In this issue of Blood, Dulucq and colleagues report that genetic polymorphisms in the promoter of dihydrofolate reductase (DHFR) are linked to expression and outcome in childhood acute lymphoblastic leukemia (ALL).
The survival of children with ALL has improved steadily over the past 5 decades, primarily as a result of optimizing the use of recognized antileukemia drugs and stratifying patients at greatest risk of relapse using age, white cell count, immunophenotype, karyotype, and response to therapy.1 Patients with specific polymorphisms in genes responsible for metabolizing leukemia drugs can also have an increased risk of relapse or excessive treatment toxicity.1
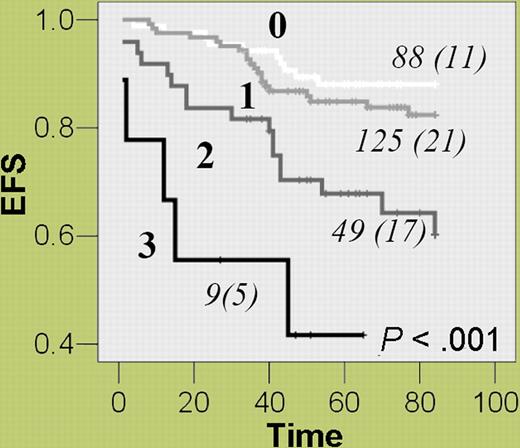
Methotrexate is a key antileukemia drug whose principal effect is the inhibition of DHFR, which impairs purine and thymidine synthesis and ultimately causes cell death.2 Dulucq and colleagues investigated whether promoter polymorphisms in DHFR affect outcome in childhood ALL. Genotyping of 48 control subjects revealed a total of 15 polymorphisms, including 3 single-nucleotide polymorphisms (SNPs)—C-1610G/T, C-680A, and A-317G—that could be used to define 5 main haplotypes. The frequency, prognostic relevance, and biological effect of these genotypes and haplotypes were ascertained in 277 children in remission from ALL. Patients homozygous for the A-317 or C-1610 alleles or who were carriers of the associated haplotype (*1) had a 70% to 80% increased risk of an adverse event compared with other patients, and this effect was independent of traditional risk factors. The authors postulate that the higher levels of DHFR mRNA associated with these genotypes were responsible for adverse patient outcome. Acknowledging the fact that numerous genes are involved in methotrexate metabolism, they investigated the effect of multiple event-predisposing genotypes. Event-free survival was inversely proportional to the number of risk genotypes—DHFR haplotype *1, thymidylate synthase 3R3R, and cyclin D1 AA870—and patients with all 3 were 8 times more likely to suffer an event compared with those without any of these genotypes (see figure).
Combined effect of multiple event-predisposing genotypes—DHFR (haplotype *1), thymidylate synthase (TS) 3R3R, and cyclin D1 (CCND1) AA870—on event-free survival in childhood ALL patients. For each group of patients with 0, 1, 2, or 3 of the specified genotypes, the number of patients (and the number of events) are given.
Combined effect of multiple event-predisposing genotypes—DHFR (haplotype *1), thymidylate synthase (TS) 3R3R, and cyclin D1 (CCND1) AA870—on event-free survival in childhood ALL patients. For each group of patients with 0, 1, 2, or 3 of the specified genotypes, the number of patients (and the number of events) are given.
This study contributes to the growing body of literature advocating a role for pharmacogenetic risk factors in the management of childhood ALL.1 Although this study has a number of strong points, there are also some important limitations. Key strengths include establishing the link between genotype and mRNA levels, providing mechanistic evidence for the association, and providing a clear rationale for the observed treatment response. When polymorphisms affecting other genes in the same pathway were taken into account, the effect increased, underlining the importance of methotrexate response in treating ALL. Although these results need independent confirmation, it is interesting to note that patients in this study were treated on 4 consecutive protocols with a consistent methotrexate dose. One limitation of the study is its lack of cytogenetic data. Only one cytogenetic subgroup, high hyperdiploidy, was considered in the multivariate analysis despite numerous others being highly relevant to prognosis.1 There is also evidence to suggest that acquired genetic abnormalities can alter germline phenotype and hence modify leukemic blast response to a drug.3
Children diagnosed with ALL today have an excellent chance of being cured,1 and new protocols are focusing on the reduction of treatment intensity. Therefore, pharmacogenetic risk factors will become increasingly important. Future studies will need to incorporate leukemic genotype into their analyses and develop algorithms for pinpointing critical genes from the plethora that govern how an individual will respond to a complex multi-drug protocol.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal