Abstract
The model of erythroleukemia caused by Spi-1/PU.1 transgenesis in mice is a multistage disease. A preleukemic step is characterized by an acute proliferation of proerythroblasts due to the arrest of differentiation provoked by Spi-1/PU.1. Later on, a blastic crisis occurs associated with somatic oncogenic mutations in the stem cell factor (SCF) receptor kit. To gain insights into the mechanisms of the leukemic progression, we performed proteomic profiling analyses of proerythroblasts isolated at the 2 stages of the disease. Our results indicate that the level of ezrin, a membrane cytoskeletal crosslinker, is increased in the leukemic cells. We show that Kit oncogenic forms are responsible for ezrin phosphorylation and that phosphorylation rather than overexpression is essential in the leukemic proerythroblasts. Using expression of dominant-negative forms of ezrin, we show that phosphorylation of ezrin on residue Y353 participates in apoptosis resistance, whereas phosphorylation on residue Y145 promotes proliferation of the leukemic cells in vitro and in vivo. Another recurrent oncogenic form of tyrosine kinases (Flt3) most frequently involved in human myeloid leukemia was also able to phosphorylate ezrin. These findings point to a new role for ezrin as signaling player in the development of leukemia, being a downstream effector of oncogenic tyrosine kinases in leukemic blasts.
Introduction
Spi-1/PU.1 transgenic mice develop an acute erythroleukemia evolving as a 2-step process.1 The first step (HS1 stage) is characterized by a large hepatosplenomegaly associated to a severe anemia. Spleen and liver are infiltrated by preleukemic proerythroblasts arrested in maturation that remain strictly dependent upon erythropoietin (Epo) for proliferation and survival in vivo as well as in vitro. Spi-1/PU.1 is an ETS transcription factor that plays a critical role in regulating the commitment of multipotent hematopoietic progenitors and the development of the B lymphoid and monocytic lineages.2-5 The pathology that occurs in Spi-1/PU.1 transgenic mice thus indicates that overexpression of Spi-1/PU.1 in erythroid precursor cells impedes their maturation. However, later on during the disease progression, proerythroblastic cells emerge that are characterized by Epo autonomous growth and tumorigenicity in vivo. These proerythroblastic cells (HS2 stage) harbor gain-of-function mutations in the Kit gene encoding the tyrosine kinase receptor for stem cell factor (SCF).6 These mutations target the Kit kinase domain and confer a ligand-independent tyrosine kinase activity to the Kit receptor. Kit mutants cause growth factor–independent proliferation of proerythroblasts, thereby resulting in neoplastic transformation. Thus, the Spi-1/PU.1 model of erythroleukemic transformation involves the collaboration of at least 2 main events, one blocking differentiation and one inducing proliferation
To further investigate the oncogenic events associated with this leukemic transformation process, we performed a proteomic profiling analysis to identify proteins differentially expressed in cells during the preleukemic (HS1) and the leukemic (HS2) stage. We found that ezrin was highly expressed in HS2 cells as compared with HS1 cells, and we focused our study on this protein. Ezrin is a member of the ezrin-radixin-moesin (ERM) protein family.7 Initially described as a cytoskeleton organizer in epithelial cells, ezrin participates to substrate adhesion, cell survival, cell motility, and formation of cell-cell junctions.8,9 The NH2-terminal region anchors ezrin in the plasma membrane, whereas the COOH-terminal domain interacts with the actin cytoskeleton. In inactive state, the actin and membrane-binding domains are masked through intramolecular and/or intermolecular self-associations.9 The functional activation of ezrin disrupts the closed conformation through a sequential process involving phosphatidyl4,5 -bisphosphate (PIP2) interaction, plasma membrane localization, and tyrosine/threonine phosphorylations.10-13 Notably, extracellular signals such as those mediated by the hepatocyte growth factor (HGF), the epidermal growth factor (EGF), or the platelet-derived growth factor (PDGF) induce phosphorylation of ezrin in epithelial cells through stimulation of their transmembrane receptors.14-16 Alternatively, ezrin can be activated by intracellular signaling factors such as the Src kinase Lck in T lymphocytes17 or the Src18 and Rho pathways10 in epithelial cells. Moreover, ezrin interacts with p85, the regulatory subunit of PI3-kinase, and activates the PI3K/AKT pathway.16 Thus, ezrin participates in signaling programs leading to its pleiotropic functions.
Several studies provide evidence that ezrin has a metastasis-promoting function in cancer. Increased ezrin expression has been associated with high metastatic potential in a variety of human and rodent cancers, including pancreatic adenocarcinomas, osteosarcomas, rhabdomyosarcomas, and breast carcinomas.19-25 In the present study, we report that a dysregulation of ezrin is related to the progression of preleukemic proerythroblasts toward malignancy. We show that disruption of ezrin function by the ezrin mutants Y145F and Y335F leads to reduced proliferation and increased death of leukemic cells. Notably, we show that ezrin is a downstream signaling effector of the oncogenic forms of Kit expressed in the leukemic proerythroblasts. Together, our data suggest that ezrin might be an important element in the oncogenic processes associated to activating mutations in tyrosine kinases.
Methods
Cell lines, growth kinetics, and colony assays
HS1 and HS2 spi-1 transgenic cell lines have been previously described.1,6 For growth kinetics, cells were plated at 2 × 105 cells/mL. PP1 and PP2 (Calbiochem, La Jolla, CA), LY294002 (PI3 kinase inhibitor; Sigma- Aldrich, Lyon, France), and imatinib mesilate (Novartis-Pharma, Basel, Switzerland) were added at the indicated concentrations in culture medium. Living cells in culture were separated from dead cells through ficoll (Histopaque-1077; Sigma-Aldrich) gradient centrifugation (30 minutes at 400g).
Clonogenic assays were performed in 1% methylcellulose medium (MethoCult M3134; Teku-bio, Le Perray en Yvelines, France) supplemented with 1% fetal bovine serum (FBS) and Epo (1 U/mL). A total of 400 growing cells were seeded into 1.5 mL medium in 30-mm dishes and colonies (50 cells at least) were counted on day 6 of incubation.
Cells were observed using a Nikon Eclipse TE300 microscope (Nikon, Champigny-sur-Marne, France) with a 40× objective. Images were acquired with a Nikon Coolpix 950 and processed using Adobe Photoshop (Adobe Systems, San Jose, CA).
DNA constructs and transfections
The human vesicular stomatitis virus glycoprotein (VSVG)–tagged ezrin constructs (wild-type [WT], N-terminal (Nter), Y145F, and Y335F) were cloned into the pEF-neo vector by standard cloning procedures. pEF-BOS-KitD814Y and pEF-BOS-KitWT were described previously.6 pMKIT-Flt3D835Y was supplied by Dr P. Dubreuil (Inserm, Marseille, France),26 and pcDNA3-myc-TEL-Jak2 was described previously.27 A total of 2 μg of plasmids were transfected in COS-7 cells with Lipofectamine-PLUS reagent according to manufacturer instructions (Invitrogen, Cergy, France). Spi-1 transgenic proerythroblasts were nucleofected with 5 μg of plasmids using an Amaxa nucleofector and G16 programs (Amaxa Biosystems, Köln, Germany). Transfectants were selected in growth medium containing 800 μg/mL G418 (Invitrogen, Cergy Pontoise, France).
Determination of apoptotic cells by Hoechst staining
Cells were fixed with phosphate-buffered saline (PBS) containing 4% paraformaldehyde for 10 minutes at room temperature, washed in PBS, and stained for 10 minutes with 50 mg/mL Hoechst 33342 (Molecular Probes, Eugene, OR) in PBS. Apoptotic cells were examined under an inverted fluorescence microscope.
Western blotting and antibodies
The following primary antibodies were used: the monoclonal anti-VSVG (VSVG P5D4 clone; Roche Diagnostics, Mississauga, ON), a polyclonal antibody directed against the carboxy-terminal domain of ezrin,28 a rabbit anti-TEL immune serum,27 a rabbit immune anti-Kit serum,26 the antiphosphotyrosine 4G10 clone (Upstate Biotechnology, Lake Placid, NY), and antibodies raised against the phosphorylated forms of Akt (S473) and Erk1/Erk2, against the constitutive Akt and Erk1/Erk2, and against the activated form of caspase 3 (D175; Cell Signaling, Beverly, MA). An anti–β-actin antibody was purchased from Sigma, anti-Flt3 (N20), anti-moesin, anti-radixin, and anti-merlin antibodies were purchased from Teku bio) and the anti-BrdU polyclonal antibody was purchased from Dako (Trappes, France).
Immunoprecipitation and Western blotting
Cells (2 × 107 cells/mL) were lysed in ice-cold TNE buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 20 mM NaF, 25 mM β-glycerophosphate, 1 mM Na-pyrophosphate, 0.1 mM Na3VO4, 1% NP40, and protease inhibitors [Amersham, Orsay, France]). Lysates were centrifuged at 18 000g for 15 minutes at 4°C. Supernatants were incubated with 1 μg of ezrin antibody and 20 μL of protein G beads (Ultra-link Immobilized protein G plus, Invitrogen) for 2 hours at 4°C, then washed extensively with TNE buffer containing 0.1% NP40. Whole-cell extracts or immunoprecipitates were fractionated by SDS-PAGE, blotted, and visualized as previously described.29
Cell-cycle analysis
Exponentially growing cells were fixed in 70% cold ethanol and treated with RNAse prior DNA staining with propidium iodure (PI). To estimate cell-cycle time, cells were treated with BrdU, fixed immediately in cold ethanol or kept at 37°C for 3 hours after BrdU removal prior to fixing. Immunodetection of incorporated BrdU, estimation of S and G2/M phases, and potential doubling time (Tpot) calculations were achieved as described.30
Flow cytometry analyses were performed using a FACScalibur (Becton Dickinson, Meylan, France). Data were analyzed with FlowJo (Treestar, San Carlos, CA) software for Akt labeling and BrdU and ModFitLT (Verity, Topsham, ME) software for G1/S/G2 determination.
Tumorigenicity
Cells (107 in 0.5 mL α-MEM containing 2% FBS) were injected subcutaneously into 8-week-old female nude mice. Tumor nodules were taken off after 3 weeks and weighed.
Results
Ezrin expression is increased in leukemic HS2 spi-1 transgenic proerythroblasts
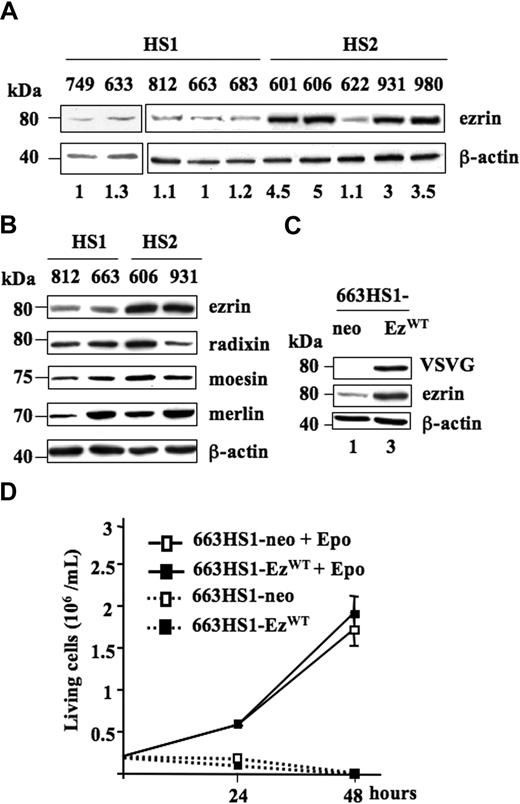
To assess changes in protein expression that could play a role during the leukemic progression of spi-1 transgenic proerythroblasts, we performed a comparative proteomic profiling between preleukemic HS1 and leukemic HS2 cells. Protein extracts were resolved by bidirectional gel electrophoresis (Supplementary materials, available on the Blood website; see the Supplemental Materials link at the top of the online article). The differentially detected protein spots were subjected to trypsin digestion, and the resulting peptides were analyzed by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry. We focused our study on ezrin, whose expression was higher in HS2 than in HS1 extracts. This observation was further confirmed by Western blot analysis of extracts prepared from independent HS1 and HS2 cell lines. Indeed, ezrin expression was 3- to 5-fold increased in extracts from HS2 cells compared with extracts from HS1 cells, except for the 622HS2 cell line (Figure 1A). Notably, among the HS2 cell lines, cell line 622 was previously characterized as autocrine for Epo.1
Expression of EzrinWT in HS1 and HS2 spi-1 transgenic proerythroblasts. (A) Whole-cell lysates from HS1 preleukemic cells (749, 633, 812, 663, and 683) and HS2 leukemic cells (601, 606, 622, 931, and 980) were subjected to Western blot analysis using an antibody raised against ezrin. The blot was stripped and reprobed with an anti-actin antibody to visualize the equal loading of proteins. Molecular weight standards are indicated on the left of the panels. The membrane was exposed in an Imager, and the resulting signal was quantified using the ImageGauge software package (Fuji, Paris, France). Values were normalized to actin expression. Basal protein level was AU for 663 HS1 cells. The ezrin expression level for each cell line is indicated. Western blot is from a representative experiment. (B) Ezrin, radixin, moesin, and merlin expression. Whole-cell lysates from HS1 preleukemic cells (812 and 663) and HS2 leukemic cells (606 and 931) were subjected to Western blot analysis using antibodies raised against ezrin, radixin, moesin, merlin, and β-actin as a loading control. (C) Expression of ezrin in whole-cell lysates from one clone of 663 HS1 cells transfected either with pEF-neo or pEF-neo EzrinWT-VSVG constructs and cultured in the presence of Epo. Lysates were analyzed by Western blotting. The membrane was first probed with an anti-VSVG antibody and then with an anti-ezrin antibody. β-actin served as a loading control. Fold-increase in ezrin expression is indicated at the bottom of each lane. (D) Enforced expression of wild-type ezrin did not change the proliferation characteristics of transfected 663 HS1 cells (663H1-EzWT versus 663HS1-neo cells) whether cells were cultured in the presence (1 U/mL) or in the absence of Epo. Numbers of living cells were monitored at 24 and 48 hours using the Trypan blue (0.2% in PBS) exclusion staining (Sigma-Aldrich) and a Vi-Cell analyzer (Beckman Coulter, Villepinte, France). The mean number of living cells and standard deviations were determined from 3 experiments.
Expression of EzrinWT in HS1 and HS2 spi-1 transgenic proerythroblasts. (A) Whole-cell lysates from HS1 preleukemic cells (749, 633, 812, 663, and 683) and HS2 leukemic cells (601, 606, 622, 931, and 980) were subjected to Western blot analysis using an antibody raised against ezrin. The blot was stripped and reprobed with an anti-actin antibody to visualize the equal loading of proteins. Molecular weight standards are indicated on the left of the panels. The membrane was exposed in an Imager, and the resulting signal was quantified using the ImageGauge software package (Fuji, Paris, France). Values were normalized to actin expression. Basal protein level was AU for 663 HS1 cells. The ezrin expression level for each cell line is indicated. Western blot is from a representative experiment. (B) Ezrin, radixin, moesin, and merlin expression. Whole-cell lysates from HS1 preleukemic cells (812 and 663) and HS2 leukemic cells (606 and 931) were subjected to Western blot analysis using antibodies raised against ezrin, radixin, moesin, merlin, and β-actin as a loading control. (C) Expression of ezrin in whole-cell lysates from one clone of 663 HS1 cells transfected either with pEF-neo or pEF-neo EzrinWT-VSVG constructs and cultured in the presence of Epo. Lysates were analyzed by Western blotting. The membrane was first probed with an anti-VSVG antibody and then with an anti-ezrin antibody. β-actin served as a loading control. Fold-increase in ezrin expression is indicated at the bottom of each lane. (D) Enforced expression of wild-type ezrin did not change the proliferation characteristics of transfected 663 HS1 cells (663H1-EzWT versus 663HS1-neo cells) whether cells were cultured in the presence (1 U/mL) or in the absence of Epo. Numbers of living cells were monitored at 24 and 48 hours using the Trypan blue (0.2% in PBS) exclusion staining (Sigma-Aldrich) and a Vi-Cell analyzer (Beckman Coulter, Villepinte, France). The mean number of living cells and standard deviations were determined from 3 experiments.
Ezrin belongs to the ERM family of proteins that includes radixin, moesin, and merlin, the product of the tumor-suppressor gene NF2. When the expression level of these different proteins was analyzed by Western blotting with appropriate antibodies, no significant variation was seen between preleukemic and leukemic cell extracts, except for ezrin (Figure 1B).
Thus, an overexpression of ezrin appears as a characteristic feature of the malignant status of proerythroblasts in spi-1 transgenic mice.
Overexpression of ezrin does not modify the proliferation of HS1 preleukemic proerythroblasts
To approach whether the overexpression of ezrin played a role in HS2 cells, we ectopically overexpressed ezrin in HS1 cells and studied the changes in proliferation. To this end, constructs encoding the VSVG-tagged wild-type ezrin (VSVG-ezrinWT) and/or the neomycine resistance gene (NeoR) were stably transfected in 663 HS1 cells and G418-selected clones were amplified. Among those, 2 clones from each transfection were studied in more details (Figure 1; Table S2). Since similar results were obtained, only data with one clone 663HS1-EzWT and one clone 663HS1-neo are shown herein. Ezrin expression was controlled by Western blotting using first an anti-VSVG antibody and then an anti-ezrin antibody (Figure 1C). The exogenous VSVG-ezrinWT and the endogenous ezrin migrate similarly as an 80-kDa protein. Ezrin quantification indicated an approximately 3-fold increase in 663HS1-EzWT compared with 663HS1-neo cells. Given the increased expression of ezrin detected in HS2 cells (Figure 1A), the expression level of enforced ezrin appeared physiologically relevant. As illustrated in Figure 1D, transduced 663HS1-EzWT cells proliferated in the presence of Epo quite similarly to control 663HS1-neo cells. Epo starvation resulted in a massive cell death, demonstrating that the survival and proliferation of 663HS1-EzWT cells remained strictly dependent on Epo. This indicated that ezrin overexpression was not sufficient to abolish the Epo dependency of HS1 cells for growth and survival. We also determined that ezrin overexpression did not induce Epo hypersensitivity in HS1 cells when cultured in the presence of limiting concentrations (0.01 and 0.05 U/mL) of Epo (data not shown).
Dominant-negative form of ezrin inhibits proliferation and induces apoptosis in HS2 leukemic cells
To gain insights into a role for ezrin in leukemic HS2 proerythroblasts, we used a dominant-negative form of ezrin, corresponding to the N-terminal domain (amino acids 1-309) of the protein.15 An expression vector for the VSVG-tagged ezrin mutant (VSVG-ezrinNter) was stably-transfected into 606HS2 cells (clone 606HS2-EzNter). Controls were 606HS2 cells overexpressing VSVG-ezrinWT (clone 606HS2-EzWT) and/or NeoR (clone 606HS2-neo). A total of 2 cell clones from each transfection were studied in Figure 2 and Table S2. A 40-kDa protein corresponding to VSVG-ezrinNter, and the 80-kDa protein corresponding to VSVG-ezrinWT were detected in transfected cells by Western blotting using an anti-VSVG antibody (Figure 2A). We then analyzed the proliferation rate of HS2 cells in which ezrinNter expression was enforced. A marked reduction of approximately 3-fold over 48 hours was seen in the number of living 606HS2-EzNter cells compared with control 606HS2-EzWT and 606HS2-neo cells (Figure 2B), indicating that the overexpression of a dominant-negative form of ezrin strongly affects the proliferation of leukemic cells. Next, we investigated the consequences of ezrinNter expression on the viability of HS2 cells. Figure 2C shows that about 35% of 606HS2-EzNter cells were dead after 48 hours of culture, whereas no mortality was observed in 606HS2-EzWT and 606HS2-neo cells. 606HS2-EzNter cells died by apoptosis as shown by the presence of fragmented nuclei detected by Hoechst staining (Figure 2D). Accordingly, the mature active form (17 kDa) of caspase-3 processed during apoptosis31 was detected in extracts from 606HS2-EzNter cells on Western blotting (Figure 2E).
Expression of EzWT and EzNter in HS2 and HS1 proerythroblasts. (A) 606HS2 cells were transfected with either pEF-neo EzWT-VSVG or pEF-neo EzNter-VSVG or pEF-neo empty vector. Whole-cell lysates from 606HS2-EzWT cells, 606HS2-EzNter cells, and 606HS2-neo cells were subjected to Western blotting using anti-VSVG and anti-ezrin antibodies. The membrane was reprobed with an anti–β-actin antibody as a loading control. The apparent molecular weight of endogenous and EzWT-VSVG (80 kDa) and the apparent molecular weight of EzNter-VSVG (40 kDa) are indicated on the left of the panel. Fold-increase in ezrin expression in 606HS2-EzNter and 606HS2-EzWT was calculated in comparison to 606 HS2-neo cells. Notably, because the anti-ezrin antibody is directed against the C terminal region of ezrin, EzNter could not be detected. (B) Proliferation of 606HS2-EzWT, 606HS2-EzNter, and 663HS1-neo cells. Data are means plus or minus SD of 4 independent experiments performed in triplicate. (C) The percentage of dead cells was determined by Trypan blue exclusion assay on 606HS2-EzWT cells, 606HS2-EzNter cells, and 606HS1-neo cells at 24 hours and 48 hours of culture. Data are issued from the experiment presented in panel B. (D) Detection of fragmented and condensed nuclei in apoptotic cells by fluorescence microscopy. Representative images after Hoechst staining of 606HS2-EzWT cells and 606HS2-EzNter cells cultured for 48 hours. Arrows indicate apoptotic bodies. A total of 3 different fields (500 cells per field) were scored by 2 investigators. Percentage of apoptosis is indicated as means plus or minus SD of 3 independent experiments. Bar corresponds to 15 μm. (E) Processing of caspase-3 in 606HS2-EzWT cells, 606HS2-EzNter cells, and 663HS1-neo cells. WCEs were subjected to Western blotting using an anti-cleaved caspase-3 p17. β-actin served as a loading control. (F) 663HS1 cells were transfected with either pEF-neo EzWT-VSVG, pEF-neo EzNter-VSVG, or pEF-neo empty vector. Whole-cell lysates were subjected to Western blot using anti-VSVG and anti-ezrin antibodies. The membrane was reprobed with an anti–β-actin antibody as a loading control. Living 663HS2-EzWT, 663HS2-EzNter, and 663HS1-neo cells were numbered, and percentages of dead cells were determined by Trypan blue exclusion assay at 24 hours and 48 hours of culture. Data are means plus or minus SD of 3 independent experiments performed in triplicate.
Expression of EzWT and EzNter in HS2 and HS1 proerythroblasts. (A) 606HS2 cells were transfected with either pEF-neo EzWT-VSVG or pEF-neo EzNter-VSVG or pEF-neo empty vector. Whole-cell lysates from 606HS2-EzWT cells, 606HS2-EzNter cells, and 606HS2-neo cells were subjected to Western blotting using anti-VSVG and anti-ezrin antibodies. The membrane was reprobed with an anti–β-actin antibody as a loading control. The apparent molecular weight of endogenous and EzWT-VSVG (80 kDa) and the apparent molecular weight of EzNter-VSVG (40 kDa) are indicated on the left of the panel. Fold-increase in ezrin expression in 606HS2-EzNter and 606HS2-EzWT was calculated in comparison to 606 HS2-neo cells. Notably, because the anti-ezrin antibody is directed against the C terminal region of ezrin, EzNter could not be detected. (B) Proliferation of 606HS2-EzWT, 606HS2-EzNter, and 663HS1-neo cells. Data are means plus or minus SD of 4 independent experiments performed in triplicate. (C) The percentage of dead cells was determined by Trypan blue exclusion assay on 606HS2-EzWT cells, 606HS2-EzNter cells, and 606HS1-neo cells at 24 hours and 48 hours of culture. Data are issued from the experiment presented in panel B. (D) Detection of fragmented and condensed nuclei in apoptotic cells by fluorescence microscopy. Representative images after Hoechst staining of 606HS2-EzWT cells and 606HS2-EzNter cells cultured for 48 hours. Arrows indicate apoptotic bodies. A total of 3 different fields (500 cells per field) were scored by 2 investigators. Percentage of apoptosis is indicated as means plus or minus SD of 3 independent experiments. Bar corresponds to 15 μm. (E) Processing of caspase-3 in 606HS2-EzWT cells, 606HS2-EzNter cells, and 663HS1-neo cells. WCEs were subjected to Western blotting using an anti-cleaved caspase-3 p17. β-actin served as a loading control. (F) 663HS1 cells were transfected with either pEF-neo EzWT-VSVG, pEF-neo EzNter-VSVG, or pEF-neo empty vector. Whole-cell lysates were subjected to Western blot using anti-VSVG and anti-ezrin antibodies. The membrane was reprobed with an anti–β-actin antibody as a loading control. Living 663HS2-EzWT, 663HS2-EzNter, and 663HS1-neo cells were numbered, and percentages of dead cells were determined by Trypan blue exclusion assay at 24 hours and 48 hours of culture. Data are means plus or minus SD of 3 independent experiments performed in triplicate.
The VSVG-ezrinNter expression construct was also stably-transfected into 663HS1 cells. The Epo-dependent proliferation rates, and the viability of 663HS1-EzNter and 663HS1-EzWT cells were compared. Figure 2F shows that the overexpression of VSVG-ezrinNter in HS1 cells induced a profound impairment of proliferation and survival.
Altogether, these findings indicate that the expression of ezrinNter acts in a dominant-negative way on HS2 as well as HS1 cells, suggesting that endogenous ezrin participitates in controlling growth and survival of proerythroblasts.
Tyrosine phosphorylation of ezrin depends on the tyrosine kinase activity of oncogenic Kit mutants in HS2 leukemic cells
Ezrin is known to be a substrate for tyrosine kinases. We recently showed that mutations in the kit gene occurred in 86% of HS2 tumors. These mutations target either amino acid residue 814 (D814Y) or 818 (D818Y), causing the autophosphorylation of the SCF receptor and consequently the activation of downstream mitogen-activated protein (MAP) kinase and PI3 kinase pathways.6
As shown in Figure 1, ezrin was found overexpressed in HS2 cells harboring Kit mutations, but not in the 622 HS2 cells that were autocrine for Epo and not mutated for Kit.6
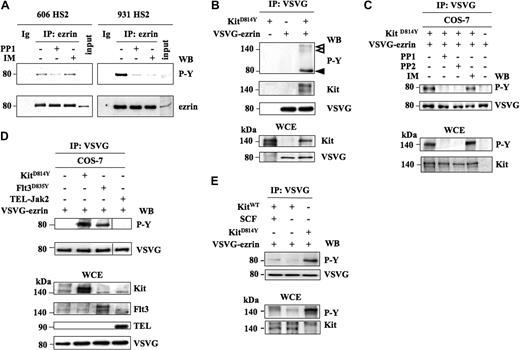
This prompted us to investigate whether Kit mutants were involved in the phosphorylation of ezrin. At first, the tyrosine phosphorylation status of ezrin was investigated in 2 HS2 leukemic cell lines, one harboring the Kit mutation D814Y (606HS2 cells) and one harboring the D818Y mutation (931HS2 cells). Ezrin was immunoprecipitated and analyzed by Western blotting using antiphosphotyrosine antibodies. Tyrosine phosphorylation of ezrin was detected in both cell lines treated with pervanadate (Figure 3A). Moreover, this phosphorylation was abolished when the 931HS2 cells were treated with PP1 or imanitib mesylate (IM), 2 compounds that we previously used as inhibitors of the KitD818Y tyrosine kinase activity.6 In the 606 HS2 cells, ezrin phosphorylation was abolished by PP1, but not altered by IM as expected from the resistance of KitD814Y to IM.6 Thus, ezrin is phosphorylated on tyrosine in HS2 cells, and this phosphorylation is associated with the Kit mutant activities.
Ezrin phosphorylation depends on the kinase activity of Kit mutants. (A) Ezrin is tyrosine-phosphorylated in 606 and 931 HS2 cells. Cells were treated or not for 4 hours with Kit inhibitors: PP1 (5 μM) and IM (1 μM). Ezrin was immunopreciptated from cell extracts treated with 0.1 mM pervanadate for 10 minutes. Immunoprecipitates were analyzed by Western blot using antiphosphotyrosine antibodies (PY) followed by an anti-ezrin antibody. Ig corresponds to a rabbit immunoglobulin immunoprecipitation. Inputs corresponded to 1:1000 immunoprecipitated cell extracts from 606 and 931 HS2 cells. (B) Tyrosine phosphorylation of ezrin by KitD814Y in COS-7 cells. COS-7 cells were transiently transfected with expression vectors coding for EzWT-VSVG and/or Kit mutants. Ezrin was immunoprecipitated from cell extracts with the anti-VSVG antibody, and the immunoprecipitates were analyzed by Western blot using antiphosphotyrosine (4G10), anti-kit, and anti-VSVG antibodies. The phosphorylated proteins are indicated by arrowheads. VSVG-Ezrin and Kit expression were controlled in WCEs from transfected cells with the indicated antibodies. (C) Sensitivity of the ezrin/Kit interaction to PP1, PP2, and IM. COS-7 cells were transiently transfected with expression vectors coding for EzWT-VSVG and/or KitD814Y and treated or not for 1 hour with PP1 (5 μM), PP2 (5 μM), or IM (1 μM). Cell extracts were treated with pervanadate (0.1 mM for 10 minutes) and subjected to immunoprecipitation with anti-ezrin antibody. The immunoprecipitates were analyzed by Western blot using antiphosphotyrosine antibodies (P-Y) and anti-VSVG antibodies. Ezrin phosphorylation was detected on Western blot with anti-PY (P-Y) and anti-VSVG antibodies. The expression and phosphorylation of Kit were controlled on WCEs with the anti-PY and anti-Kit antibodies. (D) Tyrosine phosphorylation of ezrin by the oncogenic Flt3D835Y or TEL-Jak2 kinases. COS-7 cells were transfected with expression vectors coding for KitD814Y or Flt3D835Y or TEL-Jak2 and VSVG-ezrin. Ezrin was immunoprecipitated from transfected COS-7 cell extracts with the anti-VSVG antibody, and the immunoprecipitates were analyzed by Western blot using antiphosphotyrosine (P-Y) and anti-VSVG antibodies. The expression of the transfected proteins in WCEs was confirmed by Western blotting using the antibodies indicated on the right of the blots. Vertical lines have been inserted to indicate repositioned gel lanes. (E) Tyrosine phosphorylation of ezrin by wild-type Kit. COS-7 cells were transfected with expression vectors coding for KitWT and VSVG-ezrin in the presence or in the absence of SCF. Ezrin was immunoprecipitated from transfected COS-7 cell extracts with the anti-VSVG antibody, and the immunoprecipitates were analyzed by Western blot using antiphosphotyrosine (P-Y) and anti-VSVG antibodies. The expression and phosphorylation of Kit in WCEs were confirmed by Western blotting using the anti–P-Y and anti-Kit antibodies.
Ezrin phosphorylation depends on the kinase activity of Kit mutants. (A) Ezrin is tyrosine-phosphorylated in 606 and 931 HS2 cells. Cells were treated or not for 4 hours with Kit inhibitors: PP1 (5 μM) and IM (1 μM). Ezrin was immunopreciptated from cell extracts treated with 0.1 mM pervanadate for 10 minutes. Immunoprecipitates were analyzed by Western blot using antiphosphotyrosine antibodies (PY) followed by an anti-ezrin antibody. Ig corresponds to a rabbit immunoglobulin immunoprecipitation. Inputs corresponded to 1:1000 immunoprecipitated cell extracts from 606 and 931 HS2 cells. (B) Tyrosine phosphorylation of ezrin by KitD814Y in COS-7 cells. COS-7 cells were transiently transfected with expression vectors coding for EzWT-VSVG and/or Kit mutants. Ezrin was immunoprecipitated from cell extracts with the anti-VSVG antibody, and the immunoprecipitates were analyzed by Western blot using antiphosphotyrosine (4G10), anti-kit, and anti-VSVG antibodies. The phosphorylated proteins are indicated by arrowheads. VSVG-Ezrin and Kit expression were controlled in WCEs from transfected cells with the indicated antibodies. (C) Sensitivity of the ezrin/Kit interaction to PP1, PP2, and IM. COS-7 cells were transiently transfected with expression vectors coding for EzWT-VSVG and/or KitD814Y and treated or not for 1 hour with PP1 (5 μM), PP2 (5 μM), or IM (1 μM). Cell extracts were treated with pervanadate (0.1 mM for 10 minutes) and subjected to immunoprecipitation with anti-ezrin antibody. The immunoprecipitates were analyzed by Western blot using antiphosphotyrosine antibodies (P-Y) and anti-VSVG antibodies. Ezrin phosphorylation was detected on Western blot with anti-PY (P-Y) and anti-VSVG antibodies. The expression and phosphorylation of Kit were controlled on WCEs with the anti-PY and anti-Kit antibodies. (D) Tyrosine phosphorylation of ezrin by the oncogenic Flt3D835Y or TEL-Jak2 kinases. COS-7 cells were transfected with expression vectors coding for KitD814Y or Flt3D835Y or TEL-Jak2 and VSVG-ezrin. Ezrin was immunoprecipitated from transfected COS-7 cell extracts with the anti-VSVG antibody, and the immunoprecipitates were analyzed by Western blot using antiphosphotyrosine (P-Y) and anti-VSVG antibodies. The expression of the transfected proteins in WCEs was confirmed by Western blotting using the antibodies indicated on the right of the blots. Vertical lines have been inserted to indicate repositioned gel lanes. (E) Tyrosine phosphorylation of ezrin by wild-type Kit. COS-7 cells were transfected with expression vectors coding for KitWT and VSVG-ezrin in the presence or in the absence of SCF. Ezrin was immunoprecipitated from transfected COS-7 cell extracts with the anti-VSVG antibody, and the immunoprecipitates were analyzed by Western blot using antiphosphotyrosine (P-Y) and anti-VSVG antibodies. The expression and phosphorylation of Kit in WCEs were confirmed by Western blotting using the anti–P-Y and anti-Kit antibodies.
To investigate a potential interaction between ezrin and KitD814Y, we used COS-7 cells that do not express germinal Kit. Cells were transiently transfected with expression vectors coding for VSVG-ezrinWT and/or KitD814Y, and coimmunoprecipitation experiments with an anti-VSVG antibody were performed. Tyrosine-phosphorylated proteins associated with ezrin were assessed by immunoblotting using an antiphosphotyrosine, an anti-Kit, and an anti-VSVG antibody, successively. Then, the expression of transfected proteins was controlled by immunoblotting of whole-cell extracts (WCEs) with anti-Kit and anti-VSVG antibodies. As shown in Figure 3B, a 140-kDa tyrosine-phosphorylated protein corresponding to Kit was coimmunoprecipitated with tyrosine-phosphorylated VSVG-ezrin in Kit-transfected cells. This phosphorylation of ezrin was dependent on Kit kinase activities because a treatment of COS-7 cells coexpressing ezrin and KitD814Y by PP1 or PP2 abolished the tyrosine phosphorylation of ezrin (Figure 3C), whereas an IM treatment did not.
Altogether, these data argue for ezrin as a direct substrate of Kit tyrosine kinase activity in vivo.
To test whether ezrin phosphorylation was limited to the Kit mutants, 2 constitutively activated tyrosine kinases were assayed: the mutated Flt3 receptor (Flt3D835Y) frequently identified in human acute myeloid leukemias (AML),32 and the TEL-Jak2 fusion protein expressed in chronic myeloproliferative disease33 or acute lymphoblastic leukemias.34 The expression vectors were transfected into COS-7 cells along with the VSVG-ezrin expression vector, and the expression of transfected proteins was controlled by immunoblotting of WCEs with appropriate antibodies (Figure 3D). The immunoprecipitated ezrin was tyrosine-phosphorylated when expressed with Flt3D835Y but not with TEL-Jak2 (Figure 3D; top subpanel). Altogether, the data indicate that ezrin is phosphorylated on tyrosine by either KitD814Y or Flt3D835Y, these proteins being constitutively activated mutants of the class III tyrosine kinase receptor family.
Finally, we determined that ezrin could also be a target for the wild-type Kit stimulated by its ligand SCF. In COS-7 cells cotransfected with expression vectors coding for ezrin and KitWT, ezrin is tyrosine-phosphorylated in the presence of SCF, though with a poor efficiency (Figure 3E).
Ezrin phosphorylation on Y145 and Y353 residues influences the proliferation and the survival of HS2 leukemic cells
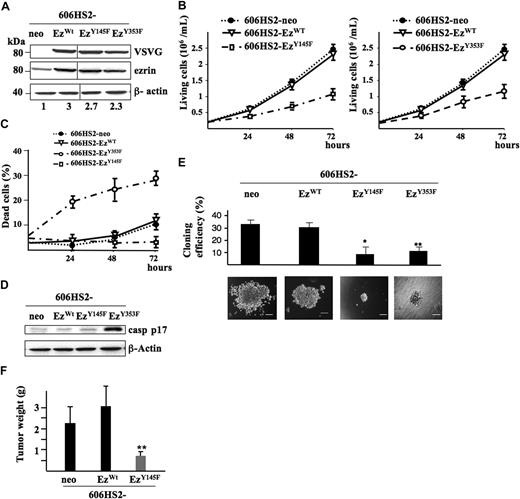
Ezrin has 2 major tyrosine residues (Y145 and Y353) that can be phosphorylated in response to stimulation by some growth factors, such as EGF, and are important for ezrin signaling in epithelial cells.12 To determine whether residues Y145 and Y353 are involved in ezrin function in HS2 cells, we generated stable transfectants of 606HS2 cells and 931HS2 cells with expression constructs coding for VSVG-ezrinY145F and VSVG-ezrinY353F. The expression level of transfected proteins were analyzed by Western blotting using an anti-VSVG and an anti-ezrin antibody, successively. A total of 2 independent clones of 606HS2-EzY145F and 606HS2-EzY353F and 2 independent clones of 931HS2-EzY145F and 931HS2-EzY353F cells were analyzed in Figure 2 and Table S2. Whatever the constructs (EzrinWT, EzrinY145F, or ErzinY353F), the expression level of ezrin was less than 3-fold over the control (Figure 4A).
Expression of ezrinWT, ezrinY145F, and ezrinY353F in HS2 leukemic proerythroblasts. (A) 606HS2 cells were transfected with pEF-neo EzWT-VSVG, pEF-neo EzY145F-VSVG, pEF-neo EzY353F-VSVG, or pEF-neo empty vector. WCEs were subjected to Western blot using anti-VSVG and anti-ezrin antibodies, and an anti–β-actin antibody as a loading control. The fold increase in ezrin expression is indicated at the bottom of each line. Vertical lines have been inserted to indicate repositioned gel lanes. (B) Proliferation of 606 cells expressing ezrinWT, ezrinY145F, or ezrinY145F. Cells transfected with the pEF-neo vector were used as control. Living cells were plated at 2 × 105 cells/mL following a ficoll gradient centrifugation to remove dead cells. Viable cells were scored daily for 72 hours. Data are means plus or minus SD of 5 independent experiments performed in triplicate. (C) Percentage of death of 606HS2-EzWT, 606HS2-EzY145F, 606HS2-EzY353F, and 606HS2-neo cells. Dead cells were scored daily by Trypan blue exclusion staining. Data are means plus or minus SD of 5 independent experiments performed in triplicate. (D) Processing of caspase-3 in 606HS2-EzWT, 606HS2-EzY145F, 606HS2-EzY353F, and 606HS2-neo cells. After 48 hours of culture, WCEs were subjected to Western blotting using an anti-cleaved caspase-3 antibody (casp p17). β-actin served as a loading control. (E) Colony formation assayed in methylcellulose. A total of 400 606HS2-EzWT, 606HS2-EzY145F, 606HS2-EzY353F, and 606HS2-neo cells were seeded onto 1% methylcellulose-containing medium, and colonies were scored after 6 days. Data represent means plus or minus SD of 6 independent experiments performed in duplicate by 2 investigators. Statistical significant differences were estimated by a 2-tailed Student t test (*P < .001, 606HS2-EzWT and 606HS2-EzY145F cells; **P < .005, 606HS2-EzWT and 606HS2-EzY353F cells). Representative images of colonies after 6 days of culture were acquired with a Nikon Eclipse TE300 microscope (Nikon, Champigny sur Marne, France). Bars correspond to 80 μm. (F) In vivo tumorigenicity. Subcutaneous tumors grown in nude mice injected with 606HS2-EzWT, 606HS2-EzY145F, or 606HS2-neo cells were extracted 3 weeks after injection and weighed. Two independent clones of 606HS2-EzY145F and 606HS2-EzWT or 606HS2-neo cells were each injected into 8 mice. Histograms are the compilation (mean ± SD) of these 2 independent experiments. A bar represents the mean-weight of 16 tumors. **Significant tumor weight reduction in 606HS2-EzY145F compared with 606HS2- EzWT (Student t test; P < .001).
Expression of ezrinWT, ezrinY145F, and ezrinY353F in HS2 leukemic proerythroblasts. (A) 606HS2 cells were transfected with pEF-neo EzWT-VSVG, pEF-neo EzY145F-VSVG, pEF-neo EzY353F-VSVG, or pEF-neo empty vector. WCEs were subjected to Western blot using anti-VSVG and anti-ezrin antibodies, and an anti–β-actin antibody as a loading control. The fold increase in ezrin expression is indicated at the bottom of each line. Vertical lines have been inserted to indicate repositioned gel lanes. (B) Proliferation of 606 cells expressing ezrinWT, ezrinY145F, or ezrinY145F. Cells transfected with the pEF-neo vector were used as control. Living cells were plated at 2 × 105 cells/mL following a ficoll gradient centrifugation to remove dead cells. Viable cells were scored daily for 72 hours. Data are means plus or minus SD of 5 independent experiments performed in triplicate. (C) Percentage of death of 606HS2-EzWT, 606HS2-EzY145F, 606HS2-EzY353F, and 606HS2-neo cells. Dead cells were scored daily by Trypan blue exclusion staining. Data are means plus or minus SD of 5 independent experiments performed in triplicate. (D) Processing of caspase-3 in 606HS2-EzWT, 606HS2-EzY145F, 606HS2-EzY353F, and 606HS2-neo cells. After 48 hours of culture, WCEs were subjected to Western blotting using an anti-cleaved caspase-3 antibody (casp p17). β-actin served as a loading control. (E) Colony formation assayed in methylcellulose. A total of 400 606HS2-EzWT, 606HS2-EzY145F, 606HS2-EzY353F, and 606HS2-neo cells were seeded onto 1% methylcellulose-containing medium, and colonies were scored after 6 days. Data represent means plus or minus SD of 6 independent experiments performed in duplicate by 2 investigators. Statistical significant differences were estimated by a 2-tailed Student t test (*P < .001, 606HS2-EzWT and 606HS2-EzY145F cells; **P < .005, 606HS2-EzWT and 606HS2-EzY353F cells). Representative images of colonies after 6 days of culture were acquired with a Nikon Eclipse TE300 microscope (Nikon, Champigny sur Marne, France). Bars correspond to 80 μm. (F) In vivo tumorigenicity. Subcutaneous tumors grown in nude mice injected with 606HS2-EzWT, 606HS2-EzY145F, or 606HS2-neo cells were extracted 3 weeks after injection and weighed. Two independent clones of 606HS2-EzY145F and 606HS2-EzWT or 606HS2-neo cells were each injected into 8 mice. Histograms are the compilation (mean ± SD) of these 2 independent experiments. A bar represents the mean-weight of 16 tumors. **Significant tumor weight reduction in 606HS2-EzY145F compared with 606HS2- EzWT (Student t test; P < .001).
The growth kinetics of 606HS2-EzY145F and 606HS2-EzY353F cells were compared with those of 606HS2-EzWT and 606HS2-neo cells. A significant reduction in cell proliferation was seen at 48 and 72 hours (Figure 4B). Then, cell viability was analyzed by trypan blue exclusion (Figure 4C). Although ezrinY145F reduced the expansion of 606HS2 cells, no alteration in cell viability was seen even after 72 hours. In contrast, 28% plus or minus 5% of cells expressing ezrinY353F were dead after 72 hours of culture. These cells died by apoptosis as deduced from the detection of cleaved caspase-3 in cell extracts (Figure 4D). These observations were confirmed in clonogenic assays. The cloning efficiency and the size of colonies derived from 606HS2-EzY145F and 606HS2-EzY353F cells were significantly reduced compared with 606HS2-EzWT and 606HS2-neo control cells (Figure 4E). In addition, morphologic examination of cells forming the colonies showed striking differences. Whereas 606HS2-EzY145F cells had no evident cell morphology alterations, 606HS2-EzY353F cells exhibited morphologic characteristics of cell death. The ability of ezrinY353F to induce cell death impeded further biological and biochemical investigations with HS2 cells transfected with EzrinY353F.
The relevance of the reduced 606HS2-EzY145F cell proliferation in vitro to tumor development in vivo was tested. 606HS2-EzY145F, 606HS2-EzWT, or 606HS2-neo cells were injected subcutaneously into nude mice, and the tumor growth was measured 3 weeks after graft. The tumors formed by 606HS2-EzY145F cells were significantly smaller (around 3-fold) than tumors formed by 606HS2-EzWT cells or 606HS2-neo cells (Figure 4F), indicating that expression of ezrinY145F mutant in HS2 cells reduced their tumorigenic potential in vivo.
Altogether, these data suggest that Y145 and Y353 phosphorylations are required for the function of ezrin in HS2 leukemic cells, and that these tyrosine phosphorylations participate in different pathways to control either the growth or the survival of leukemic proerythroblasts.
Ezrin phosphorylation at residue Y145 is involved in cell-cycle progression
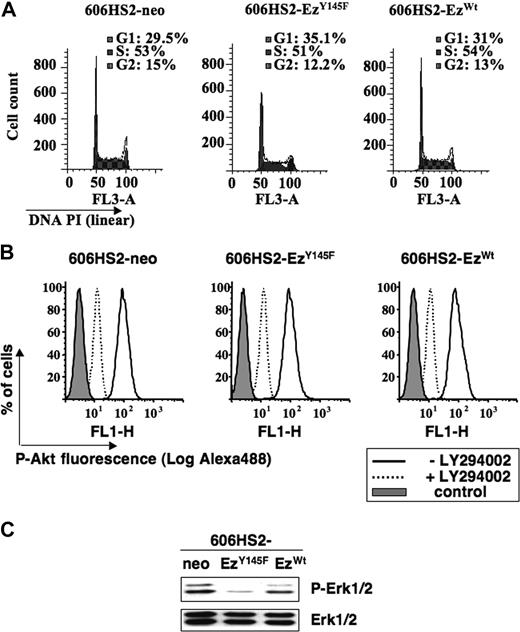
We investigated further whether the proliferative disadvantage of 606HS2-EzY145F cells was related to a modification in the cell cycle. A flow cytometric analysis of the DNA content after PI incorporation at 24 hours or at 48 hours (similar results not shown) shows that cells transfected with ezrinY145F or ezrinWT or neo vector exhibited a very similar repartition into the phases of cell cycle (Figure 5A).
Expression of ezrinY145F slows down the cell cycle of HS2 leukemic proerythroblasts. (A) Cell-cycle analysis of 606HS2-neo, 606HS2-EzY145F, and 606HS2-EzWT cells. Cells were seeded at 2 × 105 cells/mL. After 24 hours of culture, cells were ethanol-fixed, and the DNA content was estimated after PI incorporation by flow cytometry. Percentage of G0/G1, S, and G2/M phases calculated with ModFit software is indicated. Graphs (FlowJo representation) for each clone are representative of 3 independent experiments. Similar results were obtained after 48 hours. (B) Inhibition of Akt activation in 606HS2-EzWT, 606HS2-EzY145F, and 606HS2-neo cells cultured in the presence or absence of LY294002 (10 μM) for 24 hours. Cells were fixed with cytofix cytoperm buffer (BD Biosciences, Le Pont de Claix, France) and labeled with anti–phospho-Akt. The primary antibody was revealed with the anti-rabbit Alexa-488–conjugated antibody (Jackson ImmunoResearch, Montluçon, France) before flow cytometry analysis. Control corresponds to cells incubated with the secondary Alexa-488–conjugated antibody. Graphs are representative of 2 independent experiments. (C) Activation of Erk1/2 in 606HS2-EzWT, 606HS2-EzY145F, and 606HS2-neo cells. Total cell extracts were subjected to immunoblotting with antibodies indicated on the right of the blots. Western blots are representative from 3 independent experiments.
Expression of ezrinY145F slows down the cell cycle of HS2 leukemic proerythroblasts. (A) Cell-cycle analysis of 606HS2-neo, 606HS2-EzY145F, and 606HS2-EzWT cells. Cells were seeded at 2 × 105 cells/mL. After 24 hours of culture, cells were ethanol-fixed, and the DNA content was estimated after PI incorporation by flow cytometry. Percentage of G0/G1, S, and G2/M phases calculated with ModFit software is indicated. Graphs (FlowJo representation) for each clone are representative of 3 independent experiments. Similar results were obtained after 48 hours. (B) Inhibition of Akt activation in 606HS2-EzWT, 606HS2-EzY145F, and 606HS2-neo cells cultured in the presence or absence of LY294002 (10 μM) for 24 hours. Cells were fixed with cytofix cytoperm buffer (BD Biosciences, Le Pont de Claix, France) and labeled with anti–phospho-Akt. The primary antibody was revealed with the anti-rabbit Alexa-488–conjugated antibody (Jackson ImmunoResearch, Montluçon, France) before flow cytometry analysis. Control corresponds to cells incubated with the secondary Alexa-488–conjugated antibody. Graphs are representative of 2 independent experiments. (C) Activation of Erk1/2 in 606HS2-EzWT, 606HS2-EzY145F, and 606HS2-neo cells. Total cell extracts were subjected to immunoblotting with antibodies indicated on the right of the blots. Western blots are representative from 3 independent experiments.
Then, the cell-cycle time of 606HS2-EzY145F and 606HS2-EzWT cells and 606HS2-neo cells was measured after pulse labeling with bromodeoxyuridine. The DNA-BrdU flow cytometry analysis permitted to estimate the potential doubling time and the duration of cell-cycle phases as described by Terry and White.30 Calculation in Table 1 indicates a S phase of 15 hours and a doubling time of around 24 hours for 606HS2-EzY145F cells, whereas the S phase was around 10 hours and the doubling time was less than 14 hours for 606HS2-EzWT and 606HS2-neo cells. This result is consistent with a delayed progression through each phase of the cell cycle in cells expressing ezrinY145F.
Estimation of duration of cell-cycle phases in 606HS2-neo, 606HS2-EzY145F, and 606HS2-EzWT
| Duration . | 606HS2-neo . | 606HS2-EzY145F . | 606HS2-EzWT . |
|---|---|---|---|
| T S, h | 9.8 ± 1.2 | 15.1 ± 0.5 | 10.1 ± 2.0 |
| T G2/M, h | 0.9 ± 0.1 | 2.0 ± 0.2 | 1.1 ± 0.2 |
| Tpot, h | 13.3 ± 1.0 | 23.7 ± 1.6 | 13.8 ± 1.7 |
| Duration . | 606HS2-neo . | 606HS2-EzY145F . | 606HS2-EzWT . |
|---|---|---|---|
| T S, h | 9.8 ± 1.2 | 15.1 ± 0.5 | 10.1 ± 2.0 |
| T G2/M, h | 0.9 ± 0.1 | 2.0 ± 0.2 | 1.1 ± 0.2 |
| Tpot, h | 13.3 ± 1.0 | 23.7 ± 1.6 | 13.8 ± 1.7 |
Duration in hours of S (T S) and G2/M (T G2/M) phases and potential doubling time (Tpot) were calculated for each experiment after a BrdU pulse (3 nM for 15 minutes) followed by a chase of 3 hours. Values represent means plus or minus SD of 3 independent experiments.
We previously showed the importance of PI3K/Akt and Erk1/Erk2 signaling pathways in the proliferation and survival of HS2 cells.29 We thus evaluated whether the expression of ezrinY145F would influence the phosphorylation of Akt and Erk1/Erk2. As shown by fluorescence-activated cell sorter (FACS) analysis (Figure 5B), the expression of ezrinY145F did not change the levels of Akt phosphorylation, agreeing with the absence of apoptotic cells in Figure 4. The levels of phosphorylated Erk1/Erk2 were compared in total extracts from 606HS2-EzY145F, 606HS2-EzWT, and 606HS2-neo cells by Western blotting using appropriate antibodies. Figure 5C shows that Erk1/2 phosphorylation was strongly reduced in 606HS2-EzY145F cells compared with 606HS2-EzWT cells and 606HS2-neo cells. Thus, in agreement with the proliferation delay induced by ezrinY145F, the phosphorylation of ezrin on residue Y145 appears associated to activation of Erk1/2.
Discussion
The erythroleukemia that develops in spi-1 transgenic mice evolves as a multistep process. The early step is characterized by an accumulation of erythroblasts arrested at a proerythroblastic stage that remain strictly dependent from Epo for survival and proliferation. These preleukemic cells (HS1) result from the ectopic overexpression of the transcription factor Spi-1/PU.1 in the proerythroblast. Later on, leukemic proerythroblasts (HS2 cells) characterized by autonomous growth and tumorigenicity emerge as a consequence of acquired somatic mutations in the tyrosine kinase receptor Kit. In the present work, we show that ezrin expression is enhanced in leukemic HS2 proerythroblasts compared with preleukemic HS1 cells, and we provide evidence that the oncogenic forms of Kit are responsible for ezrin phosphorylation. By gene transfer of dominant-negative ezrin variants, we demonstrate that inhibition of ezrin function promotes a delay in cell proliferation and alters the apoptosis-resistant phenotype of the HS2 leukemic cells. Together, these findings point to a new role for ezrin as a signaling player in the development of hematopoietic malignancies.
Functions of ezrin are well-defined in epithelial cells. Ezrin is a membrane cytoskeletal cross-linker that participates in several growth factor receptors signaling leading to cell survival, differen-tiation, motility, invasion, and cell adhesion. However, the role played by ezrin during normal erythroid differentiation remains poorly documented. No abnormality during erythroid development has been reported in ezrin knockout (vil2-null) mice, which die with an intestinal epithelium disorganization.35 Nevertheless, the protein is detected in the marginal band of chicken erythrocytes, suggesting a role in the cytoskeletal organization.36 Ezrin is expressed in murine erythrocytes (our personal unpublished data, May 2005), and the data reported herein show that ezrin, like other members of the ERM family, is expressed in spi-1 transgenic proerythroblasts. Strikingly, an enforced expression of the N-terminus domain of ezrin in both preleukemic and leukemic cells resulted in a reduction in cell number and the appearance of apoptotic cells, indicating that ezrin may play a crucial role in proerythroblasts by participating to the viability and proliferation. Because of unanticipated effects of this dominant-negative mutant, future experiments are needed to determine the precise function of ezrin in erythoid cells.
A recurrent feature found in HS2 leukemic cells was that ezrin was overexpressed in leukemic cells, suggesting a distinct role for this protein in the progression toward malignancy. This finding is consistent with several studies that have linked an increase in ezrin expression to a high metastatis potential in a variety of sarcomas and carcinomas.19-25 In these solid tumors, elevated levels of ezrin have been attributed to a transcriptional up-regulation of the ezrin (vil2) gene. Notably, we observed that the transcription pattern of the vil2 gene was similar in HS1 and HS2 cells (our unpublished data from Northern blot and RQ-PCR analysis, March 2004). Moreover, nucleotidic sequencing did not reveal modifications in the primary structure of ezrin in HS2 cells, excluding the idea that mutations induce changes in the protein stability. The mechanism(s) by which ezrin become up-regulated during leukemogenesis remains unknown.
Strikingly, an ectopic overexpression of ezrin in preleukemic proerythroblasts is not sufficient to change the behavior of the cells in terms of Epo dependency for growth and survival. Altogether, these observations were consistent with the idea that the contribution of ezrin overexpression to oncogenesis required additional stimuli specific to leukemic cells.
Besides the observation that ezrin was overexpressed in HS2 leukemic proerythroblasts, we found that ezrin was tyrosine-phosphorylated. Activating mutations in the SCF receptor Kit is a hallmark of HS2 cells.6 Because ezrin overexpression was associated to Kit mutation in HS2 cells (Figure 1A), mutant Kit appeared as a potential player for the tyrosine phosphorylation of ezrin. As a first corroboration, we observed that the tyrosine phosphorylation of ezrin was abolished during treatments of HS2 cells by drugs (PP1 and IM) known to abolish the kinase activities of Kit.6 Consistent with a functional interaction, ezrin and Kit mutants were coimmunoprecipitated in extracts prepared from COS-7 cells cotransfected with both constructs and the phosphorylation of ezrin was sensitive to inhibitors of the kinase activity of Kit, confirming that the Kit kinase activity was important for the tyrosine phosphorylation of ezrin.
In epithelial cells, ezrin is phosphorylated by some tyrosine kinases. Specifically, the tyrosines 145 (Y145) and 353 (Y353) are phosphorylated in response to the stimulation by HGF.15 Phosphorylation of Y353 induces the activation of the PI3 kinase and its downstream effector Akt, providing a mechanism for the function of ezrin in cell survival.16 Notably, Y353 is not present in radixin and moesin. Therefore, the antiapoptotic function associated to Y353 phosphorylation appears specific for ezrin. Phosphorylation of Y145 participates in signaling involved in the control of cell spreading and proliferation.18 Y145 is a substrate for various kinases of the Src family, including Lck in T cells in response to T-cell receptor (TCR) activation17 and Src in epithelial cells in response to EGF stimulation.18 To address the importance of Y145 and Y353 in ezrin function in HS2 leukemic cells, we expressed ezrin mutants point-substituted (Y145F and Y353F) that mimic dephosphorylated ezrin. The massive apoptosis resulting from ezrinY353F expression shows that phosphorylation on residue Y353 induces a survival signal in leukemic cells. In contrast, expression of ezrinY145F in leukemic cells caused a major defect in proliferation both in vitro and in vivo due to an increase in the potential doubling time of the cells, which was associated with an extinction in MAP kinases Erk1/2 activation. Thus, Erk may be an effector of ezrin in cell proliferation, as reported by others in breast carcinoma cells.25 The phosphorylation of both Y145 and Y353 residues provide distinct regulatory signals in controlling cell proliferation and survival, and these findings are in line with previously reported behavior of ezrin in epithelial cells. How Kit phosphorylates ezrin is unknown. Clearly, ezrin phosphorylation is not a usurped pathway for mutated Kit, since ezrin can also be phosphorylated downstream of SCF-stimulated Kit. Kit and ezrin coimmunoprecipitation in COS-7 cells makes ezrin a possible substrate for Kit, but phosphorylation of ezrin can also be carried out by a second kinase activated by Kit. Formally, it will be important to determine whether the tyrosine residues 145 and 353 of ezrin are substrates for Kit kinase in leukemic cells.
This study is the first that emphasizes a role for oncogenic kinases in the phosphorylation of ezrin in tumors. How might ezrin phosphorylation contribute to erythroleukemia associated to Kit mutations? Given the increasing evidences that ezrin is a signal transducer in functions as diverse as cell morphogenesis, adhesion, motility, proliferation, and survival depending on the cell stimuli and cell type,9,37 it is likely that ezrin also behaves as a signal transducer in leukemic cells. The persistent signaling mediated by Kit mutants could block ezrin in a constitutively active form. Phosphorylated tyrosines of ezrin may serve as anchoring residues, enabling the building of constitutive multicomponent transmembrane complexes involved in the activation of signaling pathways including the MAP kinase pathway.
Phosphorylation on Y145 depends on the conformational activation of ezrin through binding to polyphosphoinositodes and threonine phosphorylation downstream of the Rho or protein kinase C (PKC) signaling pathways.10,38 Although threonine 567 is phosphorylated in HS2 cells (data not shown), the mechanism of its phosphorylation is unknown. We previously showed that the constitutive activation of MAP kinases Erk1/2 in HS2 cells is determined by a PKC-dependent mechanism.29 It is tempting to speculate that PKC participates in the process of ezrin activation during the signaling cascade initiated from Kit mutants and leading to Erk1/2 phosphorylation.
Besides ezrin phosphorylation by Kit, we found that the oncogenic form of Flt3 receptor (Flt3D835Y) can also induce the phosphorylation of ezrin on tyrosine. Given that ezrin is also phosphorylated downstream to the PDGF receptor,14 it is possible to postulate that ezrin is a substrate for the class III tyrosine kinase receptors; 2 of them, Kit and Flt3, play a crucial role in hematopoiesis.39 In this regard, the role of phosphorylated ezrin in proliferation and survival might be seen as a general process when tyrosine kinases are activated in cancer cells. The mutant forms of Kit in the HS2 tumors are also found in human mastocytosis and acute myeloid leukemia (AML). Gastrointestinal stromal tumors (GISTs) in humans are associated with Kit-activating mutations that affect the juxtamembrane domain. In these tumors, an up-regulation of ezrin has been correlated to the expression of Kit mutants through a transcriptional process.40,41 As the ezrin phosphorylation level has not been determined in those tumor samples, it is possible that ezrin phosphorylation downstream of Kit mutants occurs similarly to what is observed in murine erythroleukemia. Concerning malignant hemopathies, gain-of-function mutations targeting Flt332,42 and Kit43,44 are detected respectively in approximately 40% and 5% of AML, and are responsive of the acute proliferation of blasts. It will be of interest to make an extensive analysis of the level of expression and phosphorylation of ezrin in human leukemic samples.
Kit may play a role in hematopoietic cells through mechanisms similar to those involving Met in invasiveness and metastatic migration in solid tumors. Met signaling toward Erk is dependent on the formation of a complex with ezrin and adhesion molecules such as the hyaluronic acid receptor CD44.45 CD44 has been implicated in metastasis in a variety of sudies.46 CD44 is expressed in hematopoietic cells and has a role in homing and adhesion.47 Its overexpression in malignant hematopoietic cells is frequent48 and indicative of a bad prognostic. The constitutive anchorage of ezrin in the membrane may promote its interaction with signaling partners such as CD44. This hypothesis remains to be validated in future studies.
These past years, several works have underlined the importance of ezrin overexpression during cell transformation, invasion, and metastasis. Our findings show that tyrosine phosphorylation rather than overexpression is essential in a murine erythroleukemic process. They identify ezrin as an effector of oncogenic tyrosine kinases during a leukemic process and open new perspectives regarding the disruption of ezrin function as an approach for targeting leukemia cell growth and survival. More studies are required to elucidate which downstream pathways are affected by ezrin activation and which genes are regulated in response to this type of signaling in cancer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Z. Maciorowski (Cytometry Department at Institut Curie) for helpful assistance with flow cytometry and cell-cycle analysis and Janssen-Cilag (Issy-les-Moulineaux, France) for the gift of recombinant human Epo. We are grateful to C. Guillouf, P. Rimmelé, O. Kosmider, and D. Buet for many helpful discussions and F. Wendling for her comments on the manuscript.
This work was supported by the Institut National de la Santé et de la Recherche Médicale (Inserm) and Institut Curie, and by the Association pour la Recherche sur le Cancer (grant no. 3822), Fondation de France (grant no. 4778), Ligue contre le Cancer (équipe labellisée 2006 to F.M.-G.), Institut National du Cancer (INCa), and Association Christelle Bouillot. R.M. was a postdoctoral researcher supported by the Fondation de France.
Authorship
Contribution: R.M. performed proteomic profiling, phenotypic characterization of the cell lines, molecular cloning, FACS, and cell-cycle analysis, and COS-7 transfection experiments and tumorigenic assays. L.H. performed proteomic profiling analysis. A.N. contibuted purification of anti-ezrin antibodies and co-immunoprecipitation assays. I.G. performed COS-7 transfection experiments. M.A. provided expertise in ezrin function. P.M. provided expertise in proteomic profiling. F.M.-G. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Françoise Moreau-Gachelin, Inserm U830–Institut Curie, 26 rue d'ulm, 75005 Paris, France; e-mail: framoreau@curie.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal