Abstract
Lyn kinase functions as a regulator of imatinib sensitivity in chronic myelogenous leukemia (CML) cells through an unknown mechanism. In patients who fail imatinib therapy but have no detectable BCR-ABL kinase mutation, we detected persistently activated Lyn kinase. In imatinib-resistant CML cells and patients, Lyn activation is BCR-ABL independent, it is complexed with the Gab2 and c-Cbl adapter/scaffold proteins, and it mediates persistent Gab2 and BCR-ABL tyrosine phosphorylation in the presence or absence of imatinib. Lyn silencing or inhibition is necessary to suppress Gab2 and BCR-ABL phosphorylation and to recover imatinib activity. Lyn also negatively regulates c-Cbl stability, whereas c-Cbl tyrosine phosphorylation is mediated by BCR-ABL. These results suggest that Lyn exists as a component of the BCR-ABL signaling complex and, in cells with high Lyn expression or activation, BCR-ABL kinase inhibition alone (imatinib) is not sufficient to fully disengage BCR-ABL–mediated signaling and suggests that BCR-ABL and Lyn kinase inhibition are needed to prevent or treat this form of imatinib resistance.
Introduction
BCR-ABL is a constitutively active tyrosine kinase expressed as a consequence of 9:22 chromosomal translocation (Philadelphia chromosome) in a majority of chronic myelogenous leukemia (CML) patients and a subset of patients with acute lymphocytic leukemia (ALL).1 The role for BCR-ABL in these diseases has been well established by both molecular and animal models2 and is the basis for target-specific therapy by the tyrosine kinase inhibitor, imatinib (Gleevec, STI-571; Novartis AG, Basel, Switzerland). Imatinib provides very effective control of BCR-ABL–positive leukemias, especially in newly diagnosed patients in whom hematologic and cytogenetic responses are common.3 Patients with more advanced disease (accelerated phase or blast crisis) have a lower response rate and frequently progress on therapy.4,5 Reduction in imatinib activity has been linked to changes in BCR-ABL structure and expression (through mutations, deletions, or amplification),6-8 although recent observations have demonstrated that these changes alone do not account for all imatinib failures.9-13 Other mechanisms, such as engagement of other signaling cascades, loss of tumor suppressor function, acquisition of stem cell–like characteristics, or cellular pharmacology may also play a role in disease progression and imatinib activity.14-18
CML cell model studies have shown that expression or activation of Src-family kinases (SFKs) may also play a role in imatinib resistance.19-21 Resistance to imatinib in K562 cells occurs in the absence of BCR-ABL point mutations or gene amplification, but correlates with overexpression of Lyn kinase, a SFK whose activity is unaffected by imatinib.19 Lyn kinase activation increases BCR-ABL phosphorylation, and may prevent BCR-ABL from adopting the inactive conformation that is a prerequisite for imatinib binding.22-25 Lyn overexpression is also reported to increase expression of survival genes and suppress apoptosis through a BCR-ABL–independent mechanism.21 Analysis of clinical specimens demonstrated that Lyn and other SFKs (Hck) are highly activated in blasts from CML patients in a BCR-ABL kinase–independent fashion, suggesting that persistent Lyn (or other SFK) activation or overexpression may play a role in imatinib resistance.19 However the nature of Lyn signaling complexes and survival regulatory activities in CML cells has not been described.
In this report, we describe key regulatory activities of Lyn in CML cells overexpressing this kinase. Lyn phosphoprotein complexes were analyzed in CML cell lines and primary specimens obtained from imatinib-resistant patients (without mutations in BCR-ABL). We detected persistent activation of Lyn kinase and incomplete suppression of BCR-ABL and substrate phosphorylation (CrkL) following imatinib incubation. Incomplete suppression of BCR-ABL signaling was associated with formation of a Lyn:Gab2:BCR-ABL complex and persistent tyrosine phosphorylation of both BCR-ABL (Y177) and Gab2 in Lyn-overexpressing cells. Lyn or Gab2 silencing induced apoptosis in CML cells with elevated Lyn expression. Lyn was also tightly complexed with another tyrosyl-phosphoprotein, identified as c-Cbl, which was stabilized when Lyn expression was inhibited. Although c-Cbl stability was regulated by Lyn, its phosphorylation was primarily mediated by BCR-ABL. Our results suggest that Lyn plays a regulatory role in formation of BCR-ABL protein complexes, and its overexpression or activation may lead to inadequate control of BCR-ABL signaling and CML disease progression on imatinib therapy.
Methods
Cell lines
K562, K562R,19 and Cos-7 cells were grown and maintained in RPMI-1640 with 10% fetal bovine serum (Hyclone, Logan, UT). Cell lysates were prepared from PBS-washed cells as previously described.19 Washed cell pellets were solubilized in lysis buffer consisting of 50 mM HEPES, pH 7.0, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 100 mM NaF, 10 mM sodium pyrophosphate, 10% glycerol, 1% Triton X-100, 1 mM phenylmethylsulphonyl fluoride (PMSF), 1 mM Na3VO4, 10 μg/mL leupeptin, and 10 μg/mL aprotinin. Immunoprecipitation of proteins from clinical specimens was performed with lysates containing additional phosphatase and protease inhibitor cocktails (catalog nos. P5726 and P8340, respectively; Sigma-Aldrich, St Louis, MO).
Inhibitors and reagents
Imatinib was synthesized26 and kindly provided by Dr William Bornmann (Department of Experimental Diagnostic Imaging, M. D. Anderson Cancer Center). Synthesized product was identical to the material previously provided by Dr E. Buchdunger (Novartis AG). Dasatinib27 was synthesized and provided by Bristol-Myers Squibb (Princeton, NJ). Antibodies used in this study included Hck/Lyn activation site–specific anti–pY411-Hck; pY396-Lyn, Hck, and Lyn (Santa Cruz Biological, Santa Cruz, CA); c-ABL (8E9 clone; Pharmingen, San Diego, CA); pY (clone 4G10), CrkL, c-Cbl (clone 7G10), and Gab2 (UBI, Lake Placid, NY); pY-CrkL, pY177-BCR-ABL, pY774-c-Cbl, Fyn, Src, and PARP (Cell Signaling, Beverley, MA); and β-actin (Sigma). Yes antibody was obtained from Wako Chemical (Richmond, VA). In some studies, rabbit monoclonal pY396-Lyn (Epitomics, Burlingame, CA) was used to detect activated Lyn.
Patient samples
Blood samples were collected from CML patients as part of an institutionally approved tissue sample collection protocol, and after patients signed an informed consent in accordance with the Declaration of Helsinki. All specimens used in this study were derived from patients who were progressing on imatinib therapy at the time of collection and analysis and did not express a mutation in the BCR-ABL kinase domain. All patients received dasatinib in a phase 1 clinical study that was reviewed and approved by the M. D. Anderson Cancer Center Institutional Review Board. Mononuclear cells were isolated from blood samples by density centrifugation (Ficoll-Hypaque) and washed with PBS, and aliquots were lysed immediately (for RNA extraction) or resuspended in cell culture media (RPMI-1640, 10% fetal bovine serum) and incubated overnight at 37°C in a 5% CO2 incubator before treatment with tyrosine kinase inhibitors.
Analysis of BCR-ABL mutations
BCR-ABL mutations were analyzed in CML patient specimens as previously described.7,8 In brief, total RNA was extracted (RNeasy Protect Mini Kit; Qiagen Valencia, CA) and used to prime a one-step reverse-transcription–polymerase chain reaction (RT-PCR) reaction (Invitrogen, Carlsbad, CA) using the following primers: CM10 (5′-GAAGCTTCTCCCTGACATCCGT-3′, BCR: 2609–2630) and 3′ ABL KD (5′GCCAGGCTCTCGGGTGCAGTCC-3′, ABL: 1292–1271), resulting in a 1.3-kb fragment. The 1.3-kb gel-purified fragment was used as a template to prime a second PCR reaction (5′ ABL KD [5′GCGCAACAAGCCCACTGTCTATGG-3′[and 3′ ABL KD) resulting in amplification of the ABL kinase domain. Second PCR reactions were performed using PCR SuperMix High Fidelity (catalog no. 10790–020; Invitrogen). The resultant 0.6-kb fragment was isolated by QIAquick Gel Extraction Kit (catalog no. 28704) and subcloned into the pGEM-T vector (catalog no. A3600; Promega, Madison, WI). At least 10 clones containing the ABL kinase domain were directly sequenced using an ABI377 automated sequencer.
