Abstract
Tissue-specific silencing of genes may be used for genetic engineering in mice and has possible therapeutic applications in humans. Current strategies in mice rely on Cre/loxP technology requiring the generation of multiple transgenic lines and breeding strategies. Here, we describe the selective silencing of CD18, a leukocyte-specific integrin in neutrophils using a micro RNA (miRNA) strategy that requires the generation of one transgenic line. CD18-specific miRNA hairpin driven by the myeloid specific human MRP8 promoter resulted in the generation of transgenic lines with 75% to 95% reduction in CD18 protein levels in neutrophils and monocytes. Minimal decreases in T cells and a partial diminution in macrophages were observed. Neutrophil CD18 silencing resulted in neutrophilia, splenomegaly, and significant defects in neutrophil trafficking with the degree of alterations correlating with the extent of CD18 silencing. Thus, our data demonstrate the utility of using miRNA approaches to silence genes in neutrophils, which are terminally differentiated cells with a short half-life that largely precludes their genetic manipulation in vitro. Furthermore, the mouse models provide a valuable tool to examine the contribution of CD18 on neutrophils to leukocyte adhesion deficiency type I (LAD-I), a complex inherited disorder in which reduced or absent CD18 expression in multiple leukocyte subsets leads to impaired innate and adaptive immune responses.
Introduction
Homologous recombination combined with the Cre/loxP system has been used to achieve conditional or tissue-specific targeting of gene expression in mice. This approach requires the generation of homologous targeted embryonic stem cells, and multiple transgenic lines and breeding strategies.1 Recent advances in RNA interference (RNAi) have provided an attractive alternative to silencing genes in vivo that is more rapid and also has possible therapeutic applications. RNAi is a posttranscriptional gene-silencing mechanism that is induced by double-stranded RNA. Introduction of chemically synthesized small interfering RNAs (siRNAs) and/or transfections of DNA-based vector systems driving the expression of short hairpin RNAs (shRNAs) by RNA pol III promoters (U6 and H1) have been used as powerful tools to examine the function of specific genes. Endogenous micro RNAs (miRNA) are small single-stranded RNAs that regulate genes required for a wide range of cellular functions. miRNAs are produced by the processing of primary transcripts (pri-mRNA) by the RNAse III type enzyme Drosha into pre-miRNAs (approximately 70 nucleotide stem-loop RNAs), which are exported to the cytoplasm and further cleaved by the cytoplasmic RNase III Dicer into miRNA-containing RNA duplexes. In the case of near-perfect complementarity to the targets, the outcome is endonucleolytic cleavage of the target mRNA,2-5 while binding to partially complementary sites, predominantly in the 3′UTR, causes mRNA degradation or repression of translation.6-8 The expression of artificial miRNAs by RNA Pol II promoters has been successfully used to silence genes in vitro, which exploits the machinery used to generate naturally occurring miRNAs.8,9 Vector-based strategies to generate in vivo expression of shRNA have been reported using pol III promoters, although the efficiency of silencing has been low.10,11 On the other hand, the use of the pol II promoters encoding synthetic miRNAs has resulted in more widespread knockdown, and the phenotypes observed resemble those of their knockout counterparts.12 Reports of tissue-specific silencing are, however, very limited.13
The goal of this study was to silence genes in neutrophils by expressing synthetic miRNAs by the myeloid-specific human promoter hMRP8 (human migration inhibitory factor-related protein 8, S100A8), which exhibits neutrophil expression of transgenes.14 As proof of principle, we targeted the CD18 integrin family of adhesion molecules. The CD18 integrins, composed of a common CD18 chain in complex with unique alpha subunits (CD11a-d), are present on all leukocytes and play critical roles in innate and adaptive immune responses. An absence or mutation in the CD18 subunit leads to leukocyte adhesion deficiency type I (LAD-I) in humans. LAD-I patients present with neutrophilia, delayed wound healing, skin lesions, and enhanced susceptibility to bacterial infections.15 While heterozygotes are normal, patients with less than 1% CD18 expression are severely immunocompromised. Those with moderate CD18 levels (2.5%-31%) have relatively fewer severe abnormalities, with the severity of clinical symptoms directly related to the degree of CD18 deficiency.15 Two mouse models of CD18 integrin deficiency generated by gene targeting, one resulting in CD18 deficiency and the other in hypomorphic CD18 expression, exhibited features of LAD-I.16-18 CD18-deficient mice displayed massive neutrophilia, splenomegaly, delayed wound healing, T cell–dependent psoriasis, and significantly reduced neutrophil recruitment in a number of inflammatory models.19
Here we generated mice with a deficiency in CD18 in neutrophils by placing a synthetic miRNA hairpin that targets murine CD18 downstream of the hMRP8 promoter shown to drive transgene expression primarily in mature neutrophils and monocytes.14,20 Classical symptoms of LAD-I patients and CD18-deficient mice include neutrophilia and defects in neutrophil trafficking,15,19 while splenomegaly is observed in CD18-deficient mice17,21 and occasionally in LAD-I humans.22 Transgenic lines exhibited mild neutrophilia, splenomegaly, and a marked reduction in neutrophil trafficking with the extent of each correlating with the level of CD18 silencing. We anticipate that the robust nature of gene knockdown and the high degree of cell specificity will allow our RNAi-transgenic approach to gain wide acceptance as a reverse genetics tool in the study of neutrophil function. This approach represents a significant advance in the field since neutrophils are short lived, terminally differentiated cells that are not only resistant to gene or siRNA delivery in vitro, but also easily activated by in vitro manipulations. The generation of animals with CD18 selectively silenced in neutrophils provides novel models to understand the role of CD18 in this leukocyte subset in vivo.
