B-cell–activating factor of the TNF family, (BAFF), and a proliferation-inducing ligand (APRIL) regulate B-lymphocyte survival and activation. We report that BAFF, but not APRIL, increased the chemotactic response of primary human B cells to CCL21, CXCL12, and CXCL13. The BAFF-induced increase in B-cell chemotaxis was totally abolished by blockade of BAFF-R and was strongly dependent on the activation of PI3K/AKT, NF-κB, and p38MAPK pathways. BAFF had similar effects on the chemotaxis of naive and memory B cells in response to CCL21 but increased more strongly that of memory B cells to CXCL13 than that of naive B cells. Our findings indicate a previously unreported role for the BAFF/BAFF-R pair in mature B-cell chemotaxis. The synergy between CXCL13 and BAFF produced by stromal cells and follicular dendritic cells may have important implications for B-cell homeostasis, the development of normal B-cell areas, and for the formation of germinal center–like follicles that may be observed in various autoimmune diseases.
Introduction
B cell–activating factor belonging to the TNF family (BAFF) has emerged as an important regulator of B-cell homeostasis and survival: it acts alone or in combination with B-cell receptor (BCR), IL-4, or CD40 ligands.1,,–4 BAFF binds 3 different TNF receptors: BCMA (B-cell maturation),5,6 TACI (transmembrane activator and CAML interactor),7 and BAFF-R/BR3 (BLys receptor 3).8 A highly similar homolog (called “a proliferation-inducing ligand” or APRIL)1 also binds TACI and BCMA but not BAFF-R.9 BCMA, TACI, and BAFF-R are mostly found on B lymphocytes,10,–12 whereas BAFF-R is also present on a subset of T cells.11,13 Accordingly, BAFF produced by antigen-presenting cells provides T-cell costimulation.13 The BAFF/BAFF-R pair is essential for survival of immature T2, B2, and marginal zone (MZ) B cells,14,–16 but not for that of B1 cells,17,18 whereas TACI exerts a negative control over BAFF-mediated B2 cell survival.19,20 BCMA has no obvious effect on mature B-cell survival, but is important for long-term plasma cell biology10,12 and antigen presentation.21
BAFF- or BAFF-R–deficient mice form only rudimentary germinal centers (GCs) and produce low levels of IgG in response to T-dependent (TD) antigens.22,23 In contrast, TACI-deficient mice display an impaired response to type II T cell–independent antigens, suggesting that TACI is required for B1 cell survival.24 BAFF-R and TACI provide signals for isotype switching toward IgG and IgE, but the switch to IgA is mainly controlled by TACI.17,25 Many BAFF transgenic mice show signs of autoimmune-like diseases,2,26 whereas aged APRIL-transgenic mice display a progressive expansion of B1 cells infiltrating the peritoneum and lymphoid organs.27 These various observations support a major role for the TACI/APRIL and BAFF-R/BAFF pairs in B1 and B2 cell physiology, respectively.
Like CD40L, BAFF mainly promotes NF-κB and MAPK activation.28,29 Triggering BAFF-R results in activation of NF-κB2 and NF-κB1 pathways, whereas triggering BCMA and TACI only activate the NF-κB1 pathway.7,9,28,30,31 Different sets of MAPK and transcription factors are activated downstream from BCMA, TACI, and BAFF-R.29,32,33 In particular, it has been shown that p38MAPK but not ERK is stimulated early after BAFF-R triggering.34
Lymphocyte recirculation, which is essential for maintaining an effective immune system, is tightly regulated by the expression of adhesion molecules, chemoattractant receptors, and environmental cytokines.35,36 Trafficking of human naive and memory B cells is mainly orchestrated by CXCR4/CXCL12, CXCR5/CXCL13, and CCR7/CCL21 (or CCL19) pairs.37,38 The efficiency of the humoral response depends on the chemotactic response of mature B cells that is modulated by BCR- and IL-4–receptor triggering and CD40/CD40L interactions.37,39,,–42 In particular, BCR triggering enhances the chemotactic response of naive B cells to CCL21 but decreases that to CXCL13. In contrast, CD40L enhances the migration of memory B cells to CXCL13 without modifying that of CXCL12, CCL21, or CCL19.37,40 These modifications allow a coordinated relocalization of B cells during the various phases of the TD humoral response, characterized by GC foundation. There are substantial similarities between CD40 and BAFF-R signaling pathways and their biological effects on the B-cell response, so it is plausible that BAFF also modulates B-cell chemotaxis. We therefore analyzed the effect of human BAFF on the expression of CCR7, CXCR5, and CXCR4 by primary B cells and on their migratory capacity in response to CCL21, CXCL13, and CXCL12. We found that BAFF enhanced the migration of primary B cells to these chemokines through BAFF-R triggering. BAFF did not modify the basal expression or the ligand-induced internalization of the chemokine receptors studied but modulated their chemokine-induced signaling. BAFF-induced BAFF-R stimulation led to the phosphorylation of IκBα, PI3K/AKT, and p38MAPK, and the processing of NF-κB2 (p100) to p52. The use of specific inhibitors confirmed that PI3K/PDK1, p38MAPK, and NF-κB pathways activated downstream from BAFF-R were essential for the effect of BAFF on primary B-cell chemotaxis. These findings establish a novel role for the BAFF/BAFF-R pair in the regulation of B-cell chemotaxis during the normal humoral response and potentially during the recruitment of pathologic B cells into ectopic follicles.43
Methods
Flow cytometry
Cell-surface antigens were analyzed by single-parameter or multiparameter flow cytometric analysis using the following monoclonal antibodies (mAbs): CD19-phycoerythrin (PE), IgD-PE, and CD44-FITC (all from BD Biosciences, Le Pont de Claix, France). CCR7-PE, CXCR5-PE, and CXCR4-PE mAbs were purchased from R&D Systems (Abingdon, United Kingdom). Mouse isotype-matched FITC- and PE-conjugated control IgG1 and IgG2a were purchased from BD Biosciences. The expression of receptors for APRIL and BAFF was detected by sequential addition of Flag-APRIL and Flag-BAFF ligands (Alexis Biochemicals, Lausen, Switzerland, Coger, Paris, France; 100 ng/mL), mouse anti–Flag M2 antibody (Sigma-Aldrich Chimie, Isle d'Abeau Chesnes, France), and PE-conjugated goat F(ab′)2 anti–mouse IgG (Beckman Coulter, Marseille, France) as previously described.44 Cell-surface expression of BCMA and TACI was detected with rat anti–human BCMA (Vicky 1; Alexis) and rat anti–human TACI (1A1; Alexis) Ab and FITC-conjugated mouse anti–rat IgG (BD Biosciences). Rat IgG1 (BCMA) and IgG2a (TACI) were purchased from Beckman Coulter. BAFF-R expression was detected with goat anti–BAFF-R (R&D Systems) and PE-conjugated donkey F(ab′)2 anti–goat IgG (Beckman Coulter). A FACScan flow cytometer was used for data acquisition and CellQuest software (BD Biosciences) was used for data analysis. After gating on viable cells, 10 000 cells/sample were analyzed.
B-cell preparation
B cell–enriched populations were obtained from palatine tonsils as previously described.41 Briefly, after one cycle of rosette formation, residual T cells and monocytes were depleted with CD2 and CD14 magnetic beads (Dynabeads M-450; Dynal AS, Oslo, Norway). The total B-cell population was depleted of CD38+ GC B cells by Percoll gradient separation as previously described.41 The resulting B-cell population, including only naive and memory B cells, is herein referred to as “B cells” and was 98% (± 6%) CD19+, 93% (± 3%) CD44+, and 2% (± 3%) CD3+ (n = 20). The viability of these cells was consistently higher than 98%.
B-cell cultures
For in vitro culture assays, B cells (2 × 106 cells/mL) were cultured in RPMI 1640–glutamax medium (Invitrogen SARL, Cergy-Pontoise, France) containing 10 mM HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM sodium pyruvate, and 10% heat-inactivated fetal calf serum (FCS) for various periods, with or without 25 to 100 ng/mL FLAG-APRIL, recombinant human BAFF (AbCys, Paris, France), or 100 ng/mL CD40MegaLigand (CD40MGL; Alexis). The concentration of endotoxin in the culture medium and in the reagents used was consistently below 1 ng/mL as indicated by the manufacturers.
In some experiments, neutralizing goat anti–BAFF-R Ab or goat IgG (R&D Systems), 100 nM wortmannin (WM; a PI3K/PI4K inhibitor), 10 μM SB203580 (SB; p38MAPK inhibitor), or a combination of these were added with BAFF for 16 hours before assaying B-cell chemotaxis.
In vitro chemotaxis assay
Chemotaxis assays were carried out as described.41 In brief, 5 × 105 B cells in 100 μL of prewarmed RPMI 1640 containing 10 mM HEPES and 1% FCS were transmigrated through 5-μm pore size bare filters Transwell inserts (Costar, Cambridge, MA) for 3 hours at 37°C in response to chemokines (250 ng/mL CXCL12 or CCL21 or 500 ng/mL CXCL13). After exclusion of cell debris by forward and side scatter gating, the migrated cells were counted with a FACScan for 60 seconds. Results are shown as the percentage of specific migration, from which background migration to control medium was subtracted. To determine the phenotype of migrating cells, input cells and migrating cells were stained with CD44-FITC and IgD-PE mAbs and separately analyzed among gated sIgDhigh and sIgD− B cells by flow cytometry. The number of cells migrating to the lower chamber is expressed as a percentage of the naive or memory B cells added at the start of the assay (“input cells”).
To test the effect of inhibitors on the chemotaxis, B cells (2 ×106 cells/mL) were cultured with medium (mock treatment) or 25 ng/mL BAFF for 16 hours, washed, and incubated for 1 hour with 1 μM WM, 10 μM PD98059 (PD; MEK1/2 inhibitor), 10 μM SB, 250 nM U73122 (PLC inhibitor) or its inactive control (U73343), 1 μM chelerythrine chloride (CC; inhibitor of all types of PKC), 1 μM SN50 (inhibitor of NF-KB1 [p50] nuclear translocation), 100 ng/mL Pertussis toxin (PTX; inhibitor of Gαi proteins) and 20 μM Y27632 (ROCKI inhibitor; all from Calbiochem, San Diego, CA), 10 μM SH5 (PDK1 inhibitor; Alexis, Coger) or DMSO as a control. The samples were then subjected to the chemotaxis assay. Alternatively, B cells were incubated with medium, 100 nM WM (dose-selectively inhibiting the class I PI3Ks41 ), 10 μM SB, or both in the presence or absence of 25 ng/mL BAFF for 16 hours at 37°C before being subjected to the chemotaxis assay.
Ligand-induced chemokine receptor internalization
B cells (2 × 106 cells/mL) were cultured with 25 ng/mL BAFF or medium (mock treatment) for 16 hours at 37°C. BAFF- and mock-treated B cells were subsequently incubated for 60 minutes with medium or 100 ng/mL chemokine at 37°C. After washing in ice-cold medium, cells were stained with CD44-FITC and PE-CXCR4, PE-CXCR5, PE-CCR7, or PE–control IgG antibody for 30 minutes at 4°C. Fluorescence-activated cell sorter (FACS) analysis was performed on 10 000 viable cells after gating on CD44+ cells.
Western blots
Freshly isolated B cells were resuspended at a density of 107cells/mL in prewarmed RPMI 1640 without FCS, starved for 2 hours, then stimulated for 2 minutes at 37°C with medium, 100 ng/mL CXCL13, 100 ng/mL CCL21, or 50 ng/mL BAFF. Alternatively, mock- and BAFF-treated B cells were resuspended at a density of 107 cells/mL in prewarmed RPMI 1640 without FCS, starved for 2 hours, then stimulated for 2 minutes at 37°C with medium or 100 ng/mL chemokine. Lysates were prepared as previously described.41 Equal amounts of total cellular protein were loaded in each lane of SDS–polyacrylamide gels and subjected to electrophoresis. Subsequently, Western blotting was performed using antibodies recognizing phospho-AKT (S473), AKT, phospho-ERK1/2 (T202/Y204), phospho-IκBα (S32/S36), phospho-PLCβ3 (S537), PLCβ3, phospho-p38MAPK (T180/Y182), p38MAPK, and pan–phospho-PKC (all from New England Biolabs, Ozyme, France), PKCα/β/γ, IκBα, NF-κBp52, ERK1/2, phospho-PKCθ, PKCθ, or actin (from Santa Cruz Biotechnology, Tebu, France), followed by incubation with horseradish peroxidase (HRP)–conjugated secondary antibodies. Protein bands were detected using enhanced chemiluminescence (ECL) reagents (Supersignal Westpico chemilumiscent substrate; Pierce Biotechnologies, Illkirch, France), and the ECL signal was recorded on ECL Hyperfilm. To quantify band intensities, films were scanned, saved as TIFF files, and analyzed with National Institutes of Health (NIH) Image J free software.
Immunoprecipitation
Lysates (corresponding to 800 μg total protein) were incubated overnight at 4°C with 50 μL of packed agarose-conjugated antiphosphotyrosine beads (4G10; Millipore SAS, St Quentin-en-Yvelines, France). Immune complexes were washed 3 times before being analyzed by Western blot using a PLCγ2 antibody (from Santa Cruz Biotechnology). A total of 40 μg protein of each total lysate was also analyzed by Western blot to determine the total PLCγ2 content.
Statistical analysis
Data are expressed as means plus or minus SD unless otherwise indicated. Differences between groups were assessed using the Wilcoxon sum rank test and P values less than .05 were considered significant.
Results
BAFF, but not APRIL, increases the chemotaxis of human primary B cells
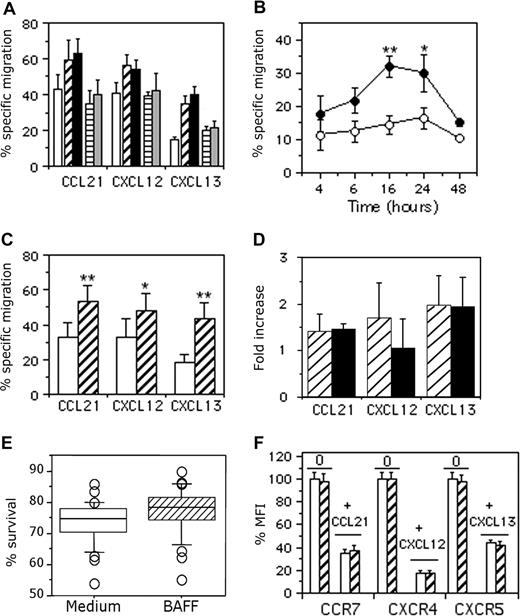
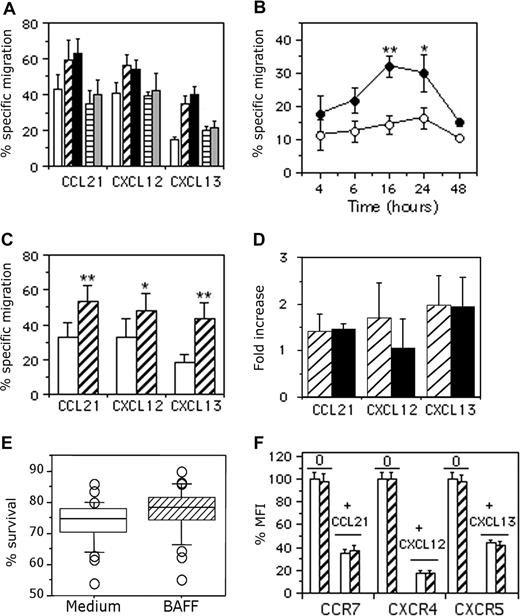
We assessed the chemotactic response of B cells to CCL21, CXCL12, and CXCL13 following incubation for 16 hours with medium (mock treatment), 25 ng/mL or 100 ng/mL BAFF, and 25 ng/mL or 100 ng/mL APRIL. In the absence of chemokine, BAFF and APRIL had no significant effect on B-cell chemotaxis (data not shown). However, BAFF, but not APRIL, increased the chemotactic response to these 3 chemokines. BAFF at 25 ng/mL increased the percentage of B cells specifically migrating to CCL21 by 1.4-fold (16.7% ± 5.1% increase; n = 6), to CXCL12 by 1.3-fold (16% ± 3% increase; n = 3), and to CXCL13 by 2.4-fold (19.7% ± 2.5% increase; n = 3; Figure 1A).
Dose- and time-dependent effect of BAFF on B-cell chemotaxis. (A) B cells were incubated for 16 hours with medium (□), 25 ng/mL (▫) or 100 ng/mL (■) BAFF, and 25 ng/mL (▤) or 100 ng/mL ( ) APRIL, and were then tested for migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, or 500 ng/mL CXCL13. Basal migration of B cells was 5% (± 1.4%, medium-treated), 4.7% (± 1.1%, 25 ng/mL BAFF–treated), 5.4% (± 0.5%, 100 ng/mL BAFF–treated), 4.3% (± 2%, 25 ng/mL APRIL–treated), and 5% (± 3%, 100 ng/mL APRIL–treated) Results are expressed as the mean percentage plus or minus SD of cells specifically migrating in response to each chemokine. (B) B cells were incubated for up to 48 hours with medium or 25 ng/mL BAFF. Mock-treated (□) and BAFF-treated (■) B cells were analyzed for migration to 500 ng/mL CXCL13. The experiment was performed using samples from 4 different donors, and basal migration ranged from 3% to 4% and 3.3% to 4.7% for mock- and BAFF-treated B cells, respectively. Results are expressed as the percentage plus or minus SD of specific chemotaxis. *P < .05; **P < .005. (C) B cells were treated for 16 hours with medium (□) or 25 ng/mL BAFF (▫) and analyzed for their migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, and 500 ng/mL CXCL13. Basal migration was 4.4% (± 1.2%) and 4.3% (± 1.1%) for mock- and BAFF-treated B cells, respectively. Results are expressed as the mean percentage plus or minus SD of specifically migrating cells obtained for each donor. *P < .05; **P < .005. (D) B cells were treated for 16 hours with medium (data not shown), 25 ng/mL BAFF (▫), or 100 ng/mL CD40MGL (■) and analyzed for their migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, and 500 ng/mL CXCL13. The experiment was performed using samples from 3 different donors, and basal migration was 3.3% (± 2.1%), 2.3% (± 1.5%), and 1.3% (± 0.6%) for mock-, BAFF-, and CD40MGL-treated B cells, respectively. Results are expressed as mean fold increase plus or minus SD. (E) The percentage of B-cell recovery after overnight culture with medium or 25 ng/mL BAFF was calculated for each donor. Results are expressed as median values obtained from 28 independent experiments. Error bars correspond to 95% confidence intervals about the median. (F) B cells from 3 different donors were incubated for 16 hours at 37°C with medium (□) or 25 ng/mL BAFF (▫) and then incubated for 60 minutes at 37°C with medium or 100 ng/mL chemokines. Cells were washed in ice-cold medium and stained with PE-conjugated CCR7, CXCR4, or CXCR5 mAb for 30 minutes at 4°C. The mean channel fluorescence intensity (MFI) values for mock-treated cells were between 57 and 123 for CCR7, 356 and 1067 for CXCR4, and 64 and 81 for CXCR5. Each of these individual values was considered as 100% expression. Data are expressed as the mean plus or minus SD percentage of MFI values for remaining surface expression.
) APRIL, and were then tested for migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, or 500 ng/mL CXCL13. Basal migration of B cells was 5% (± 1.4%, medium-treated), 4.7% (± 1.1%, 25 ng/mL BAFF–treated), 5.4% (± 0.5%, 100 ng/mL BAFF–treated), 4.3% (± 2%, 25 ng/mL APRIL–treated), and 5% (± 3%, 100 ng/mL APRIL–treated) Results are expressed as the mean percentage plus or minus SD of cells specifically migrating in response to each chemokine. (B) B cells were incubated for up to 48 hours with medium or 25 ng/mL BAFF. Mock-treated (□) and BAFF-treated (■) B cells were analyzed for migration to 500 ng/mL CXCL13. The experiment was performed using samples from 4 different donors, and basal migration ranged from 3% to 4% and 3.3% to 4.7% for mock- and BAFF-treated B cells, respectively. Results are expressed as the percentage plus or minus SD of specific chemotaxis. *P < .05; **P < .005. (C) B cells were treated for 16 hours with medium (□) or 25 ng/mL BAFF (▫) and analyzed for their migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, and 500 ng/mL CXCL13. Basal migration was 4.4% (± 1.2%) and 4.3% (± 1.1%) for mock- and BAFF-treated B cells, respectively. Results are expressed as the mean percentage plus or minus SD of specifically migrating cells obtained for each donor. *P < .05; **P < .005. (D) B cells were treated for 16 hours with medium (data not shown), 25 ng/mL BAFF (▫), or 100 ng/mL CD40MGL (■) and analyzed for their migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, and 500 ng/mL CXCL13. The experiment was performed using samples from 3 different donors, and basal migration was 3.3% (± 2.1%), 2.3% (± 1.5%), and 1.3% (± 0.6%) for mock-, BAFF-, and CD40MGL-treated B cells, respectively. Results are expressed as mean fold increase plus or minus SD. (E) The percentage of B-cell recovery after overnight culture with medium or 25 ng/mL BAFF was calculated for each donor. Results are expressed as median values obtained from 28 independent experiments. Error bars correspond to 95% confidence intervals about the median. (F) B cells from 3 different donors were incubated for 16 hours at 37°C with medium (□) or 25 ng/mL BAFF (▫) and then incubated for 60 minutes at 37°C with medium or 100 ng/mL chemokines. Cells were washed in ice-cold medium and stained with PE-conjugated CCR7, CXCR4, or CXCR5 mAb for 30 minutes at 4°C. The mean channel fluorescence intensity (MFI) values for mock-treated cells were between 57 and 123 for CCR7, 356 and 1067 for CXCR4, and 64 and 81 for CXCR5. Each of these individual values was considered as 100% expression. Data are expressed as the mean plus or minus SD percentage of MFI values for remaining surface expression.
Dose- and time-dependent effect of BAFF on B-cell chemotaxis. (A) B cells were incubated for 16 hours with medium (□), 25 ng/mL (▫) or 100 ng/mL (■) BAFF, and 25 ng/mL (▤) or 100 ng/mL ( ) APRIL, and were then tested for migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, or 500 ng/mL CXCL13. Basal migration of B cells was 5% (± 1.4%, medium-treated), 4.7% (± 1.1%, 25 ng/mL BAFF–treated), 5.4% (± 0.5%, 100 ng/mL BAFF–treated), 4.3% (± 2%, 25 ng/mL APRIL–treated), and 5% (± 3%, 100 ng/mL APRIL–treated) Results are expressed as the mean percentage plus or minus SD of cells specifically migrating in response to each chemokine. (B) B cells were incubated for up to 48 hours with medium or 25 ng/mL BAFF. Mock-treated (□) and BAFF-treated (■) B cells were analyzed for migration to 500 ng/mL CXCL13. The experiment was performed using samples from 4 different donors, and basal migration ranged from 3% to 4% and 3.3% to 4.7% for mock- and BAFF-treated B cells, respectively. Results are expressed as the percentage plus or minus SD of specific chemotaxis. *P < .05; **P < .005. (C) B cells were treated for 16 hours with medium (□) or 25 ng/mL BAFF (▫) and analyzed for their migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, and 500 ng/mL CXCL13. Basal migration was 4.4% (± 1.2%) and 4.3% (± 1.1%) for mock- and BAFF-treated B cells, respectively. Results are expressed as the mean percentage plus or minus SD of specifically migrating cells obtained for each donor. *P < .05; **P < .005. (D) B cells were treated for 16 hours with medium (data not shown), 25 ng/mL BAFF (▫), or 100 ng/mL CD40MGL (■) and analyzed for their migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, and 500 ng/mL CXCL13. The experiment was performed using samples from 3 different donors, and basal migration was 3.3% (± 2.1%), 2.3% (± 1.5%), and 1.3% (± 0.6%) for mock-, BAFF-, and CD40MGL-treated B cells, respectively. Results are expressed as mean fold increase plus or minus SD. (E) The percentage of B-cell recovery after overnight culture with medium or 25 ng/mL BAFF was calculated for each donor. Results are expressed as median values obtained from 28 independent experiments. Error bars correspond to 95% confidence intervals about the median. (F) B cells from 3 different donors were incubated for 16 hours at 37°C with medium (□) or 25 ng/mL BAFF (▫) and then incubated for 60 minutes at 37°C with medium or 100 ng/mL chemokines. Cells were washed in ice-cold medium and stained with PE-conjugated CCR7, CXCR4, or CXCR5 mAb for 30 minutes at 4°C. The mean channel fluorescence intensity (MFI) values for mock-treated cells were between 57 and 123 for CCR7, 356 and 1067 for CXCR4, and 64 and 81 for CXCR5. Each of these individual values was considered as 100% expression. Data are expressed as the mean plus or minus SD percentage of MFI values for remaining surface expression.
) APRIL, and were then tested for migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, or 500 ng/mL CXCL13. Basal migration of B cells was 5% (± 1.4%, medium-treated), 4.7% (± 1.1%, 25 ng/mL BAFF–treated), 5.4% (± 0.5%, 100 ng/mL BAFF–treated), 4.3% (± 2%, 25 ng/mL APRIL–treated), and 5% (± 3%, 100 ng/mL APRIL–treated) Results are expressed as the mean percentage plus or minus SD of cells specifically migrating in response to each chemokine. (B) B cells were incubated for up to 48 hours with medium or 25 ng/mL BAFF. Mock-treated (□) and BAFF-treated (■) B cells were analyzed for migration to 500 ng/mL CXCL13. The experiment was performed using samples from 4 different donors, and basal migration ranged from 3% to 4% and 3.3% to 4.7% for mock- and BAFF-treated B cells, respectively. Results are expressed as the percentage plus or minus SD of specific chemotaxis. *P < .05; **P < .005. (C) B cells were treated for 16 hours with medium (□) or 25 ng/mL BAFF (▫) and analyzed for their migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, and 500 ng/mL CXCL13. Basal migration was 4.4% (± 1.2%) and 4.3% (± 1.1%) for mock- and BAFF-treated B cells, respectively. Results are expressed as the mean percentage plus or minus SD of specifically migrating cells obtained for each donor. *P < .05; **P < .005. (D) B cells were treated for 16 hours with medium (data not shown), 25 ng/mL BAFF (▫), or 100 ng/mL CD40MGL (■) and analyzed for their migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, and 500 ng/mL CXCL13. The experiment was performed using samples from 3 different donors, and basal migration was 3.3% (± 2.1%), 2.3% (± 1.5%), and 1.3% (± 0.6%) for mock-, BAFF-, and CD40MGL-treated B cells, respectively. Results are expressed as mean fold increase plus or minus SD. (E) The percentage of B-cell recovery after overnight culture with medium or 25 ng/mL BAFF was calculated for each donor. Results are expressed as median values obtained from 28 independent experiments. Error bars correspond to 95% confidence intervals about the median. (F) B cells from 3 different donors were incubated for 16 hours at 37°C with medium (□) or 25 ng/mL BAFF (▫) and then incubated for 60 minutes at 37°C with medium or 100 ng/mL chemokines. Cells were washed in ice-cold medium and stained with PE-conjugated CCR7, CXCR4, or CXCR5 mAb for 30 minutes at 4°C. The mean channel fluorescence intensity (MFI) values for mock-treated cells were between 57 and 123 for CCR7, 356 and 1067 for CXCR4, and 64 and 81 for CXCR5. Each of these individual values was considered as 100% expression. Data are expressed as the mean plus or minus SD percentage of MFI values for remaining surface expression.
Kinetic studies showed that the BAFF-mediated increase in B-cell chemotaxis reached a maximum at around 16 to 24 hours and decreased thereafter, suggesting that the BAFF-mediated increase of B-cell chemotaxis was transitory. After 16 hours, specific migration to CXCL13 was 14% (± 3%) and 32% (± 3%, 2.3-fold increase; n = 4) for mock- and BAFF-treated cells, respectively (Figure 1B). Similar kinetics were observed in response to CXCL12 and CCL21 (data not shown). Given the well-known variability of chemokine responsiveness among individuals, B cells from more donors were tested and responses similar to those shown on Figure 1B were obtained consistently. After 16 hours, BAFF increased the chemotactic response to CCL21 and CXCL12 by 1.6-fold (19.7% ± 4.6% increase; n = 18, P < .005; and 18.8% ± 5.9% increase; n = 9, P < .05, respectively) and to CXCL13 by 2.4-fold (24% ± 5.9% increase; n = 20, P < .005; Figure 1C). CD40MGL and BAFF similarly increased the chemotaxis to CCL21 (1.5- and 1.4-fold increase, respectively) and CXCL13 (1.9- and 2-fold increase, respectively), whereas CD40MGL is less efficient than BAFF in increasing the chemotactic response to CXCL12 (1-fold vs 1.7-fold increase, respectively; Figure 1D). Viable cell recovery was similar in medium- and BAFF-treated B cells (73% ± 5% and 77% ± 5%, respectively) after 16 hours of culture (Figure 1E), so this increase was not due to the antiapoptotic effect of BAFF.
We tested whether the BAFF-mediated increase of human B-cell chemotaxis reflected increased chemokine receptor expression. In the absence of their respective ligands, CCR7, CXCR4, and CXCR5 were similarly expressed on mock- and BAFF-treated B cells (Figure 1F). CCL21-, CXCL12-, and CXCL13-induced internalization decreased the expression of CCR7, CXCR4, and CXCR5 by 65% (± 3%), 82% (± 1.9%), and 56% (± 3.1%) in mock-treated B cells and by 63% (± 4.9%), 83% (± 2.8%), and 58% (± 3.2%, n = 3) in BAFF-treated B cells, respectively. Thus, BAFF did not significantly change the basal expression or the ligand-induced internalization of CCR7, CXCR4, or CCR5. Similarly, BAFF did not significantly modulate the expression of various adhesion molecules, including α4, β7, or β1 integrins (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
BAFF enhancement of B-cell chemotaxis is dependent on BAFF-R
Incubation with BAFF but not APRIL enhances B-cell chemotaxis, so we compared the expression of TACI, BCMA, and BAFF-R (Figure 2A) and the binding of FLAG-BAFF and FLAG-APRIL (Figure 2B) on freshly isolated B cells and after 16 hours of culture. Freshly isolated B cells strongly bound FLAG-BAFF but not FLAG-APRIL. Weak expression of both BCMA and TACI was detected on the membrane, and in contrast, BAFF-R was strongly expressed. After culture in medium, the binding of FLAG-BAFF and the expression of BAFF-R and BCMA were slightly higher, whereas both the binding of FLAG-APRIL and the expression of TACI were substantially higher. The addition of a neutralizing BAFF-R Ab totally abolished the BAFF-mediated increase in B-cell chemotaxis (Figure 2C), suggesting that the effect of BAFF on B-cell chemotaxis mainly resulted from BAFF-R stimulation.
BAFF increases B-cell chemotaxis through interactions with BAFF-R. (A) Surface expression of BCMA, TACI, and BAFF-R was analyzed by flow cytometry on freshly isolated B cells (0 hours) and on B cells incubated for 16 hours with medium. MFI of specific staining (positive minus IgG control) was 17 (BCMA), 17 (TACI), and 55 (BAFF-R) at 0 hours and 28 (BCMA), 32 (TACI), and 67 (BAFF-R) at 16 hours. Data are representative of 4 separate experiments. (B) Similarly, the ability of BAFF-FLAG and APRIL-FLAG to bind B cells was analyzed by flow cytometry using both freshly isolated B cells (0 hours) and B cells incubated for 16 hours with medium. MFI of specific staining was 7 (APRIL) and 39 (BAFF) at 0 hours and 44 (APRIL) and 121 (BAFF) at 16 hours. Data are representative of 4 separate experiments. (C) B cells were incubated for 16 hours with medium (□), 10 μg/mL anti–BAFFR Ab (■), 25 ng/mL BAFF (▫), or 10 μg/mL anti–BAFF-R mAb and 25 ng/mL BAFF (▤). B cells were analyzed for migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, and 500 ng/mL CXCL13. The experiment was performed with samples from 3 different donors and results are expressed as the percentage plus or minus SD of specific chemotaxis. *P < .05.
BAFF increases B-cell chemotaxis through interactions with BAFF-R. (A) Surface expression of BCMA, TACI, and BAFF-R was analyzed by flow cytometry on freshly isolated B cells (0 hours) and on B cells incubated for 16 hours with medium. MFI of specific staining (positive minus IgG control) was 17 (BCMA), 17 (TACI), and 55 (BAFF-R) at 0 hours and 28 (BCMA), 32 (TACI), and 67 (BAFF-R) at 16 hours. Data are representative of 4 separate experiments. (B) Similarly, the ability of BAFF-FLAG and APRIL-FLAG to bind B cells was analyzed by flow cytometry using both freshly isolated B cells (0 hours) and B cells incubated for 16 hours with medium. MFI of specific staining was 7 (APRIL) and 39 (BAFF) at 0 hours and 44 (APRIL) and 121 (BAFF) at 16 hours. Data are representative of 4 separate experiments. (C) B cells were incubated for 16 hours with medium (□), 10 μg/mL anti–BAFFR Ab (■), 25 ng/mL BAFF (▫), or 10 μg/mL anti–BAFF-R mAb and 25 ng/mL BAFF (▤). B cells were analyzed for migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, and 500 ng/mL CXCL13. The experiment was performed with samples from 3 different donors and results are expressed as the percentage plus or minus SD of specific chemotaxis. *P < .05.
BAFF increases the CXCL13-dependent chemotaxis of memory B cells more strongly than that of naive B cells
Using the transmigration assay, we compared the ability of naive (CD44+IgD+) and memory (CD44+IgD−) B cells from 6 donors to migrate in response to chemokines following a 16-hour incubation with medium or with 25 ng/mL BAFF (Figure 3). The percentages of memory B cells in mock- and BAFF-treated input populations were similar after a 16-hour incubation period (26% ± 6.1% and 25.6% ± 7.1%, respectively) and comparable to that of untreated B cells (28.5% ± 6.7%; data not shown).
The BAFF-mediated increase of CXCL13-dependent chemotaxis is stronger with memory B cells than with naive B cells. Mock- and BAFF- treated B cells were analyzed for their migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, and 500 ng/mL CXCL13. Input populations and migrated cell populations were stained with CD44-FITC and IgD-PE. Representative dot-plots of input cells (A,B) and transmigrated cells to medium or CXCL13 (C,D) are shown. The numbers of cells in the input and transmigrated populations from each B-cell subset are given in each quadrant (A-D). The percentages of naive (dotted bars) and memory B cells (hatched bars) that specifically migrated to the indicated chemokines are shown in panels E and F for mock- and BAFF-treated cells, respectively. The percentages of total B cells migrating to each chemokine are indicated below the histograms (E,F). Data from one representive experiment of 6 are shown (A-F). The experiment was repeated with samples from 6 different donors, and results are expressed as the mean BAFF-induced percentage increase plus or minus SD of total (■), naive (dotted bars), and memory B cells (hatched bars) that specifically migrated to the indicated chemokines. Basal migration was 4.8% (± 0.8%), 4% (± 0.6%), and 7.6% (± 1.8%) for total, naive, or memory B cells in mock-treated cultures, and 4.9% (± 0.7%), 3.8% (± 1%), and 7.9% (± 2.9%) in BAFF-treated cultures, respectively. (G). *P < .05.
The BAFF-mediated increase of CXCL13-dependent chemotaxis is stronger with memory B cells than with naive B cells. Mock- and BAFF- treated B cells were analyzed for their migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, and 500 ng/mL CXCL13. Input populations and migrated cell populations were stained with CD44-FITC and IgD-PE. Representative dot-plots of input cells (A,B) and transmigrated cells to medium or CXCL13 (C,D) are shown. The numbers of cells in the input and transmigrated populations from each B-cell subset are given in each quadrant (A-D). The percentages of naive (dotted bars) and memory B cells (hatched bars) that specifically migrated to the indicated chemokines are shown in panels E and F for mock- and BAFF-treated cells, respectively. The percentages of total B cells migrating to each chemokine are indicated below the histograms (E,F). Data from one representive experiment of 6 are shown (A-F). The experiment was repeated with samples from 6 different donors, and results are expressed as the mean BAFF-induced percentage increase plus or minus SD of total (■), naive (dotted bars), and memory B cells (hatched bars) that specifically migrated to the indicated chemokines. Basal migration was 4.8% (± 0.8%), 4% (± 0.6%), and 7.6% (± 1.8%) for total, naive, or memory B cells in mock-treated cultures, and 4.9% (± 0.7%), 3.8% (± 1%), and 7.9% (± 2.9%) in BAFF-treated cultures, respectively. (G). *P < .05.
Results from one representative experiment are shown in Figure 3A-F. A total of 15% of all B cells treated with medium (Figure 3E) and 43% of all B cells treated with BAFF (Figure 3F) specifically migrated in response to CXCL13. This BAFF-induced increase was due to an additional 19% of naive B cells (14% in mock-treated B cells vs 33% in BAFF-treated B cells) and 35% of memory B cells (19% in mock-treated B cells vs 54% in BAFF-treated B cells) specifically migrating to CXCL13 (Figure 3E,F). The percentages of total B cells specifically migrating in response to CCL21 was 29% (medium) and 46% (BAFF), with an additional 16% of naive and memory B cells in the migrating population. Similarly, the percentage of total B cells migrating in response to CXCL12 was 35% in medium and 55% in those treated with BAFF, with 19% and 21% more naive and memory B cells, respectively, in the migrating population (Figure 3E,F).
Similar results were obtained in 6 independent experiments showing that BAFF similarly increased the chemotaxis of naive and memory B cells to CCL21 and CXCL12. However, the BAFF-dependent increase of chemotaxis to CXCL13 was 1.8-fold higher for memory B cells than naive B cells (19.7% ± 5.3% naive and 34% ± 8.2% memory B cells migrated to CXCL13; P < .05; Figure 3G).
The BAFF-mediated increase in B-cell chemotaxis requires p38MAPK, PI3K/PDK1, ROCK I, and NF-κB1
To determine which effectors are involved in the BAFF-induced increase in B-cell chemotaxis, we tested the effects of various inhibitors added prior to the chemotaxis assay. Migration toward CXCL13 was similarly inhibited by the addition of 100 ng/mL PTX, 250 nM U73122, and 1 μM CC to B cells treated with and without BAFF from 5 different donors (Figure 4A). The addition of 250 nM U73343, the inactive form of the PLC inhibitor U73122 (data not shown), or of 10 μM PD98059 did not inhibit the chemotaxis of mock- or BAFF-treated B cells. In contrast, the addition of 10 μM SB203580 did not impair the CXCL13-mediated migration of mock-treated B cells, but inhibited by 38% (± 12%, P < .005) that of BAFF-treated B cells (Figure 4A). The addition of 1 μM WM (inhibiting all classes of PI3K), 10 μM SH5, 20 μM Y27632, or 1 μM SN50 consistently inhibited the CXCL13-mediated specific migration of BAFF-treated cells more strongly than that of mock-treated cells. Similar results were obtained for CCL21-mediated chemotaxis (Figure 4B). This suggested that BAFF-treated B cells are more dependent than mock-treated B cells on NF-κB and on the downstream effectors of PI3K, including PDK1 and ROCK I.
PI3K/AKT, p38MAPK, Rho-kinase, and NF-κB participate differently in the chemotaxis of mock- and BAFF-treated B cells. Mock-treated (□) and BAFF-treated (▫) B cells from 6 different donors were incubated for 1 hour at 37°C with various inhibitors or DMSO and were then subjected to the chemotaxis assay to 500 ng/mL CXCL13 (A) or 250 ng/mL CCL21 (B). Results are expressed as the mean percentage of inhibition plus or minus SD of the specific migration. *P < .05; **P < .005. B cells from 3 different donors were incubated with medium, 100 nM WM, 10 μM SB203580, or both in the presence (▫) or absence (□) of 25 ng/mL BAFF for 16 hours at 37°C and were then subjected to the chemotaxis assay to 500 ng/mL CXCL13 (C) or 250 ng/mL CCL21 (D). Results are expressed as the mean percentage plus or minus SD of specific migration.
PI3K/AKT, p38MAPK, Rho-kinase, and NF-κB participate differently in the chemotaxis of mock- and BAFF-treated B cells. Mock-treated (□) and BAFF-treated (▫) B cells from 6 different donors were incubated for 1 hour at 37°C with various inhibitors or DMSO and were then subjected to the chemotaxis assay to 500 ng/mL CXCL13 (A) or 250 ng/mL CCL21 (B). Results are expressed as the mean percentage of inhibition plus or minus SD of the specific migration. *P < .05; **P < .005. B cells from 3 different donors were incubated with medium, 100 nM WM, 10 μM SB203580, or both in the presence (▫) or absence (□) of 25 ng/mL BAFF for 16 hours at 37°C and were then subjected to the chemotaxis assay to 500 ng/mL CXCL13 (C) or 250 ng/mL CCL21 (D). Results are expressed as the mean percentage plus or minus SD of specific migration.
We next tested the effects on B-cell chemotaxis of PI3K and p38MAPK inhibitors added with BAFF. Neither 100 nM WM (selectively inhibiting the class I PI3Ks) nor SB203580 had any effect on mock-treated B-cell chemotaxis (data not shown). The chemotactic response of BAFF-treated B cells to CXCL13 was reduced by 57% by 100 nM WM, 62% by SB203580 and 86% by both (Figure 4C); similarly, the BAFF-mediated increase of B-cell chemotaxis to CCL21 was reduced by 65%, 57%, and 100% by the same inhibitors (Figure 4D). These findings suggested that class I PI3Ks and p38MAPK are activated downstream from BAFF-R and are key effectors of the BAFF-mediated increase of B-cell chemotaxis.
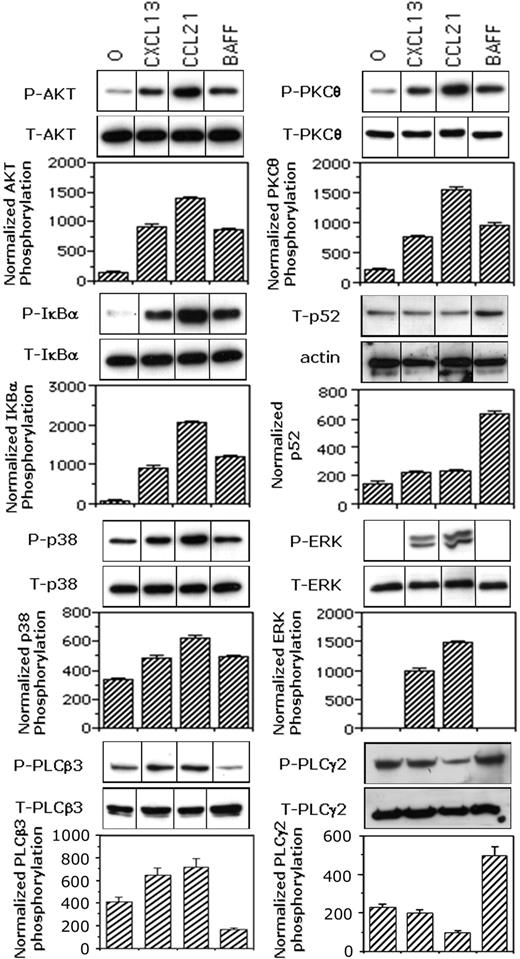
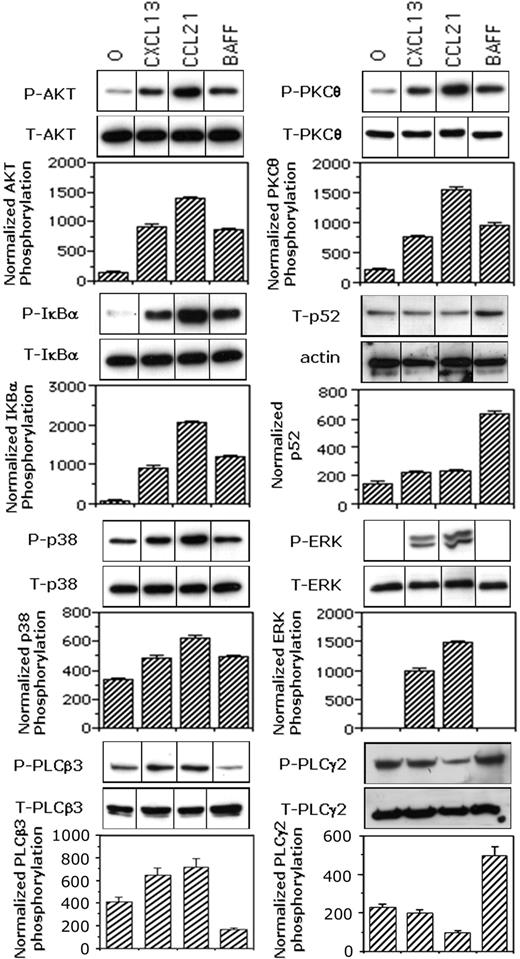
BAFF and chemokines differently regulated the phosphorylation of p38MAPK, ERK1/2, AKT, and IκBα as well as the processing of NF-κB2 (p100) to p52
Consistent with our previous findings for B cells,41 we observed that CCL21 increased the phosphorylation of AKT by 9.2-fold, of IκBα by 16-fold, and of p38MAPK by 2.1 fold (Figure 5). Similarly, CXCL13 increased the phosphorylation of AKT by 6-fold, of IκBα by 7.6-fold, and of p38MAPK by 1.5-fold. Both chemokines strongly induced the phosphorylation of ERK (1000-fold or greater increase) and of PKCθ (7.4- and 3.6-fold with CCL21 and CXCL13, respectively). Preliminary experiments showed that 50 ng/mL BAFF was optimal for assessing its effects on the phosphorylation of downstream effectors by biochemical approaches (data not shown). As expected from previous studies in mice and humans, BAFF induced both the phosphorylation of IκBα (7.6-fold increase) and the processing of NF-κB2 (p100) to p52 with a 4.7-fold increase of p52 content. This NF-κB2 (p100) processing was not significantly observed in response to chemokines. More surprisingly, BAFF also induced phosphorylation of AKT (4-fold), PKCθ (3.6-fold), p38MAPK (1.8-fold), and PLCγ2 (4.2-fold), but not that of ERK or PLCβ3.
BAFF and chemokines differently activate NF-κB1 and NF-κB2 pathways. Mock-treated B cells were stimulated for 2 minutes with medium, 100 ng/mL CCL21, 100 ng/mL CXCL13, or 50 ng/mL BAFF. The p52 contents of total cell lysates were corrected for the actin contents. Phosphorylation of AKT, IκBα, p38MAPK, ERK1/2, PKCθ, and PLCβ3 were corrected for total relevant protein. Blots from one representative experiment are shown. Values reported for p52 contents or phosphorylation are mean values plus or minus SD from 2 (PLCβ3) or 3 independent experiments. Values reported for PLCγ2 phosphorylation were calculated as the ratio between PLCγ2 contents in 4G10 immunoprecipitates and those in total lysates. Results are mean values plus or minus SD from 2 independent experiments. Black dividing lines between lanes represent the cuts resulting from the grouping of images from different parts of the same gel in order to optimize the order of the bands and/or remove lanes corresponding to conditions not relevant to the present work.
BAFF and chemokines differently activate NF-κB1 and NF-κB2 pathways. Mock-treated B cells were stimulated for 2 minutes with medium, 100 ng/mL CCL21, 100 ng/mL CXCL13, or 50 ng/mL BAFF. The p52 contents of total cell lysates were corrected for the actin contents. Phosphorylation of AKT, IκBα, p38MAPK, ERK1/2, PKCθ, and PLCβ3 were corrected for total relevant protein. Blots from one representative experiment are shown. Values reported for p52 contents or phosphorylation are mean values plus or minus SD from 2 (PLCβ3) or 3 independent experiments. Values reported for PLCγ2 phosphorylation were calculated as the ratio between PLCγ2 contents in 4G10 immunoprecipitates and those in total lysates. Results are mean values plus or minus SD from 2 independent experiments. Black dividing lines between lanes represent the cuts resulting from the grouping of images from different parts of the same gel in order to optimize the order of the bands and/or remove lanes corresponding to conditions not relevant to the present work.
Thus, in primary human B cells, BAFF triggered both the alternative and classic NF-κB pathways, whereas the chemokines CXCL13 and CCL21 only stimulated the classic pathway. In addition, we report the first evidence in primary B cells that BAFF activated the PI3K/PDK1 pathway: the induction of AKT and PKCθ phosphorylation.
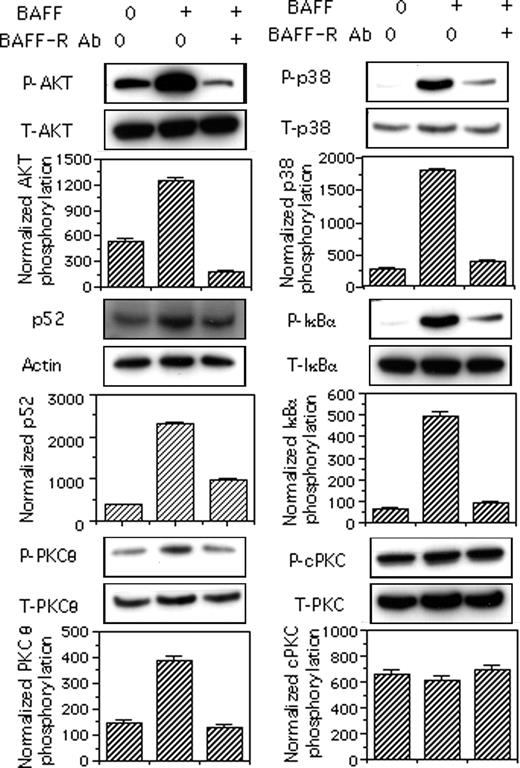
Neutralizing BAFF-R Ab abolishes BAFF-induced phosphorylation of AKT, p38MAPK, and IκBα, and p52 production
To confirm that BAFF-induced phosphorylation of the various effectors described occurred downstream from BAFF-R, we compared the BAFF-induced phosphorylation of these effectors following the incubation of B cells with or without a neutralizing anti–BAFF-R or with an isotype-control Ab. Incubation with the neutralizing anti–BAFF-R Ab but not goat IgG (data not shown) prior to BAFF stimulation totally prevented the phosphorylation of AKT, p38MAPK, IκBα, and PKCθ, and strongly decreased BAFF-induced p52 production (Figure 6).
BAFF-induced phosphorylation of p38MAPK, AKT, IκBα, and PKCθ depends on BAFF-R triggering. Mock-treated B cells were incubated for 30 minutes at 4°C with medium or 10 μg/mL anti–BAFF-R Ab and then stimulated with medium or 50 ng/mL BAFF. The p52 contents of total-cell lysates were corrected for actin content. Phosphorylation of AKT, IΚBα, p38MAPK, classical PKC, and PKCθ were each corrected for total relevant protein. Blots from one representative experiment are shown. Values reported for p52 contents and phosphorylation are mean values plus or minus SD from 2 independent experiments.
BAFF-induced phosphorylation of p38MAPK, AKT, IκBα, and PKCθ depends on BAFF-R triggering. Mock-treated B cells were incubated for 30 minutes at 4°C with medium or 10 μg/mL anti–BAFF-R Ab and then stimulated with medium or 50 ng/mL BAFF. The p52 contents of total-cell lysates were corrected for actin content. Phosphorylation of AKT, IΚBα, p38MAPK, classical PKC, and PKCθ were each corrected for total relevant protein. Blots from one representative experiment are shown. Values reported for p52 contents and phosphorylation are mean values plus or minus SD from 2 independent experiments.
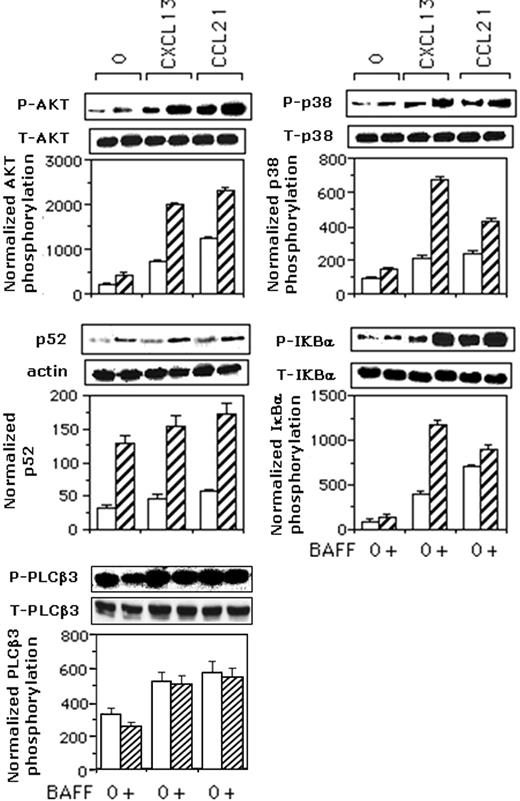
BAFF increases the chemokine-induced phosphorylation of IκBα, p38MAPK, and AKT
We compared the chemokine-induced phosphorylation profiles of various effectors in mock- and BAFF-treated B cells. The production of p52 was similarly increased in BAFF-treated and mock-treated cells, independent of their stimulation by medium (4.3-fold) or by chemokines (3.4- and 3.1-fold in the presence of CXCL13 and CCL21, respectively; Figure 7). In contrast, CXCL13- and CCL21-induced phosphorylation of IκBα were differently increased by B-cell pretreatment with BAFF (by 3- and 1.3-fold, respectively). BAFF also increased the CXCL13- and CCL21-induced phosphorylation of AKT by 2.8- and 1.9-fold and of p38MAPK by 3.2- and 1.8-fold, respectively. BAFF- and CCL21-induced phosphorylation of p38MAPK and IκBα were mostly additive; there was a synergistic effect between BAFF- and CXCL13-induced phosphorylation. BAFF did not modulate the chemokine-induced phosphorylation of PLCβ3.
BAFF increases CXCL13- and CCL21-induced phosphorylation of PI3K/AKT, p38MAPK, and IκBα. Mock- and BAFF-treated B cells were stimulated for 2 minutes with medium, 100 ng/mL CXCL13, or 100 ng/mL CCL21. Phosphorylation of AKT, p38MAPK, PLCβ3, and IκBα was corrected for total relevant protein. The p52 contents in total-cell lysates were corrected for actin contents. Blots from one representative experiment are shown. Values reported for p52 contents and phosphorylation of AKT, p38MAPK, and IκBα are mean values plus or minus SD from 3 or 2 (PLCβ3) independent experiments.
BAFF increases CXCL13- and CCL21-induced phosphorylation of PI3K/AKT, p38MAPK, and IκBα. Mock- and BAFF-treated B cells were stimulated for 2 minutes with medium, 100 ng/mL CXCL13, or 100 ng/mL CCL21. Phosphorylation of AKT, p38MAPK, PLCβ3, and IκBα was corrected for total relevant protein. The p52 contents in total-cell lysates were corrected for actin contents. Blots from one representative experiment are shown. Values reported for p52 contents and phosphorylation of AKT, p38MAPK, and IκBα are mean values plus or minus SD from 3 or 2 (PLCβ3) independent experiments.
Discussion
BAFF has a major role in B-cell maturation and survival, and we show here that it also enhances the chemotactic response of mature B cells to CXCL12, CXCL13, and CCL21. This effect is dose dependent, reaching a maximum at 25 ng/mL of BAFF. Like the CD40L-induced modulation of B-cell chemotaxis, the effect of BAFF is transitory and peaks at around 16 to 24 hours.37 Although a short period of incubation in medium (mock treatment) progressively increases the chemotaxis of freshly isolated B cells,37,39,41 25 ng/mL of BAFF further increases B-cell chemotaxis by 1.6-fold (CXCL12, CCL21) or 2.4-fold (CXCL13) after 16 hours of incubation. A comparable increase in CXCL13- and CCL21-mediated chemotaxis was observed in the presence of CD40MGL. Consistent with BAFF-R being the major receptor for BAFF on mature B cells, the BAFF-mediated increase in B-cell chemotaxis is totally abolished by the addition of a neutralizing anti–BAFF-R Ab. In agreement with previous work,12 BAFF-R was similarly expressed on naive and memory B cells (data not shown), and its expression was slightly increased by incubation. Accordingly, BAFF binding to freshly isolated B cells was substantial and only increased slightly after B-cell incubation. BCMA was barely detectable both before and after the incubation period, whereas the expression of TACI was up-regulated during incubation. The binding of APRIL increased substantially during incubation but remained 10-fold lower than that of BAFF. Despite this increased binding, APRIL does not modulate B-cell chemotaxis, suggesting differences in the signaling pathways downstream from BAFF-R and TACI. Although the percentage of naive and memory B cells was not modified by the incubation, the BAFF-mediated increase of CXCL13-dependent chemotaxis was greater for memory B cells than for naive B cells. This phenomenon was observed despite the fact that memory and naive B cells similarly express CXCR5, TACI, and BAFF-R (data not shown). This difference in sensitivity between memory and naive B cells was, however, specific to CXCL13: BAFF similarly increased the chemotactic response of naive and memory B cells to CXCL12 and CCL21. These findings are reminiscent of data reported by Roy et al showing that a 16-hour incubation with CD40L specifically increases the CXCL13-mediated chemotaxis of memory B cells.37,42 Similarly, we have previously described a hyper-responsiveness to CCL20 of memory B cells relative to naive B cells,40 although they express similar levels of CCR6, and a preferential relative increase in the chemotaxis to CCL20, CXCL12, and CCL21 of type I IFN-treated memory B cells.41 The similarities between the BAFF and CD40L signaling pathways are well known, so the same effectors may be responsible for the preferential enhancement of CD40L- and CXCL13-induced chemotaxis in memory B cells.
CXCL13 is mainly produced by stromal cells and follicular dendritic cells (FDCs) in normal tissue and plays a unique role both in B-cell entry into the lymphoid follicles36 and in the GC organization during the TD response.45 CCL19 and CCL21 are mainly produced by dendritic cells within T-cell zones, and the ratio between CCL19/21 and CXCL13 is determinant for the relocalization of naive B cells during the initiation phase of the humoral response.45 As BAFF similarly increases the chemotactic response of naive B cells to CXCL13 and CCL21, it is unlikely that BAFF impedes their relocalization, although it may speed up their entry into lymphoid organs. In contrast, by increasing the chemotactic response of memory B cells to CXCL13 over that to CCL21, BAFF might favor their sequestration within follicles or their re-entry into follicles during a secondary response. The synergy between BAFF and CXCL13 might also favor the recruitment of pathological B cells and their sequestration in follicle-like structures during autoimmune diseases where CXCL13 and BAFF are simultaneously overproduced.43 Interestingly, patients with Sjögren syndrome have reduced numbers of memory B cells in the blood, whereas these cells accumulate in the salivary glands.46
The BAFF-mediated increase of B-cell chemotaxis does not correlate with the increase in CXCR4, CXCR5, or CCR7 expression or with the impairment of chemokine-induced receptor internalization. This suggests that BAFF/BAFF-R interactions modulate signaling downstream from the chemokine receptors. Using various inhibitors of downstream effectors, we show that chemokines induce the activation of PLCβ3, PI3K/AKT, RhoA/ROCKI, IκBα, p38MAPK, and ERK1/2 in primary B cells. All but p38MAPK and ERK1/2 are crucial for B-cell chemotaxis.41 BAFF modifies the requirements for B-cell chemotaxis, such that it becomes dependent on p38MAPK and more dependent on PI3K/PDK1, RhoA/ROCKI, and NF-κB1 (Figure 4). In contrast, BAFF does not modify the involvement of Gαi, PLC, or PKC in B-cell chemotaxis, and this is consistent with the observation that BAFF does not stimulate the heterotrimeric G proteins or the types of PLC involved in chemotaxis (ie, PLCβ; Figure 5). However, we observed that BAFF/BAFF-R interactions induce the phosphorylation of PLCγ2 in primary B cells. This agrees with data from Hikida et al showing that NF-κB is not activated downstream from BAFF-R in PLCγ2-deficient mice.47
The BAFF-mediated activation of p38MAPK was totally abolished by the presence of a BAFF-R–neutralizing mAb, demonstrating the requirement for interaction between BAFF and BAFF-R. Consistent with the involvement of p38MAPK in the BAFF-induced increase of B-cell chemotaxis, preincubation with SB203580, an inhibitor of p38MAPK activity, inhibited the BAFF-mediated enhancement of chemotaxis by 50% (Figure 4). The involvement of p38MAPK activity in the chemotaxis of BAFF-treated cells but not of mock-treated cells is puzzling because both BAFF and chemokines induce its phosphorylation. However, these 2 pathways may have subcellular distributions, leading to the phosphorylation of a different set of effectors, as previously reported for ERK.41,48 In addition, we show that BAFF, through the triggering of BAFF-R, induces the activation of the PI3K/PDK1 pathway (evidenced by AKT and PKCθ phosphorylation) and the phosphorylation of IκBα. In the light of recent studies of TRAF6-dependent PI3K recruitment downstream from CD40,49 our results suggest that a similar mechanism occurs downstream from BAFF-R. Patke et al also report the activation of AKT downstream from BAFF-R in mice.50 As a consequence, activated PI3K/PDK1 may participate in the activation of RhoA/ROCK I and IκBα. Whereas 100 nM WM and SB203580 added separately inhibited the effect of BAFF on B-cell chemotaxis by 57% to 62%, their coaddition led to stronger inhibition (86% or greater), indicating that these 2 pathways are at least partially independent. In agreement with previous findings, BAFF-R triggering led to the activation of NF-κB1 and the processing of NF-κB2 (p100) to p52 in resting B lymphocytes.4 Indeed, addition of a neutralizing anti–BAFF-R Ab decreased both the phosphorylation of IκBα and the production of p52 protein. Data obtained in the presence of SN50 and MG132 (a proteasome inhibitor; data not shown) also indicate that NF-κB participates in the BAFF-mediated enhancement of B-cell chemotaxis. Therefore, PI3K/PDK1, p38MAPK, and NF-κB pathways appear to act in concert to mediate the BAFF-induced effect on B-cell chemotaxis.
Our study demonstrates a previously unreported role for BAFF in stimulating B-cell chemotaxis and reveals a particular synergy between CXCL13 and BAFF on memory B cells. In physiologic conditions, this BAFF-dependent effect might favor the sequestration or re-entry of memory B cells into follicles during primary or secondary immune responses, respectively. During autoimmune diseases (systemic lupus erythematosis, Sjögren syndrome, or rheumatoid arthritis), this synergy might favor the formation of ectopic follicles. This work also improves our knowledge of signaling pathways downstream from BAFF-R and, for the first time, evidences the BAFF-mediated activation of p38MAPK and PI3K/PDK1 in primary human B cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr G. Gras for critical reading of the manuscript.
This work was supported by grants from Inserm and the Fondation de la Recherche Médicale (FRM). G. Borhis is supported by a SIDACTION fellowship.
Authorship
Contribution: G. Badr and G. Borhis performed the research and wrote the paper. E.A.L., N.C., F.D., V.D., and G.L. participated in research. A.T. and Y.R. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yolande Richard, Service of Immuno-Virology, CEA, DSV/iMETI, 18 route du Panorama, 92260 Fontenay-aux-Roses, France; e-mail: yolande.richard-clausse@cea.fr.
References
Author notes
G. Badr and G. Borhis contributed equally to this work.







 ) APRIL, and were then tested for migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, or 500 ng/mL CXCL13. Basal migration of B cells was 5% (± 1.4%, medium-treated), 4.7% (± 1.1%, 25 ng/mL BAFF–treated), 5.4% (± 0.5%, 100 ng/mL BAFF–treated), 4.3% (± 2%, 25 ng/mL APRIL–treated), and 5% (± 3%, 100 ng/mL APRIL–treated) Results are expressed as the mean percentage plus or minus SD of cells specifically migrating in response to each chemokine. (B) B cells were incubated for up to 48 hours with medium or 25 ng/mL BAFF. Mock-treated (□) and BAFF-treated (■) B cells were analyzed for migration to 500 ng/mL CXCL13. The experiment was performed using samples from 4 different donors, and basal migration ranged from 3% to 4% and 3.3% to 4.7% for mock- and BAFF-treated B cells, respectively. Results are expressed as the percentage plus or minus SD of specific chemotaxis. *P < .05; **P < .005. (C) B cells were treated for 16 hours with medium (□) or 25 ng/mL BAFF (▫) and analyzed for their migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, and 500 ng/mL CXCL13. Basal migration was 4.4% (± 1.2%) and 4.3% (± 1.1%) for mock- and BAFF-treated B cells, respectively. Results are expressed as the mean percentage plus or minus SD of specifically migrating cells obtained for each donor. *P < .05; **P < .005. (D) B cells were treated for 16 hours with medium (data not shown), 25 ng/mL BAFF (▫), or 100 ng/mL CD40MGL (■) and analyzed for their migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, and 500 ng/mL CXCL13. The experiment was performed using samples from 3 different donors, and basal migration was 3.3% (± 2.1%), 2.3% (± 1.5%), and 1.3% (± 0.6%) for mock-, BAFF-, and CD40MGL-treated B cells, respectively. Results are expressed as mean fold increase plus or minus SD. (E) The percentage of B-cell recovery after overnight culture with medium or 25 ng/mL BAFF was calculated for each donor. Results are expressed as median values obtained from 28 independent experiments. Error bars correspond to 95% confidence intervals about the median. (F) B cells from 3 different donors were incubated for 16 hours at 37°C with medium (□) or 25 ng/mL BAFF (▫) and then incubated for 60 minutes at 37°C with medium or 100 ng/mL chemokines. Cells were washed in ice-cold medium and stained with PE-conjugated CCR7, CXCR4, or CXCR5 mAb for 30 minutes at 4°C. The mean channel fluorescence intensity (MFI) values for mock-treated cells were between 57 and 123 for CCR7, 356 and 1067 for CXCR4, and 64 and 81 for CXCR5. Each of these individual values was considered as 100% expression. Data are expressed as the mean plus or minus SD percentage of MFI values for remaining surface expression.
) APRIL, and were then tested for migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, or 500 ng/mL CXCL13. Basal migration of B cells was 5% (± 1.4%, medium-treated), 4.7% (± 1.1%, 25 ng/mL BAFF–treated), 5.4% (± 0.5%, 100 ng/mL BAFF–treated), 4.3% (± 2%, 25 ng/mL APRIL–treated), and 5% (± 3%, 100 ng/mL APRIL–treated) Results are expressed as the mean percentage plus or minus SD of cells specifically migrating in response to each chemokine. (B) B cells were incubated for up to 48 hours with medium or 25 ng/mL BAFF. Mock-treated (□) and BAFF-treated (■) B cells were analyzed for migration to 500 ng/mL CXCL13. The experiment was performed using samples from 4 different donors, and basal migration ranged from 3% to 4% and 3.3% to 4.7% for mock- and BAFF-treated B cells, respectively. Results are expressed as the percentage plus or minus SD of specific chemotaxis. *P < .05; **P < .005. (C) B cells were treated for 16 hours with medium (□) or 25 ng/mL BAFF (▫) and analyzed for their migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, and 500 ng/mL CXCL13. Basal migration was 4.4% (± 1.2%) and 4.3% (± 1.1%) for mock- and BAFF-treated B cells, respectively. Results are expressed as the mean percentage plus or minus SD of specifically migrating cells obtained for each donor. *P < .05; **P < .005. (D) B cells were treated for 16 hours with medium (data not shown), 25 ng/mL BAFF (▫), or 100 ng/mL CD40MGL (■) and analyzed for their migration to 250 ng/mL CCL21, 250 ng/mL CXCL12, and 500 ng/mL CXCL13. The experiment was performed using samples from 3 different donors, and basal migration was 3.3% (± 2.1%), 2.3% (± 1.5%), and 1.3% (± 0.6%) for mock-, BAFF-, and CD40MGL-treated B cells, respectively. Results are expressed as mean fold increase plus or minus SD. (E) The percentage of B-cell recovery after overnight culture with medium or 25 ng/mL BAFF was calculated for each donor. Results are expressed as median values obtained from 28 independent experiments. Error bars correspond to 95% confidence intervals about the median. (F) B cells from 3 different donors were incubated for 16 hours at 37°C with medium (□) or 25 ng/mL BAFF (▫) and then incubated for 60 minutes at 37°C with medium or 100 ng/mL chemokines. Cells were washed in ice-cold medium and stained with PE-conjugated CCR7, CXCR4, or CXCR5 mAb for 30 minutes at 4°C. The mean channel fluorescence intensity (MFI) values for mock-treated cells were between 57 and 123 for CCR7, 356 and 1067 for CXCR4, and 64 and 81 for CXCR5. Each of these individual values was considered as 100% expression. Data are expressed as the mean plus or minus SD percentage of MFI values for remaining surface expression.