Semaphorin 3A (Sema3A), a known inhibitor of axonal sprouting, also alters vascular patterning. Here we show that Sema3A selectively interferes with VEGF- but not bFGF-induced angiogenesis in vivo. Consistent with this, Sema3A disrupted VEGF- but not bFGF-mediated endothelial cell signaling to FAK and Src, key mediators of integrin and growth factor signaling; however, signaling to ERK by either growth factor was unperturbed. Since VEGF is also a vascular permeability (VP) factor, we examined the role of Sema3A on VEGF-mediated VP in mice. Surprisingly, Sema3A not only stimulated VEGF-mediated VP but also potently induced VP in the absence of VEGF. Sema3A-mediated VP was inhibited either in adult mice expressing a conditional deletion of endothelial neuropilin-1 (Nrp-1) or in wild-type mice systemically treated with a function-blocking Nrp-1 antibody. While both Sema3A- and VEGF-induced VP was Nrp-1 dependent, they use distinct downstream effectors since VEGF- but not Sema3A-induced VP required Src kinase signaling. These findings define a novel role for Sema3A both as a selective inhibitor of VEGF-mediated angiogenesis and a potent inducer of VP.
Introduction
Class 3 semaphorins, a family of secreted proteins, are implicated in a variety of biologic functions. Originally identified as axonal guidance cues, class 3 semaphorins also regulate the cardiovascular, immune, and respiratory systems.1 The prototype member of this family of proteins is semaphorin 3A (Sema3A) because it was the first family member shown to cause growth cone collapse. Within the cardiovascular system, Sema3A was recently found to modulate vessel formation by inhibiting integrin activity.2 Accordingly, in vitro Sema3A suppressed extracellular matrix–mediated adhesion and migration of endothelial cells, and disrupted VEGF-mediated endothelial cell migration.3 In zebrafish, either overexpression or a deficiency in Sema3A orthologs led to vessel-patterning defects.4,5 Thus, Sema3A plays a critical yet complicated role in vessel patterning that may be based on its capacity to regulate integrin function in endothelial cells.
While the antiangiogenic effects of Sema3A are associated with the suppression of integrin function, neovascularization requires coordinated signaling between integrins and growth factor receptors because cellular responses to growth factors depend on ligation of specific integrins. For instance, VEGF- and bFGF-mediated angiogenesis can be selectively disrupted by antagonists of αvβ5 and αvβ3, respectively.6 Currently, it is unclear whether Sema3A functions as a general inhibitor of angiogenesis or whether it influences specific angiogenic growth factor signaling pathways. Therefore, we set out to address whether Sema3A inhibits VEGF- and/or bFGF-mediated angiogenesis.
Sema3A and VEGF share a common coreceptor, Nrp-1, providing a potential mechanism by which Sema3A regulates VEGF-induced angiogenesis.3,7 Moreover, Nrp-1 is necessary for vessel development, since Nrp-1 knockouts have impaired angiogenesis and cardiovascular development8,9 and antibodies directed against Nrp-1 abrogate vessel remodeling.10 While Nrp-1 is not required for VEGF function, it can enhance signaling of VEGF through one of its receptor tyrosine kinases VEGFR2.7 However, Nrp-1 is necessary for Sema3A-mediated signal transduction, since Sema3A must bind Nrp-1 to then complex with plexins (plexinA1-A4 or plexinD1), the signaling receptors for semaphorins.11
Because VEGF also functions as a permeability factor, we examined whether Sema3A can also inhibit VEGF-induced permeability. Generally, vascular permeability (VP) is associated with pathological conditions such as inflammation, cancer, and ischemic injury and typically leads to the leak of serum proteins and cells into surrounding tissues. In ischemic conditions that occur during myocardial infarction or stroke, increased VP leads to severe tissue damage. In fact, we previously showed that genetic or pharmacological inhibition of Src and Yes suppressed VEGF-induced VP, thereby protecting animals from ischemic injury following myocardial infarction and stroke.12,13 Therefore, the finding that Sema3A suppressed VEGF-mediated VP prompted us to investigate its role as a regulator of VP.
Here we demonstrate that Sema3A acts as a selective inhibitor of VEGF-mediated angiogenesis yet also acts as a potent inducer of microvascular permeability via activation of Nrp-1. In vivo, Sema3A inhibited VEGF- but not bFGF-induced angiogenesis, which may be due to selective inhibition of VEGF signaling to FAK and Src, known mediators of both integrin and growth factor activity. These findings expand our understanding of the role Sema3A plays in regulating vascular form and function.
Methods
In vitro endothelial cell treatments
Low-passage 90% confluent human umbilical vein endothelial cells (HUVECs p4-6; Lonza, Basel, Switzerland) were starved in serum-free MCDB 131 media for 16 hours and pretreated with recombinant Sema3A (R&D Systems, Minneapolis, MN) for 15 minutes prior to growth factor stimulation with VEGF165 (50 ng/mL; Peprotech, Rocky Hill, NJ) or bFGF (50 ng/mL; Millipore, Billerica, MA). Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer and immunoblotted with the following primary antibodies: anti–P-FAK (Invitrogen, Carlsbad, CA), anti–P-Src (Invitrogen), anti–P-ERK1/2 (Cell Signaling, Danvers, MA), anti-Src (Millipore); and anti-FAK and ERK2 (Santa Cruz Biotechnology, Santa Cruz, CA). Film was scanned on an Epson 1680 scanner (Epson, Long Beach, CA) using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Chick chorioallantoic membrane angiogenesis assay
Chorioallantoic membrane (CAM) angiogenesis assays were performed as described previously.14 Filters were treated with PBS, VEGF165 (40 μg/mL), and bFGF (40 μg/mL) with or without Sema3A (50 μg/mL). To count vessels, CAMs were photographed using a research stereoscope (model SZH10; Olympus, Melville, NY) with a SPOT camera (model 2.2.1; Diagnostic Instruments, Sterling Heights, MI). PBS vessel count was subtracted as background.
In vivo permeability assay
Permeability assays were performed in 8- to 12-week-old Balb/c (Harlan, Indianapolis, IN), Yes−/− or wild-type littermate mice as described previously.14 Balb/c mice were injected with 1% Evans blue dye (Sigma-Aldrich, St Louis, MO). All other mice were injected with 1.5% Evans blue dye. An equal volume of VEGF165 (40 ng/μL), Sema3A (10, 30, or 100 ng/μL), or PBS was then injected intradermally. Recombinant Sema3F and Sema3B were used at 30 ng/μL. The amount of dye extravasation in response to PBS was subtracted as background from each experiment. Images were acquired as described in “Chick chorioallantoic membrane angiogenesis assay.”
VE-cadherin phosphorylation
Confluent HUVECs (p4-6) were starved in serum-free EBM-2 media (Lonza) for 16 hours prior to 10-minute stimulation with Sema3A (25, 100, or 400 ng/mL) or VEGF165 (50 ng/mL). Immunoprecipitation of VE-cadherin was performed as described previously15 followed by immunoblotting with antiphosphotyrosine (Millipore) and anti–VE-cadherin (Santa Cruz).
Inducible, endothelial-specific Nrp-1 knockout mice
Conditional Nrp-1fl/fl mice (The Jackson Laboratory, Bar Harbor, ME) were crossed with inducible-Cre transgenic mice where the tamoxifen-inducible Cre-ERT recombinase is driven by the 5′ endothelial enhancer of the stem cell leukemia (EC-SCL) locus16 to generate tamoxifen-inducible, endothelial-specific knockout mice. To knock down Nrp-1 expression, EC-SCL-Cre-ERT–positive or –negative Nrp-1fl/fl mice were injected with tamoxifen (20 mg/kg intraperitoneally; Sigma-Aldrich) or corn oil (Sigma-Aldrich), as vehicle, every other day for 14 days prior to in vivo permeability assays. To monitor Nrp-1 expression, hearts from these mice were excised from mice and lysed in RIPA buffer or embedded in OCT for frozen sectioning. Lysates were then immunoblotted with anti–Nrp-1 (2 μg/mL; Sigma-Aldrich) and anti-ERK2 (0.5 μg/mL; Santa Cruz). For confocal imaging, 5-μm heart sections were incubated with anti–Nrp-1 (1 μg/mL; R&D Systems) and an anti-EC marker cocktail (1 μg/mL; BD Biosciences, San Diego, CA) containing antibodies against CD31, CD144, and VEGFR2, followed by 568 antigoat and 488 antirat secondaries (Invitrogen). Images were acquired as a channel series using laser scanning confocal microscopy with 20×/0.75 NA objective (Nikon C1si; Nikon Instruments, Melville, NY).
Pharmacological agents
For in vivo permeability studies, the Src inhibitor SKI-606 (5 mg/kg intraperitoneally),17 the PI3Kγ/δ inhibitor TG100–115 (5 mg/kg intraperitoneally; TargeGen, San Diego, CA),18 or vehicle controls were delivered 30 minutes prior to injection of Evans blue dye. Anti–Nrp-1 monoclonal antibody and IgG control (50 μg/mL intravenously; R&D Systems) were also injected 30 minutes prior to Evans blue injection.
Statistical analysis
Data are presented as means plus or minus SEM, with statistical significance determined from Student t tests.