Methods
Generation of the miRNA construct and transgenic mice
Four different pre-miRNA sequences, targeting mouse CD18 and designed according to Invitrogen's RNAi Designer tool, were cloned into pcDNA 6.2-GW/EmGFP-miR (Invitrogen, Carlsbad, CA). This vector transcribes 5′ and 3′ sequences derived from the murine mir-155 and an optimized stem-loop structure for gene knockdown under the control of a cytomegalovirus (CMV) promoter (CMV-miR). Expression of GFP from this construct allowed the assessment of transfection efficiency. Of the different sequences tested, the stem-loop targeting the 21-nt sequence CTCATCAAGAATGCCTACTAT (nt's 1063 to 1083) of murine CD18 mRNA (miR-CD18) was cloned downstream of the hMRP8 promoter cassette.14 For this, a linker containing SacI and XhoI sites was inserted into the BglI and PstI sites located in exon 2 of the human MRP8 cassette. After excision of the GFP cDNA using the DraI sites from CMV-miR-CD18, a SacI-XhoI fragment that contains the pre-miRNA that targets murine CD18 was cloned into the linker region. Control vectors CMV-miR-Con (predicted not to target any known vertebrate gene) and CMV-miR-Luc (targeting the firefly luciferase gene) were obtained from Invitrogen. A HindIII-EcoR1 fragment containing the transgene cassette was excised and injected into zygotes from C57Bl/6J mice. Transgenic mice were generated in the Brigham and Women's Hospital transgenic facility (Boston, MA). Forward and reverse polymerase chain reaction (PCR) primers used for identification of the transgene in tail DNA were 5′-TGGTTTGGTTATTTGGAGAGTG-3′ and 5′-TCCAGGATCCACTGGTCGAC-3′, respectively.
Transient transfection, generation of cell lines
MPRO cell line clone 2.123 was purchased from ATCC (Manassas, VA) and cultured in Iscoves modified Dulbecco medium, 20% horse serum, and 8 ng/mL murine granulocyte-macrophage colony-stimulating factor (GM-CSF; Invitrogen).
Transient transfection was carried out by electroporation using Nucleofection (setting T20; Amaxa Biosystems, Cologne, Germany) at a cell density of 5 × 106 cells/100 μL. For stable cell line selection, the growth medium was supplemented with 50 μg/mL blasticidin (Invitrogen) 24 hours after electroporation. To obtain single-cell clones, the cells were seeded at a calculated one cell per well density in 96-well plates. Clones derived from single-cell wells were further amplified.
Luciferase assay
Twenty-four hours after transfection with CMV-miR-Luc or hMRP8-miR-Luc and pGL3-luciferase reporter plasmid (Promega, Madison, WI), cells were washed with PBS (without Ca2+ and Mg2+) containing 0.1 mM di-isopropylfluorophospate (DFP; Sigma-Aldrich, St Louis, MO). Luciferase assays were performed using the Luciferase Reporter Assay System (Promega). Lysis buffer was supplemented with 0.1 mM DFP and 1 μM pepstatin A (Sigma-Aldrich). Luciferase activity in supernatants was measured in triplicate with the Monolight 2010 (Analytical Luminescence Laboratory, San Diego, CA).
Isolation and treatment of leukocyte populations
Blood sampled from the retro-orbital plexus of mice was collected in EDTA-containing tubes followed by red blood cell (RBC) lysis with ice-cold H2O to obtain peripheral blood leukocytes. Murine bone marrow cells were collected from femurs and tibias and isolated as previously reported.24 Peripheral blood neutrophils (PBNs) were isolated using neutrophil isolation medium (Cardinal Associates, Santa Fe, NM) as described.25 Stimulation with PMA (100 ng/mL) was for 10 minutes at 37°C. Bone marrow–derived macrophages were generated by culturing bone marrow cells as previously reported.26 Macrophages were lavaged from the peritoneum in cold PBS and plated for 18 hours in 10% FCS in DMEM on polystyrene dishes, and the adherent cells were removed for analysis.
Flow cytometry analysis
All antibodies were from BD Biosciences-Pharmingen (San Diego, CA) unless otherwise indicated. Murine CD18, CD11a, and CD11b expression on peripheral blood leukocytes was characterized using FITC antimouse CD18 (M18/2 or C71/16) or CD11a (2D7; Biolegend, San Diego, CA) and APC antimouse CD11b (M1/70; eBioscience, San Diego, CA). Cell populations were identified using PE anti–Gr-1 (RB6–8C5) for neutrophils, PE anti-CD115 (AFS98; eBioscience) for monocytes, and PE-Cy7 antimouse CD3ϵ (145–2C11) for T cells. For analysis of peripheral blood neutrophils, cells were stained with FITC antimouse CD18 (C71/16) or CD16/32 (2.4G2), PE antimouse CD162 (2PH1), and APC anti–Gr-1 (Invitrogen). For analysis of macrophages, cells were stained with APC anti-F4/80 (AbD Serotec, Raleigh, NC). Geometric mean fluorescent intensity was determined by Cell Quest software (Becton Dickinson, Franklin Lakes, NJ).
Peripheral blood leukocyte count
Anticoagulated peripheral blood samples from the retro-orbital plexus of mice were analyzed with a CDC HEMAVET 850 hematology analyzer (Drew Scientific, Dallas, TX).
Croton oil–induced irritant dermatitis
Croton oil (10 μL of 2%; Sigma-Aldrich) in acetone/olive oil was applied to each side of one ear of anesthetized mice as reported.27 After 6 hours, the ear was fixed in 3.7% formalin and paraffin embedded. Sections (6-μm each) were subjected to the chloroacetate esterase reaction to identify neutrophils as described.28 The number of emigrated neutrophils was counted in an area covering 1.62 mm from the tip of the ear. Neutrophils in the vessel lumen and mast cells identified by their morphology and intensity of staining were excluded. Tissues were imaged using a Nikon Microphot-FXA microscope (Melville, NY), 100×/1.40 NA oil plan Apo. Images were acquired with the Nikon DS-SM-VI Digital Photomicrographic camera system and Nikon ACT-2U software. Ear widths were measured using spring-loaded calipers and cutaneous edema was quantified as the percentage of increase in croton oil–treated ear width compared with the untreated ear.
Statistical analysis
Data were presented as means plus or minus SEM. Data from different groups, wild-type C57Bl/6 versus each strain of miR-CD18 transgenic (Tg) mice were analyzed by Student t test.
Results
Design and characterization of synthetic miRNA-expressing constructs in neutrophil-like cells in vitro
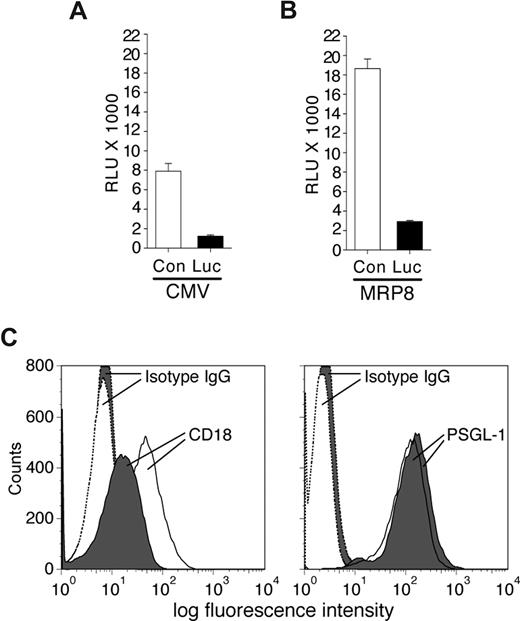
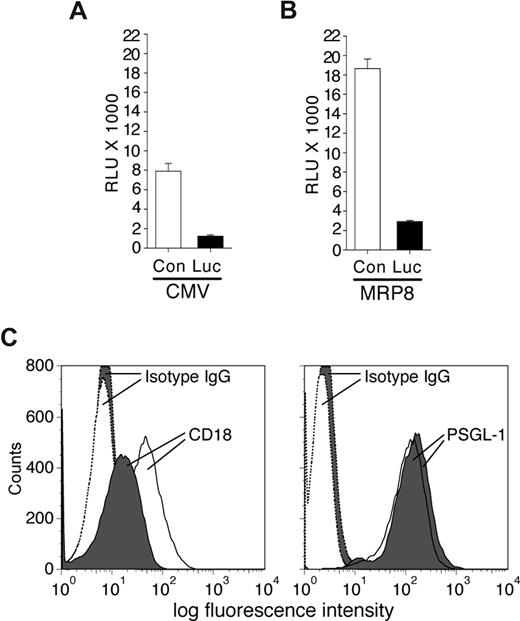
To determine the effectiveness of synthetic miRNA hairpins in silencing gene expression in neutrophils, the vector CMV-miR-Luc designed to generate a stem-loop miRNA targeting Luciferase was cotransfected with a luciferase reporter vector (pGL3) into the MPRO murine neutrophil cell line, and luciferase expression was evaluated. This analysis revealed efficient silencing of luciferase compared with control miRNA (Figure 1A). Similar results were obtained following MPRO cell differentiation with retinoic acid (data not shown). These results indicate that exogenous miRNA hairpins can be efficiently processed in neutrophils to elicit gene silencing. To examine neutrophil-specific expression of synthetic miRNA, we tested the ability of the MRP8 promoter to drive expression of miR-Luc hairpin in MPRO cells. The fragment containing the Drosha processing sites and the stem-loop precursor of the miR-Luc or the irrelevant control miRNA (miR-Con) were cloned downstream of the hMRP8 promoter within exon 2.14 Expression of the hMRP8-miR-Luc efficiently silenced the luciferase signal from the cotransfected pGL3 vector in MPRO cells compared with the hMRP8-miR-Con (Figure 1B).
Characterization of miRNA vectors in the MPRO cell line. MPRO cells were transiently transfected with pGL3-luciferase reporter vector and plasmids encoding specific miRNA sequences targeting the luciferase enzyme (Luc) or a control sequence (Con) either using a CMV promoter (A) or the MRP8 promoter (B). Results from luciferase assays are reported as relative light units (RLUs). The miRNA targeting Luc efficiently reduced luciferase expression/activity compared with a control sequence. (C) MPRO cells were transfected with CMV-miR-Luc or CMV-miR-CD18 and selected using blasticidin. Shown is an example of blasticidin-resistant miR-CD18 (shaded) and miR-Luc (open) clones subjected to FACs analysis for CD18 (left panel) and PSGL-1 (right panel) expression. The histograms for isotype controls are indicated.
Characterization of miRNA vectors in the MPRO cell line. MPRO cells were transiently transfected with pGL3-luciferase reporter vector and plasmids encoding specific miRNA sequences targeting the luciferase enzyme (Luc) or a control sequence (Con) either using a CMV promoter (A) or the MRP8 promoter (B). Results from luciferase assays are reported as relative light units (RLUs). The miRNA targeting Luc efficiently reduced luciferase expression/activity compared with a control sequence. (C) MPRO cells were transfected with CMV-miR-Luc or CMV-miR-CD18 and selected using blasticidin. Shown is an example of blasticidin-resistant miR-CD18 (shaded) and miR-Luc (open) clones subjected to FACs analysis for CD18 (left panel) and PSGL-1 (right panel) expression. The histograms for isotype controls are indicated.
Next, several miRNA sequences targeting murine CD18 were generated and cloned into the CMV-miR expression vector that contains the selectable marker blasticidin. After transient transfection of these plasmids into 293 cells along with murine CD18 cDNA, fluorescence-activated cell sorting (FACs) analysis was performed to detect surface expression of the protein. One (miR-CD18) of the 4 sequences demonstrated reproducible reduction in CD18 levels (data not shown) and was evaluated further for its ability to silence endogenous CD18 in MPRO cells. Due to low transfection efficiency of these cells, MPRO cell lines were established after blasticidin selection. Of 29 cell lines examined, 6 exhibited a more than 75% reduction in surface CD18 expression. Similarly established CMV-miR-Luc–expressing cells exhibited no reduction in CD18 levels in 36 cell lines examined. Levels of PSGL-1, another surface receptor, were equivalent in both sets of cell lines (Figure 1C).
Generation of transgenic mice with neutrophil-selective expression of miR-CD18
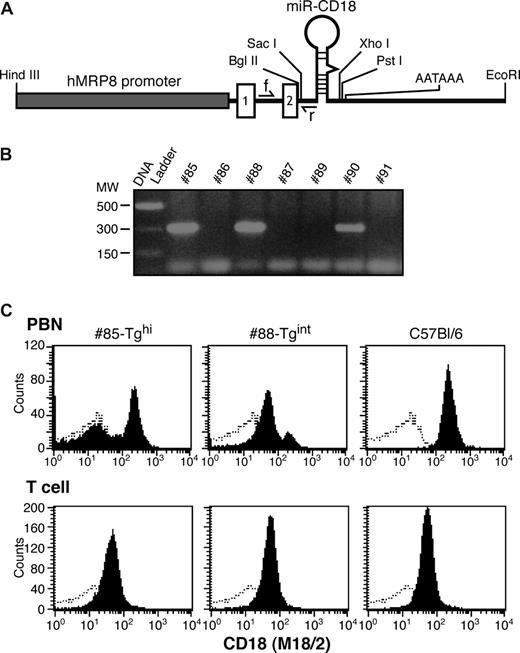
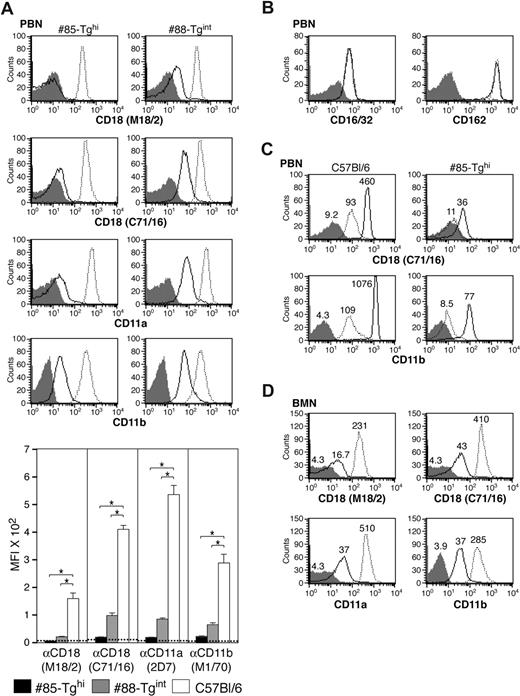
The miR-CD18 hairpin was cloned downstream of the hMRP8 promoter (hMRP8-miR-CD18), as depicted in Figure 2A, and was injected into oocytes to generate transgenic (Tg) animals. Three founders were identified by PCR analysis (Figure 2B). FACs analysis of CD18 surface expression in peripheral blood revealed a marked reduction of CD18 levels in peripheral blood neutrophils (PBNs) in a significant proportion of cells in Tg #85 and #88 mice, while no knockdown was observed in CD3+ T cells (Figure 2C). These founders were used to establish independent mouse lines. Offspring of Tg #88 animals (#88-Tgint) exhibited a significant but partial reduction (75%) in surface CD18 levels in PBNs as detected by antibodies to CD18. Tg #85 (#85-Tghi) demonstrated a greater than 95% decrease in CD18 surface expression. Similar results were obtained using antibodies to the CD11a or CD11b subunits, which detect LFA-1 and Mac-1, respectively (Figure 3A). Levels of 2 other surface proteins, FcγRs (CD16/32) and L-selectin (CD162), were comparable in Tg and wild-type animals (Figure 3B). In addition to being present at the cell surface, CD11b/CD18 is stored in intracellular granules of unstimulated cells and increases 5- to 10-fold on the surface after stimulation with inflammatory mediators.29 We observed a marked increase in CD18 and CD11b subunits on PMA-stimulated wild-type PBNs that was significantly reduced in #85-Tghi transgenic neutrophils (Figure 3C). Bone marrow–derived neutrophils (BMNs) isolated from mice are often used as a source for in vitro functional and biochemical analyses due to the limited number of PBNs that can be harvested from mice. Thus the efficiency of silencing in this neutrophil population was examined. BMNs exhibited on average a 90% reduction in CD18 (Figure 3D).
Design of the MRP8-miR-CD18 transgene expression vector and generation of transgenic founders. (A) A synthetic miRNA hairpin targeting CD18 was cloned in exon 2 of the hMRP8 cassette containing the hMRP8 promoter. The location of the forward (f) and reverse (r) PCR primers for genotyping is indicated. The forward primer is in the intron between exons 1 and 2, and the reverse primer partially overlaps with the 5′miR flanking region. (B) Genotypes of mice were determined by PCR analysis of tail DNA. The expected PCR product of 300 bp for the transgene was observed in 3 animals. (C) FACs analysis of peripheral blood neutrophils (PBNs) and T cells for CD18 (shaded) and isotype control (open) from #85-Tghi and #88-Tgint founders is shown.
Design of the MRP8-miR-CD18 transgene expression vector and generation of transgenic founders. (A) A synthetic miRNA hairpin targeting CD18 was cloned in exon 2 of the hMRP8 cassette containing the hMRP8 promoter. The location of the forward (f) and reverse (r) PCR primers for genotyping is indicated. The forward primer is in the intron between exons 1 and 2, and the reverse primer partially overlaps with the 5′miR flanking region. (B) Genotypes of mice were determined by PCR analysis of tail DNA. The expected PCR product of 300 bp for the transgene was observed in 3 animals. (C) FACs analysis of peripheral blood neutrophils (PBNs) and T cells for CD18 (shaded) and isotype control (open) from #85-Tghi and #88-Tgint founders is shown.
Evaluation of CD18 expression in neutrophils of miR-CD18 animals. (A) Mouse CD18, CD11b, and CD11a expression was analyzed by flow cytometry in peripheral blood neutrophils (PBNs) in miR-CD18 #85-Tghi (solid line), #88-Tgint (solid line), and wild-type C57Bl/6 (dotted line) mice. Data using isotype IgG are shown as shaded area in histograms. Efficiency of CD18 knockdown was determined using the average geometric mean fluorescence intensity (MFI) plus or minus SEM (graph, bottom) (n = 7-9 for each strain). Dotted line indicates average MFI in CD18-null mice (n = 4). *P < .001. (B) Mouse CD16/32 (left) and CD162 (right) expression on PBNs in #85-Tghi and wild-type mice and (C) mouse CD18 and CD11b expression in PBNs isolated from wild-type and #85-Tghi mice and stimulated in vitro without (dotted line) or with PMA (solid line) are shown. The MFI of each sample is also given. (D) Analysis of mouse CD18, CD11b, and CD11a on bone marrow neutrophils (BMNs). The MFIs of miR-CD18 #85-Tghi mouse (solid line), wild-type mouse (dotted line), and isotype control (shaded area) samples are indicated.