Transfection
Cos-7 cells were transfected with expression vectors for wild-type (bcr-abl) or kinase-inactive (K271A/bcr-abl) BCR-ABL (provided by Dr R. Arlinghaus, Department of Molecular Pathology, M. D. Anderson Cancer Center), Lyn (pcDNA3.1-Lyn) (provided by Dr S. Corey, Department of Pediatrics, M. D. Anderson Cancer Center), c-Cbl (Cbl) (provided by Dr B. Darnay, Department of Experimental Therapeutics, M. D. Anderson Cancer Center), or a combination of these vectors using FUGENE. A total of 1 μg DNA was used for each transfection (FUGENE (Roche Diagnostics, Indianapolis, IN)). Twenty-four hours after transfection, cells were harvested or treated with tyrosine kinase inhibitor for 1 hour before preparation and analysis of cell lysates.
Lyn cDNA (EcoR1 digest of the pcDNA3.1-Lyn expression vector) was also subcloned into the EcoR1 site of the pMX-IRES-GFP vector modified to include a puromycin selection marker.28,29 The pMX-Lyn-IRES-GFP construct (pMX-Lyn) or an empty vector (pMX) (5 μg) was electroporated into freshly washed 2.5 × 106 K562 cells in solution T on a setting of 017, according to the manufacturer's (AMAXA, Gaithersburg, MD) instructions. Two days after electroporation, cells were cultured in 10 μg/mL puromycin and sorted for GFP positivity (after 5 days) by flow cytometry (fluorescence-activated cell sorting [FACS]). Empty vector–derived GFP-positive cells and GFP-positive cells from pMX-Lyn transfectants were examined for Lyn expression and c-Cbl protein levels after 48 hours of growth in the absence of puromycin.
Lyn, Gab2, c-Cbl, and BCR-ABL silencing with short interfering RNA (siRNA)
K562 and K562R cells were electroporated with 100 nM siRNA against Lyn, Gab2, c-Cbl, or a scrambled control sequence using the AMAXA nucleoporation system. All siRNA was purchased from DHARMACON (Lafayette, CO), except BCR-ABL siRNA, which was custom designed and synthesized as previously described.30,31 Electroporation was performed with freshly washed 2.5 × 106 cells in solution T on a setting of 017, according to the manufacturer's (AMAXA) instructions; approximately 50% of cells are electroporated under these conditions. Forty-eight to 72 hours after electroporation, lysates were prepared and analyzed for effects on protein expression, signaling, caspase activation, or cell survival (trypan blue exclusion or MTT assays as described in Bartholomeusz et al31 ).
Results
Lyn alters imatinib-mediated phosphoregulation
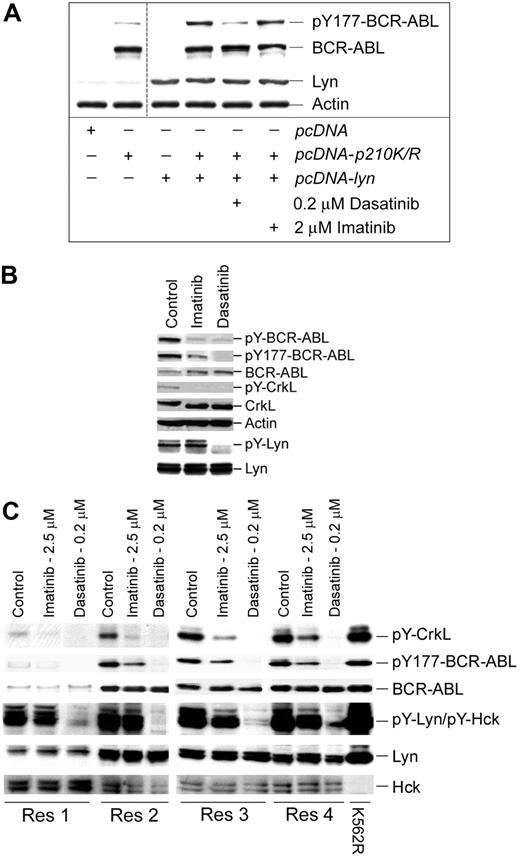
Previously, we reported that Lyn kinase expression and activation are increased as CML cells transition to an imatinib-resistant phenotype,19 and similar observations have been reported by other laboratories.20,21 More recent studies suggest that Lyn activation is BCR-ABL independent in some models of leukemogenesis.32 As shown in Figure 1, Lyn expression was elevated (> 8-fold) in imatinib-resistant (K562R) versus imatinib-sensitive (K562) cells (inset), and sensitivity to kinase inhibition was distinct in these populations. Hck is not expressed in K562 or K562R cells (data not shown), and screening for elevated or differential expression of additional SFKs (Src, Yes, Fyn) detected only minor changes.
Cellular and biochemical response to tyrosine kinase inhibitors in imatinib-sensitive and -resistant cells. (A) K562 and K562R cells were treated with imatinib (left) or dasatinib (right) at the indicated concentration for 48 hours before assessment of cell viability as previously described.19 Each point represents the average plus or minus SEM (error bar) of 4 determinations. (Inset) Immunoblot analysis of BCR-ABL, Lyn, Src, Yes, Fyn, and actin in equal protein lysates from K562 and K562R cells. BCR-ABL sequencing did not detect mutations in the Abl kinase domain in either cell line (data not shown). (B) K562 (left) or K562R (right) cells were incubated with imatinib (5 μM) or dasatinib (0.5 μM) for 0, 2, or 24 hours before lysates were analyzed for tyrosine-phosphorylated (activated) BCR-ABL, Lyn, and CrkL. Lyn and PARP protein (uncleaved [uPARP] = 116 kDa or cleaved [cPARP] = 85 kDa) were also detected by immunoblotting. (C) K562 and K562R cells were left untreated (control) or electroporated (AMAXA) with control (Con) or Lyn siRNA and analyzed for activated Lyn (pY396-Lyn) or Lyn protein levels by immunoblotting after 48 hours. Lysates were also probed for PARP as a marker of apoptosis. Table 1 summarizes the effect of siRNA on viability in K562 and K562R cells. The results represent the average and standard deviation from 3 independent experiments.
Cellular and biochemical response to tyrosine kinase inhibitors in imatinib-sensitive and -resistant cells. (A) K562 and K562R cells were treated with imatinib (left) or dasatinib (right) at the indicated concentration for 48 hours before assessment of cell viability as previously described.19 Each point represents the average plus or minus SEM (error bar) of 4 determinations. (Inset) Immunoblot analysis of BCR-ABL, Lyn, Src, Yes, Fyn, and actin in equal protein lysates from K562 and K562R cells. BCR-ABL sequencing did not detect mutations in the Abl kinase domain in either cell line (data not shown). (B) K562 (left) or K562R (right) cells were incubated with imatinib (5 μM) or dasatinib (0.5 μM) for 0, 2, or 24 hours before lysates were analyzed for tyrosine-phosphorylated (activated) BCR-ABL, Lyn, and CrkL. Lyn and PARP protein (uncleaved [uPARP] = 116 kDa or cleaved [cPARP] = 85 kDa) were also detected by immunoblotting. (C) K562 and K562R cells were left untreated (control) or electroporated (AMAXA) with control (Con) or Lyn siRNA and analyzed for activated Lyn (pY396-Lyn) or Lyn protein levels by immunoblotting after 48 hours. Lysates were also probed for PARP as a marker of apoptosis. Table 1 summarizes the effect of siRNA on viability in K562 and K562R cells. The results represent the average and standard deviation from 3 independent experiments.
