Abstract
The activated B cell–like (ABC) subgroup of diffuse large B-cell lymphoma (DLBCL) is characterized by constitutive activation of the nuclear factor-κB (NF-κB) pathway. In this study, we showed that the NF-κB pathway induced the expression of the cytokines interleukin (IL)-6 and IL-10 in ABC DLBCL cell lines, which also have high levels of total and phosphorylated signal transducer and activator of transcription (STAT) 3 protein, suggesting autocrine signaling. Using RNA interference for STAT3, we defined a gene expression signature of IL-6 and IL-10 signaling through STAT3. Based on this signature, we constructed a molecular predictor of STAT3 signaling that defined a subset of ABC DLBCL tumors with high expression of STAT3, IL-6, and/or IL-10 and their downstream targets. Although the STAT3-high and STAT3-low subsets had equivalent expression of genes that distinguish ABC DLBCL from germinal center B cell–like DLBCL, STAT3-high ABC DLBCLs had higher expression of signatures that reflected NF-κB activity, proliferation, and glycolysis. A small-molecule inhibitor of Janus kinase signaling, which blocked STAT3 signature expression, was toxic only for ABC DLBCL lines and synergized with an inhibitor of NF-κB signaling. These findings suggest that the biological interplay between the STAT3 and NF-κB pathways may be exploited for the treatments of a subset of ABC DLBCLs.
Introduction
The signal transducer and activator of transcription (STAT) proteins comprise of a family of transcription factors that regulate diverse cellular events, such as differentiation, proliferation, and cell survival.1 A variety of cytokines and growth factors activate STAT factors by binding to cell surface receptors, which triggers the activity of receptor-associated Janus kinase (JAK) family members, including JAK1, JAK2, JAK3, and TYK2. JAKs phosphorylate STAT proteins, leading to their dimerization and transit to the nucleus. The transcriptional targets of STAT proteins play roles in cell-cycle progression (eg, cyclin D1, c-myc, p21) as well as cell survival (eg, BCL-XL, MCL1, BCL-2).1 Constitutively active STATs (particularly STAT3 and STAT5) contribute to the malignant phenotype in both human cancer cell lines and primary tumors.2
Gene expression profiling studies have identified at least 3 molecular subtypes of diffuse large B-cell lymphoma (DLBCL) that differ in the expression of several hundred genes and have distinct cure rates after cyclophosphamide/doxorubicin/vincristine/prednisone chemotherapy.3 The GCB DLBCL subgroup expresses genes characteristic of normal germinal center B cells such as BCL-6.4-6 In contrast, the activated B cell–like (ABC) DLBCL subgroup expresses a subset of the genes that are characteristic of plasma cells, including IRF-4, XBP-1, and genes encoding endoplasmic reticulum and Golgi proteins involved in secretion.4-6 These observations led to the hypothesis that ABC DLBCLs may be blocked in differentiation between the germinal center B-cell and plasma cell stages of differentiation.4 One potential mechanism for this differentiation block is translocation of BCL-6, because BCL-6 has been shown to repress PRDM1, which encodes a key mediator of plasmacytic differentiation, Blimp-1.7,8 Indeed, BCL-6 translocations are almost 3 times more common in ABC DLBCLs than in GCB DLBCLs.9 A second mechanism of differentiation block is chromosomal deletion or mutational inactivation of PRDM1.10,11 These observations imply that the biology of ABC DLBCLs may reflect an origin from postgerminal center plasmablasts.
Constitutive activation of the nuclear factor-κB (NF-κB) signaling pathway is crucial for survival of cell lines of the ABC DLBCL but not GCB DLBCL subtype.12 ABC DLBCL lines rely upon the CARD11/MALT1/BCL10 signaling complex to activate the central kinase in the NF-κB pathway, IκB kinase β (IKKβ).13 Accordingly, a specific small-molecule inhibitor of IKKβ is specifically toxic for ABC DLBCL cell lines.14
We now report that IL6 and IL10 are key downstream target genes of NF-κB signaling in ABC DLBCL lines. IL-6 was originally identified as a T cell–derived cytokine that induced terminal maturation of B cells into plasma cells.15,16 IL-6 may have a widespread role in cancer, in that it is produced by tumor cells and/or stroma and can stimulate the growth of neoplastic cells.17 Autocrine or paracrine production of IL-6 contributes to the growth of multiple myeloma cells,18,19 a plasmacytic malignancy that shares several phenotypic characteristics with ABC DLBCL, including expression of the transcription factors IRF-4 and XBP-1. Indeed, previous studies demonstrated IL-6 secretion by the OCI-Ly3 cell line, which is now classified as an ABC DLBCL.20 IL-10 can also promote the proliferation of normal B cells that have been activated through the B cell receptor or CD40.21 Mouse B-cell lymphomas that arise spontaneously in the New Zealand Black strain produce IL-10, and IL-10 is required for their generation.22 IL-6 and IL-10 are secreted by malignant cells purified from some patients with non-Hodgkin lymphoma (NHL), and this phenotype correlates with circulating levels of the corresponding cytokines in serum.23
Binding of IL-6 and IL-10 to their surface receptors activates JAK family kinases, initiating several cascades of intracellular signaling events.16,24 Many of the biological effects of these cytokines are due to JAK phosphorylation of STAT3, leading to its nuclear accumulation and the activation of target genes that typically contain “GAS” (gamma activated site) DNA motifs.16,24,25 It is noteworthy that the STAT3 promoter itself contains a GAS motif through which STAT3 can initiate a positive autoregulatory loop.26 This feed forward loop increases not only the levels of phosphorylated STAT3 but also those of unphosphorylated STAT3 (U-STAT3). Recent work has highlighted the ability of U-STAT3 to activate gene expression, principally by interacting with other transcription factors that recruit it to various regulatory regions to which they bind.27,28 Of specific interest to the biology of ABC DLBCL is the demonstration that U-STAT3 interacts with the p65 subunit of NF-κB, inhibiting the binding of IκBs.28,29 The U-STAT3/NF-κB interaction results in synergistic activation of certain target genes, including IL-6.
In the present study, we investigated which molecular subgroups of DLBCL use IL-6 and/or IL-10 signaling through STAT3. Using a gene expression signature of STAT3 activity, we identified a subset of ABC DLBCLs with high expression of STAT3 as well as IL-6 and/or IL-10. This subset of cases was further characterized by higher activity of the NF-κB pathway and increased expression of genes involved in proliferation and glycolysis. ABC DLBCL cell lines that secreted IL-6 and/or IL-10 were selectively killed by an inhibitor of STAT3 signaling in a fashion that synergized with NF-κB pathway inhibition, suggesting new strategies for therapy of this type of DLBCL.
Methods
Chemicals and cells
Recombinant human IL-6 and IL-10 were purchased from R&D Systems (Minneapolis, MN). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (St Louis, MO). JAK inhibitor I and U0126 were purchased from Calbiochem (La Jolla, CA). MLN120B was obtained from Millennium Pharmaceuticals (Cambridge, MA).
All cell lines were maintained in Iscove modified essential medium with β-mercaptoethanol (55 mmol/L), penicillin (50 units/mL), streptomycin (50 mg/mL), and 20% heparinized normal human plasma. Cells were grown in a 37°C incubator in the presence of 5% carbon dioxide. IL-6 (50 mg/mL stock) and IL-10 (50 mg/mL stock) were used as a final concentration of 50 ng/mL.
Western blotting and phospho-STAT3 quantitation
Western blotting was carried out as described previously.12 All antibodies were obtained from Cell Signaling Technology (Danvers, MA). Phosphorylation of STAT3 on tyrosine 705 was quantitated with pSTAT3 kit (Meso Scale Discovery, Gaithersburg, MD) following the manufacturer's instruction. Immunohistochemical staining for total and phosphorylated (Tyr705) STAT3 was performed as described previously.30
Cell viability assays
MTT assays were performed as described previously.31 In brief, cells grown in 96-well plates were treated with JAK inhibitor and/or MLN120B for 48 hours. MTT was added to the cells 2 hours before harvesting. Cells were lysed completely in isopropanol with 1% hydrochloric acid. The plate was read with a 96-well spectrometer using a 570-nm filter. The background was subtracted using a dual-wavelength setting of 570 and 630 nm.
Synergism calculations were done with CalcuSyn software (Biosoft, Ferguson, MO) based on Chou and Talalay32 according to the manufacturer's instructions.
IL-6 and IL-10 assays
IL-6 and IL-10 amounts were determined by using the IL-6 or IL-10 Quantikine colorimetric sandwich enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems.
siRNA transfection
STAT3 siRNA (SMARTpool; Dharmacon, Lafayette, CO) was dissolved in buffer (20 mM KCl, 6 mM HEPES, pH 7.5, and 0.2 mM MgCl2). OCI-Ly10 cells were transfected with siRNA using an electroporator (Amaxa, Gaithersburg, MD) as follows: 8 × 106 cells were resuspended at 5 to 7 × 105 cells/mL in Solution T (Amaxa) containing siRNA or, as a control, the siRNA dilution buffer alone. Cells were electroporated using the program A-23 (Amaxa), transferred to pre-warmed medium, and incubated until harvest.
IκBα super-repressor
The retroviral vector for doxycycline-inducible expression of the IκBα super-repressor (IκBsr) has been described previously.14 OCI-Ly3 and OCI-Ly10 cells engineered to express the ecotropic retroviral receptor and the bacterial tetracycline repressor were infected with the IκBα super-repressor vector as described previously.14
Gene-expression profiling and statistical analysis
Gene-expression profiling was performed using Lymphochip DNA microarrays as described previously.31 In brief, total RNA was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. For each sample, 40 mg total RNA were used for the preparation of fluorescent probes. The raw gene expression data from each DNA microarray hybridization were normalized as described previously.31 Gene-expression profiling data are available as Figures S1 and S2 (available on the Blood website; see the Supplemental Materials link at the top of the online article) and have been deposited at the NCBI's Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE10009. Hierarchical clustering was performed using Cluster software and visualized using the Tree View software.33 Gene-expression data from primary DLBCL samples were published previously.6 Details of statistical criteria for selection of gene-expression signatures are given in Document S1. Lists of the genes that comprise each signature are given in Tables S1 through S13.
STAT3 predictor and gene set enrichment analysis
For the STAT3 analysis in samples from patients with ABC DLBCL, a Bayesian predictor of STAT3-high and STAT3-low subgroups was constructed, based on previously described methods,4 and is detailed in Document S1.
Results
Cytokine pathways regulated by NF-κB
Having demonstrated that the NF-κB pathway is constitutively active in ABC DLBCL,12 we investigated the biological consequences of NF-κB signaling by focusing on genes that are activated by NF-κB signaling in this lymphoma type. Two lymphoma cell lines, OCI-Ly3 and OCI-Ly10, are models of ABC DLBCL because they resemble primary ABC DLBCL tumors in gene expression and rely upon signaling from CARD11 to activate NF-κB.13 We blocked NF-κB signaling in these cell lines by introducing a “super-repressor” form of IκB (IκBsr) that cannot be phosphorylated by IκB kinase β (IKKβ) and hence functions as a stable inhibitor of NF-κB transcription factors. Many known NF-κB targets were down-regulated by IκBsr in both ABC DLBCL cell lines (Figure 1A). Other genes, however, were reduced in expression by IκBsr in only one of the 2 ABC DLBCL cell lines, demonstrating that these closely related cell lines nevertheless expressed different sets of NF-κB target genes (Figure 1B). IL-6 was an OCI-Ly3-specific NF-κB target gene and accordingly, the secretion of IL-6 by OCI-Ly3 cells was completely abrogated by IκBsr (Figure 1B,C). IL-10, on the other hand, was an NF-κB target gene only in OCI-Ly10 cells, and IκBsr blocked the secretion of IL-10 by this cell line (Figure 1B,D).
IL-6 and IL-10 are targets of the NF-κB pathway. (A,B) Gene expression patterns of lymphoma cell line OCI-Ly3 and OCI-Ly10 induced to express IkBsr with doxycycline for the indicated times. Each column is a single experiment comparing 2 cDNA populations; the treated sample was labeled red (Cy5) and the untreated sample was labeled green (Cy3). Each row represents data from a single cDNA microarray spot. The red-to-green (Cy5/Cy3) ratio reflects hybridization to that spot, a measure of relative gene expression; intensity reflects the magnitude of the difference between the samples according to the ratio color scale. Red indicates Cy5/Cy3 ratios greater than 1, green indicates Cy5/Cy3 ratios less than 1, black indicates no significant change in gene expression, and gray indicates that the spot did not meet data selection criteria. These ratios were depicted according to the color scale shown at the bottom. The criteria for selecting these genes are described in “Methods.” (C) Expression of IκBsr inhibits IL-6 secretion. OCI-Ly3 cells were induced to express IκBsr with doxycycline. Cells were washed before adding doxycycline. ELISA was used to determine the amount of IL-6 secreted in the medium. (D) Expression of IκBsr inhibits IL-10 secretion. OCI-Ly10 cells were induced to express IκBsr with doxycycline. Cells were washed before adding doxycycline. ELISA was used to determine the amount of IL-10 secreted in the medium. (E) Expression of IL-6 and IL-10 in ABC and GCB DLBCL cell lines was measured using the Quantikine colorimetric sandwich ELISA kit. (F) Steady-state levels of total and phosphorylated STAT3 in ABC and GCB DLBCL cell lines. Cell lysate were separated on Western blotting and immunoblotted with antibodies specific for p-STAT3 (Tyr705), pSTAT3 (Ser727), STAT3, or AKT (control). A vertical line has been inserted to indicate a repositioned gel lane. (G) Relative STAT3 mRNA levels in ABC DLBCL and GCB DLBCL cell lines as assessed by gene-expression profiling on Affymetrix U133 plus 2.0 arrays (probe ID_1123163).
IL-6 and IL-10 are targets of the NF-κB pathway. (A,B) Gene expression patterns of lymphoma cell line OCI-Ly3 and OCI-Ly10 induced to express IkBsr with doxycycline for the indicated times. Each column is a single experiment comparing 2 cDNA populations; the treated sample was labeled red (Cy5) and the untreated sample was labeled green (Cy3). Each row represents data from a single cDNA microarray spot. The red-to-green (Cy5/Cy3) ratio reflects hybridization to that spot, a measure of relative gene expression; intensity reflects the magnitude of the difference between the samples according to the ratio color scale. Red indicates Cy5/Cy3 ratios greater than 1, green indicates Cy5/Cy3 ratios less than 1, black indicates no significant change in gene expression, and gray indicates that the spot did not meet data selection criteria. These ratios were depicted according to the color scale shown at the bottom. The criteria for selecting these genes are described in “Methods.” (C) Expression of IκBsr inhibits IL-6 secretion. OCI-Ly3 cells were induced to express IκBsr with doxycycline. Cells were washed before adding doxycycline. ELISA was used to determine the amount of IL-6 secreted in the medium. (D) Expression of IκBsr inhibits IL-10 secretion. OCI-Ly10 cells were induced to express IκBsr with doxycycline. Cells were washed before adding doxycycline. ELISA was used to determine the amount of IL-10 secreted in the medium. (E) Expression of IL-6 and IL-10 in ABC and GCB DLBCL cell lines was measured using the Quantikine colorimetric sandwich ELISA kit. (F) Steady-state levels of total and phosphorylated STAT3 in ABC and GCB DLBCL cell lines. Cell lysate were separated on Western blotting and immunoblotted with antibodies specific for p-STAT3 (Tyr705), pSTAT3 (Ser727), STAT3, or AKT (control). A vertical line has been inserted to indicate a repositioned gel lane. (G) Relative STAT3 mRNA levels in ABC DLBCL and GCB DLBCL cell lines as assessed by gene-expression profiling on Affymetrix U133 plus 2.0 arrays (probe ID_1123163).
Given that constitutive NF-κB activity is a feature of ABC DLBCL but not GCB DLBCL, we next investigated the secretion of IL-6 and IL-10 in a variety of cell lines characterized as ABC DLBCL or GCB DLBCL by gene expression profiling (Figure S1). As shown in Figure 1E, the 5 ABC DLBCL cell lines tested secreted one or both of these cytokines, but 9 GCB DLBCL cell lines tested secreted little, if any of either cytokine.
IL-6, IL-10, and STAT3 signaling in ABC DLBCL
To investigate whether IL-6 and/or IL-10 produced by ABC DLBCL cell lines causes autocrine or paracrine signaling, we determined the expression of total and phosphorylated STAT3 by Western blot analysis (Figure 1F). Stimulation of cells with either IL-6 or IL-10 leads to phosphorylation of STAT on tyrosine 705 and serine 727 and causes STAT3 to translocate to the nucleus and activate its target genes.34 STAT3 protein expression levels were much higher in ABC DLBCL cell lines than in GCB DLBCL cell lines, and these levels reflected the STAT3 mRNA expression in these lines (Figure 1F,G). Moreover, the ABC DLBCL cell lines also had much higher tyrosine 705 and serine 727 phosphorylation than did GCB DLBCL cell lines (Figure 1F). It is noteworthy that the STAT3 tyrosine phosphorylation status corresponded well with the cytokine profile of each cell line (Figure 1E). These results are consistent with autocrine or paracrine signaling by IL-6 and IL-10 in ABC DLBCL cells.
We next used gene-expression profiling to define the genes that are transcriptional targets of IL-6 and IL-10 signaling in ABC DLBCL. Reasoning that the effect of a cytokine would be more pronounced in cells that do not normally express it, we treated OCI-Ly3 with IL-10 and OCI-Ly10 with IL-6. Figure 2A shows that the 46 genes induced by IL-6 in OCI-Ly10 cells can be divided into 3 categories: (1) immediate early genes that are induced in less than 3 hours and then return to normal (eg, c-jun, IRF-4); (2) genes induced after 3 hours (eg, HEB, PTP-1B); and (3) genes that are induced throughout the time course (eg, SOCS-3, EBI2). Some of these genes, such as SOCS-1 and SOCS-3, are known to be induced by IL-6 in a certain cell systems and function as negative regulators of the response to IL-6.35 More genes were up-regulated in OCI-Ly3 cells treated with IL-10 (n = 127; Figure 2B), many of which have been previously shown to be IL-10 targets.36 It is noteworthy that STAT3 mRNA was up-regulated by IL-10 treatment, in keeping with the fact that the STAT3 promoter contains a consensus STAT3 binding site, and cytokine-induced phosphorylation of STAT3 can drive synthesis of STAT3 mRNA.26
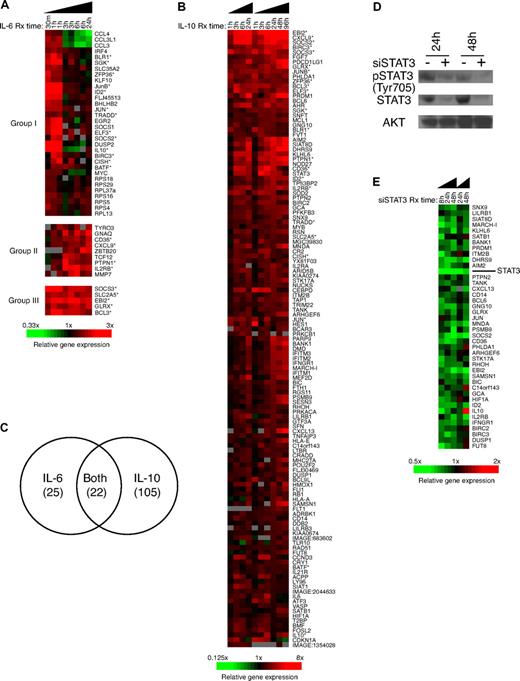
Identification of IL-10, IL-6, and STAT3 target genes in ABC DLBCL cells. (A) Gene-expression patterns of lymphoma cell line OCI-Ly10 treated with IL-6 for 0.5, 1, 3, 6, and 24 hours. Genes indicated with an asterisks are also IL-10 targets. (B) Gene-expression patterns of lymphoma cell line OCI-Ly3 treated with IL-10 for 1, 3, 6, 24, 48, and 96 hours. Genes marked with an asterisk are also IL-6 targets. (C) Comparison of the genes in response to IL-6 or IL-10 treatment. (D) Western blotting showing down-regulation of total STAT3 and phospho-STAT3 protein expression in OCI-Ly10 cell transfected with STAT3 siRNA. (E) Gene-expression patterns of lymphoma cell line OCI-Ly10 transient transfected with STAT3 siRNA pool for 8, 24, and 48 hours.
Identification of IL-10, IL-6, and STAT3 target genes in ABC DLBCL cells. (A) Gene-expression patterns of lymphoma cell line OCI-Ly10 treated with IL-6 for 0.5, 1, 3, 6, and 24 hours. Genes indicated with an asterisks are also IL-10 targets. (B) Gene-expression patterns of lymphoma cell line OCI-Ly3 treated with IL-10 for 1, 3, 6, 24, 48, and 96 hours. Genes marked with an asterisk are also IL-6 targets. (C) Comparison of the genes in response to IL-6 or IL-10 treatment. (D) Western blotting showing down-regulation of total STAT3 and phospho-STAT3 protein expression in OCI-Ly10 cell transfected with STAT3 siRNA. (E) Gene-expression patterns of lymphoma cell line OCI-Ly10 transient transfected with STAT3 siRNA pool for 8, 24, and 48 hours.
It is noteworthy that 22 genes of the IL-6 targets overlapped with the IL-10 targets (P < .001, χ2; Figure 2C), consistent with the fact that both cytokines activate similar downstream pathways, including the STAT3 pathway.1 To identify genes that are downstream targets of STAT3 in ABC DLBCL, we used siRNAs to knock down STAT3 mRNA expression in OCI-Ly10 cells. STAT3 siRNA down-regulated STAT3 mRNA by expression 3.7-fold by 8 hours after siRNA electroporation, and STAT3 mRNA and protein remained more than 3-fold decreased at 48 hours (Figure 2D,E). STAT3 targets were chosen based on their decreased expression in STAT3 siRNA-treated cells and increased expression in IL-10–treated cells (Figure 2E). In all, 41 STAT3 target genes were defined by these criteria, including the known STAT3 targets SOCS237 and BIRC3.38
STAT3 signaling distinguishes subsets of ABC DLBCL
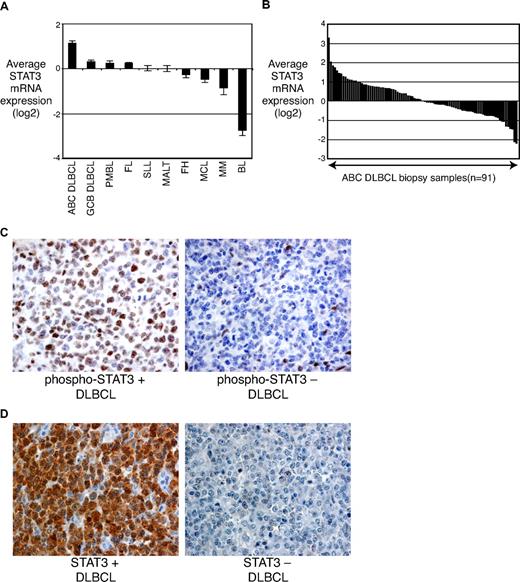
Given the close relationship of STAT3 mRNA and protein expression in DLBCL cell lines, we investigated the expression of STAT3 mRNA expression in primary patient samples that were profiled for gene expression using oligonucleotide arrays (Affymetrix, Santa Clara, CA). Among the non-Hodgkin lymphoma types tested, ABC DLBCLs had the highest average STAT3 mRNA expression and had roughly 2-fold higher STAT3 mRNA levels than GCB DLBCLs (P < .001; Figure 3A). Further, immunohistochemistry revealed intense staining for phosphorylated STAT3 in some ABC DLBCL cases, whereas others were negative (Figure 3C). Of 36 ABC DLBCLs screened, 47% had strong staining for phosphorylated STAT3, whereas 53% had weak or no staining. GCB DLBCLs had a lower frequency of strong staining for phosphorylated STAT3 (12/58, 21%; P = .007, χ2). These data suggest that cytokine-stimulated phosphorylation of STAT3 contributes to the pathogenesis of a subset of ABC DLBCLs.
STAT3 expression in primary lymphoma tumors. (A) Mean STAT3 mRNA levels in tumor samples as assessed by gene-expression profiling. PMBL, primary mediastinal B-cell lymphoma; FL, follicular lymphoma; SLL, small lymphocytic lymphoma; MALT, mucosa-associated lymphoid tissue; FH, follicular hyperplasia; MCL, mantle cell lymphoma; MM, multiple myeloma; BL, Burkitt lymphoma. (B) Histogram of STAT3 mRNA levels in individual ABC DLBCL tumor biopsies. (C) Immunostaining with a p-STAT3 (Tyr705)–specific antibody showing dense brown staining in ABC DLBCL cases. (D) Immunostaining with an antibody recognizing total STAT3 showing dense brown staining in STAT3-high ABC DLBCL cases.
STAT3 expression in primary lymphoma tumors. (A) Mean STAT3 mRNA levels in tumor samples as assessed by gene-expression profiling. PMBL, primary mediastinal B-cell lymphoma; FL, follicular lymphoma; SLL, small lymphocytic lymphoma; MALT, mucosa-associated lymphoid tissue; FH, follicular hyperplasia; MCL, mantle cell lymphoma; MM, multiple myeloma; BL, Burkitt lymphoma. (B) Histogram of STAT3 mRNA levels in individual ABC DLBCL tumor biopsies. (C) Immunostaining with a p-STAT3 (Tyr705)–specific antibody showing dense brown staining in ABC DLBCL cases. (D) Immunostaining with an antibody recognizing total STAT3 showing dense brown staining in STAT3-high ABC DLBCL cases.
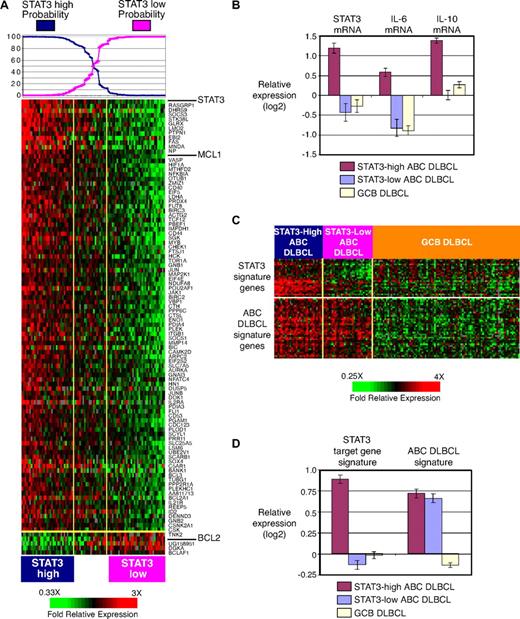
Although ABC DLBCLs had the highest average STAT3 mRNA expression among the non-Hodgkin lymphoma types (Figure 3A), STAT3 expression varied over a 35-fold range among tumors of this lymphoma subtype (Figure 3B). We therefore hypothesized that a subset of ABC DLBCL cases might be characterized by high expression of STAT3 and its target genes. To test this hypothesis, we used the list of STAT3 target genes (Figure 2E) to create a mathematical predictor of STAT3 signaling, based on Bayesian statistics (see “Methods”). We used this predictor to classify the ABC DLBCL cases into a “STAT3-high” subset (n = 33) and a “STAT3-low” subset (n = 37); the remaining 22 cases had intermediate probabilities and were declared unclassified (Figure 4A). By immunohistochemistry, strong staining for phosphorylated STAT3 was present in 57% (8/14) of the STAT3-high subset but in only 29% (4/14) of the STAT3-low subset. However, the STAT3-high gene-expression profile might not depend solely on the degree of STAT3 phosphorylation because unphosphorylated STAT3 can modulate gene expression by binding to NF-κB (see “Discussion”).27,28 Strong immunohistochemical staining for total STAT3 was found in 91% (10/11) of the STAT3-high subset but in only 23% (3/13) of the STAT3-low subset (P < .001, χ2; Figure 3D). Moreover, high expression of STAT3 protein was observed in only 7% (3/43) of the GCB DLBCL cases. These results support a role for both unphosphorylated and phosphorylated STAT3 in the STAT3-high subset of ABC DLBCLs.
