Abstract
Current knowledge about molecular mechanisms underlying disease progression and drug resistance in multiple myeloma (MM) is still limited. Here, we analyzed the potential pathogenetic role of the Y-box binding protein YB-1 in MM. YB-1 is a member of the cold-shock domain protein superfamily and involved in various cellular functions such as proliferation. Immunohistochemical analyses revealed that neither normal bone marrow (BM) plasma cells (PCs), premalignant PCs of patients with monoclonal gammopathy of unknown significance (MGUS), nor MM cells with a mature morphology showed expression of YB-1 in situ. In contrast, YB-1 was strongly expressed in situ in normal PC precursor blasts as well as in a MM subset and in vitro in all of the evaluated MM cell lines. The YB-1–expressing MM cells were characterized by an immature morphology and a highly proliferative phenotype as defined by Ki 67 expression. We observed that siRNA-mediated knockdown of YB-1 decreased proliferation and induced apoptosis in MM cells even in the presence of BM stromal cells. Furthermore, we found that overexpression of YB-1 mediated resistance toward doxorubicin-induced apoptosis in MM cells. Thus, YB-1 contributes to disease progression, survival, and drug resistance in MM and might therefore provide an attractive therapeutic target.
Introduction
Multiple myeloma (MM) is an incurable plasma cell disorder. It is caused by the growth of a malignant plasma cell clone in the bone marrow (BM), leading to severe clinical symptoms such as lytic bone lesions, impaired hematopoiesis, and renal insufficiency.1
MM is believed to evolve from a post–germinal center B cell through a multistep neoplastic transformation process culminating in oncogenic deregulation of growth and survival.2,3 This process involves acquired genetic alterations, such as translocations between Ig enhancers and proto-oncogenes, as well as the interaction between tumor cells and their microenvironment in the BM.4,5 Although most patients with MM initially respond to therapy, the disease usually recurs and progresses even under therapeutic intervention. Thus, the development of drug resistance during disease progression is considered a hallmark of MM.6 However, better insight into the molecular mechanisms underlying disease progression and drug resistance in MM is needed to prevent or attenuate this development.
The Y-box binding protein YB-1 belongs to a superfamily of cold-shock domain proteins that are highly conserved during evolution.7 Eukaryotic Y-box proteins are involved in a wide variety of cellular functions, such as regulation of DNA transcription and repair and translational control of protein synthesis.8,9 In particular, overexpression of YB-1 was found in fast proliferating cells, for example in interleukin-2 (IL-2)–stimulated T lymphocytes, or in hepatocytes of the regenerating zone after partial hepatectomy.10 We previously reported that YB-1 is overexpressed in human breast cancer and is associated with a multidrug-resistant phenotype.11 Increased levels of YB-1 expression have also been noted in other cancers, such as osteosarcoma, prostate cancer, pancreatic adenocarcinoma, ovarian carcinoma, colorectal carcinoma, and medulloblastoma.12 Recently, we demonstrated that YB-1 expression provokes breast cancer in a YB-1–transgenic mouse model with a 100% penetrance.13 Therefore, in addition to its role in drug resistance, YB-1 might act as a potent oncogene.
Because the role of YB-1 in MM is virtually unknown, we have analyzed the expression of YB-1 in normal, premalignant, and malignant plasma cells in situ and investigated its contribution to survival and drug resistance in MM cells.
Methods
Cell culture
Culture conditions for the human IL-6–dependent MM cell lines INA-6 and MM.1s were previously described.14,15 The MM cell lines used for analysis of YB-1 expression as shown in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) were also previously characterized.16 BM stromal cells (BMSCs) from a patient with MM were derived from routine diagnostic BM aspirate after informed consent of the patient was obtained in accordance with the Declaration of Helsinki and approval by the University Hospital of Würzburg Institutional Review Board. Coculture of INA-6 cells with BMSCs was performed as described previously.14
Transient transfection of INA-6 and MM.1s cells with YB-1 siRNA expression constructs
Transfection studies exploiting the INA-6 as well as the MM.1s transfection model were performed as previously described.17 Briefly, per electroporation, 5 × 106 cells were transfected with 30 μg/mL of either the pSUPER/YB-1 or the empty pSUPER vector as a mock control, and with 15 μg/mL of the expression plasmid for human truncated CD4 (pCD4Δ), as it was previously described, for subsequent enrichment of the transfected cells.18 Western blot analysis of YB-1 expression in the purified cells was performed after 72 hours, and viability was measured by annexin V–FITC/propidium iodide (PI) staining after 96 hours.
Construction of siRNA expression vectors
The pSUPER-derived siRNA expression construct against YB-1 was designed according to the guidelines described before.19 INA-6 and MM.1s cells were transiently transfected with the YB-1 siRNA expression vector, and Western blot analysis was performed to verify its efficiency. The sequence of the sense oligonucleotide used for construction of YB-1 siRNA expression plasmid (sequence derived from the actual gene in bold) was 5′-dGATCCCCCCACAAGATGGCAAAGAGATTCAAGAGATCTCTTTGCCATCTTGTGGTTTTTGGAAA-3′ (based on positions 1002-1020 of human YB-1).
Construction of an expression plasmid for siRNA-resistant YB-1
A 672-bp XbaI/BamHI fragment from a pcDNA6 expression vector that contains sequence for human YB-1, including the target sequence for the YB-1 siRNA, was subcloned into pBluescript II SK (Stratagene, Amsterdam, The Netherlands), and 5 silent mutations were introduced within the target sequence. The sequence of the sense oligonucleotide that was used in combination with the QuikChange mutagenesis kit (Stratagene) is 5′-dACCCTAAACCGCAGGACGGTAAGGAGACAAAG-3′ (based on positions 994-1025 of human YB-1, with mutated positions in italics). The insert was verified by sequencing and cloned back into the XbaI and BamHI sites of the pcDNA6/YB-1 plasmid. The resulting plasmid pcDNA6/YB-1mut was used at a concentration of 20 μg/mL in electroporations.
Western blot analysis
All procedures followed those that have previously been published.14 The cocultured INA-6 MM cells were carefully washed off to avoid contamination with BMSCs. Following separation by SDS-PAGE, proteins were transferred onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany), and stained with an antibody against YB-1. An anti–β-actin or an anti–α-tubulin antibody was used to assess equal loading.
Apoptosis assay
To assess the percentage of apoptotic and viable cell fractions, a human annexin V–FITC/PI staining kit (Bender MedSystems, Vienna, Austria) was used as previously described.14 Briefly, cells were washed in phosphate-buffered saline (PBS), incubated for 15 minutes in 100 μL binding buffer (10 mM HEPES/NaOH [pH 7.4], 140 mM NaCl, and 2.5 mM CaCl2) containing 2.5 μL annexin V–FITC and 5 μg/mL PI and analyzed by flow cytometry (FACSCalibur/CELLQuest; Becton Dickinson, Heidelberg, Germany).
Proliferation assay
To assess the percentage of the proliferating cell fraction carboxyfluorescein diacetate succinimidyl ester (CFSE; C34554; Invitrogen, Karlsruhe, Germany), a cell-permeable dye, was used according to the manufacturer's instructions. Briefly, transfected cells were incubated for 15 minutes with 10 μM CFSE immediately after enrichment 24 hours after transfection and recultivated after washing in medium for 72 hours before the CFSE content was measured by flow cytometry.
Cell-cycle analyis
Analysis of cell-cycle distribution of the transfected cells was performed as previously described.20 Briefly, 96 hours after transfection, bromodeoxyuridine (BrdU; Sigma, Deisenhofen, Germany) was added to the culture medium for 2 hours before harvesting. Cells were fixed in 70% ethanol, washed, incubated with a monoclonal anti-BrdU–FITC antibody (Becton Dickinson) for 30 minutes, washed again, resuspended in 0.5 mL PBS with PI (50 μg/mL), and analyzed in the FACSCalibur cytometer (Becton Dickinson) .
Immunohistochemical analysis
Biopsies (1 trephine and 2 extramedullary tissues with chronic inflammation) from 3 patients without MM were analyzed with regard to YB-1 expression in different normal and reactive human plasma cells, respectively. To investigate YB-1 expression in plasma cell (PC) neoplasms, 4 BM trephine biopsies from patients with monoclonal gammopathy of unknown significance (MGUS) as a putative precursor lesion and 34 biopsies from patients with overt MM (26 medullary and 8 extramedullary) were randomly retrieved from the archives of the Pathology Department of the Helios Clinics (Berlin, Germany). The routine diagnosis was established according to World Health Organization (WHO) criteria on formalin-fixed, paraffin-embedded tissue specimens, including Giemsa stain for conventional analysis of morphology and immunohistochemical stain to demonstrate PC-related antigens (CD138 and/or MUM1, Vs38C) and immunoglobulin light chain restriction (kappa, lambda).21 All diagnoses were confirmed by one of the authors (C.R.), who is an experienced hematopathologist and was trained in 1 of the national consultation centers for lymph node and hematopathology (Berlin, Germany). Additional CD20 and MIB-1 (Ki 67) stains were performed on all specimens investigated. In brief, the deparaffinated slides were subjected to an appropriate heat-induced epitope retrieval procedure according to the manufacturer's guidelines using a heat pressure cooker. YB-1 immunostaining did not require any pretreatment. Immunohistochemistry was performed using an automated immunostainer (Dako TechMate 500 plus; DakoCytomation, Hamburg, Germany). Primary antibody incubation was generally performed for 30 minutes. Antigens were localized using a detection kit (DakoCytomation ChemMate) corresponding to a labeled streptavidin-biotin method with incubation times of 30 minutes for the biotinylated bridge antibody (goat anti-mouse/rabbit anti-mouse) and the streptavidin/alkaline phosphatase conjugate, respectively, and with Neufuchsin as substrate. Counterstaining was done with hematoxylin (Merck, Darmstadt, Germany).
All staining procedures were run with positive or negative controls that were stained appropriately. The growth fraction of the PC elements was determined either by counting the MIB-1–labeled nuclei of 100 cells in solid tumor cell sheets or by estimating them in a loose PC infiltrate in comparison with corresponding high-power fields on a slide on which PCs were highlighted otherwise. For YB-1, an immunostain was rated positive if at least 10% of the PCs displayed a pronounced diffuse cytoplasmic reaction. In order to control the specificity of the polyclonal rabbit anti–YB-1 antibody, INA-6 cells were embedded in paraffin and preincubated with a 100-fold molar excess of an YB-1–derived peptide (MSSEAETQQPPA), which was originally used for immunization of the rabbit. Preincubation with this inhibitory peptide prevented YB-1 staining, verifying the specificity of the YB-1 antibody used in this study (data not shown). The microphotographs were taken with a Coolpix 5000 digital camera adapted to an Eclipse E400 light microscope (both from Nikon, Frankfurt, Germany). The images were enlarged 10× by the ocular and 40× by the objective of the microscope, and a further 3× by the optical zoom and 1.6× by the digital zoom of the camera. The insets in Figure 3 show details that were further enlarged either 2× (A,B) or 3× (C) using the digital zoom of the camera.
Confocal microscopy
Paraffin-embedded tissue sections of BM biopsies were deparaffinized and boiled in a water bath for 20 minutes in TRS (pH 6.1) buffer (DakoCytomation) for antigen retrieval. A rabbit polyclonal anti–YB-1 antibody together with mouse monoclonal anti–interferon regulatory factor 4 (IRF4; clone MUM1; DakoCytomation) antibody was applied on the sections for 1 hour at room temperature. Thereafter, the sections were washed 3 times in PBS and stained for 1 hour with combined species-specific Cy-3– and Cy-5–conjugated secondary antibodies (Jackson ImmunoResearch Labs, West Grove, PA). The nuclei were stained with DAPI (4,6 diamidino-2- phenylindole; Sigma), and the slides were mounted with Fluoromount-G (Southern Biotechnology, Birmingham, AL). The images were collected with a laser-scanning TCS SP2 Confocal System equipped with a DMRE microscope and an HCX PL APO 63×/1.40 NA oil-immersion objective lens, and were processed with Leica Confocal Software version 2.61 (all from Leica Microsystems, Mannheim, Germany). The insets were enlarged 2× using Adobe Photoshop software (Adobe Systems, San Jose, CA).
Antibodies
Antibodies used for Western blotting were diluted 1:1000 (primary antibodies: rabbit polyclonal anti–YB-1 as previously described, rat anti–α-tubulin [MCA77; Serotec, Düsseldorf, Germany], rabbit anti-HA–tag [AB9110; Abcam, Cambridge, United Kingdom], mouse anti–cyclin B1 and rabbit anti–cyclin D1 [4135 and 2922; Cell Signaling Technology, Frankfurt am Main, Germany], mouse anti-p21WAF1/CIP [sc-397; Santa Cruz Biotechnology, Heidelberg, Germany]; secondary antibodies: donkey anti-rabbit and sheep anti-mouse [Amersham, Freiburg, Germany]). Dilutions of primary antibodies for immunochemistry were rabbit polyclonal anti–YB-1 (1:50) as previously described, monoclonal anti–MIB-1 (Ki 67; 1:200), anti-CD138 (UMI15; 1:40), anti-CD20 (L26; 1:5000), anti-Vs38C (1:200), anti-kappa (R10-21-F3; 1:400), anti-lambda (N10/2; 1:600), and mouse monoclonal anti-IRF4 (clone MUM1). Biotinylated bridge antibodies were goat anti-rabbit and goat anti-mouse (all from DakoCytomation).
Results
YB-1 is strongly expressed in immature and anaplastic MM cells, but not in normal PCs, MGUS PCs, or mature MM cells
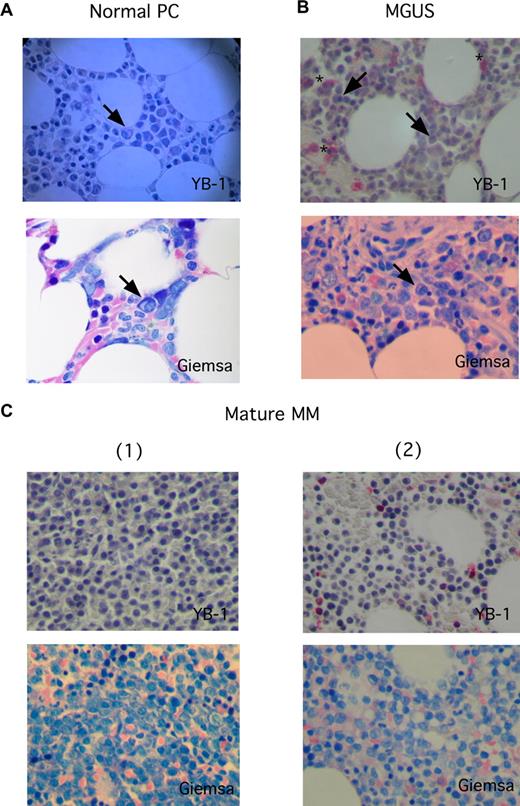
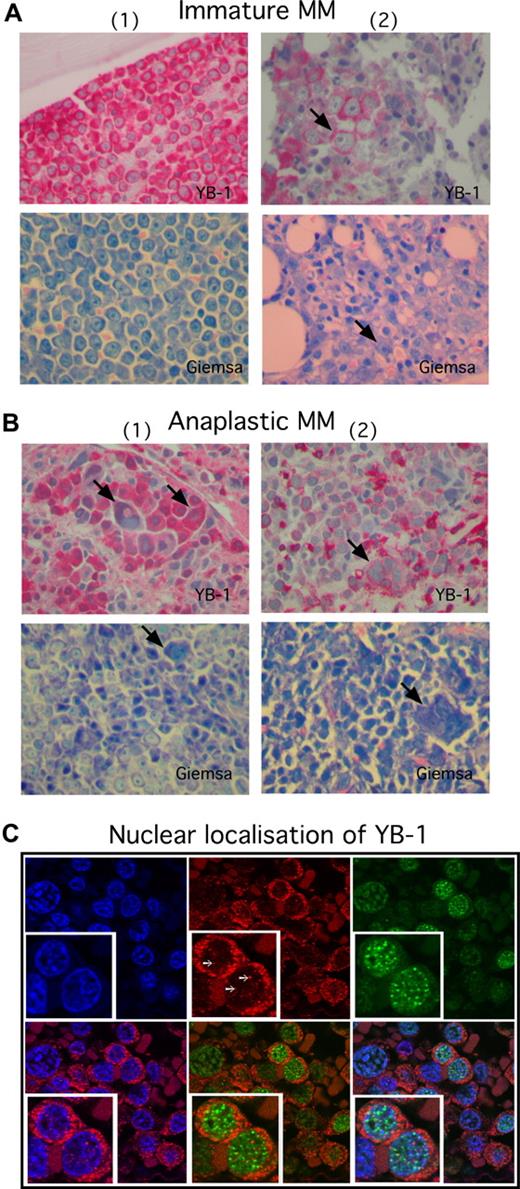
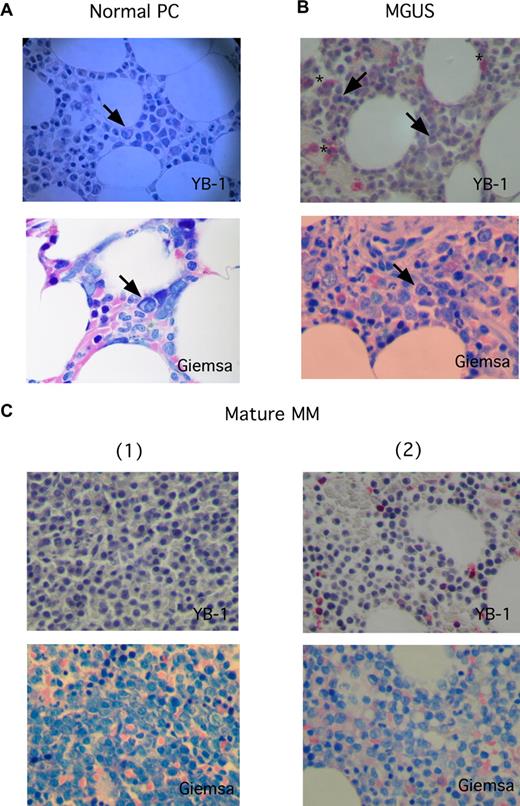
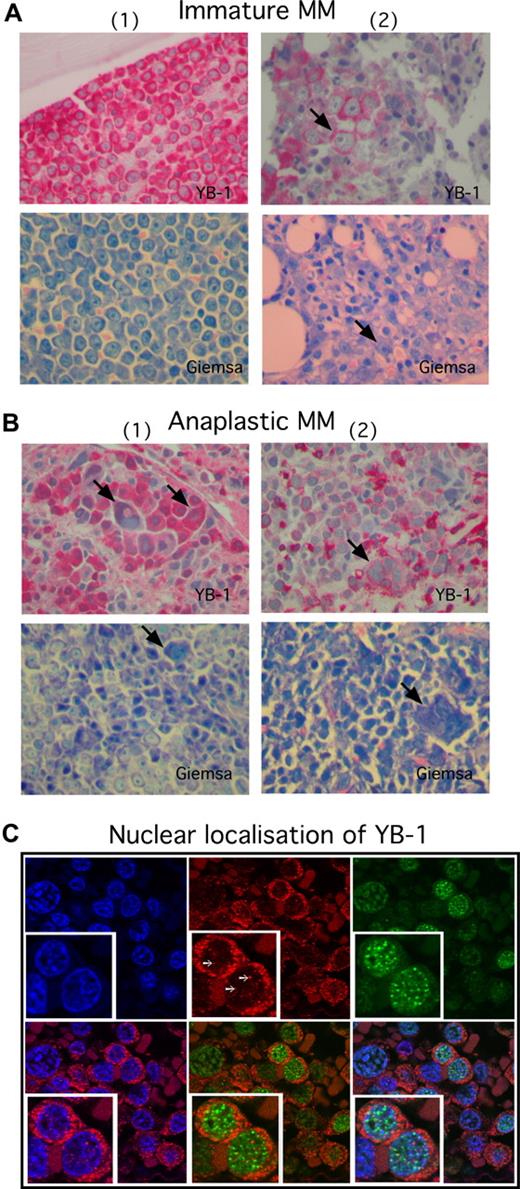
To investigate the expression of YB-1 in PCs in situ, biopsies from BM or from extramedullary myeloma manifestations were analyzed by immunohistochemistry. The staining results revealed a lack of YB-1 expression in normal or reactive polytypic PCs in BM or chronic inflammation (n = 3; Figure 1A) in PCs of patients with a precursor lesion (MGUS; n = 4) and—with 1 exception—in morphologically mature MM cells (n = 22; Figure 1B,C). In contrast, YB-1 was found strongly expressed in morphologically immature (n = 8) or anaplastic (n = 4) MM cells (Figure 2). Normal BM PCs are characterized by their round to oval form, excentrically localized nucleus devoid of visible nucleoli and with a typical chromatin structure, and their prominent perinuclear clear zone (Golgi region) in the basophil cytoplasm (Figure 2A). Although the morphology of mature PCs in patients with MM can differ from that of normal PCs (these cells may show, for example, only subtle changes in the morphology of the nucleus), most mature MM cells retain a remarkable similarity to normal PC phenotype (Figure 1C). In contrast, immature PCs display a range of different morphologic features. Immature MM cells are enlarged, immunoblast-like cells that show a more centrally localized nucleus with dispersed chromatin pattern and prominent nucleoli. The nucleo-cytoplasmic ratio is also often increased in such MM cells (Figure 2A). Anaplastic MM cells show the most impressive deviation from normal PC morphology. Dedifferentiation often leads to very large, sarcoma-like blast cells, which contain multiple and bizarre, often hyperchromatic nuclei with prominent nucleoli (Figure 2B).21 Data summarizing the PC morphology and the corresponding YB-1 expression status of all samples investigated in this study are listed in Table 1. Interestingly, whereas almost all of the mature MM samples were obtained from the BM (21 of 22 samples), 3 of 8 immature MM samples and all of the anaplastic MM samples (4 of 4 samples) were obtained from extramedullary sites. Furthermore, Western blot analysis of the YB-1 expression status of MM cell lines showed that the protein was expressed in each of the 8 cell lines tested (Figure S1).