To examine the role of Lyn in CML cell signaling and survival, its activation and expression were reduced by Lyn/BCR-ABL kinase inhibitor (dasatinib) or siRNA. Imatinib (Figure 1A left) was unable to suppress the growth or induce apoptosis of Lyn overexpressing K562R cells, whereas dasatinib induced growth suppression and apoptosis in both cell lines (Figure 1A right). The effects of these inhibitors on BCR-ABL phosphorylation, Lyn activation, and PARP cleavage were compared after short- and long-term incubation (Figure 1B). Both BCR-ABL and CrkL phosphorylation were rapidly suppressed by imatinib or dasatinib, and both drugs engaged caspase cascades resulting in PARP cleavage (left panel). In K562R cells, imatinib suppressed BCR-ABL and CrkL phosphorylation but required 24-hour incubation to achieve similar levels of kinase suppression as that detected in K562 cells. Lyn kinase was not fully suppressed by imatinib, even after long-term incubation, whereas dasatinib rapidly suppressed BCR-ABL, CrkL, and Lyn phosphorylation, and correlated with activation of PARP cleavage. These results suggest that Lyn activation is not fully inhibited by imatinib in K562R cells, and its constitutive activation delays or suppresses the effects of imatinib on BCR-ABL activation. These defects can be overcome by direct BCR-ABL/Lyn kinase inhibition with dasatinib.
Small interfering RNA was used to directly assess the impact of Lyn on cell viability and BCR-ABL kinase inhibition. Lyn siRNA reduced Lyn protein levels in both K562 and K562R cells, but apoptosis (PARP cleavage) was detected only in K562R cells (Figure 1C). Additional analysis of cell viability in siRNA-treated cells demonstrated that Lyn down-regulation resulted in a consistently greater loss of viability in K562R versus K562 cells (Table 1), suggesting that Lyn directly regulates cell survival and response to imatinib.
Regulation of BCR-ABL and Gab2 tyrosine phosphorylation by Lyn
Previous reports suggested that Lyn is downstream of BCR-ABL, but more recent studies demonstrate that Lyn phosphorylates transformation-regulatory sites on BCR-ABL.22,24,25,33,34 We conducted additional studies of Lyn-mediated phosphoregulatory control of BCR-ABL to determine its influence on imatinib sensitivity and signaling in cell lines and imatinib-resistant patient specimens. To gauge the influence of Lyn on tyrosine phosphorylation of BCR-ABL at a transformation critical site (Y177),35-37 a kinase-deficient form of BCR-ABL (K271A) was coexpressed with Lyn and treated with kinase inhibitors before analysis of BCR-ABL Y177 phosphorylation. As shown in Figure 2A, Lyn coexpression increased Y177 phosphorylation of BCR-ABL that was suppressed by dasatinib, but not imatinib.
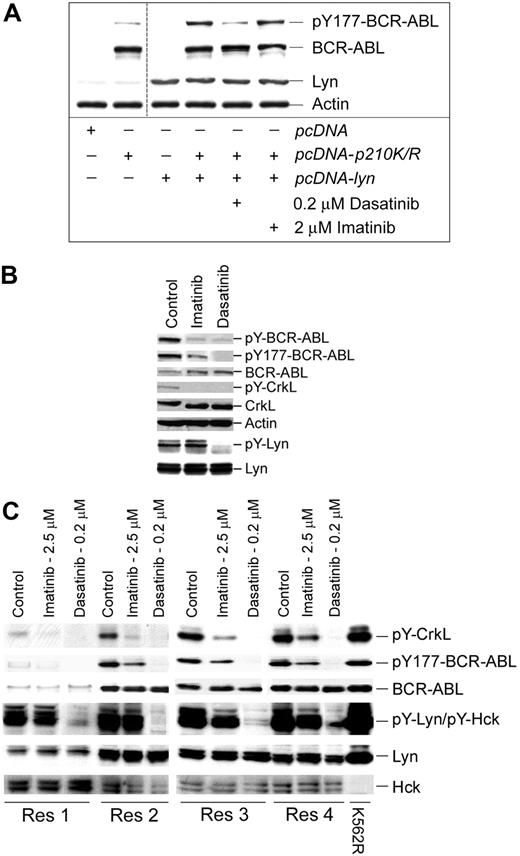
Lyn regulates BCR-ABL tyrosine phosphorylation in CML cells. (A) Cos-7 cells were transfected with a kinase-inactive mutant of BCR-ABL (K271R) or cotransfected with kinase-active Lyn. After 24 hours, cotransfectants were treated with the indicated concentration of kinase inhibitor for 2 hours. Cell lysates were prepared and immunoblotted for site-specific (Y177) BCR-ABL phosphorylation, BCR-ABL, Lyn, and actin levels. Low-level Y177-BCR-ABL was detected in BCR-ABL (K271R)–transfected Cos-7 cells (lane 2) that were not affected by imatinib or dasatinib. The vertical cut line between lanes 2 and 3 denotes merging of the original image to eliminate experimentally irrelevant sample lanes. (B) A leukopheresis specimen from a CML lymphoid blast crisis patient who progressed on imatinib therapy was left untreated (control) or was treated with imatinib (5 μM) or dasatinib (0.25 μM) for 2 hours. Equal protein cell lysates were prepared, resolved by SDS-PAGE, and immunoblotted for the antigen described. For detection of tyrosine-phosphorylated Lyn, 1 mg protein lysate from control or treated cells was subjected to Lyn immunoprecipitation and phosphotyrosine immunoblotting (pY-Lyn). The blot was stripped and reblotted for Lyn (bottom). (C) Specimens from 4 imatinib-resistant myeloid blast crisis patients (Res1-Res 4) that retained wild-type BCR-ABL expression were treated with imatinib or dasatinib (at the concentration indicated) for 2 hours. Equal protein cell lysates were subjected to immunoblotting for phospho-specific and total protein levels (as indicated). K562R cell lysate was used as a positive control for Lyn and other phosphoproteins.
Lyn regulates BCR-ABL tyrosine phosphorylation in CML cells. (A) Cos-7 cells were transfected with a kinase-inactive mutant of BCR-ABL (K271R) or cotransfected with kinase-active Lyn. After 24 hours, cotransfectants were treated with the indicated concentration of kinase inhibitor for 2 hours. Cell lysates were prepared and immunoblotted for site-specific (Y177) BCR-ABL phosphorylation, BCR-ABL, Lyn, and actin levels. Low-level Y177-BCR-ABL was detected in BCR-ABL (K271R)–transfected Cos-7 cells (lane 2) that were not affected by imatinib or dasatinib. The vertical cut line between lanes 2 and 3 denotes merging of the original image to eliminate experimentally irrelevant sample lanes. (B) A leukopheresis specimen from a CML lymphoid blast crisis patient who progressed on imatinib therapy was left untreated (control) or was treated with imatinib (5 μM) or dasatinib (0.25 μM) for 2 hours. Equal protein cell lysates were prepared, resolved by SDS-PAGE, and immunoblotted for the antigen described. For detection of tyrosine-phosphorylated Lyn, 1 mg protein lysate from control or treated cells was subjected to Lyn immunoprecipitation and phosphotyrosine immunoblotting (pY-Lyn). The blot was stripped and reblotted for Lyn (bottom). (C) Specimens from 4 imatinib-resistant myeloid blast crisis patients (Res1-Res 4) that retained wild-type BCR-ABL expression were treated with imatinib or dasatinib (at the concentration indicated) for 2 hours. Equal protein cell lysates were subjected to immunoblotting for phospho-specific and total protein levels (as indicated). K562R cell lysate was used as a positive control for Lyn and other phosphoproteins.
Additional evidence of Lyn-mediated BCR-ABL phosphorylation was also obtained through the study of cells from imatinib-resistant CML patients. We analyzed the phosphoregulatory activity of imatinib and dasatinib in 5 CML patients who failed imatinib therapy but retained expression of BCR-ABL without kinase-domain mutations. Tyrosine phosphorylation in 1 lymphoid and 4 myeloid blast crisis patients was assessed after ex vivo imatinib or dasatinib incubation at clinically relevant concentrations. Analysis of the lymphoid blast crisis specimen demonstrated that Lyn kinase activation was not suppressed by imatinib but was completely inhibited by dasatinib. Both dasatinib and imatinib effectively blocked CrkL and total BCR-ABL tyrosine phosphorylation, but Y177 site phosphorylation was only partially blocked by imatinib (Figure 2B).
To further assess the role of Lyn in phosphoregulatory control in imatinib-resistant disease, 4 additional myeloid blast crisis patient specimens were treated with inhibitors prior to analysis of BCR-ABL and substrate phosphorylation. Imatinib incompletely suppressed CrkL and Y177 BCR-ABL phosphorylation but had limited or no affect on Lyn or Hck activation (Figure 2C), whereas dasatinib fully suppressed Lyn activation and Y177-BCR-ABL phosphorylation. These results support earlier observations of BCR-ABL–autonomous activation of Lyn that is suppressed through Lyn-directed inhibition with dasatinib.
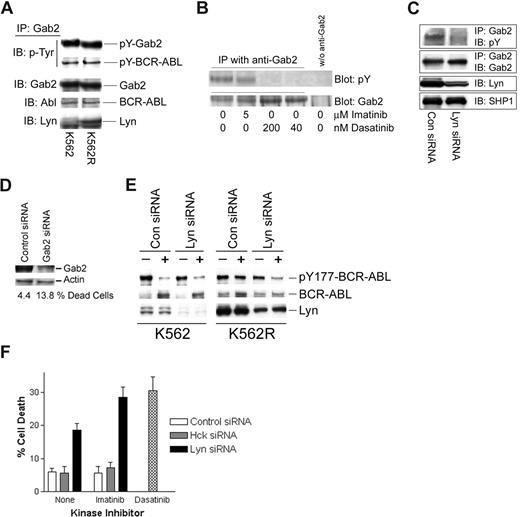
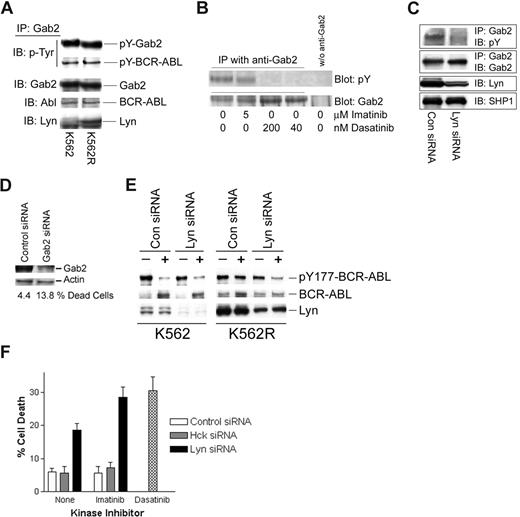
pY177-BCR-ABL serves as a binding site for Grb2, resulting in recruitment of Gab2, which is essential for BCR-ABL–mediated transformation.35-38 Through its ability to phosphorylate BCR-ABL at the Y177 site, persistently activated Lyn in imatinib-resistant cells may alter the nature of the BCR-ABL signaling complex. Gab2 has also been shown to be phosphorylated by Lyn in granulocyte colony-stimulating factor (G-CSF)–treated cells,39 suggesting that it may serve as a common downstream component in both Lyn and BCR-ABL signaling. To assess the nature of the Gab2 signaling complex in CML cells, Gab2 was immunoprecipitated from imatinib-sensitive and -resistant K562 cells, and complexing proteins were assessed by immunoblotting. As expected, Gab2 was tyrosine phosphorylated and associated with BCR-ABL in both cell lines (Figure 3A). In K562R cells, Lyn was also detected in Gab2 complexes. To assess whether Lyn plays a role in Gab2 tyrosine phosphorylation, cells were treated with imatinib, dasatinib, or Lyn siRNA prior to analysis of tyrosine phosphorylation of immunoprecipitated Gab2. As shown in Figure 3B, 5 μM imatinib did not effect Gab2 tyrosine phosphorylation, while low concentrations of dasatinib (40-200 nM) completely reduced pY-Gab2 levels. Lyn down-regulation (siRNA) reduced pY-Gab2 levels (Figure 3C), suggesting that Lyn participates in Gab2 tyrosine phosphorylation in imatinib-resistant cells. Silencing Gab2 in K562R cells resulted in a more than 3-fold increase in cell death (Figure 3D), confirming an important signaling role for this adaptor protein in imatinib-resistant cells with autonomous Lyn activation.
Lyn regulates Gab2 tyrosine phosphorylation, BCR-ABL signaling complexes, and imatinib activity in CML cells. (A) Gab2 was immunoprecipitated from K562 and K562R equal protein (1 mg) cell lysates and immunoblotted for phosphotyrosine (pY-Gab2, pY-BCR-ABL), BCR-ABL, Gab2, and Lyn. (B) K562R cells were treated with imatinib or dasatinib at the indicated concentration for 2 hours prior to Gab2 immunoprecipitation from equal protein cell lysates (0.5 mg) and blotting for phosphotyrosine (top) or Gab2 (bottom). Cell lysates subjected to immunoprecipitation media in the absence of anti-Gab2 were used to detect any nonspecific proteins (last lane). (C) K562R cells were electroporated with the indicated siRNA for 48 hours before immunoprecipitation of Gab2 and blotting for phosphotyrosine content and Gab2 levels. Lyn levels were measured by immunoblotting cell lysate. SHP1 was immunoblotted and used as a protein loading control. (D) K562R cells were electroporated with control or Gab2 siRNA (100 nM) and cell death was assessed by trypan blue exclusion (below) after 48 hours. Cell lysates were also examined for Gab2 protein levels by immunoblotting. Actin was probed as a protein loading control. (E) K562 or K562R cells were subjected to electroporation with 100 nM of either control or Lyn siRNA as described in “Methods.” After 24 hours, cells were treated with 2.5 μM imatinib (+) or vehicle alone (−) for an additional 2 hours before analysis of BCR-ABL, Lyn, and Y177-BCR-ABL levels by immunoblotting. (F) K562R cells were electroporated with 100 nM control, Hck, or Lyn siRNA (as indicated). After 24 hours, electroporated cells were treated with vehicle alone (none) or 2.5 μM imatinib for an additional 24 hours. Cells were also treated with dasatinib alone (0.2 μM) (without electroporation) for 24 hours before all cells were collected and cell death was estimated by trypan blue staining. The results represent the average plus or minus SEM of triplicate assays.
Lyn regulates Gab2 tyrosine phosphorylation, BCR-ABL signaling complexes, and imatinib activity in CML cells. (A) Gab2 was immunoprecipitated from K562 and K562R equal protein (1 mg) cell lysates and immunoblotted for phosphotyrosine (pY-Gab2, pY-BCR-ABL), BCR-ABL, Gab2, and Lyn. (B) K562R cells were treated with imatinib or dasatinib at the indicated concentration for 2 hours prior to Gab2 immunoprecipitation from equal protein cell lysates (0.5 mg) and blotting for phosphotyrosine (top) or Gab2 (bottom). Cell lysates subjected to immunoprecipitation media in the absence of anti-Gab2 were used to detect any nonspecific proteins (last lane). (C) K562R cells were electroporated with the indicated siRNA for 48 hours before immunoprecipitation of Gab2 and blotting for phosphotyrosine content and Gab2 levels. Lyn levels were measured by immunoblotting cell lysate. SHP1 was immunoblotted and used as a protein loading control. (D) K562R cells were electroporated with control or Gab2 siRNA (100 nM) and cell death was assessed by trypan blue exclusion (below) after 48 hours. Cell lysates were also examined for Gab2 protein levels by immunoblotting. Actin was probed as a protein loading control. (E) K562 or K562R cells were subjected to electroporation with 100 nM of either control or Lyn siRNA as described in “Methods.” After 24 hours, cells were treated with 2.5 μM imatinib (+) or vehicle alone (−) for an additional 2 hours before analysis of BCR-ABL, Lyn, and Y177-BCR-ABL levels by immunoblotting. (F) K562R cells were electroporated with 100 nM control, Hck, or Lyn siRNA (as indicated). After 24 hours, electroporated cells were treated with vehicle alone (none) or 2.5 μM imatinib for an additional 24 hours. Cells were also treated with dasatinib alone (0.2 μM) (without electroporation) for 24 hours before all cells were collected and cell death was estimated by trypan blue staining. The results represent the average plus or minus SEM of triplicate assays.
To determine whether Lyn affects imatinib-mediated inhibition of BCR-ABL phosphorylation at Y177, Lyn siRNA was used to suppress expression of Lyn in both K562 and K562R cells. Since Lyn silencing alone reduced K562R cell viability (Figure 1C; Table 1) early time points (24 hours after electroporation) were examined for the effect of Lyn silencing on imatinib-mediated BCR-ABL inactivation (at Y177) and cell viability. As shown in Figure 3E, Lyn expression was reduced in both populations, and imatinib (2.5 μM, 2 hours) markedly reduced Y177-BCR-ABL levels (> 90%) in both control and Lyn siRNA-electroporated K562 cells. Lyn knockdown in K562R cells resulted in recovery of imatinib-mediated Y177-BCR-ABL inhibition, suggesting that Lyn protects cells from imatinib-mediated suppression of BCR-ABL phosphorylation at Y177.
Since dasatinib overcame imatinib resistance in K562R cells, its antitumor effects were compared with imatinib in cells electroporated to reduce Lyn protein levels. Cell death in control or Hck (Hck is not expressed in K562 cells) siRNA-electroporated cells (Figure 3F) was approximately 6% to 8% and was not affected by imatinib. As in previous experiments, Lyn siRNA alone induced cell death (Figure 3F black bars; ∼ 20%), which was further increased by imatinib (∼ 30%) and approached the level of cell death detected in dasatinib-treated K562R cells (Figure 3F hatched bar). These results suggest that Lyn regulates imatinib-mediated kinase inhibition and antitumor activity in some CML cells.
Lyn negatively regulates c-Cbl protein stability
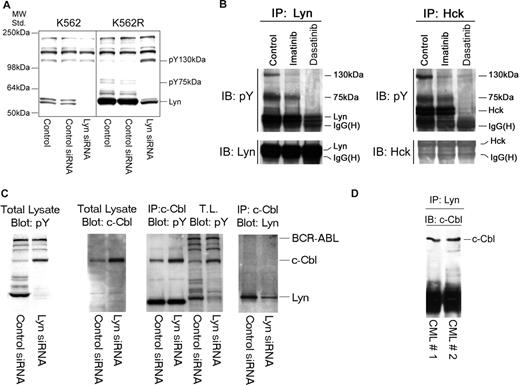
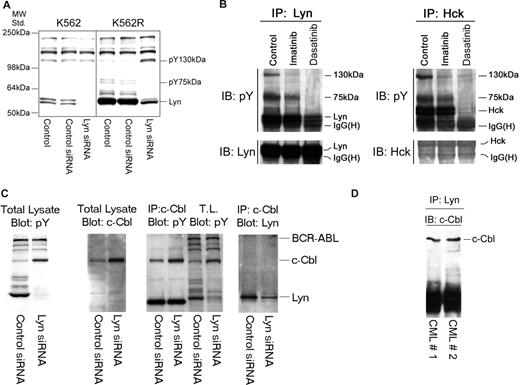
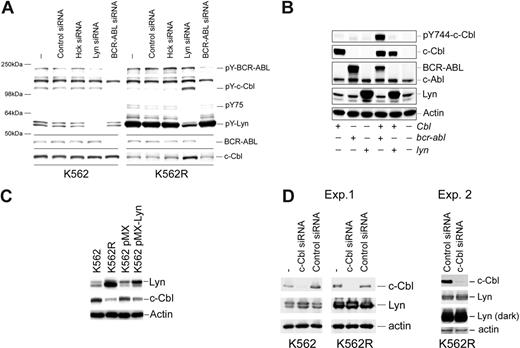
Silencing Lyn expression in K562R cells induces apoptosis (Figure 1C) and was associated with a decrease in expression and phosphorylation of Lyn and an unidentified 75-kDa phosphoprotein (Figure 4A). However, increased tyrosine phosphorylation of an approximately 130-kDa protein was also consistently noted in Lyn siRNA-treated cells. A tyrosyl-phosphoprotein of similar molecular characteristics was detected in Lyn or Hck protein complexes in cells derived from imatinib-resistant patients, and its phosphorylation/association with Lyn or Hck was affected by tyrosine kinase inhibition (Figure 4B). To identify this protein, Lyn immunoprecipitation was scaled up and the pY 130-kDa protein was detected in silver-stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels or pY blots. The gel band was excised, trypsinized, and analyzed by mass spectroscopy. This phosphoprotein was identified as c-Cbl, which was confirmed by subsequent immunoprecipitation and immunoblotting analysis of K562R cells (Figure 4C), and further analysis demonstrated c-Cbl/Lyn complexes in primary leukemic specimens (Figure 4D).
Lyn is associated with c-Cbl and regulates its stability in CML cells. (A) K562 and K562R cells were subjected to electroporation with control or Lyn-specific siRNA, and after 48 hours changes in phosphotyrosine levels were accessed in equal protein lysates by immunoblotting. Migration of molecular mass standards is shown on the left, and individual targets are shown on the right. In addition to a reduction in pY-Lyn levels in Lyn siRNA-treated cells, Lyn down-regulation in K562R cells was associated with differential regulation of 2 phosphoproteins of 75 kDa and 130 kDa. (B) Lyn and Hck were found to be highly expressed in a lymphoid blast crisis CML patient who failed imatinib therapy (Figure 2B). A specimen was obtained and treated with imatinib (5 μM) or dasatinib (0.5 μM) for 2 hours before lysates (1 mg) were prepared and subjected to Lyn (left) or Hck (right) immunoprecipitation. Immune complexes were resolved by SDS-PAGE and immunoblotted for phosphotyrosine. In addition to recovery of Lyn or Hck by immunoprecipitation, 2 phosphotyrosine protein bands (130 kDa, 75 kDa) were detected in control untreated cells. Imatinib-reduced phosphorylation or recovery of the 130-kDa protein in either Lyn or Hck immunoprecipitates, while dasatinib reduced phosphorylation or recovery of both the 130-kDa and 75-kDa phosphoproteins. In a parallel experiment, the Lyn coprecipitating 130-kDa band was excised from a silver-stained gel, subjected to trypsinization, and liquid chromatography/mass spectrometry (LC/MS) analysis. The 130-kDa protein was identified as c-Cbl. The 75-kDa protein has not yet been identified. (C) To confirm c-Cbl as a Lyn-associated and -regulated protein, K562R cells were subjected to Lyn silencing and (first panel) total lysates were immunoblotted for phosphotyrosine, (second panel) total lysates were immunoblotted for c-Cbl, (third panel) c-Cbl immunoprecipitates or total lysate (TL) was immunoblotted for phosphotyrosine, and (fourth panel) total lysates were subjected to c-Cbl immunoprecipitation and Lyn immunoblotting. The migration of specific target proteins is shown on the right. (D) Lyn was immunoprecipitated from protein lysates (250 mg) derived from 2 CML myeloid blast crisis patient specimens and immunoblotted for c-Cbl.
Lyn is associated with c-Cbl and regulates its stability in CML cells. (A) K562 and K562R cells were subjected to electroporation with control or Lyn-specific siRNA, and after 48 hours changes in phosphotyrosine levels were accessed in equal protein lysates by immunoblotting. Migration of molecular mass standards is shown on the left, and individual targets are shown on the right. In addition to a reduction in pY-Lyn levels in Lyn siRNA-treated cells, Lyn down-regulation in K562R cells was associated with differential regulation of 2 phosphoproteins of 75 kDa and 130 kDa. (B) Lyn and Hck were found to be highly expressed in a lymphoid blast crisis CML patient who failed imatinib therapy (Figure 2B). A specimen was obtained and treated with imatinib (5 μM) or dasatinib (0.5 μM) for 2 hours before lysates (1 mg) were prepared and subjected to Lyn (left) or Hck (right) immunoprecipitation. Immune complexes were resolved by SDS-PAGE and immunoblotted for phosphotyrosine. In addition to recovery of Lyn or Hck by immunoprecipitation, 2 phosphotyrosine protein bands (130 kDa, 75 kDa) were detected in control untreated cells. Imatinib-reduced phosphorylation or recovery of the 130-kDa protein in either Lyn or Hck immunoprecipitates, while dasatinib reduced phosphorylation or recovery of both the 130-kDa and 75-kDa phosphoproteins. In a parallel experiment, the Lyn coprecipitating 130-kDa band was excised from a silver-stained gel, subjected to trypsinization, and liquid chromatography/mass spectrometry (LC/MS) analysis. The 130-kDa protein was identified as c-Cbl. The 75-kDa protein has not yet been identified. (C) To confirm c-Cbl as a Lyn-associated and -regulated protein, K562R cells were subjected to Lyn silencing and (first panel) total lysates were immunoblotted for phosphotyrosine, (second panel) total lysates were immunoblotted for c-Cbl, (third panel) c-Cbl immunoprecipitates or total lysate (TL) was immunoblotted for phosphotyrosine, and (fourth panel) total lysates were subjected to c-Cbl immunoprecipitation and Lyn immunoblotting. The migration of specific target proteins is shown on the right. (D) Lyn was immunoprecipitated from protein lysates (250 mg) derived from 2 CML myeloid blast crisis patient specimens and immunoblotted for c-Cbl.
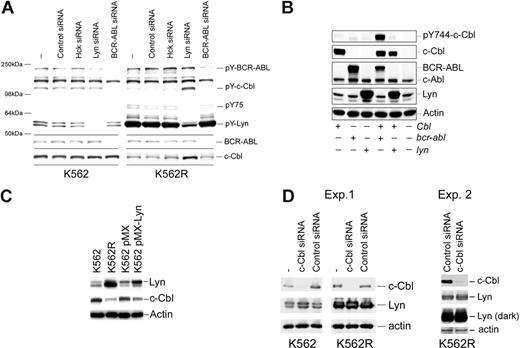
Since previous reports suggested that c-Cbl tyrosine phosphorylation was regulated by BCR-ABL,39 the effects of BCR-ABL and Lyn silencing on c-Cbl tyrosine phosphorylation and protein levels were analyzed. As shown in Figure 5A, BCR-ABL silencing reduced BCR-ABL and c-Cbl tyrosine phosphorylation in both K562 and K562R cells but did not effect c-Cbl protein levels. In contrast, Lyn suppression resulted in both an increase in c-Cbl tyrosine phosphorylation and protein levels. To further assess the role of these kinases in c-Cbl phosphoregulation, c-Cbl was coexpressed with Lyn or BCR-ABL, and tyrosine phosphorylation of a CrkL-binding site on c-Cbl (Y774)40-42 was assessed. As shown in Figure 5B, c-Cbl phosphorylation was increased when coexpressed with BCR-ABL, whereas Lyn coexpression had no effect. Real-time PCR analysis of c-Cbl mRNA from control and Lyn siRNA-treated K562R cells failed to detect distinctions, suggesting that Lyn primarily regulates c-Cbl at a posttranscriptional level (data not shown). To confirm a role for Lyn in c-Cbl regulation, Lyn was transiently overexpressed in K562 cells, and the effects on c-Cbl levels were measured. As shown in Figure 5C, Lyn overexpression correlated with a decrease in c-Cbl protein levels in K562 cells. We also noted a reduction in imatinib sensitivity in Lyn-overexpressing K562 cells (IC50 pMX-Lyn: 0.2 μM vs 0.5 μM in pMX control cells), in agreement with earlier studies.21
Kinase-mediated regulation of c-Cbl in CML cells. (A) K562 (left) and K562R (right) cells were electroporated with the indicated siRNA or left untreated (−) for 48 hours before cell lysates were analyzed for total tyrosine phosphorylation (top) or c-Cbl protein levels (bottom). Molecular mass marker migration is shown on the left, and specific target phosphoproteins are depicted on the right. (B) Cos-7 cells were transfected with cbl, bcr-abl, or lyn expression vectors alone or in combination (as indicated), and 24 hours later cells were lysed and changes in pY744-c-Cbl levels were monitored by phospho–site-specific immunoblotting. The membrane was stripped and reprobed for c-Cbl, BCR-ABL, and Lyn protein levels. Actin blotting was used as a protein loading control. (C) K562 cells were electroporated with a GFP-IRES Lyn expression vector (pMX-Lyn) or empty vector (pMX). After 7 days, cells were flow sorted for GFP positivity and equal numbers of positive cells were compared with untransfected K562 and K562R cells for Lyn, c-Cbl, and actin protein levels. (D) K562R cells were electroporated with control or c-Cbl–specific siRNA, and after 48 hours cell lysates were examined for c-Cbl, Lyn, and actin (as a protein loading control). Both a short and long exposure for Lyn detection is shown.
Kinase-mediated regulation of c-Cbl in CML cells. (A) K562 (left) and K562R (right) cells were electroporated with the indicated siRNA or left untreated (−) for 48 hours before cell lysates were analyzed for total tyrosine phosphorylation (top) or c-Cbl protein levels (bottom). Molecular mass marker migration is shown on the left, and specific target phosphoproteins are depicted on the right. (B) Cos-7 cells were transfected with cbl, bcr-abl, or lyn expression vectors alone or in combination (as indicated), and 24 hours later cells were lysed and changes in pY744-c-Cbl levels were monitored by phospho–site-specific immunoblotting. The membrane was stripped and reprobed for c-Cbl, BCR-ABL, and Lyn protein levels. Actin blotting was used as a protein loading control. (C) K562 cells were electroporated with a GFP-IRES Lyn expression vector (pMX-Lyn) or empty vector (pMX). After 7 days, cells were flow sorted for GFP positivity and equal numbers of positive cells were compared with untransfected K562 and K562R cells for Lyn, c-Cbl, and actin protein levels. (D) K562R cells were electroporated with control or c-Cbl–specific siRNA, and after 48 hours cell lysates were examined for c-Cbl, Lyn, and actin (as a protein loading control). Both a short and long exposure for Lyn detection is shown.
Stability of Lyn and other Src-family kinases was previously shown to be regulated by c-Cbl in cells from multiple lineages.43-49 To determine whether c-Cbl also regulates Lyn levels in CML cells, c-Cbl expression was reduced and Lyn levels were analyzed. As shown in Figure 5D, a reduction in c-Cbl levels in K562 cells was associated with a modest increase in Lyn protein levels. However, c-Cbl silencing had little effect on Lyn expressed in K562R cells. Additional studies in K562R cells (Exp. 2) failed to detect Lyn up-regulation following c-Cbl knockdown.