Differential STAT3 signature expression in subsets of ABC DLBCLs. (A) The expression levels of STAT3 and STAT3 target genes in the subgroup predictor in 92 ABC DLBCL tumor samples. The probabilities that the ABC DLBCL samples belong to the STAT3-high and STAT3-low subgroups based on a Bayesian predictor (see “Methods”) are graphed at the top, and the cases are arranged accordingly. The cases that belong to either the STAT3-high or STAT3-low ABC DLBCL subgroups with more than 90% likelihood are indicated. (B) Mean STAT3 mRNA, IL-6 mRNA, and IL-10 mRNA levels among STAT3-high ABC DLBCL, STAT3-low ABC DLBCL, and GCB DLBCL. (C) Expression of STAT3 target genes and ABC DLBCL signature genes in STAT3-high ABC DLBCL, STAT3-low ABC DLBCL, and GCB DLBCL. (D) Average expression of STAT3 target genes and ABC DLBCL signature genes in STAT3-high ABC DLBCL, STAT3-low ABC DLBCL, and GCB DLBCL.
Differential STAT3 signature expression in subsets of ABC DLBCLs. (A) The expression levels of STAT3 and STAT3 target genes in the subgroup predictor in 92 ABC DLBCL tumor samples. The probabilities that the ABC DLBCL samples belong to the STAT3-high and STAT3-low subgroups based on a Bayesian predictor (see “Methods”) are graphed at the top, and the cases are arranged accordingly. The cases that belong to either the STAT3-high or STAT3-low ABC DLBCL subgroups with more than 90% likelihood are indicated. (B) Mean STAT3 mRNA, IL-6 mRNA, and IL-10 mRNA levels among STAT3-high ABC DLBCL, STAT3-low ABC DLBCL, and GCB DLBCL. (C) Expression of STAT3 target genes and ABC DLBCL signature genes in STAT3-high ABC DLBCL, STAT3-low ABC DLBCL, and GCB DLBCL. (D) Average expression of STAT3 target genes and ABC DLBCL signature genes in STAT3-high ABC DLBCL, STAT3-low ABC DLBCL, and GCB DLBCL.
The STAT3-high and STAT3-low subsets differed strikingly in gene expression: based on a t test and a stringent P value cutoff (P < .001), 22% of the genes represented on the microarrays (929/4212) were differentially expressed, the top 100 of which are shown in Figure 4A. Remarkably, STAT3 was the most differentially expressed gene between these ABC DLBCL subsets, with mRNA levels that were 3.1-fold higher in STAT3-high tumors than in STAT3-low-tumors (P < .001; Figure 4B). Among the other genes that were more highly expressed in the STAT3-high subset were IL10 (2.7-fold; P < .001) and IL6 (2.7-fold; P < .001; Figure 4B). The average expression of the STAT3 target gene signature was more than 2-fold higher in STAT3-high ABC DLBCLs than in STAT3-low ABC DLBCLs (Figure 4C,D). GCB DLBCLs had relatively low expression of the STAT3 signature, which was equivalent to that of the STAT3-low ABC DLBCL subset, consistent with generally lower activity of the STAT3 pathway in GCB DLBCLs. It is noteworthy that many genes that are significantly more highly expressed in ABC DLBCL than in GCB DLBCL (P < .001), were expressed equivalently in STAT3-high and STAT-low ABC DLBCL cases (Figure 4C,D). Thus, much of the defining biology of the ABC DLBCL subtype is retained in both ABC DLBCL subsets even though they differ in STAT3 signaling. Both STAT3-high and STAT-3 low subsets of ABC DLBCL had an inferior overall survival compared with GCB DLBCLs, but they did not differ from each other in survival (data not shown).
Conversely, some genes were more highly expressed in the STAT3-low group (Figure 4A). Notable among these was BCL2, a gene that is more highly expressed in a subset of ABC DLBCLs with inferior prognosis.39 The reasons for the inverse relationship between STAT3 activity and BCL-2 are not known, but it is interesting that BCL-2 mRNA expression was rapidly down-regulated after treatment of OCI-Ly3 cells with IL-10 (Figure S2). Furthermore, high BCL-2 protein expression by immunostains (> 50% cells positive) was significantly higher in STAT3-low cases (88%) than in STAT3-high cases (35%; P = .001; χ2). Conversely, it is also notable that the mRNA levels for another antiapoptotic BCL-2 family member, MCL1, were significantly higher in the STAT3-high subset (1.9-fold, P < .001; Figure 4A).
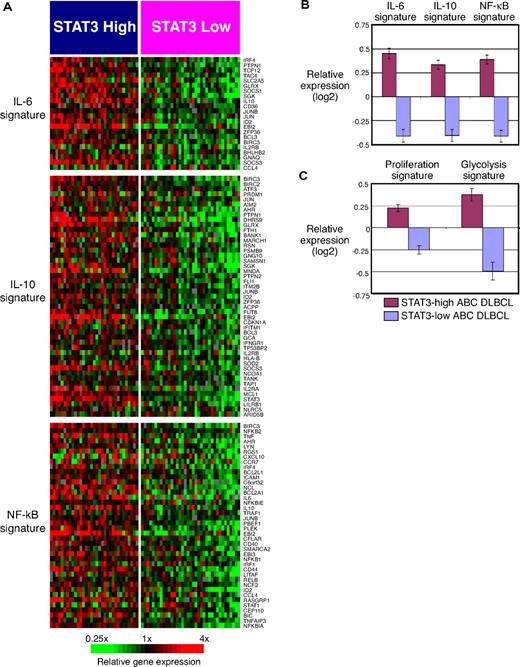
To gain further biological insight into the differences between STAT3-high and STAT3-low subsets of ABC DLBCL, we performed gene set enrichment analysis using a curated set of gene expression signatures40 (Table 1). Among the significantly enriched signatures were the IL-6 target gene signature and the IL-10 target gene signature (Figure 5A). Figure 5B shows that the average expression of these 2 signatures was greater in the STAT3-high subset than in the STAT3-low subset, in keeping with a model in which IL-6 and/or IL-10 signaling contributes to the gene expression profile of the STAT3-high subset.
Gene set enrichment analysis of gene signatures in STAT3-high ABC DLBCL versus STAT3-low ABC DLBCL cases
| Short signature name . | Signature description . | Number of coregulated genes . | Permutation P value . | Reference . |
|---|---|---|---|---|
| NF-κB-2 | NF-κB: OCILy3 and OCI-Ly10 | 30 | .001 | 41 |
| NF-κB-1 | NF-κB: OCILy3 or OCI-Ly10 | 40 | .001 | 41 |
| Proliferation-7 | Cell cycle | 99 | .001 | 41 |
| IL-6 | IL-6 targets | 22 | .003 | Current study |
| IL-10 | IL-10 targets | 47 | .003 | Current study |
| Glycolysis | Glycolytic enzymes | 5 | .003 | 60 |
| Proliferation-5 | Proliferation: DLBCL | 615 | .007 | 4 |
| Proliferation-8 | Cell cycle | 136 | .01 | 42 |
| Short signature name . | Signature description . | Number of coregulated genes . | Permutation P value . | Reference . |
|---|---|---|---|---|
| NF-κB-2 | NF-κB: OCILy3 and OCI-Ly10 | 30 | .001 | 41 |
| NF-κB-1 | NF-κB: OCILy3 or OCI-Ly10 | 40 | .001 | 41 |
| Proliferation-7 | Cell cycle | 99 | .001 | 41 |
| IL-6 | IL-6 targets | 22 | .003 | Current study |
| IL-10 | IL-10 targets | 47 | .003 | Current study |
| Glycolysis | Glycolytic enzymes | 5 | .003 | 60 |
| Proliferation-5 | Proliferation: DLBCL | 615 | .007 | 4 |
| Proliferation-8 | Cell cycle | 136 | .01 | 42 |
Gene set enrichment analysis of signatures in the STAT3-high and STAT3-low subgroups of ABC DLBCL patients. (A) Expression of IL-6 target genes, IL-10 target genes, and NF-κB target genes in the STAT3-high and STAT3-low subgroups of patients with ABC DLBCL. (B) Average expression of IL-6 target genes, IL-10 target genes, and NF-κB target genes in the STAT3-high and STAT3-low subgroups of patients with ABC DLBCL. (C) Average expression of proliferation and glycolysis genes in the STAT3-high and STAT3-low subgroups of patients with ABC DLBCL.
Gene set enrichment analysis of signatures in the STAT3-high and STAT3-low subgroups of ABC DLBCL patients. (A) Expression of IL-6 target genes, IL-10 target genes, and NF-κB target genes in the STAT3-high and STAT3-low subgroups of patients with ABC DLBCL. (B) Average expression of IL-6 target genes, IL-10 target genes, and NF-κB target genes in the STAT3-high and STAT3-low subgroups of patients with ABC DLBCL. (C) Average expression of proliferation and glycolysis genes in the STAT3-high and STAT3-low subgroups of patients with ABC DLBCL.
STAT3-high tumors had greater expression of 2 related signatures of NF-κB target genes,14 including the most highly enriched signature in this analysis (Table 1). These NF-κB target gene signatures were developed by treating ABC DLBCL cell lines (OCI-Ly3 and OCI-Ly10) with a small molecule inhibitor of IKKβ to extinguish NF-κB signaling. On average, STAT3-high tumors had a 1.7-fold higher expression of the NF-κB-2 signature than STAT3-low tumors (Figure 5B). This finding suggests an important degree of biological cross-talk between the STAT3 and NF-κB pathways in a subset of ABC DLBCLs (see “Discussion”).
Three different signatures of cellular proliferation were more highly expressed in STAT3-high ABC DLBCL cases (Table 1). Two of these (Proliferation-7 and Proliferation-8) consist of genes whose mRNA levels fluctuate in a cell cycle-dependent manner.41,42 The Proliferation-5 signature consists of genes that are co-regulated in DLBCLs, some of which are also associated with survival.6 These results are consistent with the known effects of STAT3 on cell cycle progression.34 Figure 5C illustrates greater expression of the Proliferation-5 signature in STAT3-high cases than in STAT3-low cases. A signature consisting of genes encoding glycolytic enzymes, which are also up-regulated in proliferating cells, was also higher in STAT3-high ABC DLBCLs (Figure 5C).
JAK inhibition is toxic for ABC DLBCL cells
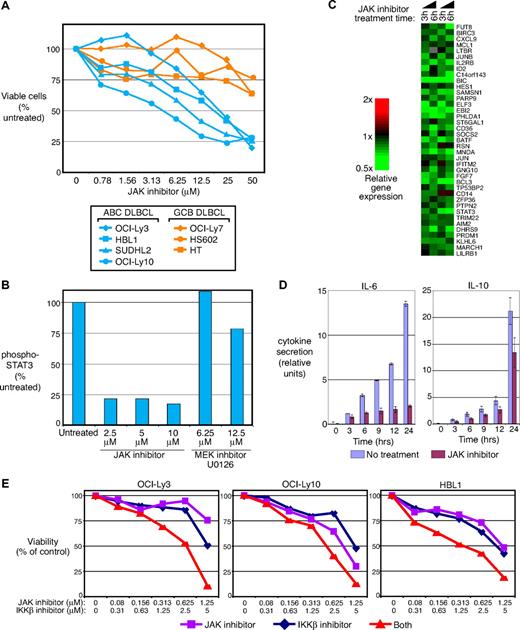
Cytokines such as IL-6 and IL-10 activate multiple JAK family kinases and STATs.43 To determine whether inhibiting these cytokine pathways affects ABC DLBCL cell proliferation and survival, we treated ABC DLBCL and GCB DLBCL cell lines with a small molecule inhibitor of JAK. This inhibitor has specific activity against each JAK but has negligible activity toward other kinases.44 The JAK inhibitor was toxic in a dose-dependent fashion to ABC DLBCL cell lines with IL-6 and/or IL-10 expression (OCI-Ly3, OCI-Ly10, HBL1, and SUDHL2) but not to GCB DLBCL cell lines that lack cytokine expression (OCI-Ly7, HS602, and HT; Figure 6A). OCI-Ly10 cells were the most sensitive to the JAK inhibitor, consistent with its having the highest levels of pSTAT3 (Tyr705; Figure 1F). Treatment of OCI-Ly10 cells with the JAK inhibitor strongly inhibited STAT3 phosphorylation, which was not observed with the mitogen-activated protein kinase kinase inhibitor U0126 (Figure 6B). Caspase 3 and/or caspase 7 activity was increased, suggesting that apoptosis was induced with JAK inhibitor treatment (data not shown). Finally, gene-expression profiling of OCI-Ly10 cells treated with the JAK inhibitor demonstrated that 38 of the IL-10 target genes were down-regulated, 22 of which were also STAT3 targets (Figure 6C). In addition, the JAK inhibitor reduced expression of many genes associated with cellular proliferation, in keeping with its toxicity for these cells (data not shown). Of special interest was the effect of the JAK inhibitor on IL-6 and IL-10 secretion. Figure 6D demonstrates that IL-6 secretion by OCI-Ly3 cells and, to a lesser degree, IL-10 secretion by OCI-Ly10 cells, were inhibited by treatment with the JAK inhibitor, suggesting that JAK/STAT3 signaling participates in a positive autoregulatory loop involving IL-6 and IL-10.
Toxicity of JAK inhibition for ABC DLBCL cell lines and synergism with NF-κB pathway inhibition. (A) ABC DLBCL (OCI-Ly3, OCI-Ly10, HBL1, and SUDHL2) and GCB DLBCL (OCI-Ly7, HT, HS602) were treated with 0 to 50 μmol/L JAK inhibitor for 2 days and assigned for viability by MTT assays. The cell numbers at each drug dose are expressed as the percentage of cell numbers obtained with untreated cells cultured in parallel. (B) OCI-Ly10 cells were treated with JAK inhibitor (0-10 μmol/L) or U0126 (0-12.5 μmol/L) for 4 hours. Cell lysate was made for the measurement of phospho-STAT3 (Tyr705). (C) Gene-expression patterns of lymphoma cell line OCI-Ly10 treated with 5 μmol/L JAK inhibitor for 3 and 6 hours. (D) JAK inhibitor treatment diminishes IL-6 and IL-10 secretion. OCI-Ly3 and OCI-Ly10 cells were washed before adding JAK inhibitor. Shown are relative cytokine units from ELISAs for IL-6 (OCI-Ly3) and IL-10 (OCI-Ly10) using supernatants of cells that were washed with fresh media immediately before addition of the JAK inhibitor. (E) OCI-Ly3, OCI-Ly10, and HBL1 cells were treated with both JAK inhibitor and MLN120B for 3 days and assigned for viability by MTT assays. The cell numbers at each drug dose are expressed as the percentage of cell numbers obtained with untreated cells cultured in parallel.
Toxicity of JAK inhibition for ABC DLBCL cell lines and synergism with NF-κB pathway inhibition. (A) ABC DLBCL (OCI-Ly3, OCI-Ly10, HBL1, and SUDHL2) and GCB DLBCL (OCI-Ly7, HT, HS602) were treated with 0 to 50 μmol/L JAK inhibitor for 2 days and assigned for viability by MTT assays. The cell numbers at each drug dose are expressed as the percentage of cell numbers obtained with untreated cells cultured in parallel. (B) OCI-Ly10 cells were treated with JAK inhibitor (0-10 μmol/L) or U0126 (0-12.5 μmol/L) for 4 hours. Cell lysate was made for the measurement of phospho-STAT3 (Tyr705). (C) Gene-expression patterns of lymphoma cell line OCI-Ly10 treated with 5 μmol/L JAK inhibitor for 3 and 6 hours. (D) JAK inhibitor treatment diminishes IL-6 and IL-10 secretion. OCI-Ly3 and OCI-Ly10 cells were washed before adding JAK inhibitor. Shown are relative cytokine units from ELISAs for IL-6 (OCI-Ly3) and IL-10 (OCI-Ly10) using supernatants of cells that were washed with fresh media immediately before addition of the JAK inhibitor. (E) OCI-Ly3, OCI-Ly10, and HBL1 cells were treated with both JAK inhibitor and MLN120B for 3 days and assigned for viability by MTT assays. The cell numbers at each drug dose are expressed as the percentage of cell numbers obtained with untreated cells cultured in parallel.
ABC DLBCL cell lines have constitutive NF-κB activity and are killed by small molecule IKKβ inhibitors of the β-carboline class.12,14 We speculated that combined treatment of ABC DLBCL cells with the IKKβ and JAK inhibitors might produce additive or perhaps synergistic toxicity for 2 reasons. First, NF-κB pathway inhibition blocks IL-6 and IL-10 production by ABC DLBCL cell lines (Figure 1) and thus should reduce autocrine and paracrine activation of JAKs. Second, STAT3 and NF-κB can interact physically and thereby synergize in transactivation of certain target genes.27,28 ABC DLBCL cell lines (OCI-Ly3, OCI-Ly10, HBL1) were treated with a range of concentrations of the JAK inhibitor and the IKKβ inhibitor MLN120B. Figure 6E shows that this combination of inhibitors was synergistic for toxicity in each of these cell lines. Synergism was formally calculated by applying the Chou-Talalay method to calculate a combination index (CI).32 Maximum synergism was noted at concentration of 1.25 μmol/L JAK inhibitor and 5 μmol/L MLN120B (CI = 0.042 for OCI-Ly3 cells, CI = 0.48 OCI-Ly10 cells, and CI = 0.228 for HBL1 cells).
Discussion
The present analysis uncovered a pathogenic role for IL-6 and IL-10 signaling through the JAK/STAT3 pathway in a subset of DLBCLs. Both IL-6 and IL-10 were targets of the constitutive NF-κB signaling that is a hallmark of the ABC DLBCL subtype. Among DLBCL cell lines, only those of the ABC DLBCL subtype secreted IL-6 and/or IL-10. Using an integrated analysis of in vitro cytokine stimulation and RNAi-mediated knockdown of STAT3, we defined a gene expression signature of STAT3 activity in DLBCL. This signature was able to functionally subdivide ABC DLBCLs into STAT3-high and STAT3-low subsets that diverged in the expression of hundreds of genes. An analysis of gene expression signatures that are differentially expressed between these 2 subsets revealed higher expression of an NF-κB target gene signature in the STAT3-high subset. JAK inhibition was toxic for ABC DLBCL cell lines, and combined treatment with an NF-κB pathway inhibitor was synergistically lethal. These results suggest a model in which a subset of ABC DLBCLs has higher NF-κB activity, enhanced IL-6 and/or IL-10 secretion, and stronger stimulation of JAK and STAT3 in an autocrine or paracrine fashion. Moreover, the synergistic toxicity of JAK and NF-κB inhibitors suggests a functional cross-talk between these 2 pathways that could be therapeutically meaningful.
Previous studies demonstrated that primary non-Hodgkin lymphoma cells can secrete IL-6 and IL-10 and respond to these cytokines, but these studies did not identify the molecular subtype of NHL with this phenotype.23 Here we show that among DLBCLs, tumors with high levels of IL-6 and IL-10 mRNA primarily belonged to the ABC rather than GCB DLBCL subtype (Figure 4B). Phosphorylated STAT3 was detected more frequently in primary tumors and cell lines of the ABC DLBCL subtype than the GCB DLBCL subtype (Figures 1F,3C). Finally, among various non-Hodgkin lymphoma types, STAT3 mRNA levels were highest in ABC DLBCL (Figure 3A). However, not all ABC DLBCLs had these phenotypes, suggesting the existence of molecularly distinct subsets within ABC DLBCL. Using a signature of STAT3 target genes, we identified STAT3-high and STAT3-low subsets of ABC DLBCL that differed with respect to expression of IL-6, IL-10, STAT3, and hundreds of other genes. Gene set enrichment analysis of gene expression signatures revealed that signatures of IL-6 and IL-10 signaling, NF-κB pathway activity, cellular proliferation, and glycolysis were more highly expressed in the STAT3-high subset of ABC DLBCL. It is important to emphasize, however, that the STAT3-high and STAT3-low subsets were both clearly ABC DLBCLs, both molecularly and clinically. A signature of genes that distinguish ABC from GCB DLBCL was equivalently expressed by these 2 subsets (Figure 4C,D) and the subsets had similarly poor overall survival. Thus, our current view is that these subsets represent ABC DLBCL variants that have differential engagement of the STAT3 and NF-κB pathways.
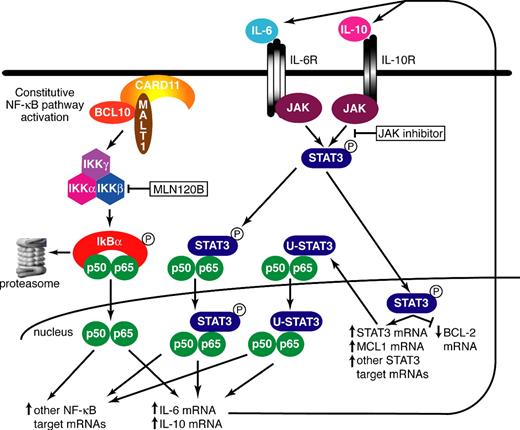
A working model of the synergism between the STAT3 and NF-κB pathways in ABC DLBCL is presented in Figure 7. ABC DLBCLs use the CARD11/MALT1/BCL10 signaling cascade to constitutively activate IKKβ, leading to nuclear accumulation of p50/p65 NF-κB heterodimers. We suggest that this is a necessary initiating event for cross-talk with the STAT3 pathway given that both IL-6 and IL-10 are targets of NF-κB signaling in ABC DLBCLs (Figure 1). Secreted IL-6 and/or IL-10 initiate STAT3 signaling in an autocrine or paracrine fashion by binding to their surface receptors and activating JAKs, which phosphorylate STAT3. One target of phosphorylated STAT3 is STAT3 itself, leading to an increase in total STAT3 protein in the cell. Both U-STAT3 and phosphorylated STAT3 can bind to the NF-κB p65 subunit and potentiate transactivation of certain NF-κB targets.28 A feed-forward loop is initiated once STAT3 and NF-κB synergistically trans-activate the IL6 gene.28 The STAT3-high subset of ABC DLBCL expressed total STAT3 protein highly in 91% of cases but expressed phopho-STAT3 protein in only 57% of cases. Therefore, it seems likely that both unphosphorylated and phosphorylated STAT3 contribute to the elevated NF-κB pathway activity in this ABC DLBCL subset (Figure 7).
Model depicting the molecular cross-talk between the STAT3 and NF-κB pathways in ABC DLBCL. See “Discussion” for details.
Model depicting the molecular cross-talk between the STAT3 and NF-κB pathways in ABC DLBCL. See “Discussion” for details.
Why ABC DLBCL subsets are different in their utilization of the NF-κB and STAT3 pathways remains unclear, but this distinction could have therapeutic import. A small-molecule inhibitor of JAK was selectively toxic to ABC DLBCL cell lines (Figure 6A). Moreover, this inhibitor synergized with an IKKβ inhibitor in killing ABC DLBCL cells (Figure 6E). Much recent interest in the clinical development of JAK inhibitors has been generated by the discovery of activating JAK2 mutations in a variety of myeloproliferative disorders.45 A pathogenetic role for STAT3 signaling may extend to many forms of cancer, adding impetus to the development of inhibitors of this pathway.34,46 In fact, kinase inhibitors that are already in clinical trials may have activity against JAKs.47 An equally compelling case exists for the development of NF-κB pathway inhibitors in cancer given its persistent activation in many types of lymphoid cancers48 and its involvement in solid tumors as well.49 JAK inhibitors that inhibit JAK2 would be expected to have on-target hematopoietic toxicities, and those that inhibit JAK3 could cause immune suppression.45 Treatment of mice with NF-κB pathway inhibitors deplete both the B- and T-cell compartments,50 presumably as a result of the homeostatic role of NF-κB signaling in preventing apoptosis.51 These considerations add importance to our observation of synergy between JAK and NF-κB pathway inhibition in killing ABC DLBCL cells (Figure 6E). In theory, lower doses of both inhibitors could be used therapeutically, allowing killing of DLBCL cells to be maintained while minimizing toxicity toward normal cells.
A potentially crucial difference between STAT3-high and STAT3-low ABC DLBCLs is in the expression of antiapoptotic BCL-2 family members. In general, STAT3-high cases were BCL-2 low/MCL1 high and STAT3-low cases were BCL-2 high/MCL1 low (Figure 4A). Recently, BH3-mimetic drugs have been developed that antagonize the antiapoptotic BCL-2 family members.52-54 ABT-737 is one prototype BH3-mimetic that blocks the action of BCL-2 but not MCL1.52 Indeed, high MCL1 expression was a principle mechanism of resistance to this compound.55,56 Our results suggest that the STAT3-low subset of ABC DLBCLs may be more responsive to ABT-737 than the STAT3-high subset.
Finally, IL-6 and/or IL-10 signaling in ABC DLBCL tumors may involve not only the malignant cell but also the immune cells within the tumor microenvironment. An important immunosuppressive role for STAT3 signaling was recently described based on a genetic analysis of mouse tumor-host interactions.57-59 In these studies, tumor-derived cytokines such as IL-6 and IL-10 up-regulated STAT3 signaling in various immune-cell subsets in the tumor microenvironment, thereby generating tolerogenic dendritic cells and regulatory T cells and compromising the function of immune effector cells. In particular, innate immune cells, including macrophages, natural killer cells, and neutrophils, became incapable of killing tumor cells. Thus, STAT3 inhibition in ABC DLBCL could have an additional therapeutic benefit deriving from enhanced immune surveillance.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the members of the Staudt laboratory for insightful discussions.
This research was supported by the Intramural Research Program of the NIH, NCI, Center for Cancer Research, an NCI Director's Challenge grant (UO1-CA84967), and the National Cancer Institute of Canada.
National Institutes of Health
Authorship
Contribution: L.T.L. designed the research, performed experiments, analyzed results, made the figures, and wrote the manuscript. G.W. analyzed results and wrote the manuscript. R.E.D. and G.L. performed experiments. P.F. and R.D.G. provided reagents and performed experiments. L.D., J.W.C. and A.R. provided reagents. L.M.S. designed the research, analyzed results, made the figures, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Louis M. Staudt, MD, PhD, 9000 Rockville Pike, Building 10, Room 4N114, Bethesda, MD 20892; e-mail: lstaudt@mail.nih.gov.