YB-1 expression is lacking in situ in normal PCs, in PCs of patients with MGUS, and in MM cells with a mature PC morphology. (A-C) Immunohistochemical analyses of YB-1 protein expression in situ, (A) in normal PCs of the BM, (B) in premalignant PCs from a patient with MGUS, or (C) malignant PCs with a mature phenotype from 2 patients with MM. PC morphology was highlighted by staining according to Giemsa. A normal PC (marked by an arrow in panel A), PCs from patients with MGUS (marked by arrows in panel B), and morphologically mature-appearing MM cells (C) do not express YB-1, which seems to be, however, sometimes expressed in granulopoietic cells (*). Images were acquired as described in “Immunohistochemical analysis.”
YB-1 expression is lacking in situ in normal PCs, in PCs of patients with MGUS, and in MM cells with a mature PC morphology. (A-C) Immunohistochemical analyses of YB-1 protein expression in situ, (A) in normal PCs of the BM, (B) in premalignant PCs from a patient with MGUS, or (C) malignant PCs with a mature phenotype from 2 patients with MM. PC morphology was highlighted by staining according to Giemsa. A normal PC (marked by an arrow in panel A), PCs from patients with MGUS (marked by arrows in panel B), and morphologically mature-appearing MM cells (C) do not express YB-1, which seems to be, however, sometimes expressed in granulopoietic cells (*). Images were acquired as described in “Immunohistochemical analysis.”
YB-1 is strongly expressed in situ in MM cells with immature and anaplastic morphology. (A,B) Immunohistochemical analysis of YB-1 protein expression in situ, or (A) in morphologically immature or (B) anaplastic PCs. Biopsies shown in panel A were obtained from the BM of 2 patients with MM; biopsies in panel B were obtained from different extramedullary sites of 2 patients with MM. Strong, diffuse predominantly cytoplasmatic expression of YB-1 is observed in morphologically immature MM cells (A) and in morphologically anaplastic MM cells (B). Each patient panel includes a Giemsa stain to highlight PC morphology. Arrows in panel A indicate a prominent nucleolus, which is a typical feature of immaturity. Arrows in panel B indicate large, multinucleated, bizarrely formed PCs, which are characteristic of an anaplastic phenotype. (C) Immunofluorescence images of primary MM samples stained for YB-1 (Cy3; red), IRF4 (Cy5; green) and nuclei (DAPI; blue). YB-1 is in situ detectable in the cytoplasm and nuclei of MM cells and is organized in dot-like structures (white arrows) inside the nucleus. Nuclear YB-1 is colocalized with transcriptionally active euchromatin (weak DAPI staining). Staining of IRF4 was performed to mark PCs. The insets show details of the picture in a 2-fold magnification. Images in panels A and B were acquired as described in “Immunohistochemical analysis,” and in panel C as in “Confocal microscopy.”
YB-1 is strongly expressed in situ in MM cells with immature and anaplastic morphology. (A,B) Immunohistochemical analysis of YB-1 protein expression in situ, or (A) in morphologically immature or (B) anaplastic PCs. Biopsies shown in panel A were obtained from the BM of 2 patients with MM; biopsies in panel B were obtained from different extramedullary sites of 2 patients with MM. Strong, diffuse predominantly cytoplasmatic expression of YB-1 is observed in morphologically immature MM cells (A) and in morphologically anaplastic MM cells (B). Each patient panel includes a Giemsa stain to highlight PC morphology. Arrows in panel A indicate a prominent nucleolus, which is a typical feature of immaturity. Arrows in panel B indicate large, multinucleated, bizarrely formed PCs, which are characteristic of an anaplastic phenotype. (C) Immunofluorescence images of primary MM samples stained for YB-1 (Cy3; red), IRF4 (Cy5; green) and nuclei (DAPI; blue). YB-1 is in situ detectable in the cytoplasm and nuclei of MM cells and is organized in dot-like structures (white arrows) inside the nucleus. Nuclear YB-1 is colocalized with transcriptionally active euchromatin (weak DAPI staining). Staining of IRF4 was performed to mark PCs. The insets show details of the picture in a 2-fold magnification. Images in panels A and B were acquired as described in “Immunohistochemical analysis,” and in panel C as in “Confocal microscopy.”
YB-1 is present in the cytoplasm and in the nucleus of YB-1+ MM cells
We analyzed the localization of YB-1 in MM cells by immunofluorescent staining and confocal microscopy. YB-1 was detectable in both the cytoplasm and the nuclei of immunopositive MM cells. YB-1 staining within the nuclei was seen as dotted pattern that colocalized with transcriptionally active euchromatin (which is only weakly stained by DAPI), supporting a role of YB-1 as transcription factor in MM (Figure 2C). To identify PCs, the biopsies were costained for IRF4, a transcription factor that plays a crucial role in PC development and is expressed in normal PCs and MM cells.22,23
YB-1 is expressed in proliferative PC precursors
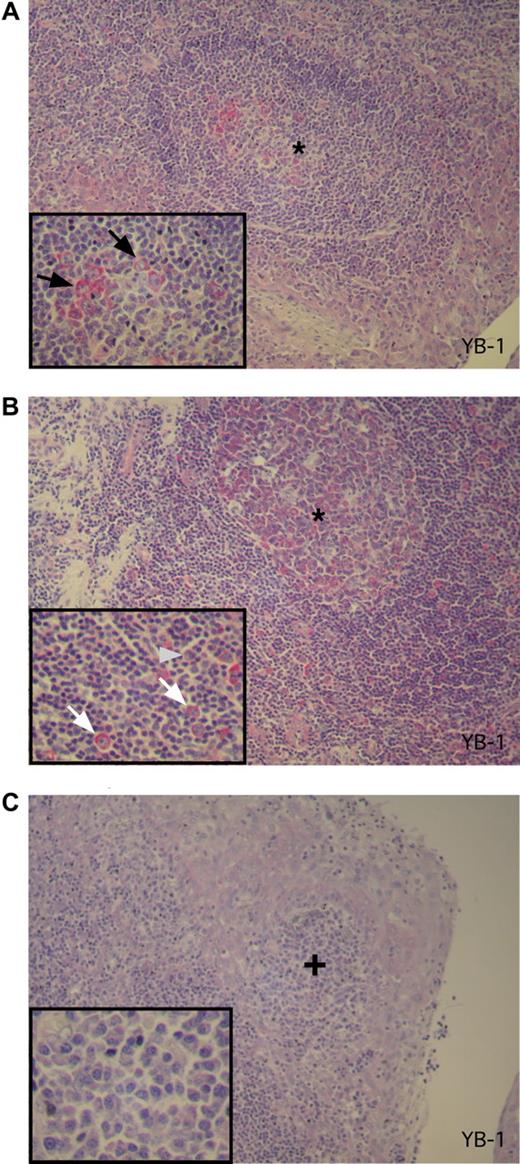
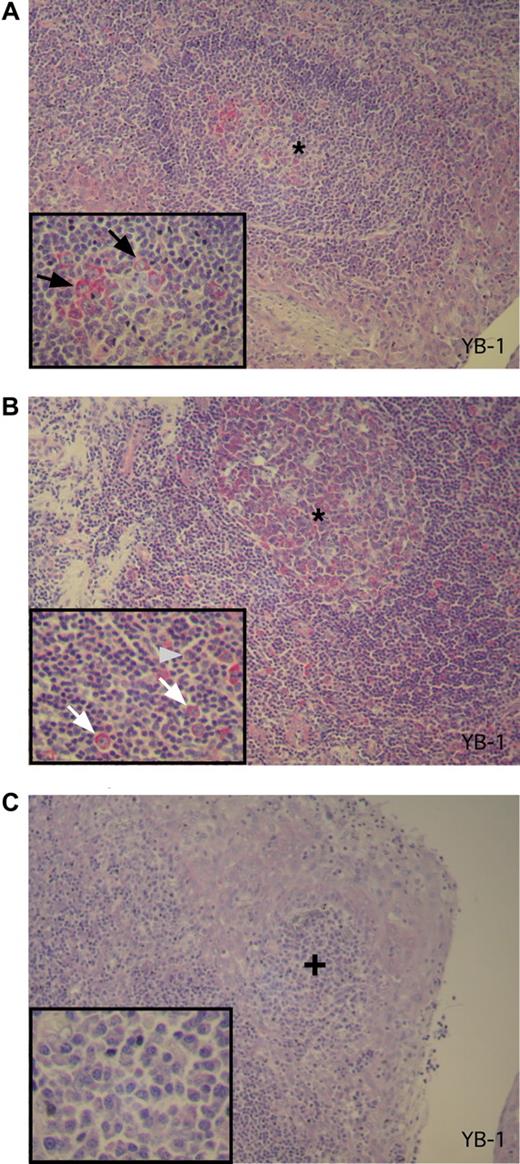
To determine expression of YB-1 in PC precursor cells, 3 tonsillectomy specimens from different patients featuring reactive changes were analyzed by immunohistochemical staining. Moderate to intense YB-1 staining was found in both a subset of germinal center B centroblasts (in secondary follicles) and in scattered activated extrafollicular B blasts within the T zone. These are activated and proliferating B cells representing precursors in PC development (Figure 3A,B). Only occasionally was a weak YB-1 expression observed in B centrocytes. In contrast, B lymphocytes of the follicle mantle T lymphocytes as well as mature-appearing PCs, located either as single cells or in subepithelial clusters in the tonsil, failed to show any YB-1 expression (Figure 3C).
YB-1 is expressed in germinal center B cells but not in mature PCs in activated tonsils. (A-C) Immunohistochemical analysis of YB-1 protein expression in situ in antigen-activated tonsils as secondary lymphoid organs. (A) Centroblasts in the germinal center (*) of the secondary follicle show a strong YB-1 expression (red). The integrated inset in the lower left corner shows a 2-fold magnification of the germinal center with YB-1–expressing centroblasts as indicated by black arrows. (B) Activated extrafollicular B-cell blasts also display expression of YB-1 protein as shown in higher magnification (2-fold) in the inset by white arrows. The gray arrowhead marked the single mature PC that is YB-1−. (C) A subepithelial PC accumulation (+) of the same tonsil PCs with a mature PC morphology (inset with a 3-fold magnification) that display no YB-1 expression. Images were acquired as described in “Immunohistochemical analysis.”
YB-1 is expressed in germinal center B cells but not in mature PCs in activated tonsils. (A-C) Immunohistochemical analysis of YB-1 protein expression in situ in antigen-activated tonsils as secondary lymphoid organs. (A) Centroblasts in the germinal center (*) of the secondary follicle show a strong YB-1 expression (red). The integrated inset in the lower left corner shows a 2-fold magnification of the germinal center with YB-1–expressing centroblasts as indicated by black arrows. (B) Activated extrafollicular B-cell blasts also display expression of YB-1 protein as shown in higher magnification (2-fold) in the inset by white arrows. The gray arrowhead marked the single mature PC that is YB-1−. (C) A subepithelial PC accumulation (+) of the same tonsil PCs with a mature PC morphology (inset with a 3-fold magnification) that display no YB-1 expression. Images were acquired as described in “Immunohistochemical analysis.”
Expression of YB-1 in MM cells is correlated with a proliferative phenotype
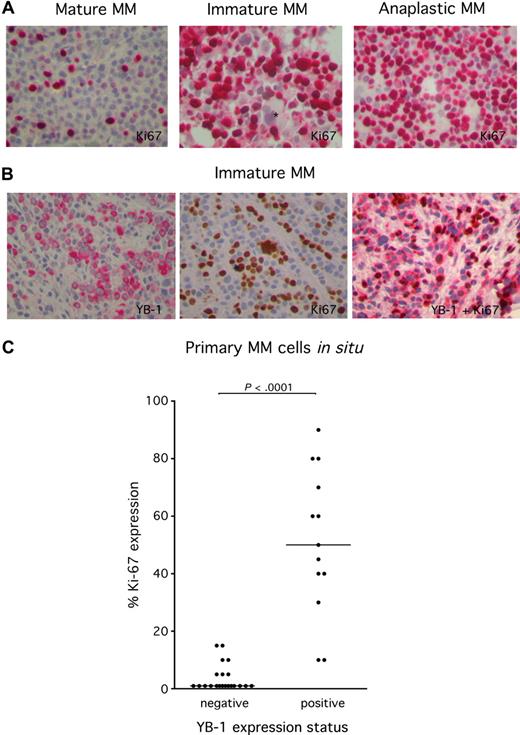
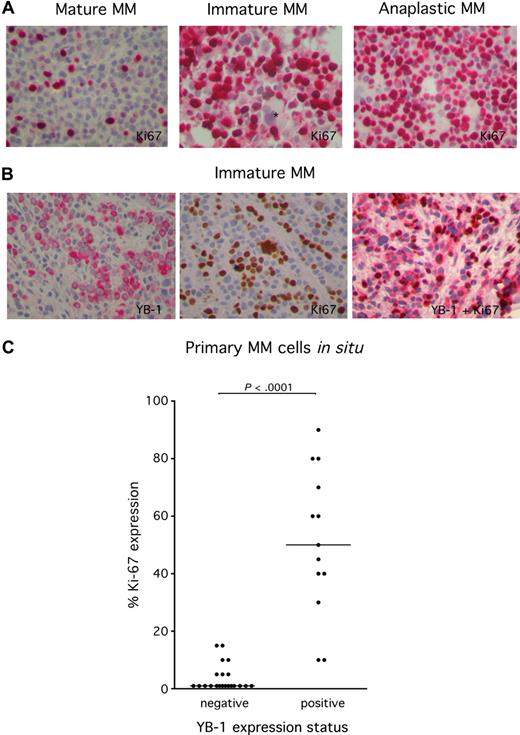
Expression of YB-1 in cell types other than PCs has been shown to be associated with an enhanced proliferation rate. In order to investigate the correlation between YB-1 expression and proliferation in MM cells, biopsy tissue was immunohistochemically stained with the antibody MIB-1, which recognizes the nuclear protein Ki 67. Because Ki 67 is expressed during the whole active phase of the cell cycle, but is absent from resting cells, it is an excellent marker for determination of the growth fraction of MM in situ. Ki 67 staining revealed that MM samples with a mature PC morphology show a lower rate of proliferating MM cells compared with MM samples with an immature or anaplastic PC morphology (Figure 4A). Costaining of YB-1 and Ki 67 underscores the finding that MM cells with strong YB-1 expression were much more likely to display Ki 67 expression than MM samples lacking YB-1 expression (Figure 4B). Statistical analysis using a 2-tailed nonpaired t test attested significance to this difference with a P value of less than .001 (Figure 4C). Thus, the expression of YB-1 in MM cells is associated with a proliferative phenotype.
High frequency of expression of the proliferation marker Ki 67 in YB-1+ MM cells. (A) Immunohistochemical analysis of Ki 67 protein expression in situ in YB-1−, mature MM cells (left), or YB-1+, immature MM cells (center) or anaplastic MM cells (right). *Negatively stained megakaryocyte. (B) Immunohistochemical analysis staining either of YB-1 (red; left) or Ki 67 protein (brown; center) or both (red and brown; right) in YB-1+, immature MM cells. Images were acquired as described in “Immunohistochemical analysis.” (C) Ki 67+ MM cells were counted to determine the growth fraction. Expression of Ki 67 is significantly more frequent in YB-1+, morphologically immature, or anaplastic PCs than in YB-1−, morphologically mature PCs. Also shown is the median of the percentage of Ki 67 staining in MM samples.
High frequency of expression of the proliferation marker Ki 67 in YB-1+ MM cells. (A) Immunohistochemical analysis of Ki 67 protein expression in situ in YB-1−, mature MM cells (left), or YB-1+, immature MM cells (center) or anaplastic MM cells (right). *Negatively stained megakaryocyte. (B) Immunohistochemical analysis staining either of YB-1 (red; left) or Ki 67 protein (brown; center) or both (red and brown; right) in YB-1+, immature MM cells. Images were acquired as described in “Immunohistochemical analysis.” (C) Ki 67+ MM cells were counted to determine the growth fraction. Expression of Ki 67 is significantly more frequent in YB-1+, morphologically immature, or anaplastic PCs than in YB-1−, morphologically mature PCs. Also shown is the median of the percentage of Ki 67 staining in MM samples.
Knockdown of YB-1 induces apoptosis in INA-6 MM cells
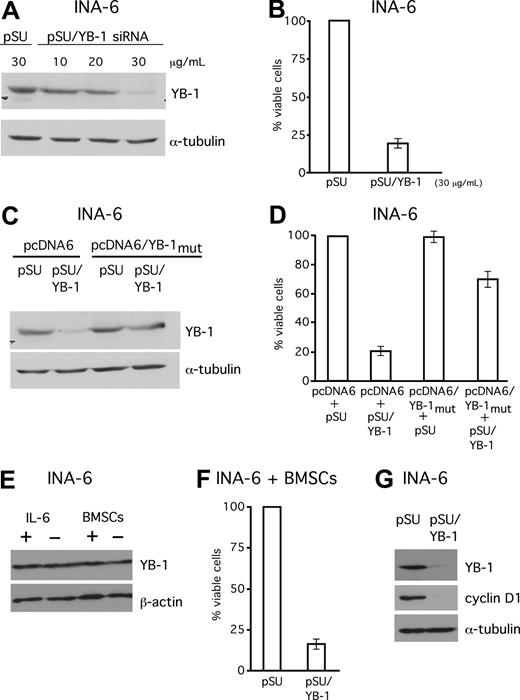
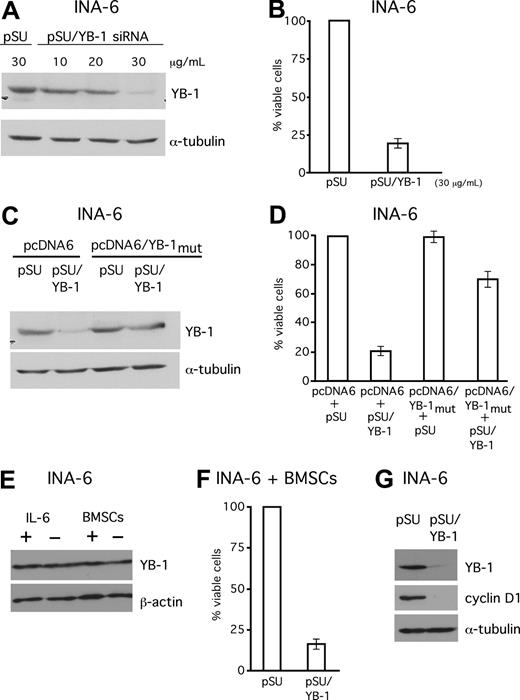
Next, we asked if expression of YB-1 might have any impact on the malignant growth of MM cells. A pSUPER-derived siRNA expression vector against YB-1 was constructed and cells from the IL-6–dependent MM cell line INA-6 were transiently transfected with this plasmid (pSUPER/YB-1; Figure 5). Transfections with the empty vector (pSUPER) served as mock controls. Western blot analysis of the transfected cells confirmed effective knockdown of YB-1 protein (Figure 5A,B). The viability of the transfected cells was determined by annexin V–FITC/PI staining. Selective knockdown of YB-1 caused a 75% decrease of the viable fraction of INA-6 cells 4 days after transfection (Figure 5B). In order to assess the specificity of this effect, the siRNA-mediated loss of endogenous YB-1 was compensated for through simultaneous expression of human YB-1 via an expression plasmid containing silent mutations within the sequence recognized by the YB-1 siRNA. The simultaneous expression of YB-1 from this plasmid in INA-6 cells was sufficient to largely antagonize the apoptotic effects of endogenous YB-1 knockdown, and increased the survival rate to 75% of control cells transfected with the empty vectors (Figure 5D). Given the importance of the BM microenvironment (BMM) for survival and drug resistance of MM cells, we next investigated whether cells or growth factors from the BMM might induce YB-1 expression. Interestingly, neither stimulation of IL-6–dependent INA-6 cells with 10 ng/mL of recombinant IL-6 nor coculture with BMSCs increased the levels of YB-1 in Western blot analyses (Figure 5E). In order to test whether the BMM provides protection against apoptosis induced by YB-1 knockdown, INA-6 cells transfected with an siRNA expression construct against YB-1 were cocultured with BMSCs. The survival of YB-1–depleted INA-6 cells was not improved in this setting (Figure 5F). Western blot analysis of 3 cyclins, which have previously been described as regulated by YB-1, showed that only cyclin D1 was down-regulated after YB-1 knockdown (Figure 5G).
SiRNA-mediated knockdown of YB-1 induces apoptosis in the MM cell line INA-6. (A) Western blot analysis of YB-1 protein expression in INA-6 cells 72 hours after transient transfection of siRNA expression constructs against YB-1. Staining of α-tubulin served as a loading control. (B) Viability of INA-6 cells in the absence of BMSCs was assayed with annexin V–FITC/PI staining 96 hours after transfection. (C,D) Coexpression of an YB-1 protein derived from an expression construct that cannot be targeted by the YB-1 siRNA (pcDNA6/YB-1mut) served as a control for the specificity of the siRNA-mediated effects. Transfection of 20 μg/mL of pcDNA6/YB-1mut into INA-6 cells resulted in normal levels of YB-1 despite siRNA treatment as detected by Western blot analysis (C) and largely protected cells from YB-1 siRNA–mediated apoptosis as assessed by annexin V–FITC/PI staining (D). (E) INA-6 cells were either kept with or without 10 ng/mL of recombinant IL-6, or kept in the presence or absence of BMSCs for 24 hours, before YB-1 expression levels were analyzed by Western blot. Staining with β-actin served as loading control. (F) Viability of INA-6 cells in the presence of BMSCs was assayed with annexin V–FITC/PI staining 96 hours after transfection with YB-1 siRNA. (G) Western blot analysis of cyclin D1 expression 72 hours after transient transfection of siRNA expression constructs against YB-1. Staining of α-tubulin served as a loading control. The error bars denote the range of values derived from 3 independent experiments.
SiRNA-mediated knockdown of YB-1 induces apoptosis in the MM cell line INA-6. (A) Western blot analysis of YB-1 protein expression in INA-6 cells 72 hours after transient transfection of siRNA expression constructs against YB-1. Staining of α-tubulin served as a loading control. (B) Viability of INA-6 cells in the absence of BMSCs was assayed with annexin V–FITC/PI staining 96 hours after transfection. (C,D) Coexpression of an YB-1 protein derived from an expression construct that cannot be targeted by the YB-1 siRNA (pcDNA6/YB-1mut) served as a control for the specificity of the siRNA-mediated effects. Transfection of 20 μg/mL of pcDNA6/YB-1mut into INA-6 cells resulted in normal levels of YB-1 despite siRNA treatment as detected by Western blot analysis (C) and largely protected cells from YB-1 siRNA–mediated apoptosis as assessed by annexin V–FITC/PI staining (D). (E) INA-6 cells were either kept with or without 10 ng/mL of recombinant IL-6, or kept in the presence or absence of BMSCs for 24 hours, before YB-1 expression levels were analyzed by Western blot. Staining with β-actin served as loading control. (F) Viability of INA-6 cells in the presence of BMSCs was assayed with annexin V–FITC/PI staining 96 hours after transfection with YB-1 siRNA. (G) Western blot analysis of cyclin D1 expression 72 hours after transient transfection of siRNA expression constructs against YB-1. Staining of α-tubulin served as a loading control. The error bars denote the range of values derived from 3 independent experiments.
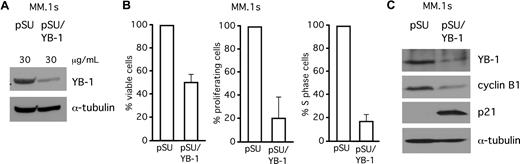
YB-1 knockdown decreases proliferation rates and increases apoptotic cell death in MM.1s cells
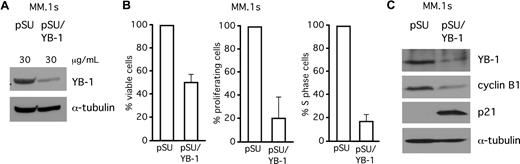
In addition to INA-6, we investigated the effects of siRNA-mediated knockdown of YB-1 in MM.1s, another MM cell line. MM.1s cells were transiently transfected either with empty pSUPER vector or with pSUPER/YB-1, and analyzed by Western blot (Figure 6A) or by staining with CFSE, BrdU/PI, or annexin V/PI (Figure 6B). At 4 days after transfection, YB-1 knockdown had caused strongly decreased proliferation rates (Figure 6B), reduced the number of cells in S phase of the cell cycle by 80% (Figure 6B), and resulted in a 50% decrease of the viable fraction of MM.1s cells (Figure 6B). Western blot analysis revealed up-regulation of p21 and down-regulation of cyclin B1 after YB-1 knockdown (Figure 6C).
SiRNA-mediated knockdown of YB-1 decreases proliferation rates and increases apoptotic cell death in MM.1s cells. (A) Western blot analysis of YB-1 protein expression in MM.1s cells 72 hours after transient transfection of siRNA expression constructs against YB-1. Staining of α-tubulin served as a loading control. (B) Viability of MM.1s cells was assayed with annexin V–FITC/PI staining (left), proliferation of MM.1s cells was detected by CFSE staining (center), and the S-phase cells of the cell cycle were detected by BrdU/PI staining, all 96 hours after transfection. (C) Western blot of p21WAF1/CIP and cyclin B1 expression levels after 72 hours after transfection with YB-1 siRNA. Staining of α-tubulin served as a loading control. The error bars denote the range of values derived from 3 independent experiments.
SiRNA-mediated knockdown of YB-1 decreases proliferation rates and increases apoptotic cell death in MM.1s cells. (A) Western blot analysis of YB-1 protein expression in MM.1s cells 72 hours after transient transfection of siRNA expression constructs against YB-1. Staining of α-tubulin served as a loading control. (B) Viability of MM.1s cells was assayed with annexin V–FITC/PI staining (left), proliferation of MM.1s cells was detected by CFSE staining (center), and the S-phase cells of the cell cycle were detected by BrdU/PI staining, all 96 hours after transfection. (C) Western blot of p21WAF1/CIP and cyclin B1 expression levels after 72 hours after transfection with YB-1 siRNA. Staining of α-tubulin served as a loading control. The error bars denote the range of values derived from 3 independent experiments.
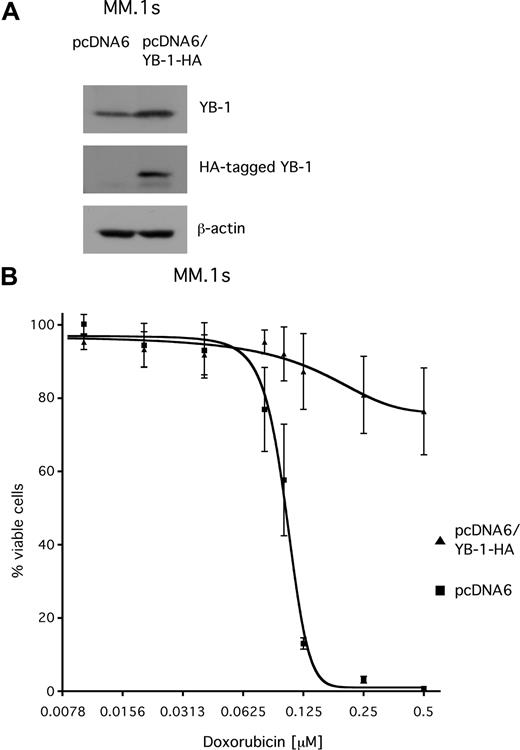
Overexpression of YB-1 in MM.1s cells confers resistance to doxorubicin
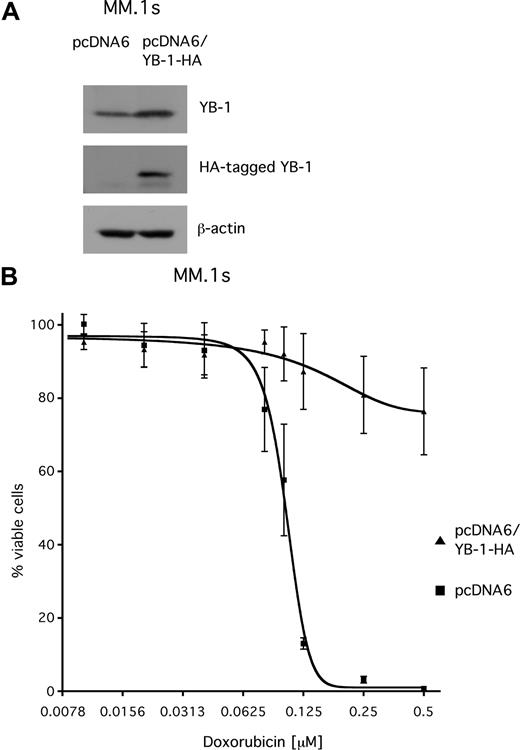
YB-1 has been reported to be involved in mechanisms that confer resistance to chemotherapeutic drugs. To investigate whether YB-1 might contribute to drug resistance in MM, the sensitivity of MM.1s cells that transiently overexpress YB-1 toward treatment with doxorubicin, a cytotoxic agent that is used in chemotherapeutic regimens for MM, was tested. MM.1s cells were transfected with either pcDNA6 vector or with an HA-tagged YB-1 expression construct (pcDNA6/YB-1-HA). Western blot analysis confirmed overexpression of the HA-tagged YB-1 protein in MM.1s cells 3 days after transfection (Figure 7A). At 2 days after transfection, cells were exposed to different concentrations of doxorubicin (up to 500 nM) and incubated for another 2 days before viability was determined by annexin V–FITC/PI staining and measured by fluorescence-activated cell sorter (FACS). The dose-response curve for MM.1s cells transfected with the empty control vector showed a steep decline, with an EC50 value of about 100 nM and complete loss of viable cells above concentrations of 200 nM of doxorubicin. In contrast, the viability of YB-1–overexpressing MM.1s cells was largely unaffected by doxorubicin, even at concentrations up to 500 nM (Figure 7B).
Transient overexpression of YB-1 protects MM.1s cells from apoptosis induced by treatment with doxorubicin. (A) Western blot analysis of endogenous YB-1 and HA-tagged YB-1 expression in MM.1s cells 48 hours after transient transfection with an expression plasmid for HA-tagged YB-1. Staining of β-actin served as a loading control. (B) MM1.s cells were either transfected with empty pcDNA6 vector as mock control or with a vector encoding HA-tagged YB-1. At 48 hours after transfection, the cells were treated with different concentrations of doxorubicin for another 48 hours. The viable cell fractions were measured by staining with annexin V–FITC/PI. The means and the standard deviations of 3 independent experiments are shown.
Transient overexpression of YB-1 protects MM.1s cells from apoptosis induced by treatment with doxorubicin. (A) Western blot analysis of endogenous YB-1 and HA-tagged YB-1 expression in MM.1s cells 48 hours after transient transfection with an expression plasmid for HA-tagged YB-1. Staining of β-actin served as a loading control. (B) MM1.s cells were either transfected with empty pcDNA6 vector as mock control or with a vector encoding HA-tagged YB-1. At 48 hours after transfection, the cells were treated with different concentrations of doxorubicin for another 48 hours. The viable cell fractions were measured by staining with annexin V–FITC/PI. The means and the standard deviations of 3 independent experiments are shown.
Discussion
Current knowledge about the molecular mechanisms underlying disease progression and drug resistance in MM is still limited. It was recently reported that stress response proteins like the heat shock proteins Hsp90α/β are overexpressed in MM and might play a role in apoptosis resistance.17,24 Like the heat-shock proteins, the cold-shock domain protein YB-1 is thought to play a role in a wide variety of environmental stress reactions.10 Increased expression of YB-1 in many solid tumors, such as breast cancer or melanoma, was found to be associated with malignant growth, drug resistance, and with poor prognosis.11,25,26
However, the role of YB-1 in MM had not yet been investigated. Here, we analyzed the expression of YB-1 in situ in normal, premalignant, and malignant PCs as well as in PC precursors. Immunohistochemical analyses revealed that YB-1 was strongly expressed in normal B blasts as well as in a subset of MM samples, whereas no expression was found in normal PCs, premalignant PCs from patients with MGUS, and in the majority of MM specimens. These findings suggests that YB-1 is up-regulated during normal B-cell development in proliferating B blasts, but down-regulated in terminally differentiated nonproliferating PCs. YB-1 expression in malignant PCs might therefore indicate dedifferentiation as part of the malignant transformation process.
The morphology of MM cells ranges from well-differentiated, near-normal plasmacytic forms, through to more dedifferentiated, larger immunoblastic (immature) PCs to bizarrely shaped sarcoma-like blast cells (anaplastic PCs).21 An immature, plasmablastic PC phenotype is considered an adverse prognostic indicator.27,28 Interestingly, we observed that YB-1+ MM cells were characterized by an immature or anaplastic morphology. In contrast, mature, plasmacytic MM cells were lacking YB-1 expression. Furthermore, YB-1+ MM—unlike YB-1− MM—often occurred at extramedullary sites. In accordance with the current pathogenetic model for MM, which suggests a multistep transformation process for cancer development, our findings indicate that expression of YB-1 in MM is a late oncogenic event, and might reflect both dedifferentiation and disease progression.3 However, further studies analyzing the longitudinal disease course of patients with MM are needed to determine exactly when during disease progression YB-1 expression is induced.
In other cell types YB-1 expression was found to be associated with enhanced proliferation.10 Ki 67, a large nuclear protein that is exclusively expressed in cycling cells, has been established as a faithful marker for proliferation in many tumor entities, and also in MM.29,30 We therefore analyzed the expression of Ki 67 in MM cells in situ, and found significantly higher growth fractions in YB-1+ than in YB-1− MM cells. Thus, expression of YB-1 in MM cells is associated with a highly proliferative phenotype.
This conclusion is underscored by a strong YB-1 expression in vitro in MM cell lines, which are derived from extramedullary manifestations and display high proliferation rates. Using the MM cell line models INA-6 and MM.1s, we investigated the consequences of siRNA-mediated knockdown of YB-1. Interestingly, we observed that YB-1 knockdown led to a growth arrest and to induction of apoptosis. The BM microenvironment is believed to essentially contribute to the malignant growth of MM cells.5 In particular, it has been demonstrated that MM cells are protected from apoptosis by coculture with BMSCs.14,18 We observed that YB-1 expression levels are not influenced by BMSCs and that YB-1–depleted MM cells underwent apoptosis even when they were cultured in the presence of BMSCs. YB-1 might therefore act as a BMM-independent survival factor. This result is in accordance with reports from other cellular systems, where it was shown that YB-1 is involved in cell-cycle regulation, and down-regulation of YB-1 resulted in reduced proliferation and increased apoptotic cell death rates.26,31-33 However, although we identified cyclin D1, cyclin B1, and p21WAF/CIP, which are all involved in cell-cycle control, as affected by YB-1 knockdown, it is currently unknown which mechanisms lead to YB-1 overexpression and how YB-1 exerts its prosurvival function in MM.
YB-1 was shown to play a role in cellular resistance to several DNA-damaging agents, including doxorubicin, which is widely used as cytotoxic drug in treatment regimens for MM. Thus, it has been demonstrated that YB-1 acts as a positive regulator of the multidrug resistance gene 1 (MDR1).11,12 Here, we could demonstrate that transient overexpression of YB-1 led to resistance toward doxorubicin-induced apoptosis in MM.1s cells, indicating that expression of YB-1 might contribute to the selection of drug-resistant MM cell clones during disease progression.
Taken together, our results show that YB-1 is expressed in immature and anaplastic MM cells, suggesting that it contributes to disease progression, survival, and drug resistance and might therefore provide an attractive therapeutic target for the treatment of an aggressively growing MM subset.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Roland Eils and Joel Beaudouin for providing access to the confocal microscope at BIOQUANT facility in Heidelberg as well as Lina Khoury, Anja Kurowski, and Charlotte Wahrendorf for excellent technical assistance.
This research was supported in part by grants from the Deutsche Forschungsgemeinschaft (SFB/TR17).
Authorship
Contribution: M.C. conceived and designed the research, performed viability assays and Western blot analyses, and wrote the manuscript; C.R. performed and evaluated the immunohisto-chemical analyses and wrote the manuscript; T.S. contributed to the construction of siRNA and YB-1 expression vectors, and wrote the manuscript; M.A. performed immunofluorescence staining and confocal microscopy; N.E. and H.D.R. produced, evaluated and provided the YB-1 antibody, and wrote the manu-script, H.L. kept the cell culture and performed the experiments with MM.1s cells; C.G. provided primary patient samples and patient characteristics; and R.C.B. established the research plan, supervised the project, wrote the manuscript, and approved the data and final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Manik Chatterjee, Department of Internal Medicine II, Division of Hematology, University Hospital of Würzburg, Josef-Schneider-Strasse 2, 97080 Würzburg, Germany; e-mail: chatterjee_m@medizin.uni-wuerzburg.de.
References
Author notes
M.C. and C.R. contributed equally to this work.