MicroRNAs (miRNAs) are negative regulators of gene expression that play an important role in hematopoiesis and tumorigenesis. We analyzed miRNA expression in classic Hodgkin lymphoma (cHL) and the influence of Epstein-Barr virus (EBV) infection on the miRNA expression profiles. The expression of 157 miRNAs in lymph nodes from 49 cHL patients and 10 reactive lymph nodes (RLNs) was analyzed by real-time polymerase chain reaction (PCR). Hierarchic clustering revealed 3 well-defined groups: nodular sclerosis cHL, mixed cellularity cHL, and RLNs. A distinctive signature of 25 miRNAs differentiated cHL from RLNs, and 36 miRNAs were differentially expressed in the nodular sclerosis and mixed cellularity subtypes. These results were validated in a set of 30 cHLs and 5 RLNs, and in 3 cHL cell lines. miR-96, miR-128a, and miR-128b were selectively down-regulated in cHL with EBV. Our findings suggest that miRNAs play an important role in the biology of cHL and may be useful in developing therapies targeting miRNAs.
Introduction
Mature microRNAs (miRNAs) are naturally occurring small noncoding RNAs that act as negative regulators of gene expression through messenger RNA interference. These molecules were described for the first time in 1993 by Ambros and colleagues in Caenorhabditis elegans (Lee et al1 ), and to date, hundreds of miRNAs have been identified in other species, including viruses.2,3 miRNAs are encoded by intronic or intergenic DNA regions, primarily as large molecules that can exceed 1 Kb, and are cleaved by an RNase complex into fragments with characteristic stem-loop structures. In the cytoplasm, a RNase called Dicer further cleaves miRNA to generate a duplex molecule of 21 to 25 nucleotides in length.4 One of the 2 chains is the mature miRNA that binds a protein complex called the RNA-induced silencing complex (RISC). When a miRNA and a messenger RNA exhibit total complementarities, RISC is capable of degrading target messenger RNA,4 whereas if an incomplete base pairing complementarity takes place, translational silencing of the target occurs. Through these mechanisms, miRNAs decrease translation of human genes.5,6
miRNAs play an important role in cellular proliferation and differentiation and embryonic development, and they also act as oncogenes or tumor suppressor genes.7,,–10 Notably, the majority of miRNAs are found in cancer-associated genomic regions or in chromosome-fragile sites,11 suggesting an important role for miRNAs in human tumorigenesis. There is also evidence that the influence of miRNAs in oncogenesis might be indirectly driven. For example, the presence of some viruses in a cell may change the host miRNA pattern.12 Viruses may participate in the origin of some tumors, such as the Epstein-Barr virus (EBV) in Hodgkin lymphoma (HL).
HL is a neoplasm characterized by the presence of relatively few tumoral cells (Hodgkin and Reed-Sternberg cells) in a nonneoplastic microenvironment.13 Hodgkin and Reed-Sternberg cells arise from germinal center B cells.14 Classic HL (cHL) is subclassified according to the morphology of Reed-Sternberg cells and the composition of the cellular background into nodular sclerosis, mixed cellularity, lymphocyte-rich, and lymphocyte depletion.15 The 2 former subtypes are the most frequent forms of cHL and contain a variable proportion of neoplastic cells.
EBV is present in the malignant cells of 40% to 60% of cHL patients. However, the precise role of the EBV in the pathogenesis of cHL is unknown. It has been reported that viruses have their own miRNA set,16 and that there is an interaction between the host miRNAs and virus miRNAs.17,18 The interaction between the virus and the malignant cells in cHL might be mediated in part by miRNAs.
To investigate whether a specific expression signature of miRNAs is associated with cHL, we assessed the expression of 156 miRNAs, the majority of which are related to hematopoiesis or tumorigenesis,7,8,11 in lymph nodes from patients with nodular sclerosis and mixed cellularity cHL and compared the expression patterns with those in reactive lymph nodes (RLNs). We also examined the influence of EBV on the expression pattern of miRNAs in cHL patients.
Methods
Approval for these studies was obtained from the Institutional Review Board of Hospital Clinic, Barcelona. Informed consent was obtained in accordance with the Declaration of Helsinki.
Tissue samples and cell lines
Forty-nine specimens of formalin-fixed paraffin-embedded lymph nodes from patients diagnosed with cHL (37 nodular sclerosis and 12 mixed cellularity) between January 1996 and June 2005 were assessed. All patients were diagnosed and followed up in a single institution. Table 1 shows the clinical and biologic characteristics of the patients. Ten RLNs were used as controls. In addition, a validation set of 30 formalin-fixed paraffin-embedded lymph nodes from cHL patients (22 nodular sclerosis and 8 mixed cellularity) and 5 RLNs were analyzed. Finally, human HL cell lines L-428 and HD-MY-Z (nodular sclerosis) and L-1236 (mixed cellularity) were analyzed to discriminate between miRNAs expressed in the cell and those present only in the microenvironment. The cell lines were cultured in RPMI 1640 containing 20% fetal calf serum (Invitrogen, Paisley, United Kingdom).
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed paraffin-embedded tissue sections, as previously described.19 Briefly, paraffin sections on silane-coated slides were dewaxed and subjected to antigen retrieval (Target Retrieval Solution; Dako, Carpinteria, CA) in a microwavable pressure cooker. Primary antibodies against CD20 (L26; Dako), CD5 (NCL-CD5; Novocastra, Newcastle-upon-Tyne, United Kingdom), CD30 (BerH2; Dako), and CD15 (Leu M1; Novocastra) were incubated, and the slides were counterstained in Gill hematoxylin and mounted in Pertex (Histolab, Gothenburg, Sweden).
RNA extraction, reverse transcription, and real-time polymerase chain reaction (PCR) quantification
Total RNA was extracted from 49 formalin-fixed paraffin-embedded cHL lymph nodes and from 10 RLNs, using RecoverAll Total Nucleic Acid Isolation (Applied Biosystems, Foster City, CA) as per the manufacturer's protocol. The same methods were used for RNA extraction in the validation data set of 30 cHL lymph nodes and 5 RLNs. RNA was extracted from the 3 cell lines using Trizol total RNA isolation reagent (Invitrogen, Carlsbad, CA) as per the manufacturer's protocol.
cDNA was synthesized from total RNA using gene-specific primers of 156 different mature miRNAs (TaqMan MicroRNA Assay Protocol, Early Access Kit; Applied Biosystems; Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Real-time PCR was performed using an Applied Biosystems 7500 Sequence detection system.
miRNA analysis by chromogenic in situ hybridization
Fluorescein (FITC) 5′-labeled locked-nuclei-acid (LNA)–incorporated miRNA ribo probes for miR-21, miR-134, miR-138, and miR-155 (miRCURY LNA detection; Exiqon, Woburn, MA) were used in formalin-fixed, paraffin-embedded tissue sections on silane-coated slides (Vision BioSystem, Mount Waverley, Australia). Chromogenic in situ hybridization was performed in an automated platform Bond Max (Vision BioSystems). Slides were pretreated with protease 1 for 10 minutes at 37°C. A total amount of 300 μL 25-nM probe was hybridized in 1× sodium chloride–sodium citrate hybridization buffer (SSC) (Innogenetics, Gent, Belgium) up to 50°C for 2 hours. We used a prediluted mouse anti-FITC antibody (Vision BioSystems) for 20 to 60 minutes followed by a biotin-free, polymeric horseradish peroxidase (HRP)–linker antibody conjugate system (Refine Detection System; Vision BioSystems). DAB was used as a chromogen reacting for 10 minutes and hematoxylin was used as a counterstain.
EBV analysis
The presence of EBV in cHL lymph nodes was examined by in situ hybridization for EBV RNA in an automated platform BenchMark XT (EBER 1 and 2, Inform EBER; Ventana Medical Systems, Tucson, AZ), and real-time PCR using specific primers and probe for the highly conserved segment BamH1W of EBV.20
Statistical analysis
miRNA expression data were normalized by 2 different approaches: global-median normalization and let7-a miRNA as previously described.21 Data were analyzed using BRB Array Tools version 3.5.0 software (Biometric Research branch, National Cancer Institute, National Institutes of Health; http://linus.nci.nih.gov/BRB-ArrayTools.html) and TIGR Multiexperiment viewer version 4.0 software (Dana-Farber Cancer Institute, Boston, MA; http://www.tm4.org). Hierarchic clustering was performed using average linkage and Euclidean distance. To identify miRNAs with significant differential expression between the 2 histologic subgroups and those that might be influenced by the presence of EBV, 2 multivariate permutation tests were performed: significance analysis of microarrays (SAM) and Student t test based on multivariate permutation (with random variance model). Differences between miRNAs were considered statistically significant if the P value was less than .001. The prediction analysis of microarrays (PAM) and class prediction methods (BRB Array Tools) were used to determine a set of miRNAs able to classify the samples into cHL and RLNs and to differentiate between the 2 histologic subgroups. To identify functional interactions of putative target genes of miRNAs, obtained from Mirbase,22 TargetScan,23 and Tarbase24 databases, we used DAVID database25 and GENIG (ALGGEN; Technical University of Catalonia, Barcelona, Spain; http://alggen.lsi.upc.edu).
Results
miRNA patterns in cHL patients and in RLNs
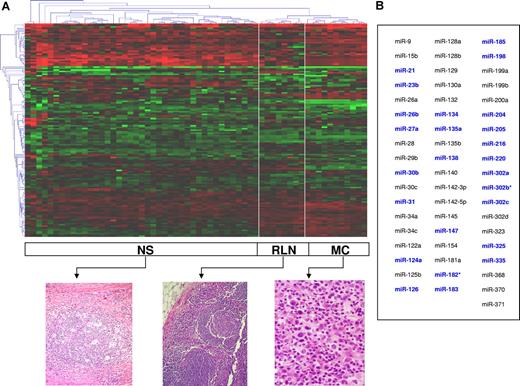
Unsupervised hierarchic clustering of the cHL and RLN samples was performed on the entire set of unfiltered data. The heat map of miRNA expression categorized 3 well-defined clusters corresponding to nodular sclerosis cHL, mixed cellularity cHL, and RLNs (Figure 1). Thirty-eight miRNAs were differentially expressed in cHL versus RLNs (Figure 2; Table S2), and a set of 55 miRNAs was able to further classify all samples into nodular sclerosis cHL, mixed cellularity cHL, or RLNs. (Figure 1). A specific miRNA expression signature consisting of 25 of these 55 miRNAs precisely differentiated between cHLs and RLNs (Figure 1; Table 2). In addition, 36 miRNAs were differentially expressed in nodular sclerosis and mixed cellularity subtypes (Figure 2). These findings were confirmed in the validation set of 30 cHLs and 5 RLNs (Figure S1).
miRNA expression pattern in cHL. (A) Unsupervised hierarchic cluster analysis categorized 3 clusters corresponding to nodular sclerosis (NS), mixed cellularity (MC), and reactive lymph nodes (RLNs). A corresponding typical histology is shown. The data were presented as log10 of relative quantification normalized in regard to global median and relative to the 10 reactive lymph node median as calibration method. (B) A set of 55 miRNAs classified all samples into NS, MC, or RLN (PAM analysis). In blue, the 25 miRNAs comprising the miRNA expression signature capable of distinguishing between cHLs and RLNs.
miRNA expression pattern in cHL. (A) Unsupervised hierarchic cluster analysis categorized 3 clusters corresponding to nodular sclerosis (NS), mixed cellularity (MC), and reactive lymph nodes (RLNs). A corresponding typical histology is shown. The data were presented as log10 of relative quantification normalized in regard to global median and relative to the 10 reactive lymph node median as calibration method. (B) A set of 55 miRNAs classified all samples into NS, MC, or RLN (PAM analysis). In blue, the 25 miRNAs comprising the miRNA expression signature capable of distinguishing between cHLs and RLNs.
Differential expression of miRNAs in cHL subtypes and in reactive lymph nodes. (A) Nodular sclerosis (NS) versus reactive lymph nodes (RLN). (B) Mixed cellularity (MC) versus reactive lymph nodes. (C) NS versus MC. (D) All cHL cases (NS and MC) versus RLN. miRNAs overexpressed (in red) or underexpressed (in green) are shown in boxes (SAM analysis).
Differential expression of miRNAs in cHL subtypes and in reactive lymph nodes. (A) Nodular sclerosis (NS) versus reactive lymph nodes (RLN). (B) Mixed cellularity (MC) versus reactive lymph nodes. (C) NS versus MC. (D) All cHL cases (NS and MC) versus RLN. miRNAs overexpressed (in red) or underexpressed (in green) are shown in boxes (SAM analysis).
Analysis of miRNA expression in human HL cell lines
cHL tumors are composed of different reactive cell types and tumor cells represent a minority. To elucidate whether different signatures might discover different tumor compositions rather than be specific for tumor cells, we analyzed the 25-miRNA signature that discriminated between cHLs and RLNs in 3 different human HL cell lines: L-428, HD-MY-Z, and L-1236 (Figure 3). We found that 20 of the 25 miRNAs analyzed were expressed by the human HL cell lines and were therefore likely to have been expressed by the tumor cells rather than the microenvironment. The 5 miRNAs (miR-220, miR-302a, miR-302b, miR-302c, and miR-325) that were not expressed in the cell lines but were overexpressed in the cHL cases may have been expressed by the reactive microenvironment. MiR-21, miR-27a, miR-147, miR-182, miR-183, and miR-216 were the most strongly up-regulated miRNAs in the HL cell lines (Figure 3A).
Analysis in human Hodgkin lymphoma (HL) cell lines: L-428 and HD-MY-Z (nodular sclerosis) and L-1236 (mixed cellularity). (A) 25-miRNA signature. This figure shows the log2 of fold change (−ΔΔCt) of the 13 miRNAs most differently expressed in the cell lines. The dotted line indicates a 4-fold difference in expression compared with the mean of expression of reactive lymph nodes. (B) Thirty-two of the 36 miRNAs differentially expressed in lymph nodes of nodular sclerosis and mixed cellularity subtypes were also differentially expressed in the cell lines. Panel B depicts only the 11 miRNAs with at least a 2-fold difference in expression between the 2 subtypes. Error bars represent SD.
Analysis in human Hodgkin lymphoma (HL) cell lines: L-428 and HD-MY-Z (nodular sclerosis) and L-1236 (mixed cellularity). (A) 25-miRNA signature. This figure shows the log2 of fold change (−ΔΔCt) of the 13 miRNAs most differently expressed in the cell lines. The dotted line indicates a 4-fold difference in expression compared with the mean of expression of reactive lymph nodes. (B) Thirty-two of the 36 miRNAs differentially expressed in lymph nodes of nodular sclerosis and mixed cellularity subtypes were also differentially expressed in the cell lines. Panel B depicts only the 11 miRNAs with at least a 2-fold difference in expression between the 2 subtypes. Error bars represent SD.
Moreover, we analyzed the 36 miRNAs that were differentially expressed in the nodular sclerosis and mixed cellularity subtypes and found that 32 were expressed in the cell lines; miR-122a, miR-154, miR-302d, and miR-371 were not expressed in the cell lines, suggesting differences in the reactive microenvironment. MiR-34a, miR-128b, miR-129, and miR-200a were the most strongly differentially expressed miRNAs between L-428 and HD-MY-Z nodular sclerosis HL cell lines versus L-1236 mixed cellularity HL cell line.
miRNA analysis by chromogenic in situ hybridization
To examine whether miRNAs that were more highly expressed in cHLs than in RLNs were detected preferentially in tumor or reactive cells, we analyzed 20 cHL lymph nodes using highly sensitive chromogenic in situ hybridization. We first analyzed miR-155 as a positive control, as previously described in cHL.26 We observed a cytoplasmic signal in Hodgkin and Reed-Sternberg cells, as well as in scattered reactive lymphocytes and activated histiocytes, as recently reported27 (Figure 4). Based on functional and target analyses and on the chromosomal locations of the miRNAs (Table 2), we selected 3 miRNAs with a potential role in tumorigenesis and analyzed them by chromogenic in situ hybridization in the 20 cases: miR-21 (validated target PTEN, encoded in 17q32.228 ); miR-134 (putative target J-chain, encoded in 14q32.3128 ); and miR-138 (putative target PU.1, encoded in 7q32.229 ). In all 20 cases, a cytoplasmic signal was observed in Hodgkin and Reed-Sternberg cells, and we also observed a certain degree of miRNA expression in surrounding lymphocytes. Moreover, a nuclear signal was identified in some reactive tumor-infiltrating lymphocytes for miR-21 and miR-138 (Figure 4).
Chromogenic in situ hybridization of cHL cases. Cytoplasmatic expression in Hodgkin and Reed-Sternberg cells of miR-21 (A), miR-134 (B), miR-138 (C), and miR-155 (D). miR-155 was used as positive control of the hybridization technique. (Microscope, Olympus BX51 [Olympus, Center Valley, PA]; camera, Olympus DP70; lens, UPIanFI 40×/0.75; software, Olympus DP Controller.)
Chromogenic in situ hybridization of cHL cases. Cytoplasmatic expression in Hodgkin and Reed-Sternberg cells of miR-21 (A), miR-134 (B), miR-138 (C), and miR-155 (D). miR-155 was used as positive control of the hybridization technique. (Microscope, Olympus BX51 [Olympus, Center Valley, PA]; camera, Olympus DP70; lens, UPIanFI 40×/0.75; software, Olympus DP Controller.)
Effect of EBV infection on miRNA expression in cHL
Ten miRNAs were differentially expressed in EBV+ cHL compared with EBV− cHL. In EBV+ cases, miR-96, miR-128a, miR-128b, miR-129, and miR-205 were underexpressed and miR-28, miR-130b, miR-132, miR-140, and miR-330 were overexpressed (Figure 5). In the subgroup of nodular sclerosis cHL, 3 miRNAs (miR-96, miR-128a, and miR-128b) were significantly underexpressed in EBV+ cHL compared with EBV− cHL. All but one of the mixed cellularity cHLs were EBV+.
Association between miRNA expression and clinical parameters
We analyzed a possible association of miRNA expression with clinical and biologic patient characteristics: age, sex, B symptoms, bulky mass, anemia, leukocytosis, lymphocytopenia, hypoalbuminemia, ESR, LDH level, β-2-microglobulin level, extranodal involvement, stage, Hasenclever index, positivity for CD15, positivity for CD20, and density of tumoral cells and T lymphocytes in the tumor microenvironment. We found that miR-138 was overexpressed in Ann Arbor stage I-II disease (P = .003), while miR-328 was overexpressed in Ann-Arbor stage III-IV disease (P = .004). To further investigate this possible association between miRNA expression and disease stage, we analyzed the expression of miR-138 and miR-328 in 30 additional patients from the validation data set (18 stage I-II; 12 stage III-IV). When results for both the original set and the validation set were combined, we found that miR-138 was overexpressed in stage I-II disease but not in stage III-IV (P = .001; Figure S2), but no association was observed between miR-328 expression and disease stage.
Discussion
Prior studies have shown that a small subset of miRNAs may define tumor entities better than microarray expression data from thousands of messenger RNAs.30 In the present study, we have characterized for the first time a 25-miRNA signature that can differentiate between cHLs and RLNs. In addition, a small number of miRNAs were differentially expressed in mixed cellularity and nodular sclerosis subtypes. Finally, overexpression of one miRNA (miR-138) was related with Ann Arbor stage I-II cHL. miR-138 has been reported to be overexpressed in other tumors,31 where it seems to be associated with an undifferentiated state.32
The differential miRNA expression observed between cHLs and RLNs may be explained by cytogenetic changes in the Hodgkin and Reed-Sternberg cells. The genomic region 17q has previously been associated with frequent gains in cHL,28 and miR-21 is encoded in this chromosomic region (Table 2). Other previously described chromosomal gains in cHL28 include 2p, where mirR-216 is encoded, 22q, where miR-185 is encoded, and 14q, where miR-134 is encoded. One of the most frequent losses involves 4q, where miR-302a, miR-302b, and miR-302c are encoded,28,33 and 3p, where miR-135a is encoded.34
The analysis of the 25-miRNAs cHL signature in cell lines showed a set of strongly up-regulated miRNAs (miR-21, miR-27a, miR-147, miR-182, and miR-216) and a set of down-regulated miRNAs (miR-126, miR-135a, and miR-204) supporting our observations in patient samples. However, other miRNAs showed a different expression pattern in the patient samples compared with the cell lines. Those differences could be due to the expression of miRNAs by the reactive microenvironment in patient samples or to the molecular and chromosomic alterations of cell lines produced by the immortalization process.35
Chromogenic in situ hybridization showed a preferential expression of miR-21, miR-134, and miR-138 in the cytoplasm of Hodgkin and Reed-Sternberg cells, suggesting that miRNA silencing may be biologically relevant in cHL tumor cells; moreover, the miRNA expression observed in lymphocytes provides some evidence that miRNA expression in the tumor microenvironment is also important to the biology and clinical behavior of cHL.13,36,37 The cHL tumor microenvironment differs among the histologic subtypes, and in the present study, a differential pattern of miRNA expression was observed in mixed cellularity and nodular sclerosis subtypes. The subsequent analysis of cell lines revealed that some miRNAs were also differentially expressed in the Hodgkin and Reed-Sternberg cells in the 2 histologic subtypes. Although prior studies had shown that miR-155 is overexpressed in cHL,26 the molecular signature in the present study does not include miR-155. However, chromogenic in situ hybridization showed that miR-155 was constantly expressed in atypical cells in all the cHL cases analyzed as well as in reactive lymphocytes and activated macrophages.26,27
Since miRNAs differentially expressed in cHL cases target putative and validated genes involved in survival, apoptosis, and B-cell functions, our findings can provide the basis for a better understanding of the complex mechanisms of transformation of a normal B cell into a tumor cell. One of the most striking features of cHL tumor cells is their acquisition of survival advantages while largely lacking expression of most of the B cell–associated genes.14,38,39 miR-21, which was overexpressed both in cHL lymph nodes and in the human HL cell lines, has been reported to favor cell survival by indirect up-regulation of antiapoptotic genes.40,,,–44 cHL is a neoplasm of B cells characterized by an incomplete B-cell phenotype, due to down-regulation of some transcription factors crucial to the full development of a mature B-cell program.14 The mechanisms causing down-regulation of the transcriptional program in cHL cells are not totally understood, but some epigenetic events are proving to be important in the silencing of B-cell genes.45 miRNAs may be a new regulatory epigenetic event, with an important role regulating the translation of a number of different genes in the complicated regulatory network that leads normal B cells from the germinal center46 to Hodgkin and Reed-Sternberg cells.
Prior reports had suggested that virus miRNAs can play an important role in the host-pathogen interaction networks. Moreover, viruses might trigger changes in the host miRNA expression pattern, thus favoring cancer development.12,47,48 We identified a subset of 10 host miRNAs whose expression was influenced by the presence of EBV. The effect of EBV on host miRNAs might explain the reported association of EBV with the clinical course of cHL patients.49 Interestingly, only one of the miRNAs differentially expressed in EBV+ cases was included in the 25-miRNA expression signature differentiating cHL from RLN, leading us to speculate that EBV is not a primary transforming event in cHL, a concept that is also supported by the fact that the majority of the cHL cases were EBV−.49
In summary, cHLs express a characteristic miRNA signature different from that of normal RLNs, with a small number of miRNAs differentially expressed in mixed cellularity and nodular sclerosis and one differentially expressed in early- and advanced-stage disease. Some of these miRNAs were expressed in Hodgkin and Reed-Sternberg cells but not in reactive cells. In addition, EBV influences host miRNA expression in cHL. These findings suggest that miRNAs may play an important role in the biology of cHL, and they may be useful in the development of therapies targeting miRNAs in tumor cells in cHL patients.40,50
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Isabel Sanchez and Misael Nolasco, Centro Nacional de Investigaciones Oncológicas (CNIO), Madrid, for providing the 3 cell lines; to Ingrid Lopez and Dolors Fuster for their excellent histology work; to Silvia Pairet for technical assistance; to Dr Beatriz Zafra from the Spanish Division of Menarini Diagnostics for her support in the situ hybridization development; to Dr Eva Bandres for her support in the data analysis; and to Renee O'Brate for her assistance in writing the paper.
This work was supported in part by RETICS 07 from Instituto de Salud Carlos III, by Program Project grants (BM-05/219) from La Caixa, and by FISS 050209 and FISS 060087 from the Spanish Ministry of Public Health. O.B. is a fellow supported by the Instituto de Salud Carlos III.
Authorship
Contribution: A.N. designed and performed the research, analyzed the data, and wrote the paper; A.G. designed the research, selected cases, analyzed the clinical data, and wrote the paper; A.M. designed and performed the research, analyzed the data, and wrote the paper; A.U.-I. and M.M. designed the research, analyzed the data, and wrote the paper; A.P. and O.B. performed the research and analyzed the data; B.G. analyzed the data and performed the statistical analysis; P.A. and A.L.-G. analyzed the clinical data; R.A. analyzed the data; and E.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mariano Monzo, Department of Human Anatomy and Embryology, School of Medicine, University of Barcelona, Casanova 143, Barcelona 08036 Spain; e-mail: mmonzo@ub.edu.
References
Author notes
A.N. and A.G. contributed equally to this article.



![Figure 4. Chromogenic in situ hybridization of cHL cases. Cytoplasmatic expression in Hodgkin and Reed-Sternberg cells of miR-21 (A), miR-134 (B), miR-138 (C), and miR-155 (D). miR-155 was used as positive control of the hybridization technique. (Microscope, Olympus BX51 [Olympus, Center Valley, PA]; camera, Olympus DP70; lens, UPIanFI 40×/0.75; software, Olympus DP Controller.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/5/10.1182_blood-2007-06-096784/2/m_zh80050815660004.jpeg?Expires=1767819076&Signature=0Vi57WT1~4bFNkqoakDWaTUA0mqpNkpDDezjIF3uIe-nEmXDiYgpfSQW9wIXDKK9qR7TvaD3rqABwmxAXidXVEykL~OE2k21GtR9okjI2EvaxB~aFw1lwCgaPH0uW2LUlW4VTFxocJWv03fls8~n9ZNHKinPZwAXB8S5voniYDYHMXMV044PBfptH0v5Wjfu5RvAeZy55D4vrdZarufEZEZoPC-2GDkgmjH7cpuE58doR81mtmqsZ0t5leonLKqnWrIX49VuqX~MBJpZaT-0fvI-ZsfzG-PDeJjwH5SG~Atz2ojBWR0BMv1fw~QQKRMzg~vTrX0taLS4fGruB163tA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

