Abstract
Targeted RNases (TRs) are immunoenzymes with ribonucleases as cytotoxic effector domains, which are less immunogenic as plant or bacterial toxin components of classical immunotoxins. In this study, we show the generation and production of the first entirely human TR (huTR) directed against CD30+ lymphomas. The scFv-Fc-RNase construct was produced in human embryonic kidney (HEK) 293T cells, yielding up to 4 mg/L soluble protein after purification by protein A affinity chromatography. Size exclusion chromatography revealed a homodimer of the predicted molecular mass. Surface plasmon resonance analysis revealed an affinity to CD30 of KD of less than 1 nM for both the scFv-Fc and the scFv-Fc-RNase proteins. Internalization of the scFv-Fc-RNase protein by CD30+ Karpas-299 cells was demonstrated by confocal microscopy. Proliferation of the CD30+ lymphoma cell line Karpas-299 was strongly inhibited by CD30-specific huTR protein (IC50 = 3.3 nM). The huTR is a promising candidate for the immunotherapy of CD30+ lymphomas because of its expected low immunogenicity, good production yields, and potent effector function upon target cell binding and internalization. Its modular design is set to target other internalizing tumor antigens using different antibody domains.
Introduction
Therapy with immunotoxins (ITs; bacterial or plant-derived toxins fused to antibodies) was hampered by systemic toxicity, vascular leak syndrome, hepatocyte injuries, and high immunogenicity, resulting in humoral immune responses lowering serum half-life and efficacy after a single dose.1-3 Antibody humanization, phage display, and human IgG transgenic mice have alleviated the immunogenicity problem for the targeting component.4 Ranpirnase (Onconase Alfacell, Bloomfield, NJ), a frog analog of human pancreatic RNase A with low immunogenicity, presently is in Phase 3 clinical trials as a single-agent therapeutic for unresectable mesothelioma.5 Pioneered by Susanna Rybak, chemical conjugates of antibodies with ranpirnase were developed (targeted RNases [TRs]).6 The antineoplastic potency of a ranpirnase::anti-CD22–IgG TR matched that of a conventional anti-CD22 immunotoxin using Pseudomonas exotoxin in tumor-bearing severe combined immunodeficient (SCID) mice, but the total cumulative dose could be increased by more than an order of magnitude.7,8 To avoid production and purification issues associated with chemical conjugation, genetic fusions of antibody and RNase were proposed, including RNases of human origin, which cause less concern about immunogenic response.9-12
Despite the safety concerns about the use of constructs with bacterial and plant toxins for the repeated treatments usually required in cancer therapy, respective fusion proteins are usually difficult to produce. In general, ITs and TRs had to be produced in prokaryotic expression systems due to their cytotoxicity on eukaryotic cells. The necessary refolding protocols lead to suboptimal yields of functional protein within the low microgram per liter range.13,14 Purification of soluble IT from the periplasm of Escherichia coli only slightly increased the yield, but the yield remained below milligram per liter.15,16 Only very recently have there been 2 reports describing TR production of CD30-scFv-angiogenin and CD22scFv-angiogenin in mammalian expression systems for the first time, but still with a yield not indicated or below milligram per liter.17,18
CD30, a 120-kDa transmembrane type I glycoprotein, was described for the first time as being an immunopathologic relevant tumor marker for Hodgkin and Reed-Sternberg cells of Hodgkin lymphomas (HLs).19 The CD30 expression is not restricted to HL, but is also present on highly malignant anaplastic large cell lymphomas (ALCLs) and adult T-cell lymphomas (ATLs) categorized as non-Hodgkin lymphomas (NHLs). Beside neoplasms, expression of CD30 in normal cells is restricted to activated B and T lymphocytes with no expression on nonhematolymphoid cells and resting hematolymphoid cells.19,20 Due to its characteristic repetitive cystein-rich regions within the extracellular domain, CD30 belongs to the tumor necrosis factor receptor (TNFR) superfamily.21 Although inducing cell proliferation and activation by activating the transcription factor NF-κB of HL cells, CD30 signaling may induce inhibition of cell growth of ALCL and other CD30+ cells.22-25 The frequency of CD30 malignancies is 4 (HL) to 15 (NHL) patients per 100 000 people per year. Standard treatment of CD30+ lymphomas is radiation therapy and chemotherapy such as BEACOPP (bleomycin, etoposide, adriamycin, cyclophosphamide, oncovin, procarbazine, prednisone) or ABDV (adriamycin, bleomycin, dacarbazine, and vinblastine).26-29 However, 20% of patients with HL either relapse after first treatment or are refractory to initial treatment.30,31 In cases of NHL, 20% to 30% of the patients with ALCL relapse within 5 years.32,33 Therefore, alternative therapies such as anti-CD30 monoclonal Antibodies (mAbs) and anti-CD30 immunoenzyme fusion proteins could be beneficial for patients in whom standard treatments of CD30 lymphomas fail. Clinical approaches to engage murine mAbs (BerH2), bispecific antibodies, or toxin-conjugated antibodies were unsuccessful as they had no long-term benefit, provoked an immune response, or induced toxic side effects.34-36 Very recently, a fusion protein of an anti-CD30 murine scFv (derived from mAb Ber-H2) was fused to human pancreatic RNase, the fusion protein was successfully produced in Drosophila S2 cells, and inhibited tumor growth both in vitro and in vivo.37
In this study, a novel fully human antibody::ribonuclease fusion protein (huTR) was generated, which was remarkably well expressed in mammalian cell culture. Its specific antigen recognition, enzymatic ribonuclease activity, cellular uptake, and in vitro inhibition of CD30+ tumor cell line proliferation make it a promising candidate to overcome the adverse safety issues of immunotoxins for the treatment of CD30+ malignancies.
