Abstract
Inside-out signaling regulation of the β2-integrin leukocyte function–associated antigen-1 (LFA-1) by different cytoplasmic proteins, including 14-3-3 proteins, is essential for adhesion and migration of immune cells. Here, we identify a new pathway for the regulation of LFA-1 activity by Cbl-b, an adapter molecule and ubiquitin ligase that modulates several signaling pathways. Cbl-b−/− mice displayed increased macrophage recruitment in thioglycollate-induced peritonitis, which was attributed to Cbl-b deficiency in macrophages, as assessed by bone marrow chimera experiments. In vitro, Cbl-b−/− bone marrow–derived mononuclear phagocytes (BMDMs) displayed increased adhesion to endothelial cells. Activation of LFA-1 in Cbl-b–deficient cells was responsible for their increased endothelial adhesion in vitro and peritoneal recruitment in vivo, as the phenotype of Cbl-b deficiency was reversed in Cbl-b−/−LFA-1−/− mice. Consistently, LFA-1–mediated adhesion of BMDM to ICAM-1 but not VLA-4–mediated adhesion to VCAM-1 was enhanced by Cbl-b deficiency. Cbl-b deficiency resulted in increased phosphorylation of T758 in the β2-chain of LFA-1 and thereby in enhanced association of 14-3-3β protein with the β2-chain, leading to activation of LFA-1. Consistently, disruption of the 14-3-3/β2-integrin interaction abrogated the enhanced ICAM-1 adhesion of Cbl-b−/− BMDMs. In conclusion, Cbl-b deficiency activates LFA-1 and LFA-1–mediated inflammatory cell recruitment by stimulating the interaction between the LFA-1 β-chain and 14-3-3 proteins.
Introduction
Leukocyte extravasation to the site of infection or inflammation is a well-organized cascade of adhesive events, including selectin-dependent rolling, chemokine-dependent leukocyte activation, and integrin-mediated firm adhesion and diapedesis.1 Leukocyte function–associated antigen-1 (LFA-1; αLβ2; CD11a/CD18) is fundamental during firm endothelial adhesion of leukocytes by interacting with endothelial counterligands such as ICAM-1.1-3
LFA-1 integrin activation is crucial for inflammatory cell adhesion and is regulated by complementary mechanisms involving affinity alterations due to rapid conformational changes, as well as affinity-independent mechanisms such as integrin lateral mobility, resulting in valency/avidity increases.4,5 LFA-1 activation can occur through inside-out signaling (ie, by intracellular signaling pathways; eg, triggered by chemokines). These pathways include protein kinases,6 lipid kinases,7 and small GTPases, such as Rap1 and its effector RAPL.8-10 In addition, the actin cytoskeleton is integral to LFA-1 activation (eg, the interaction of the cytoskeletal protein talin with the cytoplasmic tail of the LFA-1 β-chain stimulates integrin conformational changes and activation).11
During inside-out signaling activation of LFA-1, phosphorylation of the cytoplasmic tails of the α and β chains of the integrin can regulate their interactions with cytoplasmic factors.6,12,13 Constitutive phosphorylation of LFA-1 on the αL-chain Ser-1140 regulates integrin affinity changes such as the ones mediated by Rap1.13 LFA-1 β-chain phosphorylation occurs on several residues upon cell stimulation. Phosphorylation of the TTT motif (residues 758-760) upon phorbol ester treatment or through T-cell receptor activation14 has been implicated in LFA-1–mediated cell adhesion to ICAM-1 and modulation of cell spreading,15,16 mainly by mediating cytoskeletal interactions of the integrin and recruitment of 14-3-3 proteins to the integrin.13,16 14-3-3 proteins are multifunctional adaptor proteins that recognize phosphoserine- or phosphothreonine-containing motifs in proteins.17 However, the functional importance of the 14-3-3/LFA-1 interaction in inflammatory cell recruitment has not been defined.
The Cbl family consists of 3 homologs, c-Cbl, Cbl-b, and Cbl-3, that are adaptor molecules undergoing multiple interactions with protein tyrosine kinases and SH2 and SH3 domain– containing proteins.18 They are E3 ubiquitin ligases, which function to negatively regulate a diverse repertoire of surface receptors and downstream signaling proteins.18,19 c-Cbl and Cbl-b are predominantly expressed in hematopoetic cells.19,20 Cbl-b was identified as a key regulator in autoimmune diseases, as Cbl-b−/− mice were more susceptible to autoimmunity.21,22 Inflammatory cell recruitment is crucial to the pathogenesis of autoimmune diseases; however, whether Cbl-b and/or Cbl-b deficiency influence extravasation-related inflammatory cell functions such as adhesion remains incompletely addressed. In a recent report, Cbl-b–deficient T cells displayed increased Rap1 activity and enhanced LFA-1–mediated adhesion of T cells to ICAM-1 in vitro.23 These findings prompted us to investigate whether Cbl-b deficiency affects leukocyte recruitment in vivo as well as extravasation-related processes such as integrin-mediated adhesion of monocytes/macrophages in vitro and to analyze the underlying mechanisms.
Methods
Reagents and antibodies
Thioglycollate broth, phorbol 12-myristate 13-acetate (PMA), and β-actin antibody were purchased from Sigma-Aldrich (St Louis, MO). Okadaic acid and calyculin A were from LC Laboratories (Woburn, MA), and the protein kinase C (PKC) inhibitor Gö6983 was from Calbiochem (San Diego, CA). Recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF) was obtained from Endogen (Rockford, IL). Human ICAM-1, mouse ICAM-1, and mouse VCAM-1 were from R&D Systems (Minneapolis, MN). The blocking monoclonal antibody (mAb) to mouse LFA-1 (M17/4) and blocking mAb to mouse VLA-4 (R1.2) were from Biolegend (San Diego, CA). The activating mAb to human CD11a, MEM-83, and mAb C71/16 against CD18 were from Abcam (Cambridge, United Kingdom). Phosphospecific polyclonal antibody against phosphothreonine 758 in CD18 and monoclonal R2E7B against human β2 subunit were previously described.24 Allophycocyanin (APC)–conjugated antibody against mouse CD11b was obtained from BD Biosciences (San Jose, CA). AlexaFluor488 anti-mouse CD18 (M18/2) and FITC-conjugated anti-human CD11a (HI111) were purchased from Biolegend (San Diego, CA). Anti-rabbit Alexa 488 and anti-rat Alexa 568 were from Molecular Probes (Eugene, OR). Antibodies to 14-3-3β (K-19) and Cbl-b (G-1 and H-121) were from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell lines and cDNA constructs
Mouse endothelioma b.End.3 cells and HEK293 cells were from American Type Culture Collection (ATCC; Manassas, VA) and grown as described by the supplier. 293 cells stably transfected with LFA-1 were previously described.25 All cell culture reagents were purchased from Invitrogen (Carlsbad, CA) except otherwise described. The wild-type (WT) αL-integrin, WT β2-integrin, and the mutant T758A β2-integrin constructs were previously described.13 The EGFP-R18wt and EGFP-R18mut plasmids were gifts from T. Pawson (University of Toronto, ON) and previously described.17 The EGFP-R18wt construct blocks 14-3-3 interactions with its cellular ligands by binding to the phosphopeptide-binding groove in 14-3-3, whereas the EGFP-R18mut construct does not bind to 14-3-3 proteins.
Isolation of BMDMs
Bone marrow–derived monocyte isolation was performed as described26 with some modification. Briefly, bone marrow cells were flushed from the bones with ice-cold RPMI containing 10% fetal calf serum (FCS). After red blood cell (RBC) lysis, the cells were washed and then resuspended in complete RPMI 1640 medium. Cells were adjusted at a density of 106 cells/mL and then cultivated on petri dishes in the presence of murine GM-CSF (mGM-CSF; 10 ng/mL). After 4 days of incubation, floating cells were removed and adherent cells were washed twice with phosphate-buffered saline (PBS). The adherent bone marrow–derived mononuclear phagocyte (BMDM) population was verified by fluorescence-activated cell sorter (FACS) analysis as CD11b+ cells. Cells were trypsinized for use in further experiments.
siRNA-mediated knockdown and transfection
293 cells were transfected with siRNA against human Cbl-b or control siRNA (Dharmacon, Lafayette, CO) using Lipofectamine 2000 (Invitrogen); after 24 hours, electroporation was performed (300 V, 10 ms) using an Electro Square Porator ECM 830 (BTX, San Diego, CA) for transient transfection with human WT LFA-1 α-chain together with either WT β2-integrin or mutant T758A-β2 integrin. Western blotting was used to confirm Cbl-b knockdown, whereas Western blotting and flow cytometric analysis were used to analyze total and cell surface expression, respectively, of the αL- and β2-chains.
The transfection of primary WT and Cbl-b−/− BMDM with EGFP-R18wt and EGFP-R18mut was performed engaging a lentiviral system. The EGFP-R18wt and EGFP-R18mut were amplified by polymerase chain reaction (PCR) using the primers 5′-CCCGAATTCTTATCTAGATCCGGTGG-3′ and 5′-CCCGAATTCTTATCTAGATCCGGTGG-3′ containing SpeI and EcoRI restriction sites, respectively. The PCR products were ligated into XbaI and EcoRI sites of the lentivirus vector pLCMV-PL3 that was kindly provided by P. Chumakov (Lerner Research Institute, The Cleveland Clinic Foundation, OH). High titer stocks of recombinant lentivirus were produced by cotransfection into 293FT cells (Invitrogen) of pLCMV-PL3-R18wt and pLCMV-PL3-R18mut constructs together with the packaging plasmids, pREV and pGagPol, and VSV-G (a kind gift from P. Chumakov) using the Lipofectamine-Plus reagent (Invitrogen). Virus-containing supernatants were collected 24 to 72 hours after transfection, and virus was concentrated by PEG precipitation. Briefly, 40% PEG-8000 (Sigma-Aldrich) was added to the collected supernatants. Supernatants were incubated on ice for at least 12 hours and then centrifuged at 1500g for 20 minutes. The pellets were resuspended in RPMI medium and filtered before they were applied onto WT or Cbl-b−/− BMDMs in the presence of 8 μg/mL Sequa-Brene (Sigma-Aldrich). Experiments were performed 3 to 4 days thereafter. The transfection efficiency of R18wt and R18mut was assessed by immunofluorescence using the EGFP contained in both constructs and was approximately 50% to 60%.
Mice
In vivo peritonitis model
Thioglycollate-induced peritonitis was performed with WT C57Bl/6, Cbl-b−/−, and LFA-1−/− mice or with Cbl-b+/−LFA-1+/−, Cbl-b+/−LFA-1−/−, Cbl-b−/−LFA-1+/−, or Cbl-b−/−LFA-1−/− littermates. A previously described protocol was used.28–30 To evaluate peritoneal neutrophil or macrophage recruitment, mice were killed at 4 or 72 hours, respectively, following injection of thioglycollate. Thereafter, the peritoneal lavage was collected and the number of emigrated neutrophils or macrophages was quantified by FACS analysis by staining for Gr-1 and CD11b.30,31 Radiation bone marrow chimeras were prepared as previously described.30 WT and Cbl-b−/− recipient mice were irradiated with 9.5 Gy (950 rad) and reconstituted with 1.5 × 107 bone marrow cells from WT mice (WT → WT and WT → Cbl-b−/−).
Flow cytometric analysis
BMDMs as well as peritoneal cells were blocked with mAb 2.4G2 to Fc receptors and subsequently incubated with APC-conjugated anti-mouse CD11b antibodies in Hanks balanced salt solution (HBSS) buffer containing 0.1% bovine serum albumin (BSA) at 4°C for 1 hour. In other experiments, mouse BMDMs were stained with AlexaFluor488-conjugated anti-mouse CD18 (M18/2) and LFA-1–transfected 293 cells were incubated with FITC anti-human CD11a (HI111) antibody. For intracellular 14-3-3β staining, cultivated monocytes were fixed with 4% paraformaldehyde and then permeabilized with 0.1% Triton X-100. The cells were then incubated with antibody against 14-3-3β (K-19) for 1 hour at 4°C, washed with HBSS buffer containing 0.1% BSA, and then labeled with fluorescein-conjugated goat anti-rabbit IgG (dilution 1:40; BD Biosciences). After washing, cells were resuspended in 500 μL buffer and flow cytometry analysis was performed using FACSCalibur (Becton Dickinson, Franklin Lakes, NJ).
Cell adhesion assay
Adhesion of mouse BMDMs to mouse b.End.3 endothelial cells or immobilized mouse ICAM-1 or VCAM-1 (each 10 μg/mL; and to immobilized BSA as a control) was performed as previously described.29,30,32 Briefly, mouse endothelial cells were cultivated onto microtiter plates until confluence. Alternatively, microplates were coated with ICAM-1, VCAM-1, or BSA in PBS and blocked with 3% BSA. Fluorescence (BCECF)–labeled mouse BMDMs were washed twice in serum-free medium and plated onto the endothelial cell monolayer or the precoated wells (105/well) at 37°C for 60 minutes in the absence or presence of PMA (50 ng/mL) and blocking antibodies. In other experiments, fluorescence-labeled 293 transfectants were plated onto precoated human ICAM-1 in the absence or presence of PMA or activating mAb to LFA-1, MEM-83. Following the incubation period, the wells were washed and adhesion was quantified using a fluorescence microplate reader (BIO-TEK, Winooksi, VT). In the case of EGFP-R18– and EGFP-R18mut–transfected BMDMs, no fluorescence labeling was used, and adhesion was quantified by crystal violet staining and measuring absorbance at 590 nm.28
Immunofluorescence
A previously described protocol was used with modifications.30 BMDMs were seeded onto ICAM-1–coated coverslips in culture medium and incubated at 37°C for 5 minutes. The cells were washed and fixed with 2% paraformaldehyde for 10 minutes and permeabilized with 0.1% Triton X-100 for 5 minutes. Cells were blocked with 1% BSA and 5% goat serum in PBS for 1 hour at 22°C. Cells were then incubated with a combination of monoclonal rat anti-CD18 (C71/16) and polyclonal anti–14-3-3 (K-19) for 1 hour at 22°C and washed, followed by the incubation of secondary goat anti-rat Alexa 568 and goat anti-rabbit Alexa 488 in blocking buffer for 30 minutes at 22°C. Thereafter, cells were washed and mounted in Fluoromount-G (SouthernBiotech, Hatfield, PA). Confocal fluorescence images were captured using a Zeiss laser scanning microscope (Zeiss, Jena, Germany).30
Rap1 assay
To assess active Rap1 in mouse BMDMs or mouse splenocytes, the pull-down assay was performed with EZ-Detect Rap1 Activation Kit (Pierce, Rockford, IL) according to the manufacturer's instructions. Briefly, GST-RalGDS-RBD was added to the Swellgel Immobilized Glutathione Disc (Pierce) and 500 μg cell lysates were immediately added. The reaction mixture was incubated at 4°C for 1 hour with gentle rocking. Precipitates were then washed, resuspended in 50 μL of 2 × sample buffer, boiled for 5 minutes at 100°C, and separated by 12% NuPAGE (Invitrogen). Rap1 antibody was used to detect active Rap1. Whole-cell lysates were used to assess total levels of Rap1.
Immunoprecipitation and Western blot
A previously described protocol was used.13 Briefly, BMDMs or peritoneal macrophages were incubated with complete medium in the absence or presence of PMA (120 ng/mL) without or with okadaic acid (1 μM) at 37°C for 30 minutes. The cells were washed with ice-cold PBS and lysed in a lysis buffer containing 1% CHAPS, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM Tris-HCl (pH 7.5), 0.02% NaN3, 0.1% BSA, protease inhibitors, and 0.5 μM calyculin A. Cell lysates were incubated with monoclonal anti-mouse CD18 (C71/16) at 4°C overnight with continuous rotation and then added with UltraLink Immobilized Protein A/G (Pierce). The complexes containing antibody-bound Ag and coprecipitating proteins were pelleted and washed 5 times with lysis buffer. The bound proteins were eluted with LDS sample buffer (Invitrogen) containing β-mercaptoethanol and analyzed by Western blotting with antibodies against 14-3-3β (K-19), CD18 (C71/16), or phosphospecific T758 CD18 antibody. The blots for phosphospecific T758 CD18 were stripped and reprobed with monoclonal anti-CD18 (C71/16) antibody.
293 transfectants were washed once with ice-cold PBS and lysed in a 1% Triton X-100–containing lysis buffer, boiled, and separated by NuPAGE, followed by protein transfer to nitrocellulose membranes. Membranes were blocked for 1 hour at 22°C and incubated with antibodies against Cbl-b (G-1), β-actin, or CD18 (R2E7B). After washing, blots were incubated with appropriate peroxidase-coupled secondary antibodies (Dako, Carpinteria, CA) and developed with ECL Plus reagent (Pierce).
Statistical analysis
Data were compared using the Student t test and analysis of variance (ANOVA) with post-hoc analysis as appropriate; P values less than .05 were regarded as significant.
Results
Cbl-b deficiency increases LFA-1–dependent inflammatory cell recruitment
In order to study whether Cbl-b deficiency affects inflammatory cell recruitment in vivo, the infiltration of macrophages to the peritoneum was analyzed in WT and Cbl-b−/− mice after intraperitoneal injection of thioglycollate. As compared with WT mice, Cbl-b−/− mice displayed increased macrophage recruitment into the peritoneal cavity (Figure 1A). No difference in the baseline numbers of macrophages resident in the peritoneum (ie, in mice that received PBS) between WT and Cbl-b−/− mice was observed (Figure 1A). In addition, Cbl-b−/− mice displayed increased neutrophil recruitment compared with WT mice at 4 hours after thioglycollate injection (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Cbl-b deficiency increases macrophage recruitment in vivo. (A) The numbers of macrophages in WT (□) or Cbl-b−/− (■) mice are shown at 0 hours and 72 hours after intraperitoneal injection of thioglycollate solution. (B) Thioglycollate-induced peritonitis in WT mice sublethally irradiated and reconstituted with bone marrow cells from WT mice (WT → WT), or with bone marrow cells from Cbl-b−/− mice (Cbl-b−/− → WT). Data are expressed as absolute numbers of emigrated macrophages into the peritoneum. Data are means plus or minus SD (n = 3-9 mice/group). #P < .01; *P < .05; ns, nonsignificant.
Cbl-b deficiency increases macrophage recruitment in vivo. (A) The numbers of macrophages in WT (□) or Cbl-b−/− (■) mice are shown at 0 hours and 72 hours after intraperitoneal injection of thioglycollate solution. (B) Thioglycollate-induced peritonitis in WT mice sublethally irradiated and reconstituted with bone marrow cells from WT mice (WT → WT), or with bone marrow cells from Cbl-b−/− mice (Cbl-b−/− → WT). Data are expressed as absolute numbers of emigrated macrophages into the peritoneum. Data are means plus or minus SD (n = 3-9 mice/group). #P < .01; *P < .05; ns, nonsignificant.
The enhanced recruitment of macrophages into the peritoneum associated with Cbl-b deficiency could be due to either intrinsic differences in Cbl-b–deficient macrophages or due to differences in the extent of peritonitis induced in Cbl-b–deficient versus WT control mice. To address these possibilities, bone marrow chimera experiments were performed. Irradiated WT recipient mice were reconstituted with either WT or Cbl-b−/− bone marrow. As shown in Figure 1B, WT mice reconstituted with Cbl-b–deficient bone marrow displayed enhanced macrophage recruitment in thioglycollate-induced peritonitis, suggesting the conclusion that intrinsic differences in Cbl-b–deficient macrophages account for their enhanced recruitment into the inflamed peritoneum.
Leukocyte-endothelial adhesion is an integral component of leukocyte recruitment. Nonstimulated or PMA-stimulated adhesion of Cbl-b−/− BMDMs to endothelial cells was significantly increased as compared with WT cells (Figure 2A). The 2 major adhesive interactions involved in leukocyte endothelial adhesion are the LFA-1/ICAM-1 and VLA-4/VCAM-1 systems.1 We therefore investigated the adhesion of BMDMs to immobilized ICAM-1 and VCAM-1. Nonstimulated or PMA-stimulated LFA-1–dependent adhesion of Cbl-b−/− BMDMs to immobilized ICAM-1 was increased as compared with WT cells. In contrast, VLA-4–dependent adhesion to VCAM-1 was not altered by Cbl-b deficiency (Figure 2B,C; data with PMA stimulation not shown). Moreover, spreading of Cbl-b−/− BMDMs onto ICAM-1 was increased compared with WT cells (Figure 2D). Furthermore, transmigration of BMDMs through an endothelial monolayer was enhanced due to Cbl-b deficiency (Figure S1B).
Cbl-b deficiency increases LFA-1–dependent BMDM adhesion. (A) Adhesion of BMDMs to mouse endothelial cells is shown in the absence (□) or presence of PMA (50 ng/mL; ■). (B,C) Adhesion of BMDMs to immobilized ICAM-1 (B) or VCAM-1 (C) is shown in the absence (□) or in the presence of mAb to LFA-1 (20 μg/mL; ■) or mAb to VLA-4 (20 μg/mL; ▩). Cell adhesion is represented as percentage of adherent cells. Data are means plus or minus SD (n = 3). Similar results were observed in 3 separate experiments. (D) Analysis of spreading of WT and Cbl-b−/− BMDMs onto ICAM-1 was performed. Data are represented as percentage of spread cells. (E) The numbers of macrophages at 72 hours after intraperitoneal injection of thioglycollate in Cbl-b+/−LFA-1+/−, Cbl-b−/−LFA-1+/−, Cbl-b+/−LFA-1−/−, or Cbl-b−/−LFA-1−/− mice are shown. Data are expressed as absolute numbers of emigrated macrophages into the peritoneum. Data are means plus or minus SD (n = 4-5 mice/group). *P < .05; #P < .01; ns, nonsignificant.
Cbl-b deficiency increases LFA-1–dependent BMDM adhesion. (A) Adhesion of BMDMs to mouse endothelial cells is shown in the absence (□) or presence of PMA (50 ng/mL; ■). (B,C) Adhesion of BMDMs to immobilized ICAM-1 (B) or VCAM-1 (C) is shown in the absence (□) or in the presence of mAb to LFA-1 (20 μg/mL; ■) or mAb to VLA-4 (20 μg/mL; ▩). Cell adhesion is represented as percentage of adherent cells. Data are means plus or minus SD (n = 3). Similar results were observed in 3 separate experiments. (D) Analysis of spreading of WT and Cbl-b−/− BMDMs onto ICAM-1 was performed. Data are represented as percentage of spread cells. (E) The numbers of macrophages at 72 hours after intraperitoneal injection of thioglycollate in Cbl-b+/−LFA-1+/−, Cbl-b−/−LFA-1+/−, Cbl-b+/−LFA-1−/−, or Cbl-b−/−LFA-1−/− mice are shown. Data are expressed as absolute numbers of emigrated macrophages into the peritoneum. Data are means plus or minus SD (n = 4-5 mice/group). *P < .05; #P < .01; ns, nonsignificant.
To provide further evidence that Cbl-b deficiency specifically activates LFA-1–mediated adhesion, we generated Cbl-b/LFA-1 double-deficient mice (Cbl-b−/−LFA-1−/−). As expected, LFA-1−/− BMDMs displayed reduced endothelial adhesion (Figure 2A) and reduced adhesion to ICAM-1 (Figure 2B) but not to VCAM-1 (Figure 2C). Interestingly, the increased adhesion of BMDMs to endothelial cells or ICAM-1 due to Cbl-b deficiency was abolished by LFA-1 deficiency (ie, Cbl-b deficiency failed to increase adhesion of LFA-1−/− cells to endothelial cells or ICAM-1; Figure 2A,B). In order to verify that Cbl-b–induced LFA-1 activation was the predominant pathway for the increased macrophage recruitment in vivo, we performed thioglycollate-induced peritonitis comparing WT, Cbl-b−/−, LFA-1−/−, and Cbl-b−/−LFA-1−/− mice. LFA-1−/− mice had reduced macrophage recruitment (Figure 2E). Moreover, the increased inflammatory cell recruitment in vivo due to Cbl-b deficiency required the presence of LFA-1, as macrophage recruitment in Cbl-b−/−LFA-1−/− mice equaled recruitment of these cells in LFA-1−/− mice (Figure 2E). Taken together, Cbl-b deficiency activates LFA-1–mediated macrophage adhesion in vitro and recruitment in vivo.
Cbl-b deficiency activates LFA-1 by increasing the association of 14-3-3β with the β-chain of LFA-1
We then went on to address the underlying molecular mechanisms of LFA-1 activation in inflammatory cells due to Cbl-b deficiency. Several cytoplasmic factors, including the GTPase Rap1, talin, and 14-3-3 proteins, are involved in inside-out signaling activation of LFA-1.8,11,13 Previously, it was established that Cbl-b–deficient T cells displayed increased Rap1 activity and thereby increased LFA-1 activation.23 However, although we found increased Rap1 activity in Cbl-b−/− splenocytes as compared with WT cells, we observed no difference in Rap1 activity between WT and Cbl-b−/− BMDMs (Figure S2). In addition, by performing coimmunoprecipitation analysis, we found no difference in the association of talin with the β-chain of LFA-1 between WT and Cbl-b−/− BMDMs (data not shown).
Association of 14-3-3β with the cytoplasmic tail of the β-chain of LFA-1, which is mediated by the phosphorylation of T758 of the β2-chain in response to inside-out activating stimuli, such as phorbol ester stimulation, can also contribute to LFA-1 activation.6,13 The 14-3-3/LFA-1 interaction regulates LFA-1–mediated cell adhesion and spreading, as it has been associated with changes in the lateral mobility and clustering of the integrin, as well as with effects of LFA-1 on actin reorganization, but not with affinity changes.13,15,16 As we observed increased LFA-1–mediated cell adhesion and spreading to ICAM-1 of Cbl-b−/− BMDMs (Figure 2B,D), we assessed whether the 14-3-3/LFA-1 interaction is affected by Cbl-b deficiency. Upon PMA-stimulated adhesion of BMDMs to ICAM-1, we found colocalization of 14-3-3β with the β2-chain of LFA-1 (Figure S3A), whereas no colocalization was observed in nonadherent cells (data not shown). By quantifying the number of ICAM-1–adherent BMDMs that displayed colocalization between 14-3-3β and the β2-chain, we found this number to be significantly higher in Cbl-b−/− BMDMs compared with WT BMDMs (Figure S3B). In order to provide biochemical evidence about the increased 14-3-3/LFA-1 interaction due to Cbl-b deficiency, we performed coimmunoprecipitation analysis. Immunoprecipitation of the integrin heterodimer was performed followed by Western blot detection of 14-3-3β. In BMDMs, detectable coimmunoprecipitation of LFA-1 with 14-3-3β was found upon PMA stimulation and with pretreatment with a Ser/Thr phophatase inhibitor, okadaic acid, consistent with previous observations.13,33 As shown in Figure 3A, increased association of 14-3-3β with LFA-1 was observed in Cbl-b−/− BMDMs as compared with WT cells. In contrast, the expression levels of total (Figure 3A) and surface (Figure 3B) CD18 as well as the total expression of 14-3-3β (Figure 3B) were not different between WT and Cbl-b−/− cells. The interaction of 14-3-3β with LFA-1 was also studied in macrophages isolated from thioglycollate-induced peritonitis. WT peritoneal macrophages displayed weak coimmunoprecipitation of CD18 with 14-3-3β, especially in the presence of okadaic acid and PMA. Association of CD18 with 14-3-3β in Cbl-b−/− peritoneal macrophages was found under nonstimulated conditions, and this interaction was further stimulated by PMA in the absence or presence of okadaic acid (Figure S4). Together, these findings suggest that the interaction of 14-3-3β with the β2-chain of LFA-1 is enhanced upon Cbl-b deficiency.
Cbl-b deficiency stimulates the increased association of 14-3-3β with the β-chain of LFA-1 in BMDMs. (A) The association of β2-integrin (CD18) with 14-3-3β in BMDMs from WT and Cbl-b−/− mice was studied. Immunoprecipitation of CD18 was performed in cell lysates of PMA-treated WT and Cbl-b−/− BMDMs. The presence of immune complexes was determined by Western blot analysis for 14-3-3β (top panel). In addition, detection of CD18 by Western blot was also performed (bottom panel). (B) Expression levels of surface β2-integrin (CD18) and intracellular 14-3-3β (in fixed-permeabilized cells) were analyzed by FACS in WT and Cbl-b−/− BMDMs. Nonspecific fluorescence was determined using secondary antibodies (dotted curves).
Cbl-b deficiency stimulates the increased association of 14-3-3β with the β-chain of LFA-1 in BMDMs. (A) The association of β2-integrin (CD18) with 14-3-3β in BMDMs from WT and Cbl-b−/− mice was studied. Immunoprecipitation of CD18 was performed in cell lysates of PMA-treated WT and Cbl-b−/− BMDMs. The presence of immune complexes was determined by Western blot analysis for 14-3-3β (top panel). In addition, detection of CD18 by Western blot was also performed (bottom panel). (B) Expression levels of surface β2-integrin (CD18) and intracellular 14-3-3β (in fixed-permeabilized cells) were analyzed by FACS in WT and Cbl-b−/− BMDMs. Nonspecific fluorescence was determined using secondary antibodies (dotted curves).
In order to demonstrate that the enhanced interaction of 14-3-3β with the β2-chain of LFA-1 is responsible for the increased LFA-1-mediated adhesiveness of Cbl-b−/− BMDMs, we interfered with 14-3-3 activity. To do so, we engaged the R18wt construct that binds to the phosphopeptide-binding groove in 14-3-3, thereby preventing 14-3-3 interactions with its cellular partners, and the noninhibitory mutant construct R18mut.17 Disruption of the 14-3-3/LFA-1 interaction by the R18wt peptide abrogated the enhanced ICAM-1 adhesion of Cbl-b−/− BMDMs (Figure 4A). These findings suggest that the 14-3-3/LFA-1 interaction mediates the activation of LFA-1 induced by Cbl-b deficiency.
Increased T758 phosphorylation in the β2-chain upon Cbl-b deficiency mediates LFA-1 activation. (A) Disruption of the association of 14-3-3β with the β-chain of LFA-1 abrogates the enhanced ICAM-1 adhesion of Cbl-b−/− BMDMs. WT (□) and Cbl-b−/− (■) BMDMs were transfected with R18wt or R18mut DNAs, and allowed to adhere onto immobilized ICAM-1. Cell adhesion is represented as percentage of control. The adhesion of R18mut-transfected WT BMDMs represents the 100% control. Data are means plus or minus SD (n = 3). (B) Increased T758 phosphorylation in the β2-chain of LFA-1 due to Cbl-b deficiency. T758 phosphorylation of β2-integrin (CD18) was determined in PMA-treated WT and Cbl-b−/− monocytes. Immunoprecipitation of CD18 was performed followed by Western blot using a phosphospecific T758 CD18 antibody or an antibody against total CD18. (C) Adhesion of WT or Cbl-b−/− BMDMs to immobilized ICAM-1 is shown in the absence (□) or in the presence (■) of the PKC inhibitor Gö6983 (50 nM). Cell adhesion is represented as the percentage of adherent cells. Data are means plus or minus SD. Similar results were observed in 3 separate experiments. #P < .01; ns, nonsignificant.
Increased T758 phosphorylation in the β2-chain upon Cbl-b deficiency mediates LFA-1 activation. (A) Disruption of the association of 14-3-3β with the β-chain of LFA-1 abrogates the enhanced ICAM-1 adhesion of Cbl-b−/− BMDMs. WT (□) and Cbl-b−/− (■) BMDMs were transfected with R18wt or R18mut DNAs, and allowed to adhere onto immobilized ICAM-1. Cell adhesion is represented as percentage of control. The adhesion of R18mut-transfected WT BMDMs represents the 100% control. Data are means plus or minus SD (n = 3). (B) Increased T758 phosphorylation in the β2-chain of LFA-1 due to Cbl-b deficiency. T758 phosphorylation of β2-integrin (CD18) was determined in PMA-treated WT and Cbl-b−/− monocytes. Immunoprecipitation of CD18 was performed followed by Western blot using a phosphospecific T758 CD18 antibody or an antibody against total CD18. (C) Adhesion of WT or Cbl-b−/− BMDMs to immobilized ICAM-1 is shown in the absence (□) or in the presence (■) of the PKC inhibitor Gö6983 (50 nM). Cell adhesion is represented as the percentage of adherent cells. Data are means plus or minus SD. Similar results were observed in 3 separate experiments. #P < .01; ns, nonsignificant.
Increased T758 phosphorylation in the β-chain of LFA-1 due to Cbl-b deficiency
14-3-3 proteins recognize phosphoserine- or phosphothreonine-containing motifs, and the interaction between 14-3-3β and the β2-chain of LFA-1 is dependent on the phosphorylation of T758 in the cytoplasmic tail of the β2-chain.13 By engaging a phosphospecific antibody against the β2-chain of LFA-1 that recognizes phosphorylated T758,24 we found that T758 phosphorylation of the β2-chain was higher in Cbl-b−/− monocytes as compared with WT cells (Figure 4B). Since T758 can be phosphorylated by several PKC isoforms,6,12 we addressed whether the enhanced ICAM-1 adhesion of Cbl-b−/− BMDMs was affected by inhibition of PKC. Treatment of Cbl-b−/− BMDMs with a relatively specific PKC inhibitor, Gö6983, reversed their enhanced adhesiveness to immobilized ICAM-1 (Figure 4C).