Results
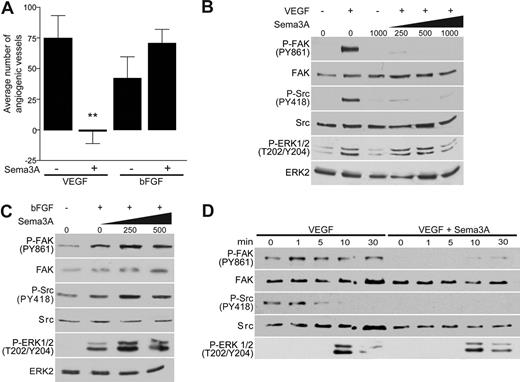
Sema3A was previously reported to disrupt angiogenesis by inhibiting integrin function2,3 ; however, it is unclear whether Sema3A inhibits one or multiple angiogenic pathways. Therefore, we set out to address how Sema3A could function as a modulator of vessel formation by determining its effects on VEGF- and bFGF-induced angiogenesis in vivo. These growth factors depend on distinct signaling pathways to induce new vessel formation.6,19 To assess the effects of Sema3A on these pathways of angiogenesis, blood vessel growth was stimulated on the chorioallantoic membranes (CAMs) of 10-day-old chick embryos with either growth factor in the presence or absence of recombinant Sema3A. While both growth factors induced a robust angiogenic response, Sema3A selectively disrupted VEGF-mediated angiogenesis (Figure 1A). In fact, we observed a somewhat enhanced vessel number in CAMs treated with both bFGF and Sema3A. Consequently, we conclude that Sema3A is a selective inhibitor of VEGF-mediated angiogenesis, since it failed to interfere with angiogenesis induced by bFGF.
Sema3A interferes with VEGF- but not bFGF-induced angiogenesis. (A) Inhibition of VEGF- but not bFGF-mediated angiogenesis by Sema3A was determined by treating CAMs with PBS, VEGF (40 μg/mL), and bFGF (40 μg/mL) with or without Sema3A (50 μg/mL) for 3 days (n = 15, **P < .01). Error bars represent SEM. (B,C) Increasing concentrations of Sema3A (ng/mL) selectively suppresses FAK and Src activation in VEGF- but not bFGF-stimulated (5 minutes) cells. Phosphorylation of FAK, Src, and ERK was determined through immunoblotting. (D) HUVECs were treated with 250 ng/mL Sema3A prior to stimulation with VEGF throughout various time points. These experiments were repeated multiple times with similar results.
Sema3A interferes with VEGF- but not bFGF-induced angiogenesis. (A) Inhibition of VEGF- but not bFGF-mediated angiogenesis by Sema3A was determined by treating CAMs with PBS, VEGF (40 μg/mL), and bFGF (40 μg/mL) with or without Sema3A (50 μg/mL) for 3 days (n = 15, **P < .01). Error bars represent SEM. (B,C) Increasing concentrations of Sema3A (ng/mL) selectively suppresses FAK and Src activation in VEGF- but not bFGF-stimulated (5 minutes) cells. Phosphorylation of FAK, Src, and ERK was determined through immunoblotting. (D) HUVECs were treated with 250 ng/mL Sema3A prior to stimulation with VEGF throughout various time points. These experiments were repeated multiple times with similar results.
Two mediators of growth factor and integrin activity are focal adhesion kinase (FAK) and Src. They are immediately activated by integrin ligation and play a role in growth factor–mediated angiogenesis.20,21 For these reasons, we investigated whether Sema3A could regulate FAK and Src activation in endothelial cells stimulated with VEGF or bFGF. Consistent with our in vivo findings, Sema3A treatment of endothelial cells inhibited VEGF-mediated phosphorylation of FAK at tyrosine 861 and Src at tyrosine 418 (Figure 1B,D). Phosphorylation of Src at this site is required for its kinase activity,22 while tyrosine 861 on FAK is a Src phosphorylation site and is linked to VEGF and integrin signaling downstream of Src.20 Sema3A alone also inhibited basal phosphorylation of these 2 kinases consistent with its role as an inhibitor of integrin function (Figure 1D).2 In contrast to VEGF, bFGF-stimulated endothelial cells exposed to Sema3A showed enhanced FAK and Src signaling relative to cells not treated with Sema3A (Figure 1C). This may account for the somewhat enhanced angiogenic response observed when bFGF is combined with Sema3A (Figure 1A).
To determine whether Sema3A was a general inhibitor of VEGF-mediated signal transduction or an inhibitor of specific downstream mediators, we examined its ability to regulate VEGF induced activation of ERK. Interestingly, Sema3A had little or no effect on VEGF-mediated signaling to ERK at doses where FAK and Src were substantially inhibited (Figure 1B,D), but enhanced ERK signaling in response to bFGF (Figure 1C). Moreover, FAK and Src phosphorylation were detected earlier than ERK activation in response to VEGF (Figure 1D), indicating that Sema3A selectively suppresses the early integrin-mediated signals associated with FAK and Src in VEGF-stimulated endothelial cells. Together with our in vivo findings, these results demonstrate that Sema3A functions as a specific inhibitor of VEGF-mediated angiogenesis via inhibition of Src and FAK.
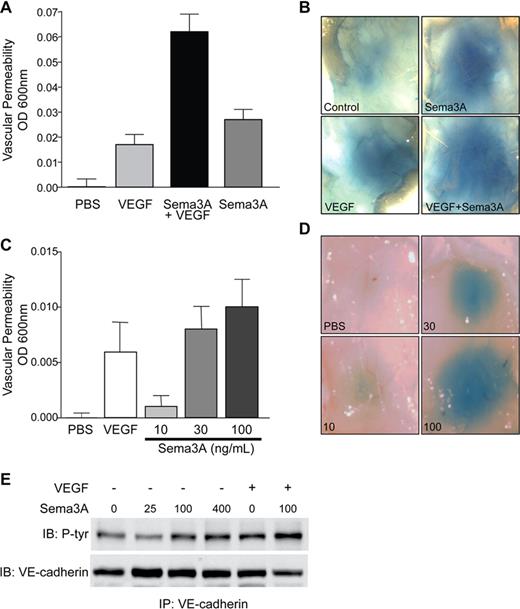
Although VEGF is a potent angiogenic growth factor, it was first described as a vascular permeability factor based on its ability to rapidly disrupt endothelial barrier function.23 Because Sema3A inhibited VEGF-induced angiogenesis, we hypothesized that it might also prevent VEGF-mediated VP. To investigate the effects of Sema3A on VEGF-induced permeability, Balb/c mice systemically injected with Evans blue were subjected to intradermal injection with an optimal dose of VEGF and/or Sema3A. To our surprise, we found that mice injected with a combination of VEGF and Sema3A actually displayed enhanced VP compared with mice injected with VEGF alone (Figure 2A,B). Importantly, injection of Sema3A alone dose-dependently caused a significant level of VP, demonstrating that Sema3A can act as a permeability factor (Figure 2C,D). Similar results were obtained using the highly homologous chicken Sema3A (data not shown), indicating that the VP effects of Sema3A are not species specific. Furthermore, in vitro Sema3A and VEGF also induce permeability to similar levels (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Therefore, Sema3A not only potentiates VEGF-mediated VP but also is approximately 5-fold more active than VEGF on a molar basis.
Sema3A potentiates VEGF-induced permeability and induces VP on its own. (A) Sema3A potentiation of VEGF-mediated VP was determined by subcutaneous injection of PBS, VEGF (40 μg/mL),17 Sema3A (30 μg/mL), or VEGF with Sema3A in Balb/c mice. Evans blue dye (1.5%) extravasation was quantitated (n = 8). Data represent means plus or minus SEM. (B) Representative images of Sema3A- and/or VEGF-induced permeability. (C) Sema3A dose-dependently induces VP as shown by injecting mice with increasing amounts of Sema3A (μg/mL), VEGF, or PBS as control (n = 6). Evans blue dye (1%) extravasation was quantitated. Data represent mean plus or minus SEM. This experiment was repeated twice with similar results. (D) Representative images of dose-dependent Sema3A-induced permeability. (E) Sema3A dose-dependently induces tyrosine phosphorylation of VE-cadherin as demonstrated by treatment of HUVECs with increasing amounts of Sema3A (ng/mL), VEGF (50 ng/mL), or both. This experiment was repeated multiple times with similar results.
Sema3A potentiates VEGF-induced permeability and induces VP on its own. (A) Sema3A potentiation of VEGF-mediated VP was determined by subcutaneous injection of PBS, VEGF (40 μg/mL),17 Sema3A (30 μg/mL), or VEGF with Sema3A in Balb/c mice. Evans blue dye (1.5%) extravasation was quantitated (n = 8). Data represent means plus or minus SEM. (B) Representative images of Sema3A- and/or VEGF-induced permeability. (C) Sema3A dose-dependently induces VP as shown by injecting mice with increasing amounts of Sema3A (μg/mL), VEGF, or PBS as control (n = 6). Evans blue dye (1%) extravasation was quantitated. Data represent mean plus or minus SEM. This experiment was repeated twice with similar results. (D) Representative images of dose-dependent Sema3A-induced permeability. (E) Sema3A dose-dependently induces tyrosine phosphorylation of VE-cadherin as demonstrated by treatment of HUVECs with increasing amounts of Sema3A (ng/mL), VEGF (50 ng/mL), or both. This experiment was repeated multiple times with similar results.
VE-cadherin regulates endothelial barrier function by maintaining adherens junctions between cells. A hallmark of VEGF- and inflammatory-induced VP is the tyrosine phosphorylation of VE-cadherin which in turn leads to the destabilization of adherens junctions.13,24,–26 Therefore, we investigated whether Sema3A-mediated VP can also alter adherens junctions. In endothelial cells, Sema3A dose-dependently increased VE-cadherin tyrosine phosphorylation (Figure 2E). Moreover, treatment of endothelial cells with both VEGF and Sema3A further enhanced the phosphorylation of VE-cadherin. This is consistent with our in vivo findings that Sema3A and VEGF additively induced VP (Figure 2A,B). Adherens junction morphology was also examined by immunostaining endothelial cells with an antibody against VE-cadherin. Upon treatment with either Sema3A or VEGF, adherens junctions appeared jagged, demonstrating that the junctions are disrupted and the cells are pulling apart. These morphologic changes are maintained for up to 1 hour after initial treatment (Figure S1B). Therefore, like VEGF, Sema3A also disrupts adherens junctions through VE-cadherin phosphorylation.
As axonal guidance cues, class 3 semaphorins are known to cause growth cone collapse.27 Similarly, various class 3 family members inhibit blood vessel formation.1 For example, Sema3F, like Sema3A, suppresses the growth of neovessels. However, it inhibits both VEGF- as well as bFGF-induced angiogenesis, indicating that it plays a broader role than Sema3A in regulating blood vessel formation.28,29 To determine if other class 3 semaphorins can induce VP, we compared the effects of Sema3A, Sema3F, and Sema3B on permeability. Unlike Sema3A, subcutaneous injection of Sema3F or Sema3B did not lead to VP (Figure 3), suggesting that Sema3B and Sema3F are functionally distinct from Sema3A with respect to their influence on the biologic properties of blood vessels.
Unlike Sema3A, Sema3F and Sema3B do not induce VP. (A) Balb/c mice were injected with 30 μg/mL Sema3A or Sema3F (n = 9), and 1% Evans blue dye extravasation was quantitated. Data represent mean plus or minus SEM. (B) Balb/c mice were injected with 30 μg/mL Sema3A or Sema3B (n = 6). Data represent means plus or minus SEM.
Unlike Sema3A, Sema3F and Sema3B do not induce VP. (A) Balb/c mice were injected with 30 μg/mL Sema3A or Sema3F (n = 9), and 1% Evans blue dye extravasation was quantitated. Data represent mean plus or minus SEM. (B) Balb/c mice were injected with 30 μg/mL Sema3A or Sema3B (n = 6). Data represent means plus or minus SEM.
Sema3A and VEGF share a common coreceptor, Nrp-1. While Nrp-1 is not required for VEGF function,7 it is necessary for Sema3A-mediated signal transduction.30,31 To determine if Nrp-1 is required for Sema3A-mediated VP, we generated tamoxifen-inducible endothelial-specific Nrp-1 knockout mice by crossing the previously described conditional Nrp-1 knockout mice (Nrp-1fl/fl)8 with inducible-Cre transgenic mice where the tamoxifen-inducible Cre-ERT recombinase is driven by the 5′ endothelial enhancer of the stem cell leukemia (EC-SCL) locus. This promoter was previously shown to induce specific expression of Cre ERT in adult endothelial cells.16 These mice are viable and tamoxifen treatment knocks down expression of Nrp-1 in heart-associated blood vessels from these animals (Figure 4A,B). After tamoxifen treatment, a deficiency in endothelial Nrp-1 prevented both Sema3A- and VEGF-induced VP in EC-SCL-Cre ERT–positive mice Nrp-1fl/fl mice compared with EC-SCL-Cre ERT–negative Nrp-1fl/fl mice (Figure 4C). Furthermore, tamoxifen-treated EC-SCL-Cre ERT–negative Nrp-1fl/fl mice showed a similar response to that of vehicle-treated EC-SCL-Cre ERT–positive Nrp-1fl/fl mice, demonstrating that tamoxifen treatment has no significant effect on Sema3A- or VEGF-induced VP (Figure 4C). These findings reveal that induction of permeability by both Sema3A and VEGF requires Nrp-1 because selective deletion of Nrp-1 in the endothelium of adult mice renders them resistant to Sema3A- and VEGF-mediated VP.
Sema3A and VEGF require Nrp-1 expression to induce vascular permeability. (A) To determine knockdown of Nrp-1 expression in inducible, endothelial-specific knockouts, mice were injected with 20 mg/mL tamoxifen (Tam) for 2 weeks to excise the floxed exon. Heart lysates from EC-SCL-CreERT–positive or –negative Nrp-1fl/fl mice were immunoblotted with antibodies against Nrp-1 or ERK2 as a loading control. (B) Cryosections (5 μm) from Tam-treated EC-SCL-CreERT–positive or –negative Nrp-1fl/fl hearts were stained with antibodies against Nrp-1 (red) and the endothelial markers (EC, green). Confocal images were taken at 400× magnification (scale bar represents 50 μm). (C) Knockdown of Nrp-1 expression disrupts Sema3A- and VEGF-mediated VP. Mice treated with Tam or vehicle were injected subcutaneously with Sema3A, VEGF, or PBS. Dye extravasation was quantitated (n = 8, *P < .05). (D) A function-blocking monoclonal antibody against Nrp-1 inhibits Sema3A- but not VEGF-induced VP. Balb/c mice were injected with an anti–Nrp-1 or IgG (50 μg/mL intravenously) 30 minutes prior to injection of VEGF, Sema3A, or PBS (n = 9, *P < .05). Error bars represent SEM.
Sema3A and VEGF require Nrp-1 expression to induce vascular permeability. (A) To determine knockdown of Nrp-1 expression in inducible, endothelial-specific knockouts, mice were injected with 20 mg/mL tamoxifen (Tam) for 2 weeks to excise the floxed exon. Heart lysates from EC-SCL-CreERT–positive or –negative Nrp-1fl/fl mice were immunoblotted with antibodies against Nrp-1 or ERK2 as a loading control. (B) Cryosections (5 μm) from Tam-treated EC-SCL-CreERT–positive or –negative Nrp-1fl/fl hearts were stained with antibodies against Nrp-1 (red) and the endothelial markers (EC, green). Confocal images were taken at 400× magnification (scale bar represents 50 μm). (C) Knockdown of Nrp-1 expression disrupts Sema3A- and VEGF-mediated VP. Mice treated with Tam or vehicle were injected subcutaneously with Sema3A, VEGF, or PBS. Dye extravasation was quantitated (n = 8, *P < .05). (D) A function-blocking monoclonal antibody against Nrp-1 inhibits Sema3A- but not VEGF-induced VP. Balb/c mice were injected with an anti–Nrp-1 or IgG (50 μg/mL intravenously) 30 minutes prior to injection of VEGF, Sema3A, or PBS (n = 9, *P < .05). Error bars represent SEM.
To extend these studies, mice were systemically treated with a monoclonal antibody directed against Nrp-1 known to inhibit VEGF binding. Injection of anti–Nrp-1 potently inhibited Sema3A-induced permeability yet had little effect on VEGF-induced permeability (Figure 4D). While our genetic studies reveal that Nrp-1 is required for VEGF-mediated VP (Figure 4C), the epitope recognized by this monoclonal antibody clearly distinguishes the VP-promoting activity of Sema3A from that of VEGF. This result is consistent with previous studies showing that a monoclonal antibody directed against the VEGF binding site of Nrp-1 has no effect on VEGF-mediated VP.10 Thus, while Nrp-1 expression may be required for Sema3A and VEGF-mediated VP, it appears that Nrp-1 may differentially induce VP by distinct pathways in response to Sema3A or VEGF due to distinct binding sites for VEGF and Sema3A on Nrp-1.32
Previous studies revealed that inhibition of the Src family kinases Src or Yes through genetic ablation or a pharmacological inhibitor blocked the VP in response to VEGF and protected mice from tissue damage after myocardial infarction or stroke.12,–14 To shed light on the mechanism by which Sema3A induces VP, mice were systemically treated with a Src inhibitor SKI-606 and then challenged with subcutaneous injection of Sema3A or VEGF. While pretreatment with SKI-606 abrogated VEGF-induced VP, it had a minimal effect on Sema3A-induced VP (Figure 5A). Accordingly, Sema3A-mediated permeability was not inhibited in Yes knockout mice that are resistant to VEGF-mediated VP (Figure 5B).14 The fact that Sema3A induces VP in a Src-independent manner is perhaps not surprising given the finding that Sema3A blocks endothelial Src activity in response to VEGF (Figure 1B). Hence, while Src kinases play a significant role in VEGF-mediated VP, they play little if any role in Sema3A-mediated VP. These results indicate that Sema3A and VEGF use different intracellular mediators to induce VP and may explain why these VP factors display additive activity (Figure 2A).
Induction of permeability by Sema3A is Src independent, but is blocked by PI3Kγ/δ inhibition. (A) Treatment with the Src family kinase inhibitor SKI-606 (5 mg/kg intraperitoneally) prior to injection with Sema3A, VEGF, or PBS significantly inhibits VEGF- but not Sema3A-mediated VP (n = 10, *P < .05). (B) Yes+/+ and Yes−/− mice show similar VP response when injected with Sema3A or PBS (n = 8). (C) Sema3A- and VEGF-induced VP depends on PI3Kγ/δ as determined by treatment of mice with the PI3Kγ/δ inhibitor TG100–115 (5 mg/kg intraperitoneally) prior to injection with Sema3A, VEGF, or PBS (n = 5, *P < .05, **P < .01). Error bars represent SEM.
Induction of permeability by Sema3A is Src independent, but is blocked by PI3Kγ/δ inhibition. (A) Treatment with the Src family kinase inhibitor SKI-606 (5 mg/kg intraperitoneally) prior to injection with Sema3A, VEGF, or PBS significantly inhibits VEGF- but not Sema3A-mediated VP (n = 10, *P < .05). (B) Yes+/+ and Yes−/− mice show similar VP response when injected with Sema3A or PBS (n = 8). (C) Sema3A- and VEGF-induced VP depends on PI3Kγ/δ as determined by treatment of mice with the PI3Kγ/δ inhibitor TG100–115 (5 mg/kg intraperitoneally) prior to injection with Sema3A, VEGF, or PBS (n = 5, *P < .05, **P < .01). Error bars represent SEM.
While VP induced by VEGF is Src dependent, inflammatory-mediated VP is independent of Src.12,14 However, both VEGF- and inflammatory-induced VP are suppressed by pharmacological inhibitors of the PI3Kγ and PI3Kδ isoforms (PI3Kγ/δ).14,18 We recently reported that systemic injection of the PI3Kγ/δ inhibitor TG100-115 in rats or mice was sufficient to limit infarct size after myocardial infarction by reducing both VEGF- and G-protein–coupled receptor–mediated inflammatory VP in vivo.18 Hence, we sought to determine if PI3Kγ/δ might also be linked to Sema3A-induced VP. Mice treated systemically with TG100-115 showed markedly diminished VP in response to either Sema3A or VEGF (Figure 5C), indicating that both factors may depend on PI3Kγ/δ to induce VP. In addition, in endothelial cells Sema3A induced phosphorylation of the known downstream mediator of PI3K signaling Akt1/2 at serine 473, providing further evidence that Sema3A can use PI3K isoforms to induce VP (Figure S2). These findings reveal that both Sema3A and VEGF-mediated VP depend on Nrp-1 and can be blocked with an inhibitor of PI3Kγ/δ; however, these processes differ in their dependence on Src kinase (Figure S3).
Discussion
Here we define a novel role for Sema3A in modulating vascular function. While Sema3A selectively disrupts VEGF-mediated angiogenesis (Figure 1A), it also induces VP independently of VEGF (Figure 2A,B). Previous studies implicated Sema3A as an inhibitor of integrin function to explain its antiangiogenic properties.2 However, our findings clearly indicate that Sema3A is not a general inhibitor of integrin function. Rather, we observed that Sema3A selectively suppressed FAK and Src phosphorylation in response to VEGF but not bFGF stimulation. Importantly, Sema3A did not suppress all functional activity associated with VEGF signaling, since ERK activity was not disrupted by Sema3A treatment at doses where FAK and Src were inhibited (Figure 1B-D). Therefore, it appears that Sema3A may interfere with the coordinate regulation of VEGF receptor and integrin function by disrupting FAK and Src.
Functionally, Sema3A may be capable of inhibiting VEGF-induced angiogenesis by binding to the VEGF receptor Nrp-1 on endothelial cells.8,10 In shifting the Nrp-1 pool away from VEGF signaling, Sema3A through its plexin receptors may be activating distinct pathways that interfere with VEGF-induced angiogenesis. The plexin receptors responsible for Sema3A signaling, plexinA1-A4 and plexinD1, are found in endothelial cells and may also serve as signaling receptors for Sema3A in blood vessels.2 Thus, activation of plexin receptors by Sema3A may explain in part how Sema3A selectively influences VEGF-mediated signaling to FAK and Src.
Our studies also reveal that Sema3A and VEGF can function additively to induce VP (Figure 2A,B). This was surprising given that Sema3A inhibited VEGF-mediated Src phosphorylation (Figure 1B). However, Sema3A together with VEGF enhances VE-cadherin phosphorylation compared with each one individually (Figure 2E), providing further evidence for an additive effect. While our previous studies show that VEGF-mediated VP depends on Src, VEGF can also use other proteins such as PI3Kγ/δ to induce VP through tyrosine phosphorylation of VE-cadherin and disruption of adherens junctions.18,33 Our results suggest that, in the presence of Sema3A, VEGF uses these alternative pathways. Importantly, these findings also indicate that Sema3A and VEGF induce VP through distinct pathways.
These results also illustrate that Sema3A is distinct among class 3 semaphorins based on its ability to promote VP and selectively suppress VEGF-mediated angiogenesis. Sema3F, another inhibitor of angiogenesis, functions as a more global inhibitor in that it blocks angiogenesis in response to both VEGF and bFGF.28,29 However, as we demonstrate, it does not induce VP (Figure 3). This may be caused by differential binding of these 2 proteins to their Nrp receptors, since Sema3A and Sema3F ligate Nrp-1 and Nrp-2, respectively.34
In the context of VP, we clearly show that Sema3A and VEGF require Nrp-1 to induce a VP response, since knockdown of Nrp-1 in the endothelium completely abrogated the VP response to both factors (Figure 4C). However, we could also selectively disrupt VP induced by Sema3A but not VEGF with a specific monoclonal antibody to Nrp-1 (Figure 4D). This, coupled with the fact that Src kinases are critical for VEGF- but not Sema3A-mediated VP, provides further support that these factors activate distinct signaling mechanisms for their VP-promoting activity (Figure 5A,B). While Sema3A and VEGF mediate VP through distinct pathways, they both depend on PI3Kγ/δ since a selective inhibitor of these isoforms interferes with both VP pathways (Figure 5C; Figure S3). Perhaps this is not surprising as PI3Kγ/δ activation influences vascular barrier function.18 Together these results begin to elucidate a mechanism by which Sema3A induces VP.
Because Sema3A-mediated VP is Src independent and inflammatory mediated VP is not blocked in Src-deficient mice,14 signaling downstream of Sema3A may be similar to that of inflammatory mediators. Although most studies implicate Src in VE-cadherin phosphorylation, it is likely that other kinases or phosphatases can also regulate the phosphorylation state of VE-cadherin, especially given that inflammatory mediators cause tyrosine phosphorylation of VE-cadherin in a Src-independent manner.14,25,26 Our studies show that Sema3A uses the PI3Kγ/δ-Akt pathway to mediate VP (Figure 5C; Figure S2). Inhibition of PI3Kγ/δ prevents tyrosine phosphorylation of VE-cadherin18 ; therefore, kinases and/or phosphatases downstream of Akt are potential candidates in regulating VE-cadherin phosphorylation and VP in response to Sema3A.
Based on the newly delineated role for Sema3A on blood vessels, it appears to be a potential target for the development of therapies against pathological conditions such as ischemic disease and cancer. Because Sema3A is secreted by blood vessels, it may function to regulate the basal permeability of certain vessels. In fact, Sema3A was found to be highly expressed in newly forming blood vessels in mice,2 which may explain why such vessels tend to be leakier than mature vessels. In pathological conditions such as wound repair or inflammation, it is possible that Sema3A suppresses VEGF-mediated angiogenesis while producing substantial VP. This may aid in the formation of a fibrin barrier around diseased tissues while suppressing vascular growth in these tissues. Recently, we observed that a number of tumors express Sema3A endogenously (data not shown). Such tumors might show extensive VP yet be resistant to VEGF-mediated angiogenesis. The capacity of tumor cells to produce Sema3A might account for their loss of sensitivity to anti-VEGF–targeted therapy and may explain why some patients develop resistance to the VEGF antibody bevacizumab.
In summary, we define a novel role for Sema3A as a selective inhibitor of VEGF-mediated angiogenesis and a potent inducer of microvascular permeability. These studies provide a basis to define how Sema3A regulates vascular form and function.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Many thanks to Glenn Begley for sharing with us the EC-SCL-Cre ERT–positive mice and TargeGen for providing us with TG100-115. We also thank Leo Barnes and Kim Lutu-Fuga for their advice and technical assistance during the preparation of this paper.
This work was supported by grants from the National Institutes of Health (NIH) to D.A.C. (HL057900 and CA50286). L.M.A. is also supported by the NIH Institutional Research and Academic Career Development Awards fellowship. We acknowledge the generosity of Michael Klagsbrun and Susan Fisher, who provided us with purified recombinant Sema3F and Sema3B, respectively.
National Institutes of Health
Authorship
Contribution: L.M.A. performed experiments and analyzed results; S.B. performed the in vitro VP studies; S.M.W. provided advice in designing experiments; J.R.G. generated the EC-SCL-Cre-ERT mice; L.M.A. and D.A.C. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David A. Cheresh, Moores UCSD Cancer Center, 3855 Health Sciences Dr, #0803, La Jolla, CA 92093-0803; e-mail: dcheresh@ucsd.edu.