Discussion
Imatinib resistance is commonly associated with mutations in BCR-ABL that effect inhibitor affinity.6-8 However, patients frequently fail imatinib therapy but retain expression of an unmutated form of BCR-ABL.9-13 In vitro selection of CML cells for imatinib resistance results in recovery of cells overexpressing tyrosine kinases that are imatinib insensitive.19-21 The most common reported alteration is autonomous activation and overexpression of Lyn kinase, and initial clinical studies suggested a role for Lyn in imatinib resistance and disease progression.19 However, although Lyn is reported to regulate CML cell survival,50 its role in this disease and in imatinib resistance is not completely understood. This study provides evidence that Lyn regulates the composition of BCR-ABL signaling complexes and influences the stability of other regulatory proteins.
Lyn was initially reported to be downstream of BCR-ABL in CML cells33,34 and was proposed to be suppressed by BCR-ABL inhibition with imatinib.19,51 However, our recent studies suggest that Lyn activation may be distinct in imatinib-responsive and -resistant cells. Lyn activation is BCR-ABL independent in imatinib-resistant patients (Figure 2B,C and Donato et al19 ) and was recently described in models of BCR-ABL–mediated leukemogenesis.32 In imatinib-resistant cells and CML patients with wild-type BCR-ABL expression (Figures 1,Figure 2–3), imatinib reduced BCR-ABL and substrate phosphorylation, but these actions were delayed or incomplete. This deficiency was corrected by dasatinib, possibly due to its direct inhibitory effects on Lyn kinase.
In vitro analysis demonstrated that Lyn directs Y177-BCR-ABL phosphorylation that is suppressed by dasatinib, but not imatinib, raising the possibility that Lyn prevents full inactivation of BCR-ABL signaling by retaining critical adaptor protein (Grb2, Gab2) binding sites on BCR-ABL.35-37 Both Y177-BCR-ABL and Gab2 are essential for leukemogenesis,36,38 and earlier reports suggested that Lyn (or other SFK) mediates phosphorylation of both proteins.22-25,39 Some previous studies predicted Gab2 as a direct substrate for BCR-ABL,52 but this conclusion was based on studies of a compound with Src/Abl inhibitory activity (PD180970). In this study, significant changes in the BCR-ABL signaling complexes were noted in cells with high expression of Lyn. For example, we noted a direct association of Lyn with Gab2 whose tyrosine phosphorylation is directed by Lyn and not inhibitable by imatinib (Figure 3A-C). Lyn-mediated phosphorylation of Y177-BCR-ABL may be facilitated through its direct association with BCR-ABL adaptor proteins (Gab2, c-Cbl) and may explain the delayed inhibition or loss of complete suppression of BCR-ABL signaling in cells with highly activated Lyn kinase. Reducing Lyn levels restores imatinib-mediated inhibition of BCR-ABL Y177 phosphorylation (Figure 3E) to a level similar to that of dasatinib treatment alone (Figure 1B). Combined Lyn suppression with imatinib treatment also reduced cell viability to a level similar to that of dasatinib (Figure 3F). Lyn silencing in CML cells may engage apoptosis, in part, by destabilizing tyrosine phosphorylation of BCR-ABL and Gab2 (Figures 1C and 3F; Table 1; and Ptasznik et al50 ).
Lyn was also complexed with tyrosine-phosphorylated c-Cbl in CML cells where it appears to regulate c-Cbl stability, but not phosphorylation. Of particular distinction to previous studies, we noted that down-regulation of Lyn resulted in an increase in c-Cbl protein levels (Figure 5A,B). c-Cbl was previously noted as a major tyrosine phosphoprotein in BCR-ABL–expressing cells.40-42 However, it was unclear whether BCR-ABL or a downstream kinase was responsible for its phosphorylation. Lyn regulates c-Cbl stability, which in turn, is tyrosine phosphorylated by BCR-ABL at Y774, a site critical for recruitment of CrkL,42 a major BCR-ABL substrate detected in CML patients.6,8,9 The reciprocal relationship between Lyn and c-Cbl may partially explain the difficulty in establishing Lyn-overexpressing CML cells as enforced Lyn expression may diminish the activities of c-Cbl (Figure 5A-D). A phosphoproteomic assessment of patients with tyrosine kinase–sensitive and –resistant disease may lead to a greater understanding of protein-protein interactions that differentiate responsive and resistant patients.53
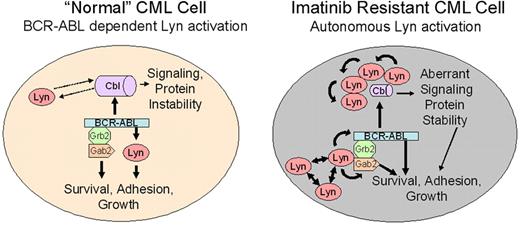
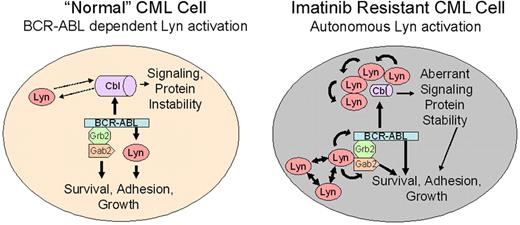
In this report, we note 2 activities associated with Lyn in CML cells that may relate to its role in imatinib resistance. These include target-specific phosphoregulation (BCR-ABL, Gab2) and association/destabilization of c-Cbl. A model of Lyn function in “normal” and Lyn-overexpressing (following clinical imatinib exposure) CML cells is shown in Figure 6. One important feature of this model is the transition from BCR-ABL–dependent to –independent Lyn activation. As described for other SFKs, Lyn overexpression alone may underlie its autonomous activation, but other upstream regulators or loss of negative regulatory control may be sufficient for its activation.58,59 We have not detected Lyn mutations in the kinase or c-terminal negative regulatory domain in imatinib-resistant patients but have preliminary evidence for site-specific Lyn tyrosine phosphorylation in some patients with autonomous Lyn activation (data not shown), suggesting that alternate upstream cascades influence Lyn activation in some CML patients. In CML patients with BCR-ABL–dependent Lyn activation, treatment with imatinib destroys sensitive cells but is less effective against those with elevated Lyn expression or BCR-ABL–independent Lyn activation. Additional treatment with imatinib results in enrichment for cells with elevated Lyn expression. Once Lyn is highly expressed or its activation is no longer controlled by imatinib, its association with Gab2 permits use of BCR-ABL as a substrate (Y177 phosphorylation). Lyn-mediated BCR-ABL and Gab2 phosphorylation stabilizes BCR-ABL signaling complexes, even in the presence of imatinib. Lyn also regulates the stability of c-Cbl, resulting in additional changes in recruitment of BCR-ABL substrates. Lyn-mediated destabilization of c-Cbl may affect the half-life of signal-carrying proteins and may mimic the phenotype of recently described loss-of-function c-Cbl mutations associated with transformation in other leukemias.56,57 An assessment of c-Cbl targets in CML cells may be necessary to fully understand the role of Lyn and its impact on imatinib activity and disease progression.
Lyn regulation in “normal” and Lyn-overexpressing CML cells. Lyn expression and activation are heterogeneous in CML cells and may be altered by imatinib therapy. In early stage or untreated (“normal”) CML, BCR-ABL is upstream of Lyn and other Src-family kinases. BCR-ABL–mediated Lyn (or related kinase) activation provides essential, possibly lineage-specific, support for BCR-ABL–mediated transformation.54,55 In this setting, BCR-ABL inhibition (with imatinib) reduces Lyn kinase activation and loss of signaling through BCR-ABL– and Lyn-mediated phosphorylation. Lyn complexes with c-Cbl, inducing partial control of Lyn stability, but Lyn does not regulate c-Cbl phosphorylation. Imatinib exposure alters Lyn expression or induces changes in upstream control of its activation. Through overexpression, autoactivation, or association with other adaptor proteins, Lyn activation acquires full or partial BCR-ABL independence, resulting in loss of control of Lyn activation with imatinib alone. Unregulated Lyn alters BCR-ABL signaling complexes, associating with Gab2, inducing its tyrosine phosphorylation, and maintaining or directing Y177 phosphorylation of BCR-ABL. These changes suppress or delay kinase inhibition by imatinib. Lyn overexpression also destabilizes c-Cbl, resulting in a reduction in its protein level. Tyrosine phosphorylation of c-Cbl is mediated by BCR-ABL in both normal and stressed CML cells, allowing it to maintain scaffold or recruitment activity. Lyn may function in a feedback loop with c-Cbl to maintain ubiquitination of substrates, altering protein stability. Stabilization of key ubiquitin-targeted proteins in leukemic cells may independently contribute to BCR-ABL–mediated transformation.56,57
Lyn regulation in “normal” and Lyn-overexpressing CML cells. Lyn expression and activation are heterogeneous in CML cells and may be altered by imatinib therapy. In early stage or untreated (“normal”) CML, BCR-ABL is upstream of Lyn and other Src-family kinases. BCR-ABL–mediated Lyn (or related kinase) activation provides essential, possibly lineage-specific, support for BCR-ABL–mediated transformation.54,55 In this setting, BCR-ABL inhibition (with imatinib) reduces Lyn kinase activation and loss of signaling through BCR-ABL– and Lyn-mediated phosphorylation. Lyn complexes with c-Cbl, inducing partial control of Lyn stability, but Lyn does not regulate c-Cbl phosphorylation. Imatinib exposure alters Lyn expression or induces changes in upstream control of its activation. Through overexpression, autoactivation, or association with other adaptor proteins, Lyn activation acquires full or partial BCR-ABL independence, resulting in loss of control of Lyn activation with imatinib alone. Unregulated Lyn alters BCR-ABL signaling complexes, associating with Gab2, inducing its tyrosine phosphorylation, and maintaining or directing Y177 phosphorylation of BCR-ABL. These changes suppress or delay kinase inhibition by imatinib. Lyn overexpression also destabilizes c-Cbl, resulting in a reduction in its protein level. Tyrosine phosphorylation of c-Cbl is mediated by BCR-ABL in both normal and stressed CML cells, allowing it to maintain scaffold or recruitment activity. Lyn may function in a feedback loop with c-Cbl to maintain ubiquitination of substrates, altering protein stability. Stabilization of key ubiquitin-targeted proteins in leukemic cells may independently contribute to BCR-ABL–mediated transformation.56,57
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients for agreeing to participate in this study and the clinical staff and faculty in the Department of Leukemia, M. D. Anderson Cancer Center, for assistance with specimen collection.
This work was supported by the National Institutes of Health (P01 CA 49639) and Leukemia and Lymphoma Society (CF01–313, 6118).
National Institutes of Health
Authorship
Contribution: J.W. and F.M. performed research and analyzed data; H.L., L.K., W.B., and Z.P. performed research; M.T. analyzed data; N.J.D. performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: M.T. is a member of the speakers' bureau for Novartis and Bristol-Myers Squibb and serves as an ad hoc advisor for both companies. N.J.D. is a past ad hoc advisor for Bristol-Myers Squibb. All other authors declare no competing financial interests.
Correspondence: Nicholas J. Donato, Associate Professor of Medicine, Division of Hematology/Oncology, Department of Internal Medicine, University of Michigan Comprehensive Cancer Center, Rm 4306 CCGC, Ann Arbor, MI 48109-0624; e-mail: ndonato@med.umich.edu.

![Figure 1. Cellular and biochemical response to tyrosine kinase inhibitors in imatinib-sensitive and -resistant cells. (A) K562 and K562R cells were treated with imatinib (left) or dasatinib (right) at the indicated concentration for 48 hours before assessment of cell viability as previously described.19 Each point represents the average plus or minus SEM (error bar) of 4 determinations. (Inset) Immunoblot analysis of BCR-ABL, Lyn, Src, Yes, Fyn, and actin in equal protein lysates from K562 and K562R cells. BCR-ABL sequencing did not detect mutations in the Abl kinase domain in either cell line (data not shown). (B) K562 (left) or K562R (right) cells were incubated with imatinib (5 μM) or dasatinib (0.5 μM) for 0, 2, or 24 hours before lysates were analyzed for tyrosine-phosphorylated (activated) BCR-ABL, Lyn, and CrkL. Lyn and PARP protein (uncleaved [uPARP] = 116 kDa or cleaved [cPARP] = 85 kDa) were also detected by immunoblotting. (C) K562 and K562R cells were left untreated (control) or electroporated (AMAXA) with control (Con) or Lyn siRNA and analyzed for activated Lyn (pY396-Lyn) or Lyn protein levels by immunoblotting after 48 hours. Lysates were also probed for PARP as a marker of apoptosis. Table 1 summarizes the effect of siRNA on viability in K562 and K562R cells. The results represent the average and standard deviation from 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/7/10.1182_blood-2007-08-109330/5/m_zh80070817770001.jpeg?Expires=1768413417&Signature=PvawdzDh0KWuhSSWvYC~Y~5I~0hQ01CmiqtdwnWxUfK6I2kqK5uXBV8eG2stdRgSO79nJDk3t~5yLfS32b5jFgH-3EDR8Uy2Boo5kbSs0qyb1tMEDuPngsfVv4qkfzk5Q9F~584qfgza4GnWSXzJgxuQeRffBlV~vb1nq04P8UcAyAw9qAbl-tvDqtftrl5-ZBC0K3olIKkgn7mC2cM3c18iCgz9T25wMwB2RLSMGSEFvKL32vMwDg5xxUsqhZ7fppHaw5Lbeeh~IXB5KdfAZuxodHZKNsEqeE7JCl3Fr9VShN9JysP0xN-EQ9noyzt6Y4fbAXikTNrFeIZzVj0usw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Cellular and biochemical response to tyrosine kinase inhibitors in imatinib-sensitive and -resistant cells. (A) K562 and K562R cells were treated with imatinib (left) or dasatinib (right) at the indicated concentration for 48 hours before assessment of cell viability as previously described.19 Each point represents the average plus or minus SEM (error bar) of 4 determinations. (Inset) Immunoblot analysis of BCR-ABL, Lyn, Src, Yes, Fyn, and actin in equal protein lysates from K562 and K562R cells. BCR-ABL sequencing did not detect mutations in the Abl kinase domain in either cell line (data not shown). (B) K562 (left) or K562R (right) cells were incubated with imatinib (5 μM) or dasatinib (0.5 μM) for 0, 2, or 24 hours before lysates were analyzed for tyrosine-phosphorylated (activated) BCR-ABL, Lyn, and CrkL. Lyn and PARP protein (uncleaved [uPARP] = 116 kDa or cleaved [cPARP] = 85 kDa) were also detected by immunoblotting. (C) K562 and K562R cells were left untreated (control) or electroporated (AMAXA) with control (Con) or Lyn siRNA and analyzed for activated Lyn (pY396-Lyn) or Lyn protein levels by immunoblotting after 48 hours. Lysates were also probed for PARP as a marker of apoptosis. Table 1 summarizes the effect of siRNA on viability in K562 and K562R cells. The results represent the average and standard deviation from 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/7/10.1182_blood-2007-08-109330/5/m_zh80070817770001.jpeg?Expires=1768537763&Signature=oJ5aRlwi6sho0puXoKMiJQ4w9u9GGy2HsobD2nvMngnm~IUkgIkKSzHyZqtWH9ynOkyEp-ReLj5naKIwRMchOIvJ5d~Iaczl2B-4Sm2L7JpY1C0ZJvqfLxyKQlj5GL~msR9RCwl9Xj5aHX6-44odOru8d0lVQU4uLwrmsc4p6HA8tqUXrFbjnwdSkW9gIuerTdyqtMo~dsXKtkQN-4dUL4eBADVM~mAb3t87QcufA6oPtaRAJWCsCnhpxkYGPSXOMk8WzCXDXFQZ4ZQdALY6bF4-7g83od6kerE8MABIMV21JSkYxEK7rJ7kU4csPzWGCQqNwkpdInxeu3EmWsoZUA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)