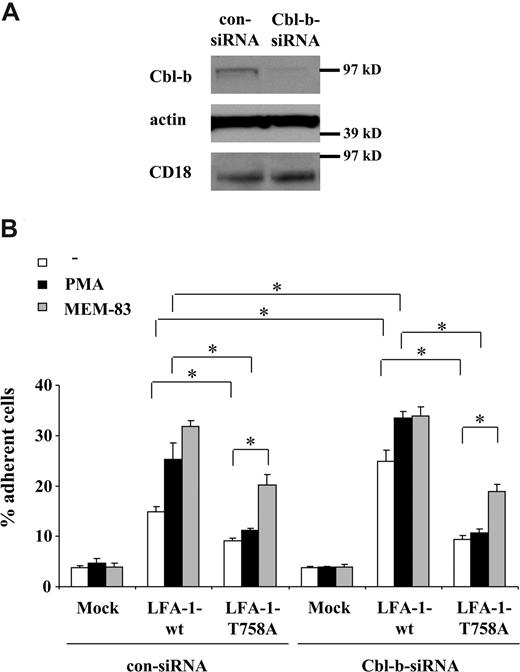
We then went on to verify in transfectants that the increased association between 14-3-3 and the β2-chain was responsible for the LFA-1–mediated adhesion activated by Cbl-b deficiency. We transfected 293 cells, which originally lack LFA-1, with WT-αL together with either WT-β2 or with mutated T758A-β2. This mutation prevents the interaction of LFA-1 with 14-3-3β.13 We performed siRNA-mediated Cbl-b knockdown in the transfected 293 cells. siRNA targeting Cbl-b but not control nontargeting siRNA decreased specifically the expression of Cbl-b but not of other proteins, including the expression of the transfected β2-chain of LFA-1 (Figure 5A). Surface expression of WT LFA-1– or mutant T758A-β2 LFA-1–transfected cells was comparable both under control conditions (upon transfection of control siRNA) and upon Cbl-b down-regulation by Cbl-b–specific siRNA (Figure S5). WT LFA-1–transfected 293 cells adhered to ICAM-1; this adhesion was further stimulated by PMA and by activating mAb to LFA-1, MEM-83, that stimulates conformational changes in LFA-1 and LFA-1 affinity.34 Consistent with previous reports, the T758A mutation in LFA-1 reduced the constitutive LFA-1–mediated adhesion to ICAM-1 by 50%, and prevented the stimulating effect of PMA on adhesion, whereas mAb MEM-83 could still significantly stimulate adhesion of mutant T758A LFA-1–transfected cells (Figure 5B), indicating that conformational changes could still take place despite the T758A mutation. Upon Cbl-b knockdown, constitutive adhesion of WT LFA-1–transfected cells to ICAM-1 was significantly up-regulated, as was PMA-stimulated adhesion, albeit to a lower extent. In contrast, Cbl-b knockdown failed to increase the constitutive or the PMA-stimulated adhesion of mutant T758A LFA-1–transfected cells to ICAM-1 (Figure 5B). The adhesion-stimulating effect of MEM-83 on both WT LFA-1–transfected and on mutant T758A LFA-1–transfected cells remained unaffected by Cbl-b knockdown (Figure 5B). Together, these results suggest that T758 in the β2-chain of LFA-1 is essential in mediating the activation of LFA-1 induced by Cbl-b down-regulation.
293 cells were transfected with siRNA targeting human Cbl-b or with control nontargeting siRNA. After 24 hours, the cells were transiently transfected with either WT human LFA-1 or mutant human T758A β2 LFA-1. The expression of WT and mutant LFA-1 were comparable and not affected by Cbl-b knockdown (Figure S5). (A) Cbl-b knockdown was verified by Western blot using an antibody to human Cbl-b. As a control, Cbl-b knockdown did not affect the expression of β-actin or of the transfected β2-integrin (CD18). (B) 293 cells were transfected with control siRNA or siRNA targeting Cbl-b and then with WT or mutant LFA-1 (αL DNA combined with WT β2 or T758A β2) or mock transfected as control, and were allowed to adhere to immobilized ICAM-1. Adhesion is shown in the absence (□) or presence (■) of PMA (50 ng/mL) or LFA-1–activating mAb, MEM-83 (10 μg/mL; ▩). Cell adhesion is represented as the percentage of adherent cells. Data are means plus or minus SD. *P < .05; ns, nonsignificant.
293 cells were transfected with siRNA targeting human Cbl-b or with control nontargeting siRNA. After 24 hours, the cells were transiently transfected with either WT human LFA-1 or mutant human T758A β2 LFA-1. The expression of WT and mutant LFA-1 were comparable and not affected by Cbl-b knockdown (Figure S5). (A) Cbl-b knockdown was verified by Western blot using an antibody to human Cbl-b. As a control, Cbl-b knockdown did not affect the expression of β-actin or of the transfected β2-integrin (CD18). (B) 293 cells were transfected with control siRNA or siRNA targeting Cbl-b and then with WT or mutant LFA-1 (αL DNA combined with WT β2 or T758A β2) or mock transfected as control, and were allowed to adhere to immobilized ICAM-1. Adhesion is shown in the absence (□) or presence (■) of PMA (50 ng/mL) or LFA-1–activating mAb, MEM-83 (10 μg/mL; ▩). Cell adhesion is represented as the percentage of adherent cells. Data are means plus or minus SD. *P < .05; ns, nonsignificant.
Discussion
In the present report, we identified a novel, previously unappreciated proinflammatory action of Cbl-b deficiency. LFA-1–dependent inflammatory cell adhesion to endothelial cells in vitro and recruitment in vivo are significantly enhanced upon Cbl-b deficiency. Therefore, LFA-1–mediated inflammatory cell adhesion joins the multitude of inflammatory pathways that are negatively regulated by Cbl-b.19 Inflammatory infiltrates are a hallmark of autoimmune diseases. Thus, the increased inflammatory cell recruitment due to Cbl-b deficiency, described here, may contribute to the higher severity of autoimmunity observed in Cbl-b−/− mice.21,22
Cbl-b deficiency specifically induced activation of LFA-1 and LFA-1–mediated adhesion by increasing phosphorylation of T758 in the β2-chain of LFA-1 and thereby enhancing the association between 14-3-3β and LFA-1. Consistently, LFA-1 deficiency reversed the increased adhesion and recruitment of Cbl-b−/− macrophages; also, disruption of the interaction between 14-3-3 and the cytoplasmic tail of the β2-integrin abrogated the enhanced ICAM-1-adhesion of Cbl-b−/− BMDMs. 14-3-3 proteins are present as dimers, and the 14-3-3αβ and δζ isoforms were previously shown to associate with the cytoplasmic tail of the β2-chain of LFA-1 through phosphorylated T758.6 Previous studies with transfected cell lines and T cells provided evidence that the 14-3-3/LFA-1 interaction participates in inside-out signaling activation of LFA-1.13 Thus, our present findings extend the importance of this interaction to inflammatory cell adhesion and recruitment. Together, Cbl-b deficiency unmasks the 14-3-3/LFA-1 interaction resulting in LFA-1 activation.
Our experiments identified the interaction between 14-3-3 and β2-chain as an intermediate step in the enhanced ability of Cbl-b−/− macrophages to adhere and home to the inflamed tissue. The affinity of the interaction between 14-3-3 and β2-integrins is poised at a low point, most likely due to the low stoichiometry of T758 phopshorylation in the tail of the β2-chain.6,33 Therefore, the phosphorylated tail of the β2-chain and 14-3-3 protein may largely dissociate during the washing procedures of immunoprecipitation, thereby resulting in difficulties in detecting the interaction, as has been previously reported for 14-3-3 interactions with other target phosphoproteins.35 However, the reversibility of the interaction between the β2-integrin and 14-3-3 protein make sense for a dynamic regulatory interaction that has to respond to activation signals.
An interaction between 14-3-3β with β1-integrin has been previously identified in nonhematopoetic cells to mediate cell spreading, adhesion, and migration.36 However, we found here no increased activation of VLA-4–mediated adhesion of inflammatory cells due to Cbl-b deficiency. A potential explanation could be the different nature of the 14-3-3/β1-integrin interaction which is independent of β1-integrin phosphorylation.36 In contrast, the 14-3-3/LFA-1 interaction is dependent on T758 phosphorylation, and this phosphorylation was increased by Cbl-b deficiency. T758 can be phosphorylated by several isoforms of the PKC in vitro, such as PKCs α, β, δ, or η (eg, upon PMA stimulation).6,12 Consistently, we found that treatment with a PKC inhibitor reversed the higher LFA-1–dependent adhesion in Cbl-b−/− cells. This result indicates that a PKC isoform may play a role in the enhanced LFA-1–dependent adhesion due to Cbl-b deficiency, although this issue requires further investigation.
Another member of the Cbl family, c-Cbl, has been implicated as an intermediate in integrin-mediated outside-in signaling. Ligation of β2-integrins as well as of β1-integrins in neutrophils and monocytes can result in tyrosine phosphorylation of c-Cbl, mediated predominantly by src family kinases.37-41 c-Cbl thereby participates in the integrin-mediated adhesion-dependent phosphatidylinositol-3-kinase activation, which is important for cell-spreading events.37,38 Distinct from these previous reports showing a participation of c-Cbl in integrin-mediated outside-in signaling, we provide here novel evidence that Cbl-b is involved in inside-out signaling activation of the β2-integrin LFA-1, but not of β1-integrins. It is intriguing that 2 homologous ubiquitin ligases, Cbl-b and c-Cbl, regulate inside-out signaling activation of β2-integrins and integrin-dependent outside-in signaling, respectively.
Previously, the T758-760 motif in the cytoplasmic tail of the β2-integrin and its phosphorylation were found to be critical for LFA-1–mediated adhesion and spreading to ICAM-1 through regulation of integrin clustering rather than affinity changes in LFA-1,15,16 most likely because this motif mediates the interaction of LFA-1 with 14-3-3 proteins.13 Consistently, we found here that Cbl-b deficiency increased constitutive and PMA-stimulated LFA-1–dependent ICAM-1 adhesion and spreading, in which integrin affinity changes are only marginally involved. In contrast, LFA-1–dependent adhesion stimulated by an activating mAb (MEM-83) that is associated with affinity changes in LFA-134 was not much affected by Cbl-b deficiency. Thus, we think that the pathway regulated by Cbl-b participates in events that strengthen LFA-1–dependent adhesion rather than in the rapid events leading to the initial affinity changes of the integrin. Moreover, LFA-1 integrin affinity can be regulated by Rap1 activity changes, which are downstream of chemokines or the T-cell receptor in T cells.3,8 We found no changes in Rap1 activity in BMDMs due to Cbl-b deficiency, although we did find increased Rap1 activity in Cbl-b−/− lymphocytes (data not shown). This is consistent with a previous report that demonstrated increased Rap1 activity in Cbl-b−/− T cells upon T-cell receptor cross-linking.23 It is likely that Cbl-b deficiency may regulate different pathways in distinct cells. Although we found no alteration of Rap1 activity in BMDMs in vitro, we cannot exclude that Rap1 is not involved in the increased inflammatory cell recruitment due to Cbl-b deficiency as observed in vivo. Nevertheless, our present findings establish that Cbl-b deficiency stimulates LFA-1–mediated inflammatory cell recruitment in vivo, and this could be attributed to an increased LFA-1 activation due to an enhanced association of 14-3-3 with LFA-1 upon Cbl-b deficiency.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr M. E. Kruhlak for help with confocal microscopy, D. Winkler for genotyping, and S. Sharrow and T. Adams for help with FACS analysis. We would also like to thank Dr J. Chiang and Dr R. Hodes for providing the Cbl-b−/− mice and for helpful discussions, Dr T. Pawson for the EGFP-R18 and EGFP-R18mut plasmids, Dr P. Chumakov for the lentiviral construct, Dr S. Shaw for critically reading the manuscript, and G. Sanchez-Howard and L. Stepanyan (Bioqual, Rockville, MD) for help with bone marrow chimera experiments.
This research was supported by the Intramural Research Program of the NIH, NCI (T.C.), the Sigrid Juselius Foundation, and the Academy of Finland (C.G.G.).
National Institutes of Health
Authorship
Contribution: E.Y.C. performed research, analyzed data, and wrote manuscript; V.V.O. performed research; S.C.F. analyzed data and provided analytical tools and vital reagents; S.M.N. and C.G.G. provided analytical tools and vital reagents; L.Z. and C.M.B. provided vital reagents; and T.C. designed and performed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: T. Chavakis, EIB, NCI, NIH, 10 Center Dr, Rm 4B17, Bethesda, MD 20892; e-mail: chavakist@mail.nih.gov.