Evaluation of CD18 expression in neutrophils of miR-CD18 animals. (A) Mouse CD18, CD11b, and CD11a expression was analyzed by flow cytometry in peripheral blood neutrophils (PBNs) in miR-CD18 #85-Tghi (solid line), #88-Tgint (solid line), and wild-type C57Bl/6 (dotted line) mice. Data using isotype IgG are shown as shaded area in histograms. Efficiency of CD18 knockdown was determined using the average geometric mean fluorescence intensity (MFI) plus or minus SEM (graph, bottom) (n = 7-9 for each strain). Dotted line indicates average MFI in CD18-null mice (n = 4). *P < .001. (B) Mouse CD16/32 (left) and CD162 (right) expression on PBNs in #85-Tghi and wild-type mice and (C) mouse CD18 and CD11b expression in PBNs isolated from wild-type and #85-Tghi mice and stimulated in vitro without (dotted line) or with PMA (solid line) are shown. The MFI of each sample is also given. (D) Analysis of mouse CD18, CD11b, and CD11a on bone marrow neutrophils (BMNs). The MFIs of miR-CD18 #85-Tghi mouse (solid line), wild-type mouse (dotted line), and isotype control (shaded area) samples are indicated.
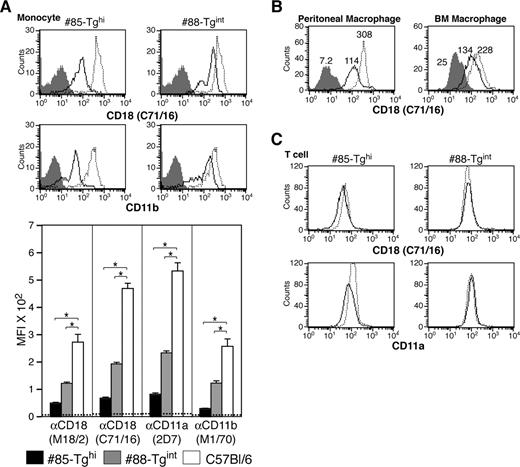
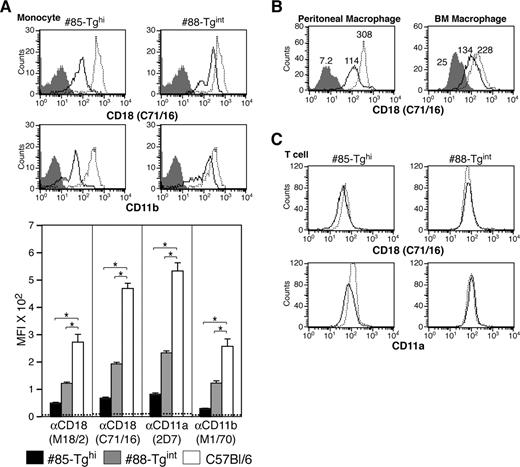
A more than 80% reduction in CD18 levels was observed in the CD115+ monocytic population in #85-Tghi mice. In #88-Tgint animals, 1 of 2 populations of CD115+ cells exhibited a 75% reduction in CD18 expression, which resulted, on average, in an overall 50% to 60% decrease in CD18 (Figure 4A). Analysis of CD18 levels in macrophages harvested from the peritoneal cavity or derived from the bone marrow revealed only a partial but reproducible reduction in CD18 levels (Figure 4B). CD18 expression in peripheral blood T cells of transgenics was minimally affected compared with wild-type animals (Figure 4C), demonstrating selectivity of the hMRP8-miR-CD18 for the myeloid lineage.
Analysis of CD18 levels in monocytes, macrophages, and T cells. (A) CD18 and CD11b expression by flow cytometry on peripheral blood monocytes (CD115+ population) of both transgenic lines: miR-CD18 #85-Tghi (solid line), #88-Tgint (solid line), and wild-type C57Bl/6 (dotted line). Data for isotype IgG (shaded area) are shown. CD18 knockdown in neutrophils was determined using the average geometric mean fluorescence intensity (MFI) plus or minus SEM (graph, bottom) (n = 6-9 for each strain). Dotted line indicates average MFI in CD18-null mice (n = 4), *P < .005. (B) Analysis of CD18 expression in #85-Tghi peritoneal (left panel) and bone marrow–derived (BM; right panel) macrophages. Histograms for miR-CD18 #85-Tghi (solid line) and wild-type C57Bl/6 (dotted line) mice, and data using isotype IgG (shaded area) are shown. (C) CD18 and CD11a expression in T cells from lines #85-Tghi (solid line), #88-Tgint (solid line), and wild-type C57Bl/6 (dotted line) are shown.
Analysis of CD18 levels in monocytes, macrophages, and T cells. (A) CD18 and CD11b expression by flow cytometry on peripheral blood monocytes (CD115+ population) of both transgenic lines: miR-CD18 #85-Tghi (solid line), #88-Tgint (solid line), and wild-type C57Bl/6 (dotted line). Data for isotype IgG (shaded area) are shown. CD18 knockdown in neutrophils was determined using the average geometric mean fluorescence intensity (MFI) plus or minus SEM (graph, bottom) (n = 6-9 for each strain). Dotted line indicates average MFI in CD18-null mice (n = 4), *P < .005. (B) Analysis of CD18 expression in #85-Tghi peritoneal (left panel) and bone marrow–derived (BM; right panel) macrophages. Histograms for miR-CD18 #85-Tghi (solid line) and wild-type C57Bl/6 (dotted line) mice, and data using isotype IgG (shaded area) are shown. (C) CD18 and CD11a expression in T cells from lines #85-Tghi (solid line), #88-Tgint (solid line), and wild-type C57Bl/6 (dotted line) are shown.
Analysis of peripheral blood counts and spleen weights
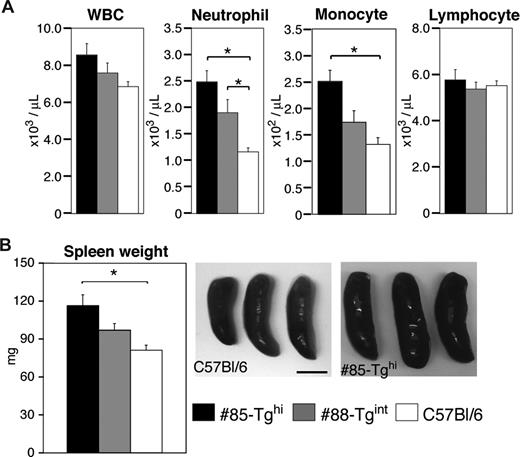
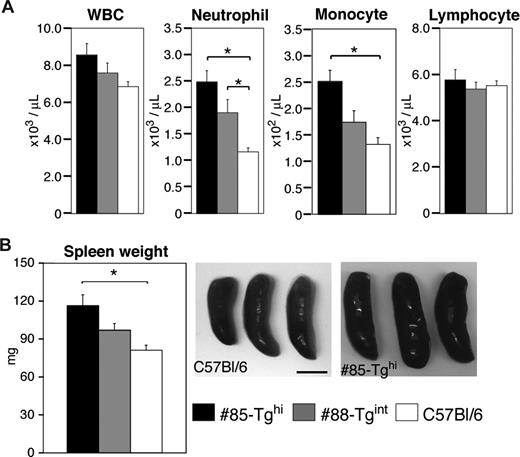
We examined total white blood cell counts and the number of neutrophils, lymphocytes, and monocytes in peripheral blood from the #85-Tghi or #88-Tgint transgenic lines compared with wild-type animals. Both lines of animals exhibited neutrophilia with the extent of neutrophilia correlating with the efficiency of CD18 silencing. Elevation of monocytes was also noted in the #85-Tghi animals, while lymphocyte counts were similar in all strains (Figure 5A).
Measurement of peripheral blood counts and spleen weights. (A) Cell counts of total peripheral white blood cells (WBCs), neutrophils, monocytes, and lymphocytes were assessed (n = 12-20 per group). *P < .005. (B) Representative pictures of spleens from miR-CD18 #85-Tghi and wild-type C57Bl/6 mice are shown. Scale bar represents 5 mm. Average spleen weights are also given (n = 7 per group). *P < .05. All data are means plus or minus SEM.
Measurement of peripheral blood counts and spleen weights. (A) Cell counts of total peripheral white blood cells (WBCs), neutrophils, monocytes, and lymphocytes were assessed (n = 12-20 per group). *P < .005. (B) Representative pictures of spleens from miR-CD18 #85-Tghi and wild-type C57Bl/6 mice are shown. Scale bar represents 5 mm. Average spleen weights are also given (n = 7 per group). *P < .05. All data are means plus or minus SEM.
Spleens were removed and weighed from WT, #85-Tghi mice, and #88-Tgint mice. Larger spleens were observed in both transgenic lines compared with wild-type mice, and the extent of splenomegaly correlated with the degree of CD18 silencing (Figure 5B). CD18-null mice exhibited a more profound splenomegaly (172.4 ± 23.3 mg, n = 5) compared with either of the transgenics.
Analysis of neutrophil trafficking in response to irritant dermatitis
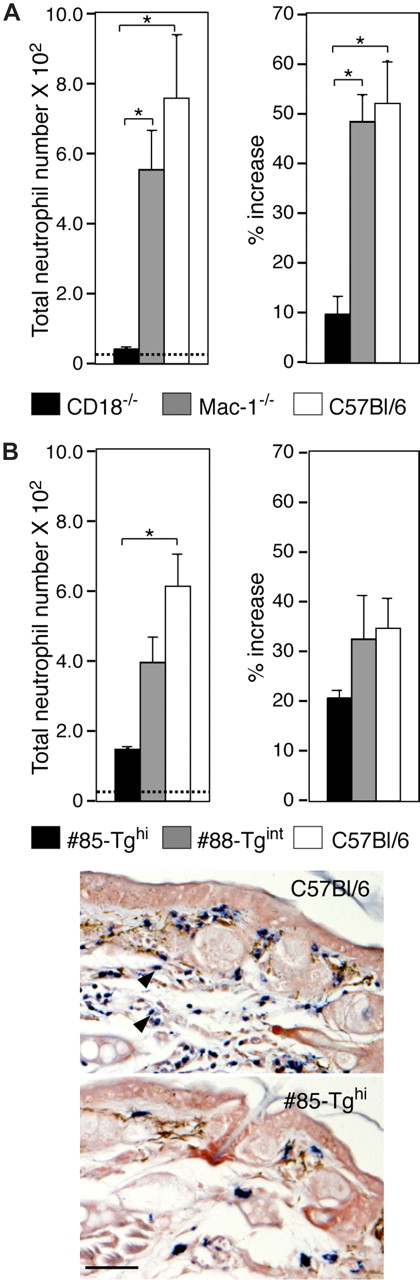
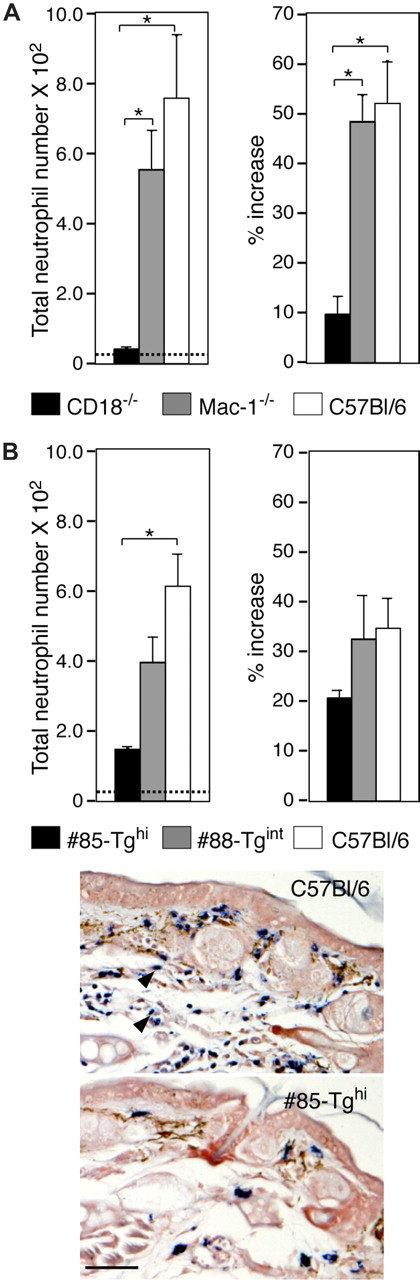
Irritant dermatitis was first induced in wild-type, CD18-null, and Mac-1–deficient mice by the topical application of croton oil to examine the dependency of the response on CD18. CD18-null mice exhibited a marked reduction in neutrophil accumulation and edema (ear swelling) compared with wild-type mice (Figure 6A) as previously reported.27 Mice lacking only Mac-1 (CD11b/CD18) had comparable responses with wild-type animals (Figure 6A), suggesting that this specific member of the CD18 integrin family is not essential for neutrophil trafficking and edema in this model. Next, #88-Tgint mice, #85-Tghi mice, and their wild-type counterparts were evaluated. Neutrophil accumulation and ear swelling were observed in the wild-type mice, while these parameters were significantly attenuated in the #85-Tghi mice and partially reduced in #88-Tgint mice (Figure 6B).
Analysis of neutrophil trafficking in a model of irritant dermatitis. (A) Croton oil was applied to either side of one ear and PBS was given to the contralateral ear of wild-type, CD18-null (CD18−/−) and Mac-1–null (Mac-1−/−) mice. After 6 hours, ear thickness was measured and recorded as the percentage increase in ear swelling of the irritant-exposed ear compared with that before the croton oil challenge. Histochemical analysis of cutaneous neutrophil infiltration by esterase staining of harvested ear specimens was also undertaken. Emigrated neutrophil counts (left panels) and ear edema (% increase) (right panels) are shown. Dotted line indicates average neutrophil counts in PBS-treated C57Bl/6 mice. *P < .005. (B) Wild-type, #85-Tghi, and #88-Tgint were subjected to irritant dermatitis and analyzed as in panel A. Representative pictures of esterase-stained neutrophils (arrowheads) following induction of dermatitis in C57Bl/6 wild-type and #85-Tghi mice are shown. Scale bar represents 50 μm. All data are mean plus or minus SEM of n = 4 to 5 per group.
Analysis of neutrophil trafficking in a model of irritant dermatitis. (A) Croton oil was applied to either side of one ear and PBS was given to the contralateral ear of wild-type, CD18-null (CD18−/−) and Mac-1–null (Mac-1−/−) mice. After 6 hours, ear thickness was measured and recorded as the percentage increase in ear swelling of the irritant-exposed ear compared with that before the croton oil challenge. Histochemical analysis of cutaneous neutrophil infiltration by esterase staining of harvested ear specimens was also undertaken. Emigrated neutrophil counts (left panels) and ear edema (% increase) (right panels) are shown. Dotted line indicates average neutrophil counts in PBS-treated C57Bl/6 mice. *P < .005. (B) Wild-type, #85-Tghi, and #88-Tgint were subjected to irritant dermatitis and analyzed as in panel A. Representative pictures of esterase-stained neutrophils (arrowheads) following induction of dermatitis in C57Bl/6 wild-type and #85-Tghi mice are shown. Scale bar represents 50 μm. All data are mean plus or minus SEM of n = 4 to 5 per group.
Discussion
Our studies demonstrate efficient neutrophil-selective silencing of a gene using an miRNA approach in transgenic animals. We anticipate that the robust nature of the interference effect and the high degree of cell specificity will allow our RNAi transgenic approach to gain wide acceptance as a reverse genetic tool for investigating gene function in neutrophils. Although miRNA-mediated gene silencing in Sertoli cells in vivo has been recently reported,13 there have been no additional reports to date of cell type–specific delivery of synthetic miRNA to other organs, or to cells of the hematopoietic compartment such as neutrophils. Indeed, successful silencing in mature neutrophils in particular may have been viewed as challenging since they are fully differentiated cells with a high turnover rate. Recently, LFA-1–targeted antibody protamine fusion proteins were shown to deliver siRNAs to LFA-1–expressing cells in vitro. Although siRNA delivery was also shown to occur when an LFA-1–expressing leukocytic cell line was engrafted in the lungs of mice, the feasibility of such a system to silence proteins in endogenous leukocyte subsets in vivo remains to be demonstrated.30
Our miRNA approach, requiring the generation of one transgenic line, is advantageous over existing conditional gene targeting techniques for tissue-specific knockdown of protein expression, as the latter requires homologous recombination in embryonic stem cells and multiple breeding strategies that are both labor and time intensive. Furthermore, the efficiency of conditional knockouts relies on the fidelity and robustness of a transgenic or knocked-in Cre construct and the sensitivity of the loxP-flanked sites to Cre-mediated recombination. Drawbacks also include cryptic or pseudo loxP sites and potential toxicity from the Cre recombinase activity.31 Another advantage of the miRNA method for silencing genes is that it allows variable silencing of the gene in different transgenic lines. This can provide models of hypomorphic expression for conditions characterized by variable protein levels. Furthermore, unlike the Cre/loxP technology, the miRNA approach exploits a nucleic acid processing activity that is intrinsic to the cell and thus likely minimizes cell toxicity.
For selective silencing in neutrophils we exploited the hMRP8 promoter, which contains tissue-specific regulatory elements responsible for expression in cells of the myeloid lineage14 (N.T. and T.N.M., unpublished data, January 2006). CD18 silencing was achieved in neutrophils and monocytes. Partial silencing of CD18 was observed in tissue- and bone marrow–derived macrophages, while it was minimal in T cells. The pattern of silencing is consistent with the reported activity of the human MRP8 promoter cassette in mice. That is, hMRP8-driven transgene expression in neutrophils has been consistently reported.14,32 On the other hand, hMRP8 activity in T cells is absent,32 is low (N.T. and T.N.M., unpublished data, January 2006) to undetectable14 in macrophages, and is intermediate (N.T. and T.N.M., unpublished data, January 2006) to high20 in monocytes. The variations in transgene expression may be dictated by the stage of monocyte/macrophage differentiation, by the activation status of the cells,33-35 and, we speculate, by the location of the transgene in different murine lines. It is noteworthy that the miRNA-mediated silencing, which relies on active transcriptional machinery, is effective in neutrophils in circulation. This, together with the recent identification of endogenous miRNAs in neutrophils,36 suggests the intriguing possibility that the endogenous miRNA pathway in neutrophils may play an important role in regulating gene expression in these terminally differentiated cells in homeostasis and inflammation.
The pathophysiology of LAD-I arises from a deficiency of CD18 in multiple leukocyte cell types. CD18 deficiency in mice leads to significant defects in T-cell extravasation resulting in altered contact hypersensitivity responses.37 Moreover, impaired accumulation and function of macrophages and neutrophils has been associated with wound-healing defects19 and enhanced susceptibility to infections. The generation of a mouse model with selective silencing of CD18 in neutrophils and monocytes as described in our study allows the evaluation of the phenotypes in the CD18-deficient subjects that are dependent primarily on CD18 on these 2 leukocyte subsets. Furthermore, generation of mice with intermediate CD18 silencing recapitulates LAD-I patients with moderate CD18 deficiency and provides the opportunity to evaluate gene dosage effects on phenotypic outcomes. A consistent finding in CD18-deficient subjects is neutrophilia. This phenotype has been attributed to ineffective neutrophil trafficking that may lead to altered granulopoiesis perhaps as a result of infection-related imbalances of cytokines, and/or defective apoptosis of blood neutrophils.29,38-40 The 2- to 3-fold neutrophilia in the miR-CD18 mice was similar to that observed in other adhesion molecule knockouts that exhibit defects in neutrophil trafficking.41 Our observation that the extent of neutrophilia correlated with the degree of CD18 silencing suggests that CD18 levels on neutrophils may be the primary determinant of neutrophilia. The significantly higher numbers of circulating neutrophils in subjects with global CD18 deficiency may be a consequence of CD18 deficiency in multiple leukocyte subsets that in turn could affect leukocytosis. However, we cannot rule out the possibility that the remaining 2% to 5% of CD18 in our transgenics is sufficient to compensate for this phenotype as hypomorphic CD18 expression in gene-targeted mice also leads to moderate neutrophilia.16 Selective CD18 silencing in neutrophils also resulted in splenomegaly, suggesting a prominent role for CD18 on this cell type in dictating this phenotype observed in CD18-null mice, which results from myeloid hyperplasia in the red pulp.17,21 Neutrophil trafficking in response to the epicutaneous application of croton oil was dependent on CD18 as previously reported.27,37 Similar to CD18-null mice, a significant reduction in neutrophil accumulation was observed in transgenic mice with more than 95% reduction in neutrophil CD18. The intermediate phenotype of neutrophilia, splenomegaly, and neutrophil trafficking in mice with 75% versus more than 95% reduction in CD18 is consistent with reports in humans that the severity of clinical complications directly correlates with the extent of CD18 deficiency15 and demonstrates that CD18 levels specifically in neutrophils may play a prominent role in shaping these phenotypes.
In summary, we describe a method for selective gene silencing in neutrophils and monocytes that has general applicability for specifically knocking-down proteins in other cell types. The approach is efficacious and also provides the opportunity to examine the effects of hypomorphic gene expression on phenotypic outcomes. In particular, the success of this approach in neutrophils has very practical applications for evaluating the role of specific genes in cells that are otherwise difficult to genetically manipulate in vitro. Retroviral- or lentiviral-mediated gene transfer to human bone marrow has been used for management of myeloid cell primary immune deficiencies.42 This prompts us to speculate that similar delivery of hMRP8-miRNA constructs that have in addition been modified to allow inducible expression may provide a means for temporal and neutrophil-selective silencing of inflammatory proteins in chronic inflammatory disease. Our analysis of mice with neutrophil-selective CD18 silencing revealed neutrophilia, splenomegaly, and neutrophil trafficking defects, suggesting a role for CD18 on neutrophils in these leukocyte adhesion deficiency type I (LAD-I) phenotypes. We anticipate that the mouse model generated in this study will continue to be a valuable tool to examine the contribution of CD18 on neutrophils to LAD-I, a complex inherited disorder in which reduced or absent CD18 expression in multiple leukocyte subsets leads to impaired innate and adaptive immune responses.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Drs Irving Weissman (Stanford School of Medicine, Palo Alto, CA) and Eric Lagasse (McGowan Institute for Regenerative Medicine, University of Pittsburgh, Pittsburgh, PA) for providing the hMRP8 promoter cassette, and to Ling Xiao for technical assistance.
This work was supported by National Institutes of Health (NIH) grant KO1 AR054984 (X.C.), an Arthritis Foundation postdoctoral fellowship (N.T.), and NIH grants RO1 HL065095 and AR050800, and PO1 HL036028 (T.N.M).
National Institutes of Health
Authorship
Contribution: X.C. designed and performed the research, analyzed and interpreted data, and drafted the paper; M.L. and N.T. designed and performed the research, analyzed and interpreted data, and performed the statistical analysis; T.N.M. designed the research, analyzed and interpreted data, and drafted the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tanya N. Mayadas, Department of Pathology, Brigham and Women's Hospital, 77 Avenue Louis Pasteur, NRB752O, Boston, MA 02115; e-mail: tmayadas@rics.bwh.harvard.edu.
References
Author notes
X.C., M.L., and N.T. contributed equally to this work.