In this issue of Blood, Saposnik and collegues show that soluble endothelial-cell protein C receptor (sEPCR) is derived not only from proteolytic ectodomain shedding, but also from alternative splicing of A3 haplotype mRNA transcripts and direct secretion of a truncated sEPCR form.
The serine protease activated protein C (APC) is a major natural anticoagulant, which proteolytically inactivates the clotting factors Va and VIIIa. In addition, APC has potent anti-inflammatory and cytoprotective capabilities.1 PC circulates in plasma as a zymogen and is activated by thrombin bound to its endothelial receptor, thrombomodulin. Another protein important for the activation of PC is the endothelial protein C receptor (EPCR), which binds protein C and further enhances its activation by the thrombin/thrombomodulin complex.
EPCR is a 46-kilodalton (kDa) type I transmembrane protein, which is expressed mainly on the luminal endothelial cell surface of large blood vessels and which is homologous to major histocompatibility complex class I/CD1 family proteins. In addition to the transmembrane EPCR, a soluble form of EPCR (sEPCR) has been described in plasma. sEPCR binds PC/APC with an affinity similar to that of membrane EPCR. Binding of APC to sEPCR interferes with binding of APC to phospholipids and inactivation of factor Va. Furthermore, binding of PC to sEPCR does not enhance APC generation, suggesting a procoagulant effect of sEPCR.2
Plasma levels of sEPCR show bimodal distribution: 80% of the healthy population have plasma levels between 75 ng/mL and 178 ng/mL, and 20% have levels between 200 ng/mL and 700 ng/mL.3 There are polymorphisms in the human EPCR gene that define at least 3 different haplotypes. One of them (haplotype A3) is associated with increased plasma levels of soluble EPCR.3 These increased plasma levels have been partially explained by increased shedding of this form.4
Saposnik and collegues have now identified a truncated form of EPCR mRNA that is much more abundant in A3 haplotype EPCR. The deleted sequence (390 base pairs) corresponds to the entire coding region of exon 4 and to a large part of its noncoding region. The truncated mRNA therefore encodes a protein that lacks the transmembrane and intracellular domains, and has a theoretical new C-terminal stretch of 56 amino acids. By transfecting cells with a cDNA that encodes the recombinant EPCR isoform, they also show that this form is not retained in the membrane, but rather is secreted. They also identify a sEPCR form in plasma samples from A3-carrying subjects, which is the same size as the protein arising from the alternatively spliced EPCR mRNA, suggesting that higher plasma levels of sEPCR in the A3-carrying population result not only from increased ectodomain shedding of membrane EPCR, but also from alternative mRNA splicing. Such a dual mechanism of soluble receptor generation has been shownalready for the generation of soluble cytokine receptors.
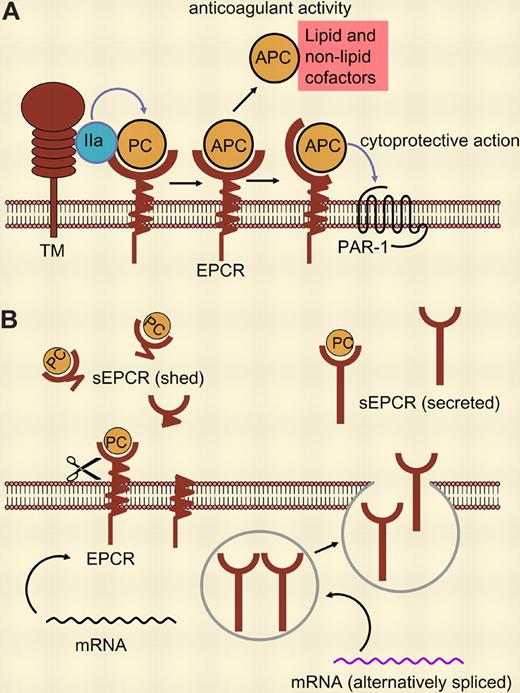
(A) Binding of protein C (PC) to its membrane receptor, EPCR, enhances its activation by thrombin (IIa) bound to thrombomodulin (TM). Activated protein C (APC) can dissociate from its receptor and—in the presence of cofactors—act as anticoagulant. Membrane-receptor–bound APC exerts cytoprotective activities mediated via PAR-1. (B) sEPCR supports neither PC activation nor APC activity. sEPCR can be generated by ectodomain shedding (left) or, as shown by Saposnik and colleagues in this issue of Blood, by alternative mRNA splicing in haplotype-A3–carrying cells, resulting in a protein that is not retained in the membrane but rather secreted (right).
(A) Binding of protein C (PC) to its membrane receptor, EPCR, enhances its activation by thrombin (IIa) bound to thrombomodulin (TM). Activated protein C (APC) can dissociate from its receptor and—in the presence of cofactors—act as anticoagulant. Membrane-receptor–bound APC exerts cytoprotective activities mediated via PAR-1. (B) sEPCR supports neither PC activation nor APC activity. sEPCR can be generated by ectodomain shedding (left) or, as shown by Saposnik and colleagues in this issue of Blood, by alternative mRNA splicing in haplotype-A3–carrying cells, resulting in a protein that is not retained in the membrane but rather secreted (right).
Biological consequences of increased sEPCR (and/or the resulting decreased membrane EPCR density) may be related to increased procoagulant activity (eg, higher risk of thrombosis). There may also be an impact on the cytoprotective activity of APC, which depends on membrane EPCR and PAR-1. For both functions of PC/APC, the A3 haplotype would be unfavorable. However, there may also be beneficial effects of decreased EPCR receptor density on the cell surface (and thus of the A3 haplotype), since it also has been shown that APC mediates breast cancer cell migration through interactions with EPCR and PAR-1.5
Conflict-of-interest disclosure: The author declares no competing financial interests. ■