Abstract
Activating mutations of the FMS-like tyrosine kinase-3 (FLT3) receptor occur in approximately 30% of acute myeloid leukemia (AML) patients and, at least for internal tandem duplication (ITD) mutations, are associated with poor prognosis. FLT3 mutations trigger downstream signaling pathways including RAS-MAP/AKT kinases and signal transducer and activator of transcription-5 (STAT5). We find that FLT3/ITD mutations start a cycle of genomic instability whereby increased reactive oxygen species (ROS) production leads to increased DNA double-strand breaks (DSBs) and repair errors that may explain aggressive AML in FLT3/ITD patients. Cell lines transfected with FLT3/ITD and FLT3/ITD-positive AML cell lines and primary cells demonstrate increased ROS. Increased ROS levels appear to be produced via STAT5 signaling and activation of RAC1, an essential component of ROS-producing NADPH oxidases. A direct association of RAC1-GTP binding to phosphorylated STAT5 (pSTAT5) provides a possible mechanism for ROS generation. A FLT3 inhibitor blocked increased ROS in FLT3/ITD cells resulting in decreased DSB and increased repair efficiency and fidelity. Our study suggests that the aggressiveness of the disease and poor prognosis of AML patients with FLT3/ITD mutations could be the result of increased genomic instability that is driven by higher endogenous ROS, increased DNA damage, and decreased end-joining fidelity.
Introduction
Acute myeloid leukemia (AML) is an aggressive form of a malignant disorder of the hematopoietic system that shows increasing incidence with age and is characterized by highly proliferative blast cells.1-3 Aberrant activity of tyrosine kinases has been shown to be present in various malignant diseases and AML is no exception. The FMS-like tyrosine kinase 3 (FLT3) gene in chromosome band 13q12 encodes a receptor tyrosine kinase that belongs to the same family as FMS, KIT, and the 2 genes encoding PDGFRα and β.4-7 It is normally expressed by hematopoietic stem/progenitor cells (HSPCs) and as hematopoietic cells differentiate FLT3 expression is lost.8-11 A large body of work has shown that FLT3 plays roles in survival, proliferation, and differentiation. Aside from its role in regulating normal hematopoiesis, FLT3 is also highly expressed in several hematologic malignancies.7 Mutations in the receptor, in the form of internal tandem duplication (ITD) of the juxtamembrane domain and point mutations of the kinase domain, both result in constitutive activation.7 These mutations occur in one third of AML patients making it one of the most commonly mutated genes in AML.12,13 Patients with FLT3/ITD mutations have been demonstrated to have very poor prognosis.5,14,15 However, the molecular basis by which FLT3/ITD mutations lead to aggressive disease and poor prognosis in AML is not yet clearly understood
The process of activation, internalization, and degradation of FLT3 occurs in a similar fashion to other members of the class III receptor-type tyrosine kinases (RTKs) family.16 Binding of FLT3 ligand (FL) causes homodimerization, tyrosine kinase activation, receptor autophosphorylation, and initiation of downstream signaling cascades.7 The FLT3 receptor kinase shares structural homology with other type III receptor kinases, such as KIT and FMS, with all 3 playing an important role in survival, proliferation, and differentiation of hematopoietic cells.17 We and other investigators have shown that STAT5 is one of the principal pathways involved in mediating gene expression in response to constitutive receptor activation through mutation. FLT3 signaling results in activation of pathways through phosphorylation of STAT5, MAPK, AKT, VAV, CBL, and BAD.18-20 Phosphorylation and activation of STAT5 by FLT3/ITD mutants are particularly strong compared with its phosphorylation in the wild-type FLT3 allele.21 STAT5 tyrosine phosphorylation mediates STAT5 protein dimerization through a mechanism involving SH2 domains and N-terminal regions and results in translocation to the nucleus where it activates transcription of a number of genes.22 However, recent data suggest that STAT5 may have other interacting partners.23,24 For example, signal-transducing adapter proteins (STAPs) have been shown to constitutively interact with inactive STAT5 in the cytoplasm but dissociate when STAT5 is phosphorylated.24 STAT5 is also phosphorylated on serine residues, possibly by the ERK1/2 proteins,25 although the functional significance of this phosphorylation event is not well understood. In Ba/F3 cells, Kawashima et al26 have shown that activated STAT5 binds to RAC1-GTP, an essential component of reactive oxygen species (ROS) producing NADPH oxidase (NOX).
Reactive oxygen species (ROS) are considered to be involved in the pathogenesis of a variety of diseases and to play an important role in carcinogenesis.27,28 Oxygen radicals are thought to be produced through the electron transport reaction in which O2 accepts a single electron, resulting in O2−. Mitochondria are believed to be the major site of ROS production.29 Another site of electron transport is the endoplasmic reticulum.30 Oxygen radicals are also generated by a family of NADPH oxidases (reviewed in Bedard and Krause31 ). The generation of ROS through the activation of the phagocyte oxidase NOX2 or gp91phox is the most well studied of the NOX family whereby NOX2 and a small subunit p22phox recruit regulatory components p47phox and p67phox (reviewed in Bedard and Krause31 ). Importantly, the small GTPase RAC proteins are essential for activating the majority of NADPH oxidase family members in the production of superoxide (reviewed in Bedard and Krause31 and Miyano et al32 ). While RAC2 seems to play a greater role in ROS production in mature neutrophils, RAC1 was shown to be involved in producing ROS in the myeloid leukemia cell line HL60 and hematopoietic stem cells.33,34 The RAC1 G-protein mediates numerous signaling pathways involved in cell growth and differentiation. RAC1 interacts with several effector proteins through a 16–amino acid Cdc42/RAC-interactive binding (CRIB) motif, although there are other examples of RAC1-interacting proteins that do not contain CRIB motifs.35
ROS have been reported to activate a variety of transcription factors and signal proteins, including NFκB, AP-1, FOS, p53, and protein kinase C (PKC).23,36-38 However, an excessive production of ROSs may cause damage to cellular components and result in lipid peroxidation, membrane injury, and DNA damage, including DNA strand breaks and base modifications.27,39 Importantly, although base modifications are a typical form of DNA damage caused by ROS, they also lead directly to the formation of double-strand break (DSB), one of the most lethal forms of DNA damage.40 By inducing DNA damage and abnormal activation of certain cell growth regulators, ROS are considered to contribute to the carcinogenic process.39,41 In turn, there is increasing evidence that oncogenic changes in cells, in particular mutations that activate RAS-MAP kinase pathways, lead to increases in ROS production. Oncogenic BCR-ABL in chronic myeloid leukemia (CML) activates RAS-MAP kinase pathways and increases ROS production by an as yet undetermined mechanism.42 In addition, activating mutations of HRAS in fibroblast cells produced large amounts of ROS.43 We have shown that overexpression of mutated NRAS leads to increased ROS production via RAC1 activation leading to genomic instability.44 Thus, reports suggest that both the NADPH oxidase complex and mitochondrial electron transport are sources of ROS in HRAS-activated fibroblasts.43,45,46
In this report, we show that both murine hematopoietic cells overexpressing FLT3/ITD and human cells carrying FLT3/ITD mutations have increased levels of ROS, and that this may be regulated at least partially through interaction of STAT5 with RAC1. Furthermore, we find that ROS production leads to increased DSBs and misrepair, which are reduced by inhibition of either FLT3/ITD signaling. These findings provide a potential mechanism for the acquisition of additional genomic alterations in myeloid malignancies containing FLT3/ITD mutations that may at least partially explain the aggressive nature of AML in patients with FLT3/ITD mutations.
Methods
Cells and reagents
The mouse hematopoietic progenitor cell lines 32D and Ba/F3, and human leukemia cell lines MOLM-14, MV-4-11 (both bearing FLT3/ITD mutations), and REH (bearing wild-type FLT3) were obtained from American Type Culture Collection (Manassas, VA). 32D cells were stably transfected with FLT3/ITD (32D/ITD) or FLT3/WT (32D/WT); Ba/F3 cells were stably transfected with FLT3/ITD (Ba/F3/ITD) or FLT3/TKD (Ba/F3/D835Y). Cells were maintained at 37°C in 5% CO2 in RPMI-1640 medium plus 10% heat-inactivated FBS (ATTC), 5% penicillin/streptomycin, and 2 mM l-glutamine. For 32D, 32D/WT, and Ba/F3 cells, recombinant murine IL-3 (Pepro Tech, Rocky Hill, NJ) was added to the medium (10 ng/mL). Primary AML samples were selected from Johns Hopkins University. All AML patient samples were tested for FLT3/ITD and other mutations, and the research was performed under IRB approval (00007145), as described previously.47 The FLT3 inhibitor, CEP-701, was kindly provided by Cephalon (West Chester, PA) and stored as a 4-mM stock solution in dimethyl sulfoxide (DMSO) at − 70°C. RAC1 inhibitor was purchased from EMD Chemicals (San Diego, CA). siRNAs were purchased from Dharmacon (Lafayette, CO). Sequences for the siRNAs are: STAT5A-AAGUGUUGUAUGGGCAGAUUUU; STAT5B-AAGCCUGGGACUCAAUAGAUCUU; the scrambled-AACAGUCGCGUUUGCGACUGGUU.
Measurement of intracellular ROS levels
Intracellular ROS levels were determined by staining with the probe 2′,7′-dichlorodihydro-fluorescein diacetate (H2DCFDA; Invitrogen, Carlsbad, CA). Cells were seeded overnight in a 12-well plate (106 cells per well) without IL-3. In case of treatment, drugs were either added at the time when cells were plated (RAC1 inhibitor) or the next day (CEP-701). Following incubation, cells were collected and centrifuged at 200g in a tabletop centrifuge (5415D; Eppendorf, Hamburg, Germany) for 5 minutes at room temperature. The pellet was washed with 1 × phosphate-buffered saline (PBS) and resuspended in 1 mL PBS. The ROS probe H2DCFDA was dissolved in 100% ethanol and added to the cell suspension to a final concentration of 10 μM, followed by incubation at 37°C for 20 minutes. The cell pellet was resuspended in 500 μL 1 × PBS. ROS level in cells was determined by flow cytometry (FACScan; Becton Dickinson, Lincoln Park, NJ).
Transfection
All transfection experiments were done using Amaxa nucleofection technology (www.amaxa.com) as per the manufacturer's instruction. Briefly, 2 × 106 cells were transfected with either 1.5 μg siRNAs (combination of STAT5A and STAT5B) or 2 μg RAC1 dominant negative construct (N-17) using nucleofection (Kit V), and immediately transferred into prewarmed culture medium. Cells were cultured for 24 to 48 hours before analysis for intracellular ROS level. For controls, an equal amount of scrambled siRNA was also used to nucleofect the cells under identical conditions. In the case of RAC1 dominant negative experiments, an empty vector plasmid was used as a control. A GFP-expressing plasmid was used to monitor the transfection efficiency.
Immunoprecipitation and immunoblotting
Immunoprecipitation for RAC1 was performed using the RAC1 Activation Assay kit (Upstate Biotechnology, Lake Placid, NY), following the manufacturer's instructions. Briefly, equal numbers of cells (5 × 107 cells) were seeded in a 25-cm2 flask containing 10 mL medium and incubated at 37°C with or without the addition of CEP-701 for the indicated time points. Cells were pelletted by centrifugation at 500g, followed by rinsing twice with ice-cold TBS (20 mM Tris, 136 mM NaCl, pH 7.4). Ice-cold MLB buffer (25 mM HEPES, pH 7.5, 250 mM NaCl, 1% Igepal CA-630, 10 mM MgCl2, 1 mM EDTA, and 2% glycerol) was used to lyse the cells (500 μL buffer/107 cells) by repeated pipetting. Cell lysate was precleared by adding 100 μL glutathione agarose (Invitrogen) per milliliter lysate and rocked for 10 minutes at 4°C. The cell lysate was pulse centrifuged for 5 seconds at 14000g, and the supernatant was collected for RAC1-GTP immunoprecipitation. Western blotting was performed using antibodies against RAC1 (provided with the kit), STAT5 (Santa Cruz Biotechnology, Santa Cruz, CA), and phosphorylated STAT5 (Cell Signaling Technology, Beverly, MA).
Immunoprecipitation for gp91phox was performed as described previously.20,48 Briefly, cells were incubated in culture medium with or without FLT3 inhibitor CEP-701 for 4 hours at 37°C, washed with ice-cold PBS, and lysed with 1% NP-40 lysis buffer. Clarified lysate (500 μg per sample) was incubated at 4°C overnight with anti-gp91phox antibody conjugated to agarose beads (Santa Cruz Biotechnology). Western blotting was performed using antibodies against RAC1 and gp91phox (Santa Cruz Biotechnology).
Western blotting of cell extracts was performed, as previously described.49 The membrane was blotted with antibodies against FLT3 (Santa Cruz Biotechnology), phosphotyrosine (Upstate Biotechnology), STAT5, and phosphorylated STAT5. Protein bands were visualized using enhanced chemiluminescence (ECL) Western blotting detection reagent (GE Healthcare, Little Chalfont, United Kingdom).
Immunocytochemistry for γH2AX foci
Immunocytochemistry for γH2AX foci was performed as described previously50 with modifications. Briefly, cells (100 000 cells/slide) were pelleted by centrifugation at 200g (5415D model; Eppendorf) for 5 minutes. The pellet was washed with TBS twice, resuspended in 1 mL TBS, cytospun onto slides, and fixed for 10 minutes in 2% paraformaldehyde prepared in TBS. Slides were rinsed twice in TBS, incubated in 70% ethanol at − 20°C for 5 minutes, and blocked for 1 hour with 4% FBS in TBS containing 0.2% Tween-20. Following washing, slides were incubated at 37°C for 1 hour with the mouse anti-γH2AX antibody (1:500 dilution in TBS containing 1% FBS and 0.2% Tween; Upstate Biotechnology). Slides were washed 3 times in TBS containing 0.2% Tween-20 and incubated at 37°C for 1 hour with anti–mouse IgG-FITC–conjugated secondary antibody (1:500 dilution in TBS containing 1% FBS and 0.2% Tween-20). After DAPI staining, foci were visualized with a Nikon fluorescence microscope and analyzed with the program NIS-Elements (Melville, NY).
In vitro DNA end-joining assay
In vitro nonhomologous end-joining (NHEJ) repair assay was performed as described previously.51 Briefly, 200 ng blunt end linear plasmid DNA (pUC18) obtained by restriction digestion using SmaI (New England Biolabs, Ipswich, MA) and 20 μg nuclear extracts from MOLM-14 and MV-4-11 cells were incubated at 25°C for 2 hours in 50 μL reaction buffer (50 mM Tris-HCl, pH 7.6, 5 mM MgCl2, 75 mM KCl, 1 mM ATP, 1 mM DTT, 5% polyethylene glycol (PEG) 8000, 1 mg/mL aprotinin, 1 mg/mL leupeptin, 10 mg/mL bestatin, 1 mM pefablock). Reactions were performed in duplicate per set of experiment. The nuclear extracts were obtained from cells without treatment or treated with CEP-701 for 4 hours. The repair reactions were stopped by the addition of 1 μL 10% SDS followed by incubation at 65°C for 15 minutes. The DNA was recovered by phenol-chloroform extraction followed by ethanol precipitation using Pellet Paint (EMD Chemicals). The second set of purified DNA products was recut with SmaI (10 U) in a 10-μL reaction buffer to investigate the correctness of the end joining. The repair products were resolved in 1% agarose gels at 100 V for 2 hours in TAE buffer (40 mM Tris-acetate, 1 mM EDTA, pH 8.3) and stained with Vistra Green (Amersham Biosciences) (1:20 000 dilution in TE buffer, pH 8.0) at 4°C for 30 minutes with gentle shaking. Digital images of the gels were obtained with a FluorImager 595 (Molecular Dynamics, Piscataway, NJ), and the data were analyzed densitometrically using GelPro 2.0 software (Media Cybernetics, Bethesda, MD).
Statistical analyses were performed using the T-Test program (Excel; Microsoft, Redmond, WA).
Results
FLT3/ITD is associated with increased ROS production
FLT3 signaling activates a number of downstream survival pathways, including RAS-MAP kinase, PI3 kinase/AKT, and STAT5.19,20,52 In AML constitutive activation of FLT3 signaling by internal tandem duplication mutation of the juxtamembrane domain is observed in a significant number of patients (∼ 30%) and is associated with poor prognosis. We have recently shown that oncogenic activation of NRAS can cause increased ROS production and genomic instability.44 Here we investigated whether FLT3/ITD activation increased ROS levels leading to increased DNA damage and misrepair of DNA DSBs that may in part explain the aggressive disease in AML bearing FLT3/ITD mutations.
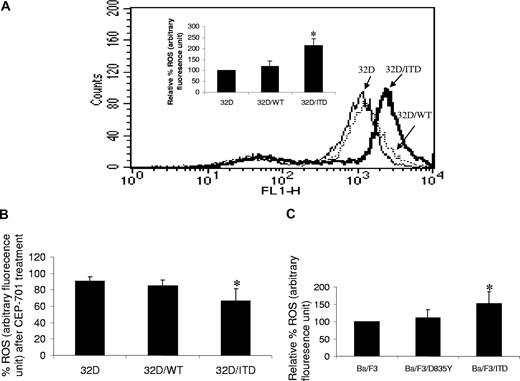
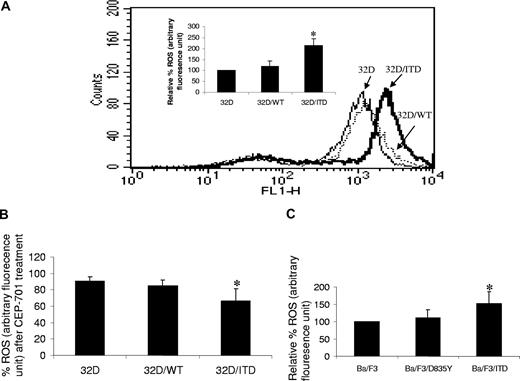
To determine whether ROS may be produced by activation of FLT3/ITD, we measured endogenous ROS production in the mouse hematopoietic progenitor cell line 32D and in 32D stably transfected with a FLT3/ITD construct, using the fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA). We found that there is significantly higher endogenous ROS in 32D/ITD cells, compared with parental 32D cells (Figure 1A; P = .01). Importantly, 32D cells stably transfected with FLT3/WT constructs show no significant increase in ROS production (Figure 1A; P = .15). To confirm the role of FLT3/ITD in increased ROS production, cells were treated with 100 nM CEP-701, a FLT3 inhibitor, for 4 hours. A significant decrease in ROS production was observed in 32D/ITD cells, compared with CEP-701–treated 32D/WT and 32D cells (Figure 1B; P = .047). No significant decrease in ROS production was observed in vehicle (0.1% DMSO)–treated cells (data not shown). Decrease in ROS production was also observed in a dose-dependent manner after 4-hour treatment in 32D/ITD cells (data not shown). These data strongly implicate FLT3/ITD signaling in ROS production.
Increased ROSs in FLT3/ITD cells. (A) Endogenous ROS was measured in 3 cell lines: mouse myeloid progenitor cell line 32D, 32D stably expressing exogenous wild-type FLT3 (32D/WT), and 32D stably expressing exogenous FLT3/ITD (32D/ITD). Shown are the relative ROS levels of the 3 cell lines, of which the 32D cell line was set at 100%. 32D/ITD cells exhibited a significantly higher ROS level than 32D and 32D/WT cells; the latter 2 showed comparable ROS levels. Flow cytometric histogram show ROS fluorescence results from one experiment. (B) All 3 cell lines were treated with CEP-701; the greatest decrease of ROS was observed in 32D/ITD cells, followed by 32D/WT cells; 32D cells showed the least decrease of ROSs. (C) The Ba/F3 cells stably transfected with FLT3/ITD construct (Ba/F3/ITD) showed higher ROS level than the cells transfected with FLT3/TKD construct (Ba/F3/D835Y) and Ba/F3 cells. The error bars represent plus or minus SEM.
Increased ROSs in FLT3/ITD cells. (A) Endogenous ROS was measured in 3 cell lines: mouse myeloid progenitor cell line 32D, 32D stably expressing exogenous wild-type FLT3 (32D/WT), and 32D stably expressing exogenous FLT3/ITD (32D/ITD). Shown are the relative ROS levels of the 3 cell lines, of which the 32D cell line was set at 100%. 32D/ITD cells exhibited a significantly higher ROS level than 32D and 32D/WT cells; the latter 2 showed comparable ROS levels. Flow cytometric histogram show ROS fluorescence results from one experiment. (B) All 3 cell lines were treated with CEP-701; the greatest decrease of ROS was observed in 32D/ITD cells, followed by 32D/WT cells; 32D cells showed the least decrease of ROSs. (C) The Ba/F3 cells stably transfected with FLT3/ITD construct (Ba/F3/ITD) showed higher ROS level than the cells transfected with FLT3/TKD construct (Ba/F3/D835Y) and Ba/F3 cells. The error bars represent plus or minus SEM.
AML patients who have acquired the FLT3/ITD mutation are associated with a poor disease prognosis, whereas those bearing the FLT3/TKD (tyrosine kinase domain) mutation do not.17 To determine whether increased ROS production is peculiar to the FLT3/ITD mutation, we measured ROS in Ba/F3 cells that express the TKD mutation (D835Y), and compared results with ROS levels measured in Ba/F3 and Ba/F3/ITD cells. We found that TKD mutation did not lead to significantly increased ROS levels (Figure 1C; P = .2).
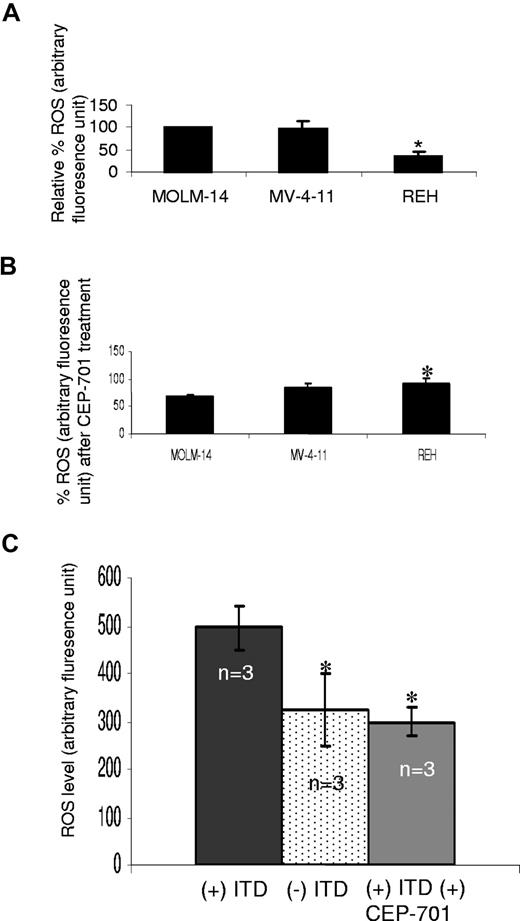
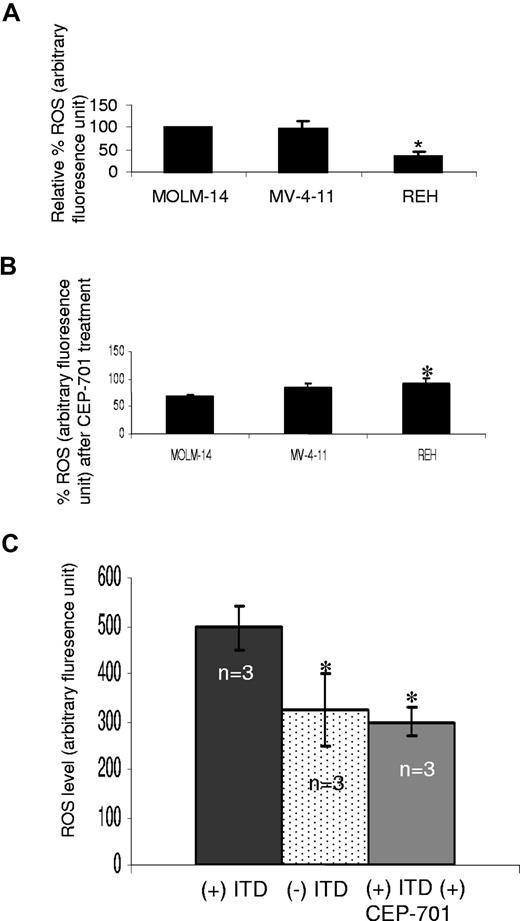
To validate our mouse cell line observations, intracellular ROS was measured in MOLM-14 and MV-4-11, 2 human AML cell lines bearing FLT3/ITD mutations, and one leukemia cell line expressing FLT/WT (REH). ROS levels are significantly less in the REH cells, compared with FLT3/ITD cells (Figure 2A; P < .001). Following treatment with 100 nM CEP-701 for 4 hours, inhibition of ROS production in REH cells is significantly less than MOLM-14 cells (P = .02; Figure 2B; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). No significant decrease in cell survival was observed in these cell lines following CEP-701 treatment (data not shown). DMSO (0.1%) alone did not decrease ROS significantly (data not shown).
Increased ROS levels in FLT3/ITD-positive human AML cell lines and patient samples. (A) Endogenous ROS was measured in 3 human leukemia cell lines: MOLM-14 and MV-4-11 (FLT3/ITD-expressing cells), and REH (FLT3/WT-expressing cell). Shown are the relative ROS levels, of which the MOLM-14 was set at 100%. REH cells exhibited a significantly lower ROS level than both MOLM-14 and MV-4-11. (B) MOLM-14 and MV-4-11 cells exhibit greater reduction in ROSs than REH cells, following CEP-701 treatment. (C) Patient samples with FLT3/ITD (n = 3) and without FLT3/ITD (n = 3) were analyzed for ROS levels, and FLT3/ITD-bearing samples were also treated with CEP-701. FLT/ITD cells exhibited significantly higher ROS, which was decreased after CEP-701 treatment. The error bars represent plus or minus SEM.
Increased ROS levels in FLT3/ITD-positive human AML cell lines and patient samples. (A) Endogenous ROS was measured in 3 human leukemia cell lines: MOLM-14 and MV-4-11 (FLT3/ITD-expressing cells), and REH (FLT3/WT-expressing cell). Shown are the relative ROS levels, of which the MOLM-14 was set at 100%. REH cells exhibited a significantly lower ROS level than both MOLM-14 and MV-4-11. (B) MOLM-14 and MV-4-11 cells exhibit greater reduction in ROSs than REH cells, following CEP-701 treatment. (C) Patient samples with FLT3/ITD (n = 3) and without FLT3/ITD (n = 3) were analyzed for ROS levels, and FLT3/ITD-bearing samples were also treated with CEP-701. FLT/ITD cells exhibited significantly higher ROS, which was decreased after CEP-701 treatment. The error bars represent plus or minus SEM.
To further confirm the involvement of FLT3 signaling in ROS production, we measured endogenous ROS levels in primary AML samples with or without FLT3/ITD mutations (n = 3). Albeit with small numbers, we find that patient samples bearing FLT3/ITD mutations show higher ROS levels, compared with those samples without FLT3/ITD mutations (Figure 2C; P < .001). In addition, treatment of FLT3/ITD patient samples with 100 nM CEP-701 (4 hours) decreased the ROS levels to those of nonmutant FLT3 AML samples (Figure 2C; P < .001). Thus, these data strongly support the result that FLT3/ITD is associated with ROS production.
FLT3/ITD signaling and ROS production through RAC1 and STAT5
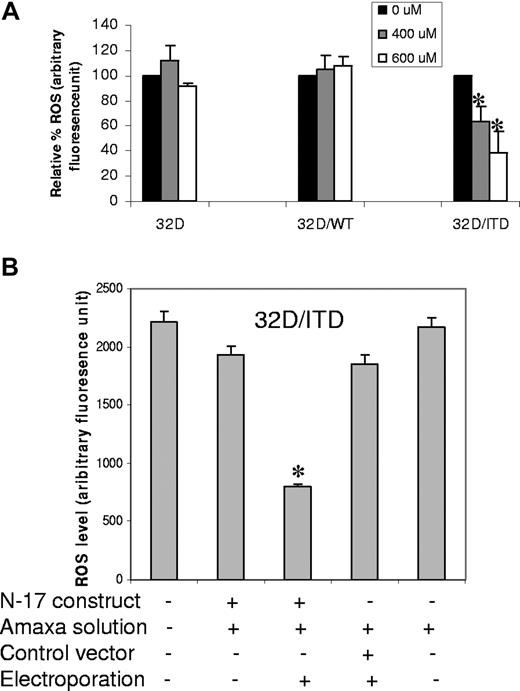
We recently reported that NRAS activation results in increased ROS production via RAC1.44 This protein is an important component and regulator of ROS producing NADPH oxidase.53 We therefore investigated whether inhibition of RAC1 activity would affect ROS production in 32D/ITD and FLT3/ITD-positive human AML cell lines. To inhibit RAC1 activity, we used a chemical RAC1 inhibitor, NSC23766 (Calbiochem, San Diego, CA), in 32D, 32D/WT, and 32D/ITD cells. A significant decrease in intracellular ROS levels was observed in 32D/ITD cells at 400 and 600 μM with overnight treatment (Figure 3A; P = .01). FLT3/ITD-positive AML cell lines (MOLM-14 and MV-4-11) also demonstrated a significant reduction in ROS following treatment with the RAC1 inhibitor (Figure S2; P = .002). Treatment with this inhibitor did not affect cell survival (data not shown). To confirm these data, 32D/ITD cells were transfected with a dominant negative construct of RAC1 (N-17).54 ROS production was significantly reduced in the transfected cells, compared with controls (Figure 3B; P = .001).
Inhibition of RAC1 activity decreases ROS production in 32D/ITD cells. (A) Inhibition of ROS production by a RAC1 inhibitor (NSC23766). RAC1 inhibitor was used at the concentrations of 400 μM and 600 μM overnight. The inhibition was represented by the percentage fluorescence unit, compared with the untreated cells. (B) 32D/ITD cells transfected with dominant negative RAC1 (N-17) resulted in significant decrease in ROS production. Transfection with vector controls show no significant changes in ROS. The error bars represent plus or minus SEM.
Inhibition of RAC1 activity decreases ROS production in 32D/ITD cells. (A) Inhibition of ROS production by a RAC1 inhibitor (NSC23766). RAC1 inhibitor was used at the concentrations of 400 μM and 600 μM overnight. The inhibition was represented by the percentage fluorescence unit, compared with the untreated cells. (B) 32D/ITD cells transfected with dominant negative RAC1 (N-17) resulted in significant decrease in ROS production. Transfection with vector controls show no significant changes in ROS. The error bars represent plus or minus SEM.
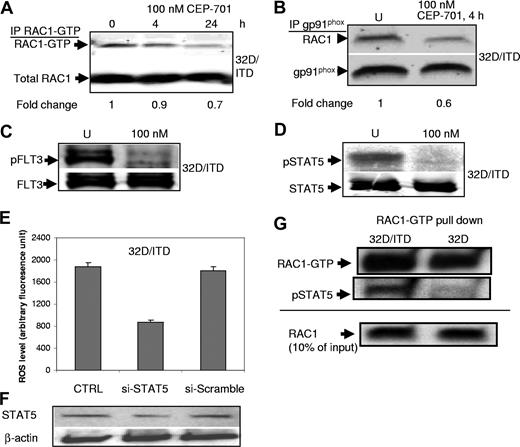
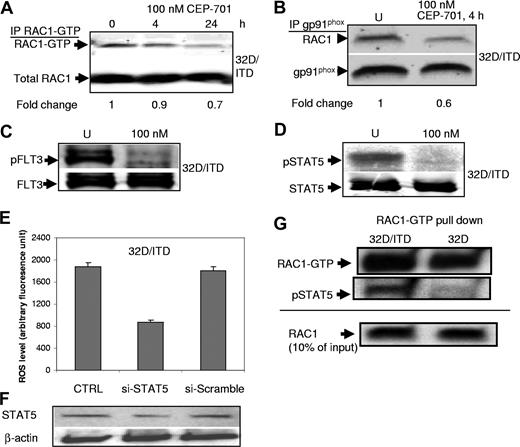
To determine whether RAC1 activity is affected by inhibition of FLT3/ITD signaling, we performed RAC1-GTP pull-down assay with 32D/ITD cells with or without CEP-701 treatment. We observed that CEP-701 treatments lead to a small decrease in RAC1 activity (Figure 4A). Active RAC1 is essential for the activity of some of the NADPH oxidases, and binds NOX proteins in the cell membrane.31 Therefore, we next examined RAC1 binding to gp91phox (NOX2) by coimmunoprecipitation assays in 32D/ITD cells before and after CEP-701 treatment. There was a significant reduction of RAC1 associated with the membrane-bound gp91phox of the NADPH complex after CEP-701 treatment, consistent with the role of RAC1 in mediating ROS production (Figure 4B).
RAC1 and STAT5 activity are involved in ROS production. (A) CEP-701–mediated inhibition of FLT3/ITD leads to a small decrease in RAC1 activity (RAC1-GTP level). RAC1 was used as a loading control. Indicated at the bottom is the fold change of RAC1-GTP relative to total RAC1. (B) Immunoprecipitation of gp91phox and Western blotting for RAC1 show decreased binding of RAC1 to gp91phox after CEP-701 treatment. gp91phox was used as a loading control. Indicated at the bottom is the fold change of RAC1 relative to total gp91phox. (C,D) CEP-701 blocks phosphorylation of FLT3, which in turn prevents STAT5 phosphorylation. Western blotting for pFLT3 (C) and pSTAT5 (D) in CEP-701–treated and untreated cells 32D/ITD cells. FLT3 and STAT5 were used as loading controls, respectively. (E,F) Partial knockdown of STAT5, which was confirmed by Western blotting, leads to significant decrease in ROS production in 32D/ITD cells. No significant decrease in ROS was observed in cells transfected with scrambled siRNA (si-Scramble) in comparison with the control without siRNA (CTRL). (G) RAC1-GTP pull-down assay in 32D and 32D/ITD cells, followed by Western blotting for RAC1 and pSTAT5. RAC1-GTP appears increased in 32D/ITD compared with 32D cells. pSTAT5 binds active RAC1 in 32D/ITD cells. Ten percent input protein is shown detected by Western blotting for RAC1.
RAC1 and STAT5 activity are involved in ROS production. (A) CEP-701–mediated inhibition of FLT3/ITD leads to a small decrease in RAC1 activity (RAC1-GTP level). RAC1 was used as a loading control. Indicated at the bottom is the fold change of RAC1-GTP relative to total RAC1. (B) Immunoprecipitation of gp91phox and Western blotting for RAC1 show decreased binding of RAC1 to gp91phox after CEP-701 treatment. gp91phox was used as a loading control. Indicated at the bottom is the fold change of RAC1 relative to total gp91phox. (C,D) CEP-701 blocks phosphorylation of FLT3, which in turn prevents STAT5 phosphorylation. Western blotting for pFLT3 (C) and pSTAT5 (D) in CEP-701–treated and untreated cells 32D/ITD cells. FLT3 and STAT5 were used as loading controls, respectively. (E,F) Partial knockdown of STAT5, which was confirmed by Western blotting, leads to significant decrease in ROS production in 32D/ITD cells. No significant decrease in ROS was observed in cells transfected with scrambled siRNA (si-Scramble) in comparison with the control without siRNA (CTRL). (G) RAC1-GTP pull-down assay in 32D and 32D/ITD cells, followed by Western blotting for RAC1 and pSTAT5. RAC1-GTP appears increased in 32D/ITD compared with 32D cells. pSTAT5 binds active RAC1 in 32D/ITD cells. Ten percent input protein is shown detected by Western blotting for RAC1.
Our previous observations21 as well as those in the present study show that STAT5 is strongly phosphorylated in cells bearing FLT3/ITD mutations and that CEP-701 blocks phosphorylation of FLT3 and its downstream target STAT5 (Figure 4C,D). Importantly, STAT5 has recently been shown to interact with RAC1.26 In an effort to delineate the pathway leading to increased ROS production, we investigated the link between STAT5 and RAC1 in FLT3/ITD-positive cells. We first determined whether ROS levels were altered by siRNA down-regulation of STAT5. Partial knockdown of STAT5 (STAT5A and STAT5B) in 32D/ITD cells resulted in decreased ROS production (Figure 4E,F). ROS levels did not change in cells transfected with scrambled oligonucleotides.
Recently, Kawashima et al showed that RAC1-GTP binds STAT5.26 Since RAC1 and STAT5 activity appear important in ROS production in cells expressing FLT3/ITD, we performed coimmunoprecipitation to determine whether binding of RAC1-GTP to phosphorylated STAT5 is increased in 32D/ITD cells, compared with 32D cells. RAC1-GTP pull-down assays were performed, followed by Western blotting for pSTAT5 (Figure 4G). The results indicate that there is increased active RAC1 in the 32D/ITD cells compared with 32D cells. Furthermore, pSTAT5 binding to active RAC1-GTP is increased in 32D/ITD, compared with 32D cells (Figure 4G). Together, these results suggest that pSTAT5 interactions with active RAC1-GTP in FLT3/ITD-expressing cells may have a role in producing ROS. These data suggest that FLT3/ITD signaling through STAT5 and RAC1 may be an important pathway for producing ROS. Further study is required to characterize the regulatory role of STAT5 and RAC1 in ROS production.
FLT3/ITD is associated with increased DSBs
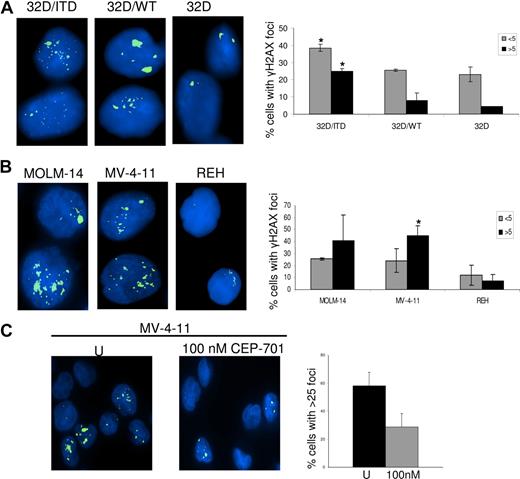
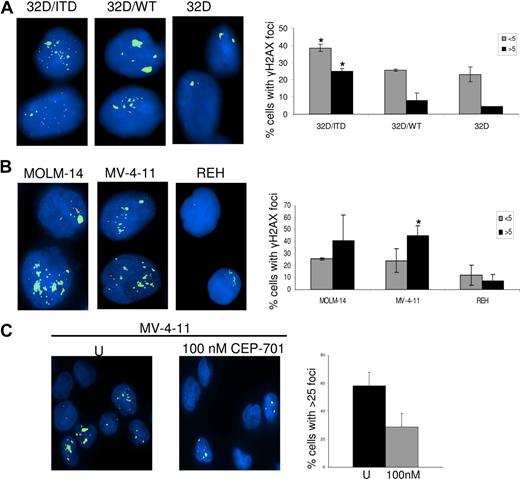
We and others have shown that increased ROS production can produce DSBs in mammalian cells.40,44 Here we investigated whether FLT3/ITD cells produce increased DSBs, and whether decreasing ROS through inhibition of FLT3/ITD signaling could lead to decreased DSBs, thereby presenting the possibility of decreasing genomic instability in AML cells bearing these mutations. We therefore examined γH2AX foci, an established measure of DSBs, in 32D/ITD cells and compared results with foci quantitated in 32D/WT and 32D parental cells. We find an increase in DSBs in 32D/ITD cells compared with both 32D/WT and 32D/control cells (Figure 5A; P = .02). This result was confirmed by examination of γH2AX foci in 2 human FLT3/ITD-positive AML cell lines (MOLM-14 and MV-4-11), compared with the FLT/WT-expressing cell line REH (Figure 5B; P = .02). To determine whether CEP-701 had an effect on the frequency of DSBs, γH2AX foci were measured in AML cell lines (MOLM-14 and MV-4-11), following 4 hours of CEP-701 treatment and results were compared with those from untreated controls. Figure 5C shows that the number of cells with more than 25 foci per cell decreases markedly in MV-4-11 cell line treated with CEP-701. Similar results were obtained for MOLM-14 (data not shown). These results suggest that inhibition of ROS by blocking FLT3 signaling leads to decreased DSBs.
DNA damage was assessed by γH2AX foci counting. (A) γH2AX foci are increased in 32D/ITD cells compared with 32D/WT and 32D cells. (B) γH2AX foci are increased in MOLM-14 and MV-4-11 cells (containing FLT3/ITD mutation) compared with REH (without FLT3/ITD mutation). Results of the foci counting are presented graphically showing the number of cells with fewer than 5 and more than 5 foci. (C) MV-4-11 cells were treated with 100 nM CEP-701 for 4 hours, followed by staining for γH2AX. Results are compared with those of untreated control. Left panel represents untreated and right panel represents CEP-701 treated cells. Graphic representation of foci showing a decrease in number of cells with more than 25 foci per cell in treated samples. The error bars represent plus or minus SEM.
DNA damage was assessed by γH2AX foci counting. (A) γH2AX foci are increased in 32D/ITD cells compared with 32D/WT and 32D cells. (B) γH2AX foci are increased in MOLM-14 and MV-4-11 cells (containing FLT3/ITD mutation) compared with REH (without FLT3/ITD mutation). Results of the foci counting are presented graphically showing the number of cells with fewer than 5 and more than 5 foci. (C) MV-4-11 cells were treated with 100 nM CEP-701 for 4 hours, followed by staining for γH2AX. Results are compared with those of untreated control. Left panel represents untreated and right panel represents CEP-701 treated cells. Graphic representation of foci showing a decrease in number of cells with more than 25 foci per cell in treated samples. The error bars represent plus or minus SEM.
Inhibition of FLT3/ITD signaling leads to increased end-joining efficiency and fidelity
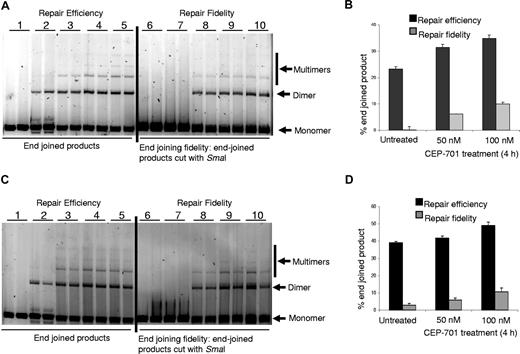
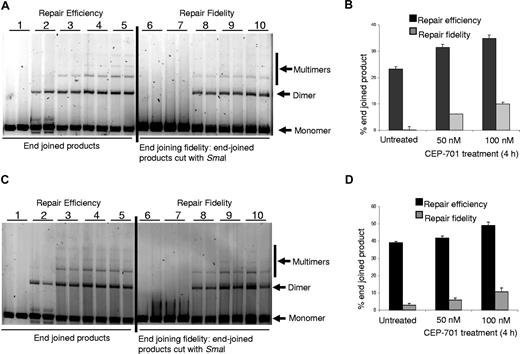
Repair of DSBs is important for maintenance of genomic integrity, as incorrect end joining leads to genomic instability.55 Since we determined that FLT3/ITD inhibition decreased DSBs in FLT3/ITD-positive cells, we wished to determine whether CEP-701 treatment might alter repair of DSBs by non-homologous end-joining (NHEJ), an error-prone repair pathway. We therefore measured end joining in an in vitro end-joining assay using nuclear extracts from 2 human FLT3/ITD-positive AML cell lines obtained with or without CEP-701 treatment, as we have previously described.56 Total end-joined product (dimers, trimers, etc) (Figure 6A,C) were measured by densitometry and presented as a percentage of total DNA in the lane (end-joined products + monomer) in Figure 6B,D. Our results show that there is a reproducible increase in end-joining efficiency following CEP-701 treatment in both FLT3/ITD-positive AML cell lines. We also measured repair fidelity by comparing percentage of end-joined products that could be cut with restriction enzyme SmaI, initially used to generate the linear substrate DNA (Figure 6A,C). The repair fidelity was calculated in the following way: [% end-joined product (on the left side of the gel) − % end-joined product left after SmaI cutting (on the right side of the gel)] = % end-joined product cut by SmaI (% correctly end joined). We observed that inhibition of FLT3/ITD leads to a reproducible increase in correct end joining indicating higher repair fidelity following FLT3/ITD inhibition-mediated ROS production (Figure 6B,D).
Inhibition of ROS production increased end-joining efficiency and fidelity. Nuclear extracts of cells treated with CEP-701 show increased end-joining efficiency and fidelity. (A) MOLM-14 nuclear extracts were used in in vitro end-joining assay using a plasmid cut with restriction enzyme SmaI. The photomicrograph shows increased repair efficiency (lanes 4,5) and fidelity (lanes 9,10) in FLT3-inhibited extracts. Left side of the gel shows efficiency, and on the right side end-joined products were recut with restriction enzyme, SmaI, used for initial cutting. Experiments were performed in duplicate. Lanes 1 and 6: negative controls; lanes 2 and 7: ligation was performed with T4 ligase; lanes 3 and 8: ligation was performed with nuclear extracts from untreated cells; lanes 4 and 9: ligation was performed with nuclear extracts from cells treated with 50 nM CEP-701; and lanes 5 and 10: ligation was performed with nuclear extracts from cells treated with 100 nM CEP-701. (B) Graphic representation of the end-joining experiment in panel A. More end-joined products were observed in CEP-701–treated samples than untreated ones. In addition, an increased amount of correctly end-joined products were observed in CEP-701–treated samples. (C,D) Similar experiments as performed for panels A,B were performed with MV-4-11 cells. The black bars represent efficiency of repair, and the gray bars represents fidelity of repair (ie, percentage of product cut by the restriction enzyme). The error bars represent plus or minus SEM.
Inhibition of ROS production increased end-joining efficiency and fidelity. Nuclear extracts of cells treated with CEP-701 show increased end-joining efficiency and fidelity. (A) MOLM-14 nuclear extracts were used in in vitro end-joining assay using a plasmid cut with restriction enzyme SmaI. The photomicrograph shows increased repair efficiency (lanes 4,5) and fidelity (lanes 9,10) in FLT3-inhibited extracts. Left side of the gel shows efficiency, and on the right side end-joined products were recut with restriction enzyme, SmaI, used for initial cutting. Experiments were performed in duplicate. Lanes 1 and 6: negative controls; lanes 2 and 7: ligation was performed with T4 ligase; lanes 3 and 8: ligation was performed with nuclear extracts from untreated cells; lanes 4 and 9: ligation was performed with nuclear extracts from cells treated with 50 nM CEP-701; and lanes 5 and 10: ligation was performed with nuclear extracts from cells treated with 100 nM CEP-701. (B) Graphic representation of the end-joining experiment in panel A. More end-joined products were observed in CEP-701–treated samples than untreated ones. In addition, an increased amount of correctly end-joined products were observed in CEP-701–treated samples. (C,D) Similar experiments as performed for panels A,B were performed with MV-4-11 cells. The black bars represent efficiency of repair, and the gray bars represents fidelity of repair (ie, percentage of product cut by the restriction enzyme). The error bars represent plus or minus SEM.
Discussion
Several studies on AML have shown that the presence of FLT3/ITD mutations resulting in constitutive activation of the FLT3 tyrosine kinase receptor portends a poor prognosis for both pediatric and adult patients.1-3 However, very little is known of the mechanisms that may lead to disease resistance to cure. Here, we show for the first time that activation of FLT3 signaling through the ITD mutation is accompanied by increased ROS production, which leads to an increase in DSBs and inaccurate repair by the error-prone NHEJ pathway. Thus, acquisition of the FLT3/ITD mutation can contribute to a cycle of genomic instability that has the potential to create further mutations, which may in turn facilitate leukemic disease resistance.
In addition to mediating numerous signaling pathways involved in cell growth and differentiation, the RAC1 G-protein is an essential component for NADPH oxidase activity and ROS production in several cell types, including stem cells and the HL60 myeloid leukemia cell line.31,33-35 We have shown that in both mouse myeloid progenitor and human myeloid leukemia cell lines containing FLT3/ITD, RAC1 is important in ROS production. Inhibition of RAC1 leads to deceased ROS production. Furthermore, inhibition of FLT3/ITD leads to a small decrease in RAC1 activity. Binding of active RAC1 to some of the NOX proteins in the cell membrane is required for ROS production, and we find a significant decrease in RAC1 binding to NOX2 (gp91phox)31 in FLT3/ITD-positive cells treated with FLT3 inhibitor, CEP-701. Therefore, NOX activity is likely to contribute at least in part to the increased ROS production in cells that have acquired FLT3/ITD mutations.
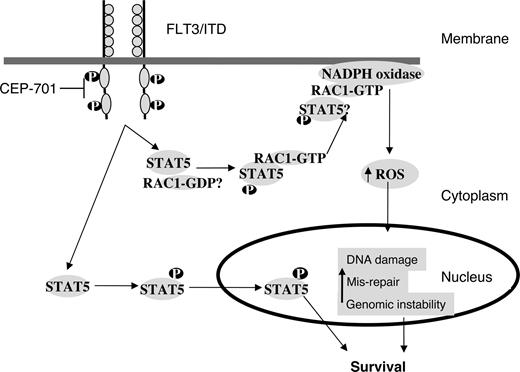
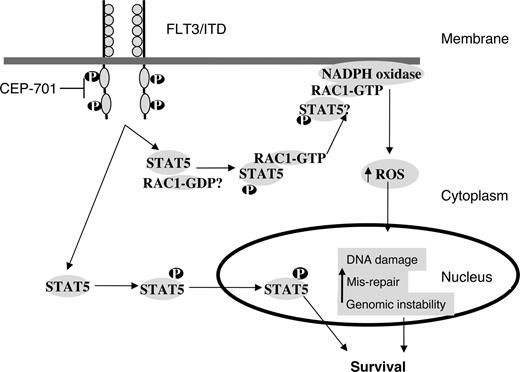
Activation of FLT3 leads to activation of RAS, PI3K/AKT, and STAT5.17 Activity of STAT5 is significantly increased in FLT3/ITD mutants.21 We have shown that signaling via STAT5 is important for ROS production because even partial siRNA down-regulation of STAT5 dramatically decreases ROS levels. Thus, increased ROS production may be a phenomenon of AML cells with activated STAT5, of which FLT3/ITD-mutated cells are just a subset. STAT5 is primarily involved in mediating gene expression in response to receptor activation. This occurs by the recruitment of STAT5 to receptor cytoplasmic domains through tyrosine phosphorylation.22 However, recent data suggest that STAT5 may interact with other proteins.24 In particular, pSTAT5 has recently been shown to bind RAC1 and is required for its passage into the nucleus.26 Thus, STAT5 may have unique nongenomic functions that regulate signaling proteins. We have shown that active RAC1-GTP binds pSTAT5 and that this binding is increased in cells expressing the FLT3/ITD mutation. Although the mechanism for this interaction is not clear at this stage, we hypothesize that pSTAT5 binding to RAC1-GTP may play an important role in regulating ROS production (Figure 7). Investigation is ongoing to further elucidate STAT5-RAC1 interactions. Identifying such protein-protein interactions may lead to the development of novel targeted therapies in the future for preventing ROS-mediated genomic instability in myeloid malignancies containing FLT3 mutations.
A working model for the role of STAT5 in regulating RAC1 activity and ROS production in FLT3/ITD-positive AML cells. FLT3/ITD signaling leads to phosphorylation of STAT5 and its subsequent translocation to the nucleus, and helps in cell survival. Our results suggest that RAC1-GTP binding to pSTAT5 is increased in FL3/ITD cells. Both RAC1 and STAT5 appear important for ROS production. Active RAC1 binding to gp91phox of the NADPH oxidase complex is required for ROS production. Inhibition of FLT3/ITD by CEP-701 leads to the inhibition of STAT5 phosphorylation, decreased RAC1 activity and binding to gp91phox, and decreased ROS levels. Further investigation is needed to clarify the STAT5/RAC1 interaction at the molecular level.
A working model for the role of STAT5 in regulating RAC1 activity and ROS production in FLT3/ITD-positive AML cells. FLT3/ITD signaling leads to phosphorylation of STAT5 and its subsequent translocation to the nucleus, and helps in cell survival. Our results suggest that RAC1-GTP binding to pSTAT5 is increased in FL3/ITD cells. Both RAC1 and STAT5 appear important for ROS production. Active RAC1 binding to gp91phox of the NADPH oxidase complex is required for ROS production. Inhibition of FLT3/ITD by CEP-701 leads to the inhibition of STAT5 phosphorylation, decreased RAC1 activity and binding to gp91phox, and decreased ROS levels. Further investigation is needed to clarify the STAT5/RAC1 interaction at the molecular level.
As mentioned above, FLT3/ITD activates other pathways in addition to STAT5, such as RAS-MAP kinases. These pathways may also contribute to ROS production. Of interest, RAC1 may be downstream of RAS,57 and we have previously shown that RAS activation leads to ROS production via RAC1.44 Thus, other signaling pathways may also contribute to ROS production and genomic instability in FLT3/ITD-positive AML. Identifying the proteins in the subpathways that are critical for generating ROS may lead to the development of novel targeted therapies for preventing ROS-mediated genomic instability in myeloid malignancies containing FLT3 mutations. ROS production through oncogenic activation provides a basis for generating genomic instability that results in the acquisition of further genomic changes that can lead to disease resistance.44 In addition, since the mitochondria is a major site for ROS production in cells, it would be important to evaluate the contribution of this subcellular compartment in FLT3/ITD-positive AML.31
One scenario for genomic instability may manifest itself through increased induction of DSBs in a cellular environment of compromised DSB repair. We previously described constitutive DNA damage and inaccurate repair of DSB in myeloid leukemias and proposed that these processes drive genomic instability in these malignancies.49,55,58 Furthermore, we have recently shown that the constitutively activated NRAS can lead to increased ROS that affect the frequency of DSBs and repair accuracy.44 We have shown for the first time that increased ROS produced in FLT3/ITD cells leads to increased DSBs, as measured by γH2AX. Inhibition of FLT3 signaling reduces the frequency of DSBs. An intriguing finding is that FLT3/ITD mutations are associated with a decreased efficiency as well as correctness of end-joining DSB repair. Treatment of cells with FLT3 inhibitors leads to increased repair efficiency and fidelity. These results are in line with data suggesting that end-joining repair proteins, such as DNA-PKCs, are sensitive to oxidation by ROS and result in reduced DSB repair efficiency.59,60 Thus, reducing ROS may ultimately lead to fewer errors and a decrease in the overall mutation rate that could lessen disease resistance in AML.
NHEJ is involved in all the phases of the cell cycle, and in contrast, the homologous recombination (HR) is involved in late S and G2 phases of the cell cycle.61 It is now accepted that there is crosstalk between the pathways and that if one pathway is compromised, compensatory repair occurs via the other pathway.56,62 Thus, if NHEJ efficiency is compromised in FLT3/ITD-positive AML, it follows that the HR pathway may be up-regulated. Studies have shown that constitutive activation of FLT3/ITD leads to up-regulation of Rad51, a key member of HR repair and may be important in resistance to therapy in AML.63,64 Our report shows basal levels of ROS in FLT3/ITD, its consequence to DNA damage and NHEJ efficiency, and modulation of these phenomena following receptor inhibition. However, the above-mentioned studies were performed following genotoxic agent treatment and measurement of DNA damage load with time. No functional assays for HR repair were performed.63,64 Thus, it would be important to determine the relative contribution of these 2 pathways in AML disease resistance following treatment with various genotoxic agents. Further investigation is needed to dissect how HR and NHEJ are regulated in AML cells and how each of these contributes to therapy resistance in AML patients.
Finally, characterization of the interactions of STAT5 and RAC1 may allow the design of inhibitors of RAC activation in FLT3-activated cancers, leading to therapeutic strategies to reduce or perhaps prevent acquisition of the genomic changes that can lead to disease progression and resistance to therapy. Determination of whether in vivo inhibition of FLT3/ITD, RAC1, and/or ROS directly in FLT3/ITD knock-in mice can decrease the accumulation of mutations and slow the rate of disease progression is essential. If ROS are part of the explanation for resistance, treatment with agents that decrease ROS may prevent further resistance mutations from accumulating in human FLT3/ITD AML patients undergoing therapy for their disease. More generally, this model for genomic instability may also be applicable to other cancers with activated RAC pathways.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Stephen Baylin, Johns Hopkins University (JHU) in Baltimore for critical reading of our paper. We thank Matt Whitehurst for data management and technical support. We thank Dr Thomas A. Winters (National Institutes of Health [NIH]) for helping us with the end-joining experiments, and Dr Mark Levis (JHU) for providing us with the AML patient samples. We thank Drs Bruce Ruggeri and Susan Bolin-Jones of Cephalon for providing the CEP-701.
This work was supported by the University of Maryland Cancer Center (F.R., K.D., A.S., and J.F.), the Cigarette Restitution Fund pilot grant (F.R. and P.S.), and grants from NIH (CA90668, CA70970; D.S.). D.S. is also supported by the Kyle Haydock Professorship.
National Institutes of Health
Authorship
Contribution: A.S., J.F., K.D., and K.-T.K. designed individual experiments, performed the research, analyzed the data, and participated in writing the paper; D.G. performed research; D.S. and P.S. contributed reagents and participated in designing some of the experiments and writing the paper; and F.R. was responsible for the overall design of the study, analysis of the data, and writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Feyruz Rassool, University of Maryland School of Medicine, 655 W Baltimore St, BRB 7-023A, Baltimore, MD 21201; e-mail: frassool@som.umaryland.edu.
References
Author notes
A.S., J.F., K.D., and K.-T.K. contributed equally to this work.