Methods
Generation of the CD30-specific antibodies and huTR variants
The CD30 specific human scFv 4E3 was obtained from a semisynthetic human antibody library (Tomlinson I + J provided by the Medical Research Council, Cambridge, United Kingdom) by phage display. The scFv antibody fragment was converted into the scFv-Fc format by fusion with the human IgG1-Fc part as well as into the human IgG1 format. Starting from these antibody formats, several CD30-specific TR variants comprising scFv-RNase, scFv-RNase-Fc, and scFv-Fc-RNase were generated and cloned into the mammalian expression vector pCMV-ER-myc (Invitrogen, Karlsruhe, Germany). The cDNA of αCD30scFv was amplified using the oligonucleotides 5′- ATATATCTCGA-GAAAAGAGAGGCTGAAGCTGAGGTGCAACTGTTGGAGTCTGG-3′ and 5′-TTTAAATCTAGACCTCCTGAGGATTTGATTTCCACCTTGGTCC-3′ (restriction sites are indicated in the oligonucleotide sequence) and cloned via XhoI and XbaI restriction sites into the Pichia pastoris expression vector pPICZαA (Invitrogen). To generate αCD30scFv-RNase TR construct, the cDNA coding for CD30-specific scFv was amplified from pIT2-CD30scFv(4E3) (Z.K., unpublished data, January 2005) using the oligonucleotides 5′-TATAAGCGCGCACTCCGAGGTGCAACTGTTGGAG-3′ and 5′-TAATAAGCTAGCGGCCGCCCGTTTGATTTCCACC-3′ and cloned by BssHII and NotI into pCMV-ER-myc. Afterward, the human pancreatic RNase cDNA amplified from pET-11d-RNase-TFR9 using the oligonucleotides 5′-TTTAAAGCGGCCGCATCCTCAGGAAAGGAATCCAAGAAATTCC-3′ and 5′- AAATTTTCTAGATTAAGCATCAAAGTGGACTGGCAC-3′ was introduced by NotI and XbaI to obtain the scFv-RNase fusion construct. The resulting vector pCMV-αCD30scFv-RNase was used for the construction of pCMV-αCD30scFv-RNase-Fc using the BamHI restriction site at the 3′ end of the scFv-RNase gene fragment. The human IgG1 Fc cDNA (hinge-CH2-CH3 domains) was amplified from pSH1–21538 using the oligonucleotides 5′-TATATAGGATCCGAGCCCAAATCTTGTGACAAAACTC-3′ and 5′-TATATATCTAGATTATCATTTACCCGGGGACAGGGAGAGGC-3′ inserted by NotI and XbaI into this vector.
The cloning procedure of the CD30 specific scFv-Fc-RNase construct was performed by 2 consecutive PCRs. In the first step, the cDNA coding for CD30-specific scFv was amplified from pIT2-CD30scFv(4E3), as described for the scFv-RNase construct, and incorporated in pCMV-hIgG1-Fc vector already containing the human IgG1 Fc domains hinge-CH2-CH3. The resulting pCMV-αCD30scFv-Fc encoding the scFv-Fc protein without RNase was used for control experiments. The second step comprised cloning of the gene fragment encoding the human pancreatic RNase to the 3′ end of the scFv-Fc construct via an additional 6–amino acid peptide spacer between the scFv-Fc and RNase. The spacer-RNase gene fragment was generated by polymerase chain reaction (PCR) using the oligonucleotides 5′-ATATATAAAGCTTGTCCCCGGGTAAAGCGGCCGCATCCTCAGGAAAGGAATCC-3′ and 5′-AAATTTTCTAGATTAAGCATCAAAGTGGACTGGCAC-3′ and cloned by HindIII and XbaI into pCMV-αCD30scFv-Fc to generate the huTR construct αCD30scFv-Fc-RNase. All cloning steps were confirmed by DNA sequencing (ABI prism 310; Applied Biosystems, Foster City, CA).
Cell lines and reagents
The transformed HEK cell line 293T (no. CRL-1 268; American Type Culture Collection [ATCC], Rockville, MD) was cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 2 mM l-glutamine, 1.5 g/L sodium bicarbonate, and 4.5 g/L glucose, 10% (vol/vol) fetal calf serum (FCS), and 1% (vol/vol) penicillin/streptomycin (PAA, Parsing, Austria). The CD30+ NHL and ALCL cell line Karpas-299 (DSMZ no. ACC 31; German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) and human HL cell line HD-MY-Z (DSMZ no. ACC346) were cultured in RPMI-1640 supplemented with 2 mM l-glutamine, 8% (vol/vol) FCS, and 1% (wt/vol) penicillin/streