ATRA and ATO.53 The ATRA/ATO combination amplifies RA signaling, as highlighted by molecules involving IFN, calcium, cAMP/PKA, MAPK/JNK/p38, G-CSF, and TNF pathways. ATRA/ATO combination strongly activates the ubiquitin-proteasome pathway and significantly represses genes/proteins promoting cell cycling or enhancing cell proliferation, such as those involved in the MAPK/JNK/p38 pathway.53 In the NB4-LR1 cell line, which is maturation resistant, ATRA exhibits antiproliferative properties through down-regulation of telomerase,89 while the ATRA/ATO combination causes a synergistic down-regulation of telomerase and shortening of telomeres, leading to subsequent cell death.90 In addition, ATO induces phosphorylation of RXRα, while ATRA amplifies ATO-induced phosphorylation of RXRα and cooperates with ATO to induce apoptosis.91 In APL cells, ATRA induces degradation of the NFκB inhibitor IκB, while ATO antagonizes IκB catabolism and consequently decreases NFκB activation.80 It is worth noting that ATO alone induces partial differentiation of APL cells,61 and the 2-step model for differentiation induction suggests that cyclic adenosine monophosphate (cAMP) should be incorporated for induction of terminal differentiation of APL cells.92 This notion was confirmed by Zhu et al93 who showed that a strong synergy exists between a low concentration of ATO (0.25 μM) and cAMP analog 8-CPT-cAMP in fully inducing differentiation of ATRA-sensitive and ATRA-resistant APL cell lines and fresh APL cells. Interestingly, ATRA rapidly triggers a marked increase in intracellular cAMP level and cAMP-dependent protein kinase (PKA) activity.94 Therefore, a crosstalk could exist between ATO and ATRA signaling pathways through a cAMP/PKA node. Importantly, enhanced degradation of PML-RARα oncoprotein might provide a plausible explanation for the superior efficacy of combination therapy in patients. The 2 agents target distinct moieties of the oncoprotein: ATO on PML, versus ATRA on RARα, and have different molecular mechanisms. In agreement with this, recent studies showed that ATRA is able to increase the cell membrane arsenic channel aquaglyceroporin 9 (AQP9) level, which allows more arsenic to enter into cells.95 Figure 5 summarizes possible focal points for the effects of ATRA in combination with ATO.
Schematic representing synergic/additive effects of ATRA and ATO. PML-NB indicates PML nuclear body; pRXRα, phosphorylated RXRα. Solid lines represent effects of induction, promotion, or amplification, while dashed lines represent action of inhibition, down-regulation, or diminution.
Schematic representing synergic/additive effects of ATRA and ATO. PML-NB indicates PML nuclear body; pRXRα, phosphorylated RXRα. Solid lines represent effects of induction, promotion, or amplification, while dashed lines represent action of inhibition, down-regulation, or diminution.
New agents for APL-targeted therapy
Humanized anti-CD33 monoclonal antibodies (mAbs).
High-density cell surface membrane expression of the CD33 differentiation antigen is detectable in almost 100% of APL patients. Gemtuzumab ozogamicin is an anti-CD33 antibody calicheamicin-conjugate. Used as a single agent for the treatment of relapsed APL, molecular remission was obtained in 9 (81.8%) of 11 patients tested after 2 doses and in 13 (100%) of 13 patients tested after the third dose.96 Another anti-CD33 mAb, HuM195, has been shown to eliminate MRD in 11 (50%) of 22 cases in a recent trial.97
FLT3 inhibitor.
The FLT3 gene encodes a type III receptor tyrosine kinase. Internal tandem duplication in the juxtamembrane domain and point mutation in the tyrosine kinase II domain can be detected in 25% to 45% of APL patients.43,98 FLT-3 inhibitor SU11657 in combination with ATRA could cause a rapid regression of leukemia in the APL mouse model,99 but to date it has not been evaluated in a clinical study.43
Conclusion and perspectives
APL has a unique and specific chromosomic aberration t(15;17) resulting in the formation of a fusion gene and protein PML/RARα, which plays a central role in APL leukemogenesis, while a common pharmacological activity is shared by ATRA and ATO, that is, to modulate and/or degrade the fusion protein PML/RARα. Therefore, the success of ATRA and ATO in APL treatment furnishes the first model of molecular target–based induction of differentiation and apoptosis, ahead of targeting therapy with imatinib mesylate for CML. The recent results of both high CR rates (90%-94%) and high 5-year DFS rates (> 90%) using ATRA/ATO/CT in APL are comparable with the best results already achieved in childhood acute lymphocytic leukemia. Because of the great efforts made by the international scientific community (Table 2), the molecular understanding of the APL disease mechanism and the mode of action of ATRA/ATO has been explored in a systematic way to establish a model of changing cellular transcriptional regulation programs in both leukemogenesis and in designing efficient therapy. All these achievements show the power of integrating Western and Eastern wisdoms and make us confident that APL status has evolved from highly fatal to highly curable. The experiences acquired in taming APL are probably useful in that they mirror the way to conquer other types of leukemia and even the nonhematologic malignancies.
Acknowledgments
The authors thank Dr Guang-Biao Zhou from the Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences, for the critical review of the article and Dr Laurent Degos from Hospital Saint Louis in Paris and Dr Samuel Waxman from Mount Sinai Medical Center in New York for their friendly long-term collaboration.
This work was supported in part by the Chinese National Key Program for Basic Research (973) and the National High Tech Program (863), National Natural Science Foundation of China, Shanghai Municipal Commission for Science and Technology, the Shanghai Municipal Commission for Education, and the Samuel Waxman Cancer Research Foundation.
Authorship
Contribution: Z.-Y.W. and Z.C. wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zhen-Yi Wang, Shanghai Institute of Hematology, Rui Jin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, 197 Rui Jin Rd II, Shanghai, 200025, China; e-mail: xiejx@public2.sta.net.cn; Zhu Chen, Ministry of Health, P.R. China, No. 1 South Xizhimenwai Rd, Xicheng District, Beijing, 100044, China; e-mail: zchen@stn.sh.cn.
References
Author notes
Z.-Y.W. and Z.C. made equal contributions in writing this article.