In this issue of Blood, Lam and colleagues report synergistic crosstalk between NF-κB and the JAK/STAT pathway in a subset of diffuse large B-cell lymphoma (DLBCL) that might have implications for future targeted therapies.
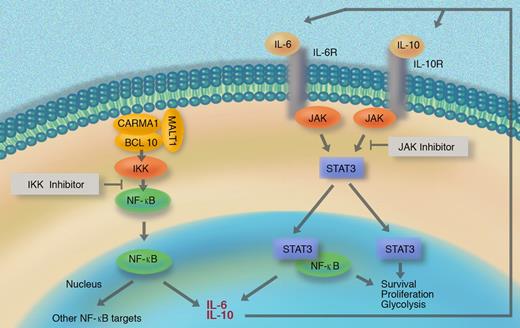
DLBCL is a genetically heterogeneous disease, and many patients are unable to be cured with conventional regimens. A better understanding of the pathobiology of DLBCL is therefore needed to develop novel, rational treatment strategies. Based on gene-expression profiling, Wiestner et al previously classified DLBCL into at least 3 clinically distinct subentities called activated B cell–like DLBCL (ABC DLBCL), germinal center–like DLBCL (GC DLBCL), and a smaller unclassified group.1 Among these, the ABC DLBCL subtype, which is characterized by aberrant activation of the NF-κB pathway, shows the most aggressive behavior. NF-κB activation in ABC DLBCL depends on the IKK kinase, which receives constitutive signaling input from the CARMA1/BCL10/MALT1 complex2 (see figure). Significantly, IKK/NF-κB signaling is essential for the survival of ABC DLBCL and for other lymphomas,3 and small-molecule inhibitors of IKK are lethal for ABC DLBCL cell lines. Treatment of animals with IKK inhibitors resulted in significant toxicities; therefore, clinical development of these inhibitors might be problematic.4
Aberrant NF-κB activity induces IL-6 and/or IL-10 production in a subset of DLBCL, which then activates the JAK/STAT pathway in an autocrine manner. The resulting synergistic NF-κB and STAT crosstalk affects lymphoma-cell survival, proliferation, and metabolism.
Aberrant NF-κB activity induces IL-6 and/or IL-10 production in a subset of DLBCL, which then activates the JAK/STAT pathway in an autocrine manner. The resulting synergistic NF-κB and STAT crosstalk affects lymphoma-cell survival, proliferation, and metabolism.
The report by Lam and colleagues in this issue of Blood investigates effector events of NF-κB signaling in DLBCL. Using cell line models, they identify IL6 and IL10 as ABC DLBCL–specific NF-κB target genes and show that IL-6 and/or IL-10 activate STAT3 via JAK kinases in an autocrine or paracrine manner. These events are associated with high levels of STAT3 phosphorylation and up-regulation of STAT3 mRNA and protein. Activated STAT3 controls a particular gene-expression pattern in ABC DLBCL lines, which allowed the authors to develop a mathematical predictor for STAT3 signaling. Turning to primary tumors, Lam et al used their predictor along with immunohistochemistry to divide ABC DLBCL cases into STAT3-high and STAT3-low subsets. Higher expression of NF-κB target genes and a differential control of survival factors, proliferation, and glycolysis regulators were detected in the STAT3-high patient group, indicating functionally relevant crosstalk between STAT3 and NF-κB in this ABC DLBCL subset.
Although the STAT3-high and STAT3-low groups do not differ in overall survival, the distinction of the 2 subentities might be significant for the design of future therapies. Lam and colleagues found that blocking STAT3 activation with a JAK kinase inhibitor induced lethality with signs of apoptosis, specifically in ABC DLBCL cells that produced IL-6/IL-10 but not in DLBCL cells that lacked cytokine expression. Notably, IKK inhibitors together with JAK inhibitors induced not only additive but synergistic toxicity in ABC DLBCL cells, potentially via down-regulation of NF-κB–dependent cytokine production and a subsequent lowering of the threshold for functional JAK/STAT pathway inhibition. Thus, the findings presented by Lam and colleagues imply that a combination of IKK and JAK inhibitors with lower individual doses and potentially reduced toxicities toward normal cells could become a treatment option for certain ABC DLBCLs. This is certainly an exciting outlook that warrants further investigations, including a detailed in vivo analysis of combined IKK and JAK inhibitor efficiency and toxicity profiles.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal