Abstract
Identification of Toll-like receptors (TLRs) and their ligands, and tumor necrosis factor–tumor necrosis factor receptor (TNF-TNFR) pairs have provided the first logical, hypothesis-based strategies to molecularly concoct adjuvants to elicit potent cell-mediated immunity via activation of innate and adaptive immunity. However, isolated activation of one immune pathway in the absence of others can be toxic, ineffective, and detrimental to long-term, protective immunity. Effective engineered vaccines must include agents that trigger multiple immunologic pathways. Here, we report that combinatorial use of CD40 and TLR agonists as a cancer vaccine, compared with monotherapy, elicits high frequencies of self-reactive CD8+ T cells, potent tumor-specific CD8+ memory, CD8+ T cells that efficiently infiltrate the tumor-burdened target organ; therapeutic efficacy; heightened ratios of CD8+ T cells to FoxP3+ cells at the tumor site; and reduced hepatotoxicity. These findings provide intelligent strategies for the formulation of multifactorial vaccines to achieve maximal efficacy in cancer vaccine trials in humans.
Introduction
The molecular identification of Toll-like receptors (TLRs) and their ligands, as well as tumor necrosis factor (TNF)–tumor necrosis factor receptor (TNFR) pairs that control adaptive immunity, has provided the first logical, hypothesis-based strategies to molecularly concoct adjuvants that elicit potent cell-mediated immunity. Paralleling TLRs in mobilizing the innate immune response, CD40 and its ligand represent the primary ligand-receptor pair essential for development of the adaptive immune response. Individually, TLR agonists1 and CD40 agonists2-4 have entered clinical trials as adjuvants for eliciting protective immune responses to cancer. Inherent in these monotherapeutic approaches are limited induction of immunity, lack of clinical efficacy and, in some cases, hepatotoxicity.3,4
TLRs are widely expressed on both hematopoietic and nonhematopoietic cells and elicit proinflammatory responses upon receptor engagement. Indeed, use of TLR agonists as solitary adjuvants triggers dendritic cell (DC) maturation, leukocyte migration, and release of chemokines and cytokines, and enhances immunity.5,6 Studies in which TLR agonists have been scrutinized for their ability to induce cross-presentation and antigen-specific CD8+ responses in vivo7 show some level of activity that is minimal compared with that observed when combined with a CD40 agonist.8,9 TLR agonists as unitary adjuvants in murine tumor models have demonstrated marginal efficacy, as reviewed,10 but have proven effective when combined with other vaccine modalities.11-13 Finally, clinical use of a TLR9 agonist in lung cancer trials has been recently suspended due to lack of clinical response.1
Studies from animal models underscore the utility of anti-CD40 (αCD40) as a unitary adjuvant.14,15 We previously demonstrated that the magnitude of immune responses elicited by TLR or CD40 agonists alone is minimal compared with the magnitude of immune responses generated by combined use of CD40 and TLR agonists.8 Less than 1% of the CD8+ T-cell population is antigen specific following immunization with αCD40 alone plus antigen, while extremely high frequencies of antigen-specific CD8+ T cells (> 25% of the total CD8+ T cells) can be generated by the coadministration of TLR and CD40 agonists plus antigen.8 This synergy was observed with all TLR agonists tested (TLR 2,3,4,6,7,9).8 Furthermore, use of CD40 agonists in the absence of any other coactivation signals leads to the early demise of antigen-specific CD8+ T cells16 and has been reported to ablate tumor-specific memory.14 Finally, phase 1/2 clinical trials implementing CD40 agonistic monotherapy have resulted in minimal therapeutic efficacy and dose-limiting toxicities.2,4
The present study comprehensively compares the impact of combination therapy with that of monotherapy on the antigen-specific immune responses to melanoma at the cellular and molecular levels. The studies presented demonstrate the profound utility of CD40 and TLR agonists when combined in an adjuvant platform in a murine model of cancer. The data show that vaccination induces extremely high frequencies of primary and memory self-reactive CD8+ T cells that infiltrate metastatic target organs and control tumor growth. Combination therapy also reduces the ratio of regulatory T cells (Tregs) to CD8+ T cells at the tumor site and allows persistent effector CD8+ T-cell function. Finally, the overt hepatotoxicity induced by CD40 monotherapy is ablated by combination therapy. Our studies show that combinatorial use of CD40 and TLR agonists provides greater therapeutic efficacy with limited toxicity and provides the principles on which to build new multifactorial adjuvants for use in clinical trials.
Methods
Mice and tumor cell lines
Male 6- to 8-week-old C57BL/6 mice were obtained from the National Cancer Institute (Bethesda, MD) and were maintained under pathogen-free conditions. All experiments were approved by the Institutional Animal Care and Use Committee of Dartmouth College. B16.F10 melanoma cells were a kind gift from Mary Jo Turk (Dartmouth-Hitchcock Medical Center, Lebanon, NH) and were maintained in complete medium (RPMI 1640 containing 10% fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM glutamine, and 50 μM 2-mercaptoethanol).
Cell lines, antibodies, and reagents
Mouse monoclonal antibodies (mAbs) to CD8 (53-6.7), CD4 (GK1.5), CD44 (IM7), CD127 (A7R34), CD122 (5H4), IL-2 (JES6–5H4), IFNγ (XMG1.2), FoxP3 (FJK-16s), Granzyme B (16G6), and the isotype control rat IgG2a were purchased from eBioscience (San Diego, CA) as were both brefeldin A and monensin. Anti-CD107a (1D4B) was purchased from BD Pharmingen (San Jose, CA). Anti-TNFα (MP6-XT22) was purchased from Invitrogen (Carlsbad, CA). Recombinant human IL-2 was purchased from Peprotech (Rocky Hill, NJ). Anti-CD40 (FGK45) was purchased from BioExpress (Lebanon, NH). Endotoxin content was less than 1 EU/mg as assessed by a quantitative chromogenic limulus amebocyte lysate kit (QCL 1000; Cambrex, East Rutherford, NJ). The TLR7 agonist S-27609 was a gift from 3M Pharmaceuticals (St Paul, MN) and has been previously described.8 Anti-CD4 (GK1.5), anti-CD8 (2.43), and anti-NK1.1 (PK136) were produced by hybridomas, and bioreactor supernatants were purified using standard methodologies. The H2Kb-restricted class I peptides Ova(257-264) (SIINFEKL) and TRP2(180-188) (SVYDFFVWL) and the modified TRP2 epitope ΔV (SIYDFFVWL) were purchased from Pepceuticals (Nottingham, United Kingdom) and were more than 90% pure. Peptides were dissolved at 5 mg/mL in DMSO and subsequently diluted in phosphate-buffered saline (PBS) for immunization.
Cell preparation
At various times after vaccination, tissues were removed for analysis. Spleens were homogenized into single cell suspensions and peripheral blood was collected into heparinized tubes via either retro-orbital bleeds or cardiac puncture. Red blood cells were lysed with ACK Lysing Buffer (BioSource, Rockville, MD). To isolate lymphocytes from metastatic target organs, lungs were removed and injected with RPMI containing 417.5 μg/mL Liberase CI (Roche, Indianapolis, IN) and 200 μg/mL DNase I (Roche), minced, and incubated at 37°C for 30 minutes before being passed through cell strainers. Cells were washed and resuspended in 80% Percoll, overlaid with 40% Percoll, and centrifuged for 25 minutes at 400g. Cells residing at the 80%/40% interface were collected, washed, and counted by Guava (Guava Technologies, Hayward, CA).
Tumor challenge and vaccinations
Mice were injected with 105 B16.F10 melanoma tumor cells intravenously to establish lung metastases. Four days later, naive or tumor-bearing mice were intravenously vaccinated with 100 μg ΔV peptide, 100 μg anti-CD40, and 100 μg of the TLR7 agonist S-27609 in various combinations as indicated. Lungs were harvested approximately 20 days later, and metastases were enumerated with the aid of a dissection microscope. Alternatively, mice were monitored for survival over the next 90 days.
In vivo depletion of cell subsets
Depletion of lymphocyte subsets was accomplished by intraperitoneal administration of 250 μg anti-CD4 (GK1.5), anti-CD8 (2.43), and anti-NK1.1 (PK136). Antibodies were delivered 4 days before the start of experimentation and weekly thereafter. Depletion was confirmed by flow cytometry and resulted in greater than 95% reduction of relevant cell types.
Flow cytometry
Single cell suspensions were incubated with antibodies labeled with FITC, PE, PerCP, PC5, or APC. Antibodies, as listed under “Cell lines, antibodies, and reagents,” were from eBioscience, BD Pharmingen, and Invitrogen. Four-color analyses were performed on a modified Becton Dickinson FACSCAN running CellQuest software (BD Bioscience).
Intracellular cytokine staining and degranulation assays
Cells from lung, spleen, or peripheral blood (peripheral blood lymphocytes [PBLs]) were incubated with 1 μg/mL Ova(257-264) or TRP2(180-188) peptide plus 10 U/mL IL-2 and 3 μg/mL brefeldin A in complete medium at 37°C for 5 to 18 hours. Cells were stained with either PerCp or PC5-labeled anti-CD8 and FITC-labeled anti-CD44 antibodies prior to being fixed and rendered permeable followed by staining with either PE- or APC-labeled anti-IFNγ (XMG1.2), PE-labeled anti-TNFα (MP6-XT22), PE-labeled anti–IL-2 (JES6–5H4), FITC-labeled anti-CD127 (A7R34), or PE-labeled anti–granzyme B (16G6). The percentage of IFNγ+ cells was calculated by subtracting the background observed with the irrelevant peptide control. For the degranulation assay, cells were treated as above but with the inclusion of monensin and 2.5 μg/mL FITC-labeled anti-CD107a (1D4B) during the initial 5- to 18-hour incubation period.
In vivo cytotoxicity assay
In vivo cytolytic activity was performed as previously described.8 Briefly, naive syngeneic splenocytes were differentially labeled with either 0.5 μM or 5 μM carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) for 10 minutes at 37°C, washed, and then pulsed for 1 hour with 20 μg/mL irrelevant Ova(257-264) (SIINFEKL) or antigen-specific TRP2(180-188) peptide, respectively. Labeled and pulsed cells were subsequently mixed at a 1:1 ratio and approximately 107 cells were injected intravenously. One day later, mice were killed and splenocytes were analyzed by flow cytometry. Specific lysis was calculated by first determining the ratio of the number of SIINFEKL-labeled targets to the number of TRP2-labeled targets for each mouse and percentage antigen-specific lysis was subsequently calculated as follows: % specific lysis = (1 − [ratio of CFSElo/CFSEhi in naive mice ÷ ratio of CFSElo/CFSEhi in immunized mice])× 100.
Serum transaminase and histologic analysis
Hepatocellular injury was biochemically assessed by measuring serum liver enzyme activity. Specifically, mice received 100 μg anti-CD40, 100 μg S-27609, or both intravenously or PBS as a control. Serum was harvested 24 to 72 hours later and levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined by standard clinical assays at the National Jewish Medical Center Core Lab (Denver, CO). For histologic analysis, livers from mice treated as above were fixed in buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) prior to being coded and scored on a 0 to 4 scale in a blinded fashion. Numeric scores were assigned as follows: liver: 0 indicates normal liver, no lesions or hepatocellular damage noted; 1, rare portal and parenchymal infiltrates but no necrosis; 2, moderate parenchymal or portal infiltrates but no necrosis; 3, frequent and/or large portal or parenchymal infiltrates with occasional isolated islands of coagulative necrosis; and 4, extensive areas of inflammation with bridging coagulative necrosis. H&E images were acquired via an Olympus BX41 microscope (Center Valley, PA) using a 20×/0.05 non-oil objective and 10× ocular attached to an Olympus DP11 digital camera and were edited with XnView for Windows, version 1.82.2 (Reims, France).
Statistical analysis
Data were expressed as the mean plus or minus SEM and differences between groups were analyzed by one-tailed ANOVA and Tukey analysis unless indicated otherwise. In the case of tumor survival experiments, statistical relevance was determined using log-rank comparison. The extent of hepatitis was scored on an arbitrary scale and the resulting nonparametric data were analyzed using the Mann-Whitney test. Probability (P) values less than .05 were considered statistically significant.
Results
High frequencies of tumor-specific, effector CD8+ T cells are elicited using CD40/TLR7 agonists and tumor-specific peptide
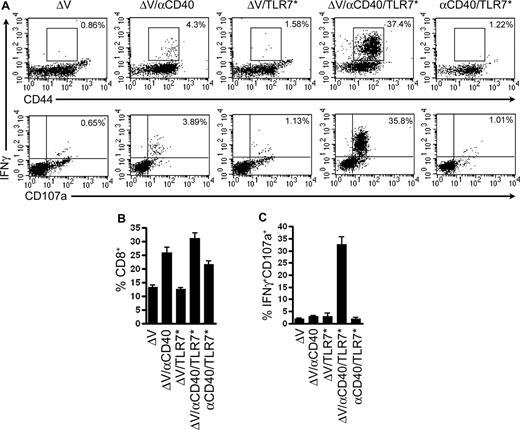
We previously demonstrated that coadministration of CD40 and TLR agonists synergistically enhances expansion of antigen-specific CD8+ T cells to foreign antigen.8 We extend these studies to show that similarly high frequencies of CD8+ T cells can be induced to self-antigens. Recently, a modified peptide variant of the H2Kb-restricted melanoma rejection self-antigen TRP2(180-188), termed ΔV (SIYDFFVWL), was shown to elicit high-affinity TRP2-specific CD8+ T cells.17 We reasoned that immunization with ΔV plus agonistic CD40 antibody (αCD40) and a TLR7 agonist (TLR7*) would magnify the ensuing CD8+ response and engender increased effector cell function. As seen in Figure 1B, αCD40 increased the relative number of CD8+ T cells in the peripheral blood of immunized mice, regardless of the addition of antigen, TLR7 agonist, or both (P ≤ .001 for ΔV/αCD40, ΔV/αCD40/TLR7*, and αCD40/TLR7* compared with ΔV alone). While αCD40 increased polyclonal CD8+ responses, it failed to generate a substantial population of TRP2-specific CD8+ T cells (Figure 1A,C). Only the combination of tumor antigen, αCD40, and TLR7 agonist resulted in the synergistic expansion of TRP2-specific T cells. To measure cytolytic potential, we assessed the ability of these cells to degranulate, which can be measured by retention of CD107a (lysosomal-associated membrane protein-1) on the cell surface.18 Cell-surface expression of CD107a is directly correlated with cytolytic activity.19,20 Only approximately 4% and approximately 2% of CD8+ cells in either the αCD40 or TLR7 agonist alone groups, respectively, expressed CD107a. However, greater than 30% of CD8+ cells primed with both αCD40 and TLR7 agonists expressed cytolytic activity by this measure (P ≤ .001 compared with ΔV alone). Combination treatment also led to increased lysis of peptide-pulsed targets in an in vivo cytotoxicity assay (data not shown). Together, these data demonstrate that the combination of αCD40 and TLR7 agonists induces high frequencies and high total numbers of self-reactive CD8+ T cells with cytolytic function.
Concomitant signaling through CD40 and TLR7 drives expansion of self-antigen–specific CD8+ T cells with enhanced cytolytic activity. C57BL/6 mice were immunized intravenously with 100 μg of the tumor-associated antigen ΔV, 100 μg αCD40 FGK45, and 100 μg S-27609 in combinations as indicated. Seven days later, mice were bled and cells were restimulated in vitro with TRP2(180-188) to assess the ability to produce IFNγ and translocate CD107a as described in “Methods.” Lymphocytes were identified by forward and side scatter and subsequently gated on all CD8+ events. (A) Representative dot plots from vaccinated mice. The numbers in the upper right corners indicate the frequency of CD8+ T cells that are positive for IFNγ and CD44 (top row) or IFNγ and CD107a (bottom row). (B) Percentage of peripheral blood lymphocytes expressing the CD8 antigen. P ≤ .001 by one-tailed ANOVA (C) Quantification of the percentages of CD8+ cells that degranulated in response to peptide restimulation. In all cases, data presented are representative of at least 3 independent experiments. Data are plotted as means plus or minus SEM (n = 8 in each group). P ≤ .001 by one-tailed ANOVA.
Concomitant signaling through CD40 and TLR7 drives expansion of self-antigen–specific CD8+ T cells with enhanced cytolytic activity. C57BL/6 mice were immunized intravenously with 100 μg of the tumor-associated antigen ΔV, 100 μg αCD40 FGK45, and 100 μg S-27609 in combinations as indicated. Seven days later, mice were bled and cells were restimulated in vitro with TRP2(180-188) to assess the ability to produce IFNγ and translocate CD107a as described in “Methods.” Lymphocytes were identified by forward and side scatter and subsequently gated on all CD8+ events. (A) Representative dot plots from vaccinated mice. The numbers in the upper right corners indicate the frequency of CD8+ T cells that are positive for IFNγ and CD44 (top row) or IFNγ and CD107a (bottom row). (B) Percentage of peripheral blood lymphocytes expressing the CD8 antigen. P ≤ .001 by one-tailed ANOVA (C) Quantification of the percentages of CD8+ cells that degranulated in response to peptide restimulation. In all cases, data presented are representative of at least 3 independent experiments. Data are plotted as means plus or minus SEM (n = 8 in each group). P ≤ .001 by one-tailed ANOVA.
αCD40/TLR7* vaccination elicits potent CD8+ T-cell memory
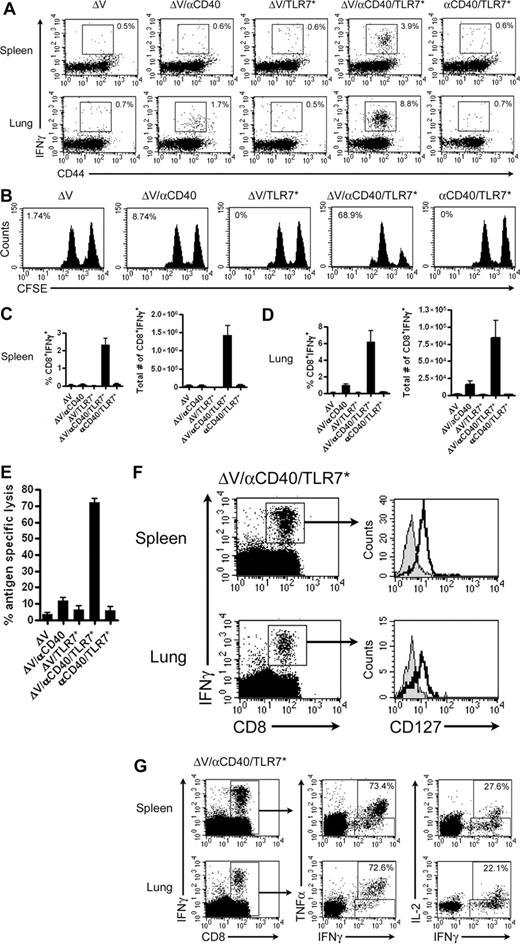
We hypothesized that coadministration of CD40 and TLR agonists would abrogate the deleterious effects of agonistic CD40–based monotherapies to engender long-term memory. To determine whether concomitant delivery of CD40 and TLR7 agonists in conjunction with tumor antigen elicits the generation of CD8+ T-cell memory, we vaccinated mice and analyzed effector functions 60+ days later. Vaccination with ΔV and αCD40 primed a minimal, persisting CD8+ effector population in the lung with limited cytolytic potential (Figure 2A,B,D). TLR7 monotherapy failed to induce a significant pool of persisting antigen-specific CD8+ T cells. In contrast, vaccination with tumor antigen, αCD40, and TLR7 agonist primed effector cells populating both spleen and lung (Figure 2A,C,D). More importantly, unlike αCD40 or TLR7* monotherapy, mice vaccinated with this regimen efficiently lysed peptide-pulsed targets when subjected to an in vivo cytotoxicity assay (Figure 2B,E; P ≤ .001, compared with either ΔV or ΔV/αCD40). In addition, the mean fluorescence intensity of IFNγ staining increased over that seen from αCD40 treatment alone (spleen: 185 ± 30 vs 310 ± 22, P = .0041; lung: 152 ± 6 vs 253 ± 25, P = .0028), demonstrating that CD8+ T cells primed by αCD40/TLR7* are more efficient in producing effector cytokines. Finally, only αCD40/TLR7* plus tumor antigen could induce autoimmune vitiligo, a response seen in approximately 36% of vaccinated mice (data not shown). To ensure the identity of the TRP2-specific memory T-cell population, we examined the CD8+ T cells for expression of CD127 (IL-7Rα), a marker shown to be selectively re-expressed upon differentiation of effector cells into memory cells.21 Indeed, TRP2-specific CD8+ T cells isolated from spleen and lung expressed CD127 (Figure 2F). Not only did the cells express CD127, but they remained fully functional, being able to produce both TNFα and IL-2. Of the IFNγ+ cells found in lung and spleen, greater than 70% secreted TNFα while greater than 20% secreted IL-2 (Figure 2G). Furthermore, since a fraction of these cells acquired the ability to secrete IL-2 and express CD127, this indicates that this vaccination regimen generates memory cells of both effector and central memory phenotype.22
In contrast to CD40 monotherapy, CD40/TLR7* therapy rescues CD8+ memory T-cell function. Mice were immunized with 100 μg each of ΔV peptide, αCD40, and S-27609 in combinations as indicated. Memory CD8+ functionality was assessed 65 days later. (A) Representative dot plots of IFNγ secretion by memory CD8+ T cells isolated from spleens and lungs of vaccinated mice. Dot plots are gated on live CD8+ cells, and numbers indicate the percentage of cells positive for both IFNγ and CD44. (B) Memory CD8+ T-cell cytolytic activity was assessed by performing an in vivo cytotoxicity assay. Numbers reflect the percentage of antigen-specific lysis. (C,D) Quantification of relative and absolute numbers of memory CD8+ cells expressing IFNγ in the spleen (C) and lung (D). Absolute numbers of positive cells were determined by multiplying the relative percentage of each cell population by the total number of cells isolated from each tissue. (E) Quantification of the in vivo cytotoxicity assay presented in panel B. P ≤ .001 by one-tailed ANOVA. (F) CD127 expression on IFNγ+-memory CD8+ T cells derived from spleens or lungs of vaccinated mice. Isotype controls are shown as filled histograms. (G) Cytokine production by memory CD8+ T cells. Cells from panel F were analyzed for the ability to produce TNFα and IL-2. Numbers reflect the percentage of CD8+IFNγ+ cells that also are positive for TNFα or IL-2. In all cases, data are pooled from at least 2 independent experiments with 4 or more mice/group per experiment and plotted as means (± SEM).
In contrast to CD40 monotherapy, CD40/TLR7* therapy rescues CD8+ memory T-cell function. Mice were immunized with 100 μg each of ΔV peptide, αCD40, and S-27609 in combinations as indicated. Memory CD8+ functionality was assessed 65 days later. (A) Representative dot plots of IFNγ secretion by memory CD8+ T cells isolated from spleens and lungs of vaccinated mice. Dot plots are gated on live CD8+ cells, and numbers indicate the percentage of cells positive for both IFNγ and CD44. (B) Memory CD8+ T-cell cytolytic activity was assessed by performing an in vivo cytotoxicity assay. Numbers reflect the percentage of antigen-specific lysis. (C,D) Quantification of relative and absolute numbers of memory CD8+ cells expressing IFNγ in the spleen (C) and lung (D). Absolute numbers of positive cells were determined by multiplying the relative percentage of each cell population by the total number of cells isolated from each tissue. (E) Quantification of the in vivo cytotoxicity assay presented in panel B. P ≤ .001 by one-tailed ANOVA. (F) CD127 expression on IFNγ+-memory CD8+ T cells derived from spleens or lungs of vaccinated mice. Isotype controls are shown as filled histograms. (G) Cytokine production by memory CD8+ T cells. Cells from panel F were analyzed for the ability to produce TNFα and IL-2. Numbers reflect the percentage of CD8+IFNγ+ cells that also are positive for TNFα or IL-2. In all cases, data are pooled from at least 2 independent experiments with 4 or more mice/group per experiment and plotted as means (± SEM).
Superior therapeutic efficacy of αCD40/TLR7* immunotherapy compared with either monotherapy in control of metastatic melanoma
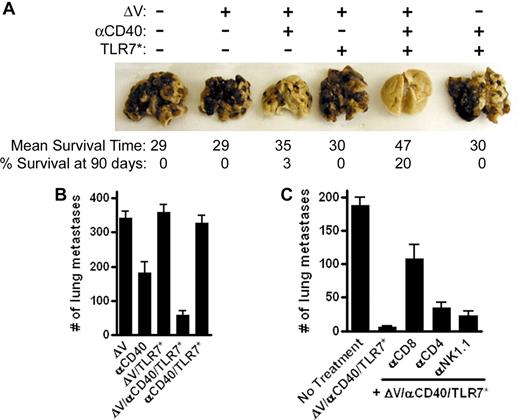
The ability of different vaccination strategies to alter the progression of metastatic melanoma was compared. Mice were intravenously inoculated with 105 metastatic B16.F10 melanoma cells and treatment was initiated 4 days later. Twenty-four days after vaccination, mice were killed and surface lung metastases were enumerated. Treatment with tumor antigen or tumor antigen plus a TLR7 agonist was ineffective in controlling tumor progression (Figure 3A,B). Immunization with tumor antigen plus αCD40 reduced the number of tumor nodules (P ≤ .001 vs ΔV alone). However, addition of a TLR7 agonist to this vaccine resulted in a 3-fold reduction in the number of metastases over αCD40 alone (Figure 3B; P ≤ .01 vs ΔV/αCD40). Furthermore, the protection afforded by αCD40/TLR7* relies upon antigen, as the removal of the H2Kb peptide, ΔV, abrogates the effect of treatment (Figure 3A,B). This protection is not unique to TLR7 agonists, as equal efficacy is observed with TLR3 and TLR9 agonists (data not shown). Moreover, changing the route of vaccination did not significantly alter the outcome of treatment (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Since αCD40/TLR7* vaccination reduced the number of lung metastases, we asked whether combination immunotherapy would afford long-term protection against metastatic disease. All mice vaccinated with tumor antigen, tumor antigen plus TLR7 agonist, or αCD40/TLR7 agonists without tumor antigen succumbed to lung failure (Figure 3A). Mean survival times were 29, 30, and 30 days, respectively. αCD40 monotherapy significantly increased survival times over tumor antigen alone (P ≤ .001) with a median survival time of 35 days and led to 3% of mice surviving greater than 90 days. However, the combination of tumor antigen plus αCD40/TLR7* greatly improved survival over αCD40 alone (P ≤ .001). Median survival times increased from 35 to 47 days with 20% of mice alive after 90 days (also see Kaplan-Meier plot in Figure S2). To determine which cellular subset mediates rejection of metastatic melanoma under this vaccination regimen, mice were depleted of CD8+, CD4+, and NK1.1+ cells prior to tumor challenge. Depletion of CD8+ cells abrogated the protective effect of vaccination (Figure 3C; P = .001 compared with vaccination without depletion). Both CD4+ and NK1.1+ cells play a partial role in tumor protection, since their depletion resulted in slightly faster, although not significant, tumor progression (Figure 3C). These data indicate that vaccination with combined immunotherapy, in the presence of antigen, leads to a CD8+ T cell–dependent immune response capable of mediating antitumor responses greater than that seen with either αCD40- or TLR-based monotherapy.
αCD40/TLR7* therapeutic intervention slows progression of metastatic melanoma. C57BL/6 mice were challenged with 105 metastatic B16.F10 melanoma cells intravenously. Four days later, mice were vaccinated with 100 μg of the tumor-associated antigen ΔV, 100 μg αCD40 FGK45, and 100 μg S-27609 in combinations as indicated. After 24 days, mice were killed, lungs were removed, and metastatic surface tumor nodules were enumerated with the aid of a dissecting microscope. (A) Photograph of macroscopically visible tumor nodules on lungs of mice, 24 days after tumor challenge. Numbers below the lungs reflect the mean survival time and long-term survival rate of mice monitored for therapeutic efficacy. Data are pooled from 3 to 4 independent experiments with greater than 8 mice per group in each experiment. (B) Enumeration of lung metastases. Data are pooled from 2 independent experiments and are presented as means plus or minus SEM (n = 16 mice in each group). Data are representative of more than 4 separate experiments with at least 6 mice in each group. (C) Enumeration of lung metastases after effector cell depletion. Mice were treated as above except for the depletion of effector cell populations prior to tumor challenge as described in “Methods.” The data are expressed as means plus or minus SEM (n = 8 mice in each group) and are representative of 3 independent experiments.
αCD40/TLR7* therapeutic intervention slows progression of metastatic melanoma. C57BL/6 mice were challenged with 105 metastatic B16.F10 melanoma cells intravenously. Four days later, mice were vaccinated with 100 μg of the tumor-associated antigen ΔV, 100 μg αCD40 FGK45, and 100 μg S-27609 in combinations as indicated. After 24 days, mice were killed, lungs were removed, and metastatic surface tumor nodules were enumerated with the aid of a dissecting microscope. (A) Photograph of macroscopically visible tumor nodules on lungs of mice, 24 days after tumor challenge. Numbers below the lungs reflect the mean survival time and long-term survival rate of mice monitored for therapeutic efficacy. Data are pooled from 3 to 4 independent experiments with greater than 8 mice per group in each experiment. (B) Enumeration of lung metastases. Data are pooled from 2 independent experiments and are presented as means plus or minus SEM (n = 16 mice in each group). Data are representative of more than 4 separate experiments with at least 6 mice in each group. (C) Enumeration of lung metastases after effector cell depletion. Mice were treated as above except for the depletion of effector cell populations prior to tumor challenge as described in “Methods.” The data are expressed as means plus or minus SEM (n = 8 mice in each group) and are representative of 3 independent experiments.
Enhancement of lung infiltrates with cytolytic potential following αCD40/TLR7* immunotherapy
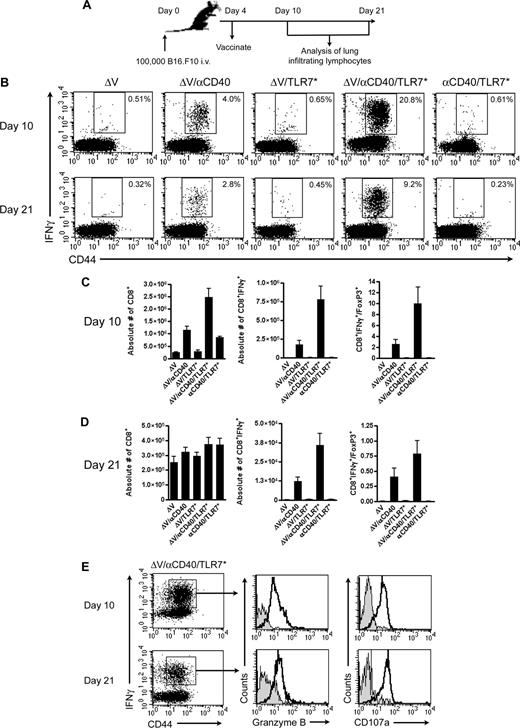
To gain insight into why αCD40/TLR7* immunotherapy mediated better antitumor immunity, we performed kinetic analyses of lung infiltrates 10 and 21 days after tumor challenge (Figure 4A). Lymphocytes isolated from tumor-bearing lungs were subjected to intracellular cytokine staining after ex vivo peptide restimulation. Only tumor antigen plus either αCD40 or αCD40/TLR7* vaccination primed tumor-specific CD8+ T cells to migrate into the metastatic target organ (Figure 4B). Flow cytometric analysis of ΔV/αCD40/TLR7* vaccinated mice revealed a 5-fold increase in the relative percentage of tumor-specific CD8+ T cells at day 10 and a 3-fold increase at day 21 over αCD40 monotherapy. On an absolute scale, αCD40 drives migration of polyclonal T cells into lungs of vaccinated mice irrespective of TLR stimulation, but this response wanes with time (Figure 4C,D). In contrast, antigen-specific cells remain elevated, with αCD40/TLR7* inducing greater absolute responses at both time points (P ≤ .001 between ΔV/αCD40/TLR7* and ΔV/αCD40 at both time points). Furthermore, cells generated from αCD40/TLR7* vaccination showed cytolytic potential as measured by degranulation and Granzyme B expression (Figure 4E).
Kinetic analysis of lung-infiltrating lymphocytes. (A) Experimental design. (B) Representative dot plots of lymphocytes isolated from metastatic target organs at day 10 or 21 after tumor challenge. Cells were isolated from tumor-bearing lungs as described in “Methods” and subjected to an in vitro restimulation with tumor peptide. Plots are gated on live, CD8+ cells. Numbers in the upper right-hand quadrant reflect the frequency of CD8+ T cells that are positive for both IFNγ and the activation marker CD44. Data are representative of 3 independent experiments with 4 mice per group in each experiment. (C,D) Quantification of lung infiltrates at either 10 (C) or 21 (D) days after tumor challenge. Data are plotted as means (± SEM) and represent pooled data from either 2 (C, n = 8 mice/group) or 3 (D, n = 12 mice/group) independent experiments, with 4 mice/group in each experiment. (E) Effector phenotype of CD8+ T cells isolated from lungs of mice vaccinated with tumor antigen plus αCD40/TLR7* at either 10 or 21 days following tumor inoculation. The dot plots are first gated on live CD8+ cells and then further gated on IFNγ+CD44+ populations. Data are representative of at least 2 independent experiments, with 4 mice/group in each experiment.
Kinetic analysis of lung-infiltrating lymphocytes. (A) Experimental design. (B) Representative dot plots of lymphocytes isolated from metastatic target organs at day 10 or 21 after tumor challenge. Cells were isolated from tumor-bearing lungs as described in “Methods” and subjected to an in vitro restimulation with tumor peptide. Plots are gated on live, CD8+ cells. Numbers in the upper right-hand quadrant reflect the frequency of CD8+ T cells that are positive for both IFNγ and the activation marker CD44. Data are representative of 3 independent experiments with 4 mice per group in each experiment. (C,D) Quantification of lung infiltrates at either 10 (C) or 21 (D) days after tumor challenge. Data are plotted as means (± SEM) and represent pooled data from either 2 (C, n = 8 mice/group) or 3 (D, n = 12 mice/group) independent experiments, with 4 mice/group in each experiment. (E) Effector phenotype of CD8+ T cells isolated from lungs of mice vaccinated with tumor antigen plus αCD40/TLR7* at either 10 or 21 days following tumor inoculation. The dot plots are first gated on live CD8+ cells and then further gated on IFNγ+CD44+ populations. Data are representative of at least 2 independent experiments, with 4 mice/group in each experiment.
Vaccine efficacy must overcome the effect of regulatory T cells, and the ratio of CD8+/FoxP3+ cells has been used to assess priming strength.23 At day 10, combination therapy resulted in a 10-fold increase in the absolute numbers of antigen-specific CD8+ T cells to FoxP3+ cells, whereas αCD40 monotherapy resulted in a 3-fold increase (Figure 4C). We have shown that optimal reduction in the conversion of FoxP3− → FoxP3+ T cells requires the maturation of DCs with both αCD40 and TLR agonists (Li Wang, Karina Pino-Lagos, Victor C. de Vries, Mohamed H. Sayegh, and R.J.N., manuscript submitted, November 2007). These data support the hypothesis that one way in which combination immunotherapy mediates increased antitumor immunity is by amplifying CD8+ T-cell numbers and effector function while decreasing the effect of immunosuppression.
αCD40-induced hepatocellular injury is reduced by coadministration of TLR7 agonist
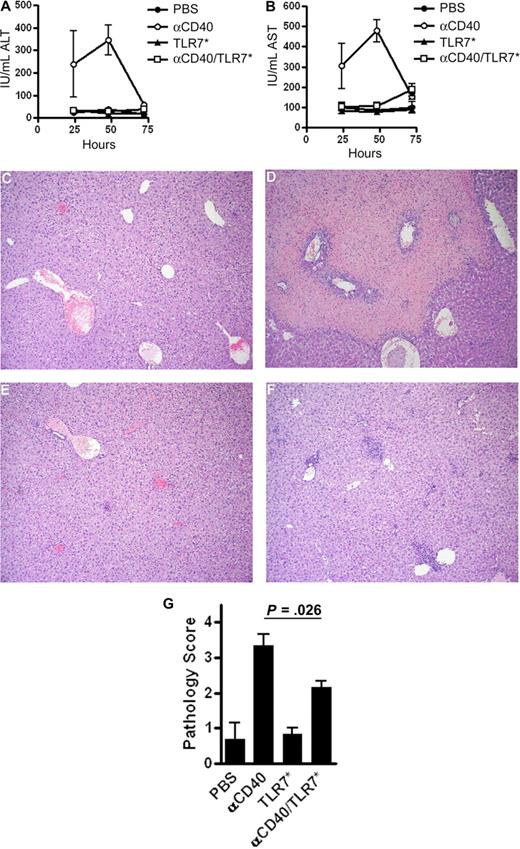
One of the significant dose-limiting safety concerns of the use of αCD40 monotherapies is liver toxicity. Several human2,4 and animal24-27 studies using CD40 agonists report elevated levels of circulating hepatocyte enzymes ALT and AST, indicative of liver damage. To examine the severity of hepatocellular damage with monotherapy versus combination therapy, we measured plasma levels of ALT and AST in mice after vaccination (Figure 5A,B). Both transaminases were significantly elevated in mice treated with αCD40, peaking at 48 hours after treatment. TLR7* had no effect on enzyme levels. In contrast to αCD40 monotherapy, αCD40/TLR7* treatment completely ameliorated the toxicity seen with αCD40 alone. Macroscopic evaluation of livers revealed substantial areas of necrosis, a finding observed only in mice treated with αCD40 (data not shown). Histologic analysis confirmed the severity of hepatocellular damage (Figure 5C-F). Normal liver architecture was seen in mice treated with PBS (Figure 5C). Livers isolated from mice treated with αCD40 exhibited widespread bridging coagulative necrosis (Figure 5D), whereas TLR7* treatment resulted in minor inflammation without any observable coagulative necrosis (Figure 5E). Livers from mice receiving αCD40/TLR7* had some foci of inflammation but little to no coagulative necrosis (Figure 5F). The extent of histologic damage was subsequently scored on a semiquantitative scale (Figure 5G). The data revealed that TLR7* significantly reduces liver toxicity associated with αCD40 monotherapy (P = .026). Although it is not clear why TLR7* attenuates αCD40-induced toxicity, we have shown that this reversion in toxicity is TLR7 dependent, as both MyD88 KO and TLR7 KO mice had similar ALT and AST enzyme levels when treated with either αCD40 or αCD40/TLR7* (data not shown). Finally, whereas the molecular and cellular mechanism for αCD40/TLR7* combination therapy in reversing toxicity remains unclear and requires further investigation, it nonetheless not only provides better therapeutic outcomes but also minimizes adverse side effects.
Hepatic toxicity associated with αCD40 monotherapy is reversed with TLR7 agonism. (A,B) Kinetic analysis of serum transaminases. Mice were treated with PBS, 100 μg αCD40, 100 μg TLR7*, or both intravenously. Serum was isolated at various time points afterward, and serum levels of alanine transaminase (A) or aspartate transaminase (B) were measured as described. Data are representative of 3 independent experiments, with n = 3 to 8 mice per group, per time point. (C-F) Histologic analysis of livers treated with PBS (C), 100 μg αCD40 (D), 100 μg TLR7* (E), or 100 μg αCD40 and 100 μg TLR7* (F) for 48 hours. (G) Semiquantitative assessment of histopathologic changes in livers from mice treated as above for 48 hours. Data are pooled from 2 independent experiments, with n = 6 mice in each treatment group. P = .026 by Mann-Whitney nonparametric test.
Hepatic toxicity associated with αCD40 monotherapy is reversed with TLR7 agonism. (A,B) Kinetic analysis of serum transaminases. Mice were treated with PBS, 100 μg αCD40, 100 μg TLR7*, or both intravenously. Serum was isolated at various time points afterward, and serum levels of alanine transaminase (A) or aspartate transaminase (B) were measured as described. Data are representative of 3 independent experiments, with n = 3 to 8 mice per group, per time point. (C-F) Histologic analysis of livers treated with PBS (C), 100 μg αCD40 (D), 100 μg TLR7* (E), or 100 μg αCD40 and 100 μg TLR7* (F) for 48 hours. (G) Semiquantitative assessment of histopathologic changes in livers from mice treated as above for 48 hours. Data are pooled from 2 independent experiments, with n = 6 mice in each treatment group. P = .026 by Mann-Whitney nonparametric test.
Discussion
Identification of molecular triggers for innate and adaptive immunity will revolutionize adjuvant platforms for vaccines. However, isolated activation of one immune pathway in the absence of others may be toxic, ineffective, and in some cases detrimental to the development of long-term, protective immunity. More effective molecularly engineered vaccines will likely include combinations of agents that trigger multiple immunologic pathways.28,29 Our studies demonstrate that CD40 and TLR agonists in combination, compared with either unitary adjuvant, elicit (1) high frequencies of self-reactive, effector CD8+ T cells, (2) potent, tumor-specific CD8+ memory, (3) CD8+ T cells that efficiently infiltrate metastatic target organs and exert effector functions, (4) superior therapeutic efficacy, (5) heightened ratios of CD8+ T cells to FoxP3+ T cells at the tumor site, and (6) reduced hepatotoxicity.
Heightened frequencies of tumor-specific CD8+ T cells have been primary end points for many human clinical trials13 and are believed to be a necessary component in the emergence of protective antitumor immunity. The frequency of antigen-specific CD8+ T cells that are elicited by the combined administration of αCD40/TLR agonist and antigen is an order of magnitude higher than that observed with almost any other adjuvant or cell-based vaccine platform, such as antigen-pulsed DCs.30-32 While the cellular and molecular basis for this striking response is incompletely understood, we have published that the expression of CD70 on DCs is critical for CD8+ T-cell expansion.9 Heightened expression of CD70 on CD8α− DCs is induced only when both CD40 and TLR agonists are coadministered. The subsequent increased signaling through CD70/CD27 could account for the superior memory responses seen after vaccination.33 Other data suggests that CD8α− DCs acquire the capacity to cross-present soluble antigen when triggered via CD40 and TLR in vivo, and this too may contribute to the extremely high frequencies of antigen-specific CD8+ T cells (A.W., R.S.N., unpublished data, 2006). Overall, our current hypothesis is that αCD40/TLR7* increases the efficiency of antigen processing and cross-presentation thereby facilitating enhanced CD8+ T-cell priming and memory. The data presented herein used peptide antigen, and as such, bypassed the cross-presentation pathway. However, it is interesting to speculate that CD40/TLR agonism may facilitate epitope spreading to alternative tumor antigens after peptide vaccination. Whether this adjuvant platform enhances epitope spreading is currently being actively evaluated.
Anti-CD40 as a unitary adjuvant has been shown to terminate both humoral34 and cell-mediated immune16 responses. While αCD40 monotherapy may provide a minimal enhancement of short-term immunity, studies have shown that it abbreviates the generation of CD8+ T-cell memory.14 Interestingly, even for humoral immunity, the use of CD40 agonists aborts long-term memory and the generation of long-lived plasma cells.17 In recent studies by Murphy and coworkers (Berner et al14 ), CD40 monotherapy resulted in the IFNγ-dependent apoptosis of tumor-specific CD4+ T cells and the inability to mount protective memory responses to tumor challenge. A number of αCD40 monoclonal antibodies have entered the clinic,2,4,35-38 only one2 of which has been reported to be a strong agonist, similar to the antimurine CD40 used herein and in a wealth of other murine studies, for example.39,40 In that phase 1 study, 4 patients, each with stage IV melanoma, were found to have a partial response on restaging at the end of study. While it may be premature to make any conclusive statements concerning agonistic αCD40 monotherapy2 as a vaccine platform, the preclinical studies in mice certainly suggest that it would be more effective as a vaccine when combined with activators of innate immunity. Even if not for clinical efficacy, the toxicity of CD40 monotherapy may be ameliorated with the addition of other immune activators. One indication where agonistic αCD40 monotherapy may be suitable is in B-cell lymphoma where, in mice, high-dose monotherapy has been shown to be extremely effective40,41
Studies in animal models reveal that as unitary adjuvants, TLR agonists can elicit robust, inflammatory responses and enhance a wide spectrum of specific immune responses.42 Results of clinical studies with TLR agonists have been mixed.43 Imiquimod, an FDA-approved topically applied TLR7 agonist, has proven extremely effective in basal cell carcinoma. Furthermore, 2 improved adult hepatitis B virus (HBV) vaccines using TLR4 agonists have been approved. However, in June 2007, Pfizer suspended a clinical program in non–small cell lung cancer for a TLR9 agonist due to lack of clinical efficacy in phase 2 and 3 trials when combined with a variety of chemotherapeutic agents.44 Our data strongly suggest that, at least in cancer indications, activators of adaptive immunity will greatly augment the therapeutic potential of TLR agonists.
It is encouraging that single-arm trials with TNFR agonists and TLR agonists have been shown to be largely safe and induce inflammatory responses. Based on emerging preclinical studies in mice using admixtures of TLR agonists, TNFR agonists, and other immune activators, it is anticipated that these admixtures will greatly improve efficacy in clinical trials, and at the same time reduce toxicity. Enhanced frequencies of primary effector T cells, potent long-term immunologic memory, and reduced regulatory T-cell functions are some of the hallmark end points that likely need to be achieved for successful therapeutic intervention. The findings of this and other studies45 provide rational strategies for the creation of multifactorial vaccines to achieve maximal efficacy in cancer vaccine trials in humans.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants CA091436 and R01CA123079 (to R.J.N).
National Institutes of Health
Authorship
Contribution: C.L.A. designed and performed research, collected and analyzed data, performed statistical analyses, and wrote the paper; A.W., S.F., A.A.S., J.D.G., and R.M.K. performed research, collected data, and analyzed data; M.J.T. and E.J.U. designed research and analyzed data; M.S.E. analyzed data; and R.J.N. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: C.L.A., R.M.K., and R.J.N. are cofounders, stockholders, and consultants of ImmuRx, a company whose technology is directed at the development of anti-CD40/TLR agonists. All other authors declare no competing financial interests.
Correspondence: Randolph J. Noelle, Dept of Microbiology & Immunology, Norris Cotton Cancer Center, Dartmouth Hitchcock Medical Center, Dartmouth Medical School, 1 Medical Center Dr, Lebanon, NH, 03756; e-mail: rjn@dartmouth.edu.