In this issue of Blood, Ghevaert and colleagues show that a mutation in the cytoplasmic domain of integrin β3 is associated with dominantly inherited macrothrombocytopenia. This mutation is predicted to disrupt a conserved salt bridge with integrin αIIb, and constitutively activates the integrin.
Large platelets are often encountered in the evaluation of thrombocytopenias, but most often these are acquired conditions. A rather long list of inherited thrombocytopenias is now appreciated.1 Among the macrothrombocytopenias, the most commonly responsible genetic variations are heterozygosity for mutations in platelet glycoprotein Ibα and the Q43P polymorphism of β1 tubulin. The molecular regulation of platelet size is poorly understood, but circumstantial evidence supports a role for the platelet cytoskeleton and microtubular system. The lion's share of this evidence stems from the study of genetic defects in inherited disorders of platelet size. Although these disorders are quite uncommon, an understanding of the molecular basis of platelet size is important because subjects with abnormally high platelet volumes have enhanced platelet reactivity and are at risk for recurrent ischemic coronary syndromes.2
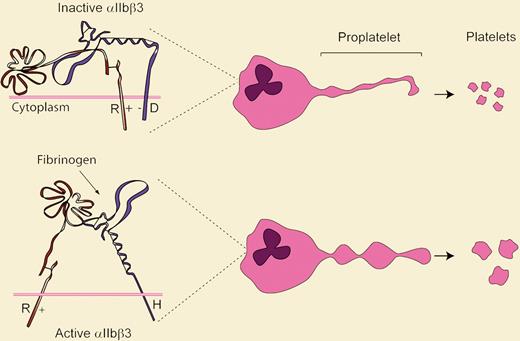
Ghevaert and colleagues performed a thorough series of studies characterizing a pedigree for macrothrombocytopenia, with the mean platelet volume of the propositus measuring a whopping 17 fL. All affected individuals had heterozygous mutations: P53L in the gene encoding GPIbα (GP1BA), and D723H in the gene encoding integrin β3 (INTB3). At least 4 syndromes (Bernard-Soulier, DiGeorge, benign Mediterranean macrothrombocytopenia, and platelet-type von Willebrand disease) are associated with macrothrombocytopenia and GP1BA mutations. However, convincing data were provided to suggest that the GP1BA P53L mutation was not responsible for the phenotype in this pedigree, and molecular modeling predicted that the GP1BA P53L substitution would not affect von Willebrand factor binding. Platelets from subjects with the INTB3 D723H mutation showed spontaneous αIIbβ3 activation, and CHO cells expressing αIIbβ3-D723H displayed enhanced binding to immobilized fibrinogen. Intriguingly, in vitro–differentiated megakaryocytes with the β3-D723H mutation exhibited larger proplatelet buds than the wild type.
It was not long ago that molecular genetic studies were rather primitive compared with the thorough multidisciplinary approaches used by Ghevaert and colleagues to characterize this pedigree. Several novel findings are presented and questions raised. First, integrin mutations should now be added to the list of causes of macrothrombocytopenia. Second, the naturally occurring mutation in β3 supports the existence and function of the conserved intracellular salt bridge between αIIb-R995 and β3-D723 that was proposed using elegant in vitro charge-reversal mutation studies (see figure).3 Third, although the interaction of αIIbβ3 with fibrinogen stimulates proplatelet development,4 a mechanism that integrates the induction of proplatelet formation due to the β3-D723H mutation with the formation of large platelets remains to be established. Perhaps this phenotype results from a simple physical effect whereby increased proplatelet adhesion to bone marrow sinusoidal fibrinogen delays platelet release while size continues to increase. Or perhaps, since integrin binding to extracellular matrix induces gene transcription,5 the enhanced adhesion of β3-D723H induces expression of genes regulating platelet size or favors cross-talk with GPIbα, tubulin, or other cytoskeletal proteins.
Model summarizing how integrin activation state may regulate platelet size. The top panel indicates how the salt bridge between wild-type αIIb-R995+ and β3-D723− constrains the integrin in an inactive conformation. The β3-D723H mutant disrupts this linkage and activates the receptor. Abnormally large platelets result via an unknown mechanism.
Model summarizing how integrin activation state may regulate platelet size. The top panel indicates how the salt bridge between wild-type αIIb-R995+ and β3-D723− constrains the integrin in an inactive conformation. The β3-D723H mutant disrupts this linkage and activates the receptor. Abnormally large platelets result via an unknown mechanism.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal