Abstract
Multiple myeloma (MM) is an incurable plasma cell malignancy with poor outcome. The most promising therapeutic options currently available are combinations of transplantation, targeted pharmacotherapy, and immunotherapy. Cell-based immunotherapy after hematopoietic stem-cell transplantation has been attempted, but with limited efficacy. Natural killer (NK) cells are interesting candidates for new means of immunotherapy; however, their potential clinical use in MM has not been extensively studied. Here, we explored the possibility of expanding NK cells from the peripheral blood of 7 newly diagnosed, untreated MM patients, using good manufacturing practice (GMP)–compliant components. After 20 days of culture, the number of NK cells from these patients had expanded on average 1600-fold. Moreover, expanded NK cells showed significant cytotoxicity against primary autologous MM cells, yet retained their tolerance against nonmalignant cells. Based on these findings, we propose that autologous NK cells expanded ex vivo deserve further attention as a possible new treatment modality for MM.
Introduction
Multiple myeloma (MM) is a malignant neoplasm characterized by clonal proliferation of plasma cells in the bone marrow (BM). This disease accounts for approximately 2% of all cancer deaths and nearly 20% of deaths caused by hematologic malignancies.1 Although allogeneic stem-cell transplantation2-5 occasionally cures these patients, and drugs such as thalidomide, lenalidomide, and bortezomib have improved outcomes,6 high-dose chemotherapy followed by autologous stem-cell transplantation (ASCT)7 still appears to be the best treatment for patients up to 70 years of age. However, the great majority of patients with MM are incurable due to the persistence of minimal residual disease.8,9 Thus, novel modalities complementing or improving current treatment options are needed
Natural killer (NK) cells are cytotoxic lymphocytes that lyse certain tumor- and virus-infected cells without any prior stimulation or immunization.10 The cytotoxic activity of NK cells is tightly controlled by a balance between activating and inhibitory signals from receptors on the cell surface. A main group of receptors that inhibit NK-cell activation is the inhibitory killer immunoglobulin-like receptors (KIRs). Upon recognition of self-MHC class I molecules on target cells, these receptors deliver an inhibitory signal that stops the activating signaling cascade, sparing cells with normal MHC class I expression from NK cell–mediated lysis. Activating receptors include the natural cytotoxicity receptors (NCRs) and NKG2D, all of which push the balance toward cytolytic action through engagement with separate ligands on the target cell surface.11,12
This role of NK cells indicates their possible use for adoptive immunotherapeutic strategies, in particular against malignancies that express low levels of MHC class I molecules.13 The aim of this study was to investigate whether, and under what conditions, NK cells from patients with MM can be expanded numerically using good manufacturing practice (GMP)–compliant components. Furthermore, we aimed to investigate if NK cells expanded ex vivo could target autologous MM cells and thus prove to be favorable candidates for immunotherapeutic approaches against MM.
Methods
Patients and acquisition of patient material
Peripheral blood and BM samples from 7 newly diagnosed patients at different stages of MM were included in this study. The patients were admitted to the Department of Hematology, Karolinska University Hospital Huddinge, Stockholm, Sweden. The study was approved by the south Stockholm research ethics committee. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Patients' characteristics at the time of blood and BM sampling are given in Table 1.
Patient characteristics at the time of blood and bone marrow sampling for this study
| Patient . | Age, y . | Sex . | MM stage, Durie & Salmon . | MM type . |
|---|---|---|---|---|
| 1 | 41 | Male | III | IgG-λ |
| 2 | 80 | Male | I | IgG-λ |
| 3 | 80 | Male | III | Light chain-λ |
| 4 | 75 | Female | II | IgG-λ |
| 5 | 66 | Female | II | IgG-λ |
| 6 | 53 | Female | I | IgG-κ |
| 7 | 64 | Male | II | Light chain-κ |
| Patient . | Age, y . | Sex . | MM stage, Durie & Salmon . | MM type . |
|---|---|---|---|---|
| 1 | 41 | Male | III | IgG-λ |
| 2 | 80 | Male | I | IgG-λ |
| 3 | 80 | Male | III | Light chain-λ |
| 4 | 75 | Female | II | IgG-λ |
| 5 | 66 | Female | II | IgG-λ |
| 6 | 53 | Female | I | IgG-κ |
| 7 | 64 | Male | II | Light chain-κ |
All the patients included in this study were newly diagnosed and had not yet started any treatment regimen at sampling time.
Peripheral blood mononuclear cells (PBMCs) as well as BM mononuclear cells (BMMCs) were isolated by gradient centrifugation, using Lymphoprep (Axis-Shield, Oslo, Norway). PBMCs and BMMCs were washed twice with phosphate-buffered saline (PBS; Gibco, Grand Island, NY), and cell viability was assessed by trypan blue exclusion. To avoid interexperimental variability, PBMCs and BMMCs were directly frozen in human serum albumin (Octapharma, Stockholm, Sweden) containing 6% DMSO (Wak-chemie Medical, Steinbach, Germany) for subsequent phenotyping and cytotoxicity experiments.
Ex vivo expansion of NK cells from PBMCs
Culture conditions for the expansion of cytotoxic cells were optimized previously using PBMCs from healthy individuals.14 Briefly, PBMCs were initially thawed and cultured in T25 flasks (TPP, Trasadingen, Switzerland) at a concentration of 0.5 × 106 cells/mL in CellGro SCGM serum-free medium (CellGenix, Freiburg, Germany) with the addition of 5% human serum (Biowhittaker-Cambrex, Walkersville, MD) and 500 U/mL rhIL-2 (Proleukin; Chiron, Emeryville, CA). For the first 5 days, the medium was supplemented with anti-CD3 antibody (Orthoclone OKT-3; Ortho Biotech, Raritan, NJ) to a final concentration of 10 ng/mL. On day 5 of culture, the OKT-3–containing medium was washed out, and fresh medium with IL-2 (500 U/mL) and 5% human serum was added. The cultures were then replenished with fresh medium every other day throughout the culture period. Total cell numbers were assessed by staining cells with trypan blue dye on days 0, 5 to 6, 9 to 10, 14 to 15, and 20 of culture. Absolute cell counts were calculated by multiplying the total number of cells by the percentage of specific subsets determined by flow cytometry. To prevent contact inhibition of cell growth,15 the cells were transferred to bigger flasks when necessary. The final products were evaluated for purity, viability, phenotype, and cytokine secretion.
For analysis of cytokine secretion, supernatants of day-0 and day-20 cell cultures from patients 1, 3, and 5 were used. Cytokine levels were measured with the human IL-6 Quantikine enzyme-linked immunosorbent assay (ELISA) Kit (R&D Systems, Minneapolis, MN) and BD OptEIA human TNF ELISA Kit (BD Biosciences, San Jose, CA) according to the manufacturers' instructions.
Flow cytometry–based phenotyping of NK cells
The cell phenotype and expansion dynamics of subpopulations were analyzed by flow cytometry on days 0, 5 to 6, 9 to 10, 14 to 15, and 20 of culture using standard procedures with fluorochrome-conjugated mAbs against CD3, CD14, CD38, CD56, and CD138.
Day-0 and day-20 cells from all patients were subjected to a more detailed immunophenotypic analysis. To avoid interacquisition variability, all frozen samples were simultaneously thawed for a detailed phenotypic characterization of the CD56+CD3− (NK) cell subset by flow cytometry. This panel included fluorochrome-conjugated mAbs against the following surface antigens: CD2 (RPA-2.10), CD3 (UCHT-1), CD4 (SK3), CD7 (M-T701), CD8 (HIT8a), CD14 (MOP9), CD16 (3G8), CD19 (HIB19), CD25 (M-A251), CD27 (M-T271), CD38 (HIT2), CD56 (B159), CD57 (NK-1), CD161 (DX12), CD183 (3D12), CD184 (12G5), CD195 (2D7/CCR5), CD197 (1C6/CXCR3), CD226 (DX11), NKB1 (DX9), LFA-1 (HI111), CD62L (DREG56), CD69 (FN50), and CD138 (MI15) purchased from BD Biosciences; CD48 (MEM102), from Biosource (Stockholm, Sweden); CD158B1/B2j (GL183), CD244(2B4) (C1.7), NKG2D (ON71), NKp30 (Z25), NKp44 (Z231), NKp46 (BAB281), LIR-1 (HP-F1), Valpha24 (C15), and Vbeta11 (C21), from Beckman Coulter (Fullerton, CA); and NKG2A (131411), NKG2C (134591), KIR2DL1 (143211), and KIR2DL3 (180701), from R&D Systems.
All antibody stainings for flow cytometry were done according to the following protocol. Fc receptors were blocked by incubation with 1 μg human IgG per 105 cells for 15 minutes on ice. The cells were then washed once with PBS and incubated with appropriate amounts of antibody at 4°C for 30 minutes followed by another wash with PBS. For both panels, LIVE/DEAD Fixable Red Dead Cell Stain (Invitrogen, Carlsbad, CA) was used for dead cell exclusion according to the manufacturer's instructions. Briefly, 1 μL dye was applied to 106 cells resuspended in 1 mL PBS and incubated on ice for 30 minutes. The labeled cells were then washed with PBS and fixed in 4% PFA prior to data acquisition. Cells were analyzed by 9-color flow cytometry (CyAn ADP LX; Dako A/S, Glostrup, Denmark) calibrated with CompBeads and appropriate isotype controls (BD Biosciences). The acquisition data were analyzed with Dako Cytomation Summit software versions 4.2 and 4.3 (Dako A/S) and FlowJo software version 7.2 for PC (Tree Star, Ashland, OR) setting appropriate SSC/FSC gates around the lymphocyte population and using LIVE/DEAD Fixable Red Dead Cell Stain negative cells. From the lymphocyte gate, NK cells were gated as the CD56+CD3− population. NK-like T cells and T cells were gated as CD3+CD56+ and CD3+CD56− populations, respectively. MM cells were gated as CD38+CD138+. In each sample a minimum of 105 cells was analyzed.
For each cell-surface receptor analyzed, mean fluorescence intensity (MFI) values were calculated for day-0 and day-20 samples. To estimate the change in receptor expression, we calculated MFI ratios (MFIday20/MFIday0) for each receptor. When the MFI for day-20 samples was higher than for day 0, the MFI ratio was higher than 1, which indicated the relative extent of up-regulation in that receptor. Likewise, an MFI ratio lower than 1 was interpreted as down-regulation in the expression of that receptor.
Evaluation of cell-mediated cytotoxicity
K562 and RPMI8226 cells were cultured in RPMI 1640 medium (Gibco) supplemented with 10% fetal calf serum (Gibco). PHA blasts were generated by stimulation of PBMCs with 10 μg/mL phytohemagglutinin and 50 U/mL rhIL-2 for 72 hours. The Direct CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec, Auburn, CA) was used according to the manufacturer's instructions for magnetic separation of CD34+ cells from BM samples of patients 1, 3, and 5. In short, following Fc receptor blocking, 150 to 300 × 106 cells were labeled with CD34 MicroBeads and incubated for 30 minutes at 4°C. Magnetically labeled cells were run once through the column. The column was washed with PBS before it was removed from the magnetic-activated cell sorting (MACS) separator and the cells were eluted with PBS. Prior to being used as targets in cytotoxicity assays, the resulting CD34-enriched populations were cultured overnight in X-vivo 15 medium (BioWhittaker-Cambrex) containing 100 ng/mL stem-cell factor to ensure the turnover of CD34 antibody on the surface.
The cytotoxic capacity of NK cells before and after expansion was evaluated in vitro against the above-mentioned targets using either a standard 4-hour 51Cr release assay or a flow cytometry–based cytotoxicity assay (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
For the 51Cr release assay, K562 target cells were labeled with 100 μCi (3.7 MBq) 51Cr for 1 hour at 37°C, washed twice with PBS, and resuspended in 1 mL RPMI medium. A total of 3 × 104 target cells in 100 μL RPMI medium was placed in triplicates into V-bottom 96-well plates and incubated for 4 hours with 100 μL effector cells at appropriate concentrations to obtain effector-target ratios from 0.3:1 to 10:1. Aliquots of supernatants were then counted using a Packard Cobra Auto-Gamma 5000 Series Counting System (Meriden, CT). The percentage of specific 51Cr release was calculated according to the formula: percentage specific release = [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100.
For the flow cytometry–based assays, we used day-0 and day-20 cells as effectors. Target autologous BM samples (all patients), magnetically separated autologous CD34+ cells (patients 1, 3, and 5), autologous PHA blasts (patients 1 and 5), as well as RPMI8226 and K562 controls were labeled with TFL4 reagent from the CytoToxiLux-PLUS kit (OncoImmunin, Gaithersburg, MD) according to the manufacturer's instructions. To compare cytotoxicity of short-term activated and expanded cells, day-5 cells from patients 2, 3, and 5 were also tested for cytotoxicity against the K562 cell line and primary autologous BM samples. In all flow cytometry–based cytotoxicity assays, 5 × 104 labeled target cells were placed in tubes together with different amounts of effector cells to obtain effector-target ratios from 0.3:1 to 10:1 in a final volume of 300 μL RPMI medium and incubated at 37°C for 4 hours. The cells were then washed once with PBS. Following Fc receptor blockade with IgG (1 μg/105 cells) on ice for 20 minutes to avoid antibody-dependent cellular cytotoxicity, the cells were incubated with appropriate amounts of fluorochrome-conjugated mAbs against CD38 and CD138 (when autologous BM samples were used as targets) or CD34 (when magnetically separated CD34+ cells were used as targets) at 4°C for 30 minutes. After washing with PBS, the cells were resuspended in 500 μL PBS containing 5 μg 7-aminoactinomycin D (7-AAD; Invitrogen) and incubated in the dark for an additional 15 minutes at 4°C before data acquisition by flow cytometry.
During analysis of the flow cytometric data, targets cells were isolated from the effector cells by TFL4 positivity and the percentage of live or dead cells was determined using 7-AAD staining on this TFL4+ population as a whole or with further gating on CD38+CD138+ cells (for BM samples as targets) and CD34+ cells (for CD34-enriched samples as targets). Cytotoxicity was assessed according to the following formula: percentage killing = [(experimental death − spontaneous death)/(maximum death − spontaneous death)] × 100.
Analysis of NK-cell degranulation
Day-0 and day-20 cells from patients 2, 5, and 7 were coincubated with target cells at a ratio of 1:1 in a final volume of 200 μL in round-bottomed 96-well plates at 37°C and 5% CO2 for 6 hours. Fluorochrome-conjugated anti-CD107a mAb or the corresponding IgG1 isotype control was added at the initiation of the assay. After 1 hour of coincubation, Monensin (GolgiStop; BD Biosciences) was added at a 1:100 dilution. Surface staining was done by incubating cells with anti-CD3 and anti-CD56 mAbs for 30 minutes on ice. The cells were then washed, resuspended in PBS, and immediately analyzed by flow cytometry.
Results
NK cells from MM patients can be expanded ex vivo
To study whether NK cells from MM patients can be expanded ex vivo using GMP-compliant components, cultures of PBMCs from MM patients were established. At the start of the culture (day 0), the mean percentage of NK cells (CD3−CD56+) was 11% (range: 7%-17%), whereas T cells constituted 56% (range: 36%-81%). Subsequently, NK-cell expansion approached log-linearity after an initial nonproliferative phase of approximately 5 days. By day 20, the total cell population had expanded on average 511-fold (range: 123- to 1545-fold; Figure 1A) and, of these, NK cells had expanded on average 1625-fold (range: 502- to 2658-fold) (Figure 1B). Because expansion of NK cells was relatively higher than that of the other cell types, NK cells dominated the culture toward the end of the incubation, reaching on average 65% of the cells by day 20 (Figure 1C). The percentage of NK-like T cells (CD3+CD56+) did not change significantly during the culture period (day 0: 12%, day 20: 11%), whereas the percentage of T cells (CD3+CD56−) declined following withdrawal of OKT3 at day 5, decreasing to an average of 22%. These results show that NK cells from MM patients can be expanded efficiently ex vivo using the 20-day culture approach described here.
Expansion dynamics of PBMC samples obtained from 7 MM patients and cultured ex vivo for 20 days. (A) Fold expansion of total cell population during the culture period. (B) Fold expansion of different subpopulations within the culture environment. (C) The dynamics of subpopulations throughout the culture period. Results are shown as mean plus or minus SD.
Expansion dynamics of PBMC samples obtained from 7 MM patients and cultured ex vivo for 20 days. (A) Fold expansion of total cell population during the culture period. (B) Fold expansion of different subpopulations within the culture environment. (C) The dynamics of subpopulations throughout the culture period. Results are shown as mean plus or minus SD.
Phenotypic analysis and cytokine production of expanded NK cells
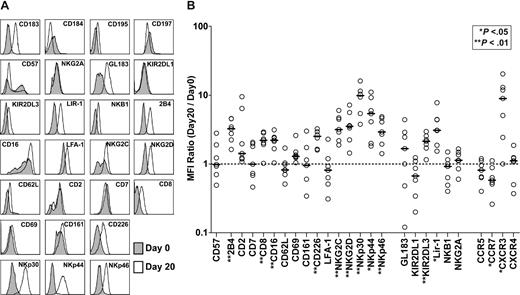
To phenotypically characterize NK cells (CD3−CD56+) in the final expansion product compared with NK cells in the starting material, a detailed flow cytometric analysis was undertaken (representative data from patient 5 appear in Figure 2A). For each receptor analyzed, we calculated MFI values on receptor-positive subsets of NK cells in samples from day-0 and day-20 cultures and used this ratio (MFIday20/MFIday0) as an indicator of the change in receptor expression. Figure 2B illustrates the MFI ratios for all receptors of all patients analyzed as well as the median values. Briefly, in the final product, we observed increased expression of the following activating receptors: 2B4, CD8, CD16, CD27, CD226, NKG2C, NKG2D, NKp30, NKp44, and NKp46. The expression of the inhibitory receptors KIR2DL3 and LIR-1 was also increased. The chemokine receptor CCR7 was expressed at significantly lower levels, whereas CXCR3 was expressed at higher levels in the final product. We observed no significant changes in the expression levels of CD2, CD7, CD57, CD62L, CD69, CD161, LFA-1, GL183, KIR2DL1, NKB1, NKG2A, CCR5, or CXCR4.
Changes in the receptor expression patterns of NK cells following expansion. (A) The surface expression levels of different receptors on day-0 and day-20 NK cells (CD3−CD56+) from all patients were assessed by multicolor flow cytometry as specified. For each receptor, analysis was made on receptor-positive subsets within the total NK-cell population. Representative data (patient 5) showing comparative phenotyping of day-0 (gray) and day-20 (white) cells. (B) Each patient's day-20/day-0 MFI ratios (○) for the cell surface receptors analyzed and the median values (—) are shown. The dashed line shows MFI ratio = 1.
Changes in the receptor expression patterns of NK cells following expansion. (A) The surface expression levels of different receptors on day-0 and day-20 NK cells (CD3−CD56+) from all patients were assessed by multicolor flow cytometry as specified. For each receptor, analysis was made on receptor-positive subsets within the total NK-cell population. Representative data (patient 5) showing comparative phenotyping of day-0 (gray) and day-20 (white) cells. (B) Each patient's day-20/day-0 MFI ratios (○) for the cell surface receptors analyzed and the median values (—) are shown. The dashed line shows MFI ratio = 1.
We also assessed the secretion cytokines including IL-6 and TNF-α in the supernatants from the final day-20 cell product and compared it with supernatants of day-0 cells from patients 1, 3, and 5. IL-6 was slightly decreased in supernatants from day-20 cells (median: 0.25 pg/mL, range: 0.24-0.33 pg/mL) compared with day-0 cells (median: 6.0 pg/mL, range: 3.8-9.9 pg/mL). The secretion of TNF-α was not altered in supernatants of cells from 2 of 3 patients, but slightly increased in supernatant of cells from one patient on day 20 (median: 18.2 pg/mL, range: 4.2-40.6 pg/mL) compared with day-0 cells (median: 2.1 pg/mL, range: 0.9-4.8 pg/mL). These data suggest that cytokine production patterns of these cells are not markedly altered after expansion. Furthermore, levels of cytokines detected in the supernatants were generally lower than serum levels of MM patients.21
NK cells expanded ex vivo show increased cytotoxicity against autologous MM cells
Cytotoxicity against the standard NK-cell target K562 was markedly elevated using effector cells from day-20 cultures, compared with effector cells from day-5 or day-0 cultures (Figure 3A). At a 10:1 effector to target cell ratio, on average 62% of the K562 targets were killed by the day-20 cells, whereas day-0 and day-5 cells killed on average only 8% and 29% of K562 targets, respectively.
Comparison of cytotoxic activity against tumor and normal cells at various time points during expansion. (A) Cytotoxicity of day-0 (all patients), day-5 (patients 2, 3, and 5), and day-20 (all patients) cells against the K562 cell line as measured by the standard 51Cr release assay, and (B) against autologous MM cells and non-MM cells from the BM as measured by the flow cytometry–based assay. (C) Representative data (patient 5) for the flow cytometry–based cytotoxicity assay showing the gating strategy used during analysis. Target cells were identified as the TFL4+ events. Further gating on CD38+ CD138+ cells was used for the analysis of MM cells within the BM samples. The percentage of live/dead cells was evaluated using 7-AAD. (D) Cytotoxicity of day-20 cells against autologous CD34+ cells (patients 1, 3, and 5), autologous PHA blasts (patients 1 and 5), and the RPMI8226 myeloma cell line (patients 1, 3, and 5).
Comparison of cytotoxic activity against tumor and normal cells at various time points during expansion. (A) Cytotoxicity of day-0 (all patients), day-5 (patients 2, 3, and 5), and day-20 (all patients) cells against the K562 cell line as measured by the standard 51Cr release assay, and (B) against autologous MM cells and non-MM cells from the BM as measured by the flow cytometry–based assay. (C) Representative data (patient 5) for the flow cytometry–based cytotoxicity assay showing the gating strategy used during analysis. Target cells were identified as the TFL4+ events. Further gating on CD38+ CD138+ cells was used for the analysis of MM cells within the BM samples. The percentage of live/dead cells was evaluated using 7-AAD. (D) Cytotoxicity of day-20 cells against autologous CD34+ cells (patients 1, 3, and 5), autologous PHA blasts (patients 1 and 5), and the RPMI8226 myeloma cell line (patients 1, 3, and 5).
If considered for clinical immunotherapy, the present NK cells would be useful only if they were able to target autologous MM cells. We thus assessed cytotoxicity against autologous MM cells by a flow cytometry–based assay. The results revealed that day-20 expanded cells provided marked cytotoxic activity against autologous MM cells, whereas neither day-0 nor day-5 cells showed more than low levels of cytotoxicity (Figure 3B). At a 10:1 effector-target ratio, 61% of autologous MM cells were killed by day-20 cells (representative data from patient 5 are shown in Figure 3C and cytotoxicity data of other patients are presented in Figure S2). Notably, no significant cytotoxicity against non-MM (CD138−) cells was observed. Cytotoxicity experiments with day-20 cells from patients 1, 3, and 5 were also performed against magnetically separated autologous CD34+ cells from BM and autologous PHA blasts as well as the MM cell line RPMI8226 as a control (Figure 3D). These studies revealed only baseline levels of cytotoxicity against CD34+ cells and PHA blasts, while a high level of cytotoxic activity against RPMI8226 cells was observed.
Autologous MM cells trigger degranulation of NK cells expanded ex vivo
To better pinpoint the active population within the final expansion product showing cytotoxicity against autologous MM cells, we shifted focus from MM cell lysis to effector cell activation by analyzing the surface expression of CD107a on different subpopulations upon contact with MM cells. CD107a expression correlates closely with degranulation and release of cytotoxic granules.22 Approximately 30% of day-20 cells expressed CD107a on the cell surface upon contact with K562 cell line. Similar degranulation was observed against autologous MM cells (representative data of one patient are shown in Figure 4A). Analysis of these expanded cells showed that NK cells (CD3−CD56+) were the main degranulating population following challenge with autologous MM cells (Figure 4B).
CD107a mAb-based degranulation assay against primary MM cells. (A) Effector cells were incubated alone, with isotype controls, or with target cells in the presence of CD107a mAb as specified. Representative data (patient 5) demonstrating the expression of CD107a on CD3− gated day-20 cells upon coculture with the K562 cell line or autologous MM cells. (B) Comparison of degranulation in different subsets of day-0, -5, and -20 effector cells upon coculture with autologous MM cells for 6 hours. The results are shown as mean percentage of CD107a+ cells (± SD; data from patients 2, 5, and 7).
CD107a mAb-based degranulation assay against primary MM cells. (A) Effector cells were incubated alone, with isotype controls, or with target cells in the presence of CD107a mAb as specified. Representative data (patient 5) demonstrating the expression of CD107a on CD3− gated day-20 cells upon coculture with the K562 cell line or autologous MM cells. (B) Comparison of degranulation in different subsets of day-0, -5, and -20 effector cells upon coculture with autologous MM cells for 6 hours. The results are shown as mean percentage of CD107a+ cells (± SD; data from patients 2, 5, and 7).
Cytotoxicity against autologous MM requires target cell interaction with activating NK-cell receptors
To determine the relative contributions of specific activating receptors to lysis of autologous MM cells, effector cells were preincubated with blocking antibodies against several individual activation receptors, or their combinations, and then coincubated with autologous MM cells. Cytotoxicity was partially inhibited by blocking 2B4, CD226 (DNAM-1), NKG2C, NKG2D, NKp30, NKp44, and NKp46 (Figure 5). This outcome indicates that several activating receptors may contribute to MM cytotoxicity in line with current knowledge of synergy among receptors for induction of cytotoxicity.23
Blocking of activating receptors on NK cells. Preincubation of day-20 effector cells with mAbs directed against NK-cell surface receptors. Relative inhibition of cytotoxicity against autologous MM cells by blocking these receptors is shown using effector cells from 3 patients.
Blocking of activating receptors on NK cells. Preincubation of day-20 effector cells with mAbs directed against NK-cell surface receptors. Relative inhibition of cytotoxicity against autologous MM cells by blocking these receptors is shown using effector cells from 3 patients.
Discussion
Ex vivo expansion and reinfusion of autologous NK cells to MM patients offers a new potentially interesting therapeutic approach to combat this disease. A prerequisite for this is the possibility to expand autologous NK cells from MM patients. In the present study, we demonstrate efficient NK cell expansion ex vivo from PBMCs of MM patients using GMP-compliant components. Furthermore, we demonstrate the ability of these NK cells to kill autologous myeloma cells. These data suggest the possibility of using autologous ex vivo–expanded NK cells for immunotherapy of MM.
Despite the rapid development of new agents, MM continues to be an incurable disease with a fatal outcome in the majority of patients, especially those in advanced stages. Thus, novel therapeutic modalities such as immunotherapy warrant exploration in an attempt to increase patient life expectancy.24 Yet, the impaired immune system in MM patients is evident in their well-recognized susceptibility to infectious complications.25 In the autologous setting, this marked immunodeficiency of MM patients must be overcome for successful induction of an anti-MM response by the patient's own immune system. Recent evidence suggests that the underlying dysfunction of the immune system in MM patients originates, at least in part, from dendritic cells26 or regulatory T cells,27,28 which may impair the immune function in MM and its premalignant condition, monoclonal gammopathy of unknown significance (MGUS). The precise nature of these defects, however, remains at present unknown. Furthermore, there is evidence that progression from premalignant gammopathy to MM is associated with a reversible defect in NKT cell function.
Although previous reports suggest that cytokine activation of NK cells may lead to a better recognition of MM cells,18,29 MM cells are considered resistant to lysis by resting and short-term activated autologous NK cells.30-32 This resistance has been explained by impaired NK cytotoxicity33,34 and increased levels of soluble IL-2 receptors35 in MM patients as well as decreased expression of activating receptors compared with those in healthy controls.36 Our results indicate that expansion and/or long-term activation of NK cells may reverse the latter dysfunction. From our assessment of degranulation measured by expression of CD107a, we conclude that expanded numbers of NK cells were the major effectors of autologous anti-MM activity under the present experimental conditions.
A concern for the use of activated NK cells is that they could cause a tissue-damaging reaction37 or may kill CD34+ cells.38 Our data indicate that the recognition of autologous MM cells by NK cells expanded ex vivo involves a certain degree of specificity. We demonstrated that day-20 cells lysed MM cells but spared nonmyeloma cells in the BM including CD34+ cells, as well as PHA blasts derived from the same patient. Therefore, the various ligands on non-MM cells and simultaneous expression of “self”-ligands for inhibitory NK-cell receptors might prevent NK-cell activation against these cells.
During the present studies, we also phenotyped the expanded NK-cell population to compare the starting material (day 0) with that at day 20 of expansion. Since a balance of activating and inhibitory signals regulates NK-cell function,39 optimal NK-cell effector function is expected when the expression of activating NK- cell receptors is adequate and not suppressed by inhibitory signals. Reduced 2B4 expression in NK cells from MM patients has been suggested to play a role in the immune escape mechanism of MM cells expressing its ligand CD48.36 However, the possibility that such escape might be a consequence of interactions between NK and MM cells cannot be excluded. The up-regulation of 2B4 after expansion ex vivo is likely one factor contributing to the cytotoxicity we observed against autologous MM cells. Furthermore, NCRs and NKG2D, which presumably take part in the recognition of MM cells by NK cells,18 are significantly up-regulated, suggesting possible pathways for autologous MM cell killing. The up-regulation of CD226 (DNAM-1), which is another activating receptor on NK cells40 and a potent inducer of cytotoxicity against many tumor cell lines of hematopoietic and nonhematopoietic origin,17,41 could also contribute to the increase in cytotoxicity. Furthermore, as previously shown, CD27low NK cells are tightly regulated by inhibitory receptors, whereas the CD27high NK-cell subset is more cytotoxic.42 The up-regulation of CD27 during expansion may also contribute to elevated levels of cytotoxicity.
To determine the relative contributions of the foregoing activating receptors, we performed cytotoxicity experiments after blocking receptors with mAbs either alone or in different combinations. We observed no specific association of NK-cell cytotoxicity with a single receptor but, rather, effects were likely mediated by several receptors. Recent data suggest that triggering of natural cytotoxicity, as assessed by studies of resting NK cells, requires coactivation by more than a single receptor.23 Presumably, recognition of tumor cells by NK cells often involves a combination of receptors that synergistically deliver activating signals. Such a phenomenon may, at least in part, explain our observations in blocking experiments and merit further investigation.
Recently published results of clinical trials testing NK cell–based immunotherapy involved the infusion of resting43,44 and short-term IL-2–activated45,46 NK cells into cancer patients. These trials have shown that adoptively transferred NK cells are well tolerated. Our results suggest that the present autologous NK cells expanded ex vivo for 20 days have a promising anti-MM potential compared with resting or short-term activated NK cells. The high levels of NK-cell expansion using GMP-compliant components is of particular importance in relation to reaching an appropriate infusion dose for immunotherapy.
In conclusion, our results demonstrate that NK cells from MM patients can be efficiently expanded ex vivo, and more importantly, NK cells expanded ex vivo showed autologous anti-MM activity in vitro. Moreover, we demonstrate no significant cytotoxicity against normal cells. Alongside research to unravel the mechanisms responsible for the phenomena reported here, the feasibility of using autologous NK cells expanded ex vivo for the management of MM patients is worthy of further exploration to verify the clinical potential of these cells. In this context, we propose that expanded NK cells can be used as a support to ASCT for preemptive treatment of relapse and better eradication of malignant cells in MM patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully acknowledge C. I. E. Smith and M. Hasan for ELISA assays; and B. Baumann, N. Björkström, and K. J. Malmberg for their assistance during the design of the multicolor flow cytometry panel and the flow cytometry–based cellular cytotoxicity assays.
This work was supported by grants from the Swedish Cancer Society, the Swedish Research Council, the Swedish Children's Cancer Foundation, the Cancer Society in Stockholm, the European Union grant Clinigene LSHB-CT-2006-018933, and the Swedish Foundation for Strategic Research.
Authorship
Contribution: E.A. and T.S. designed and performed experiments (cell culture, flow cytometric phenotyping, cytotoxicity, degranulation, and blocking assays), performed data analysis, and wrote the paper; B.B. and H.N. contributed by informing the patients and acquiring patient materials; M.G. and B.S. contributed by performing experiments (primary cell expansion, cryopreservation, and 51Cr release assays); H.C.Q. contributed by phenotyping with 4-color flow cytometry; and G.G., H.-G.L., and M.S.D. helped in designing the experiments and writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sirac Dilber, Department of Medicine, M54, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-14186 Stockholm, Sweden; e-mail: sirac.dilber@ki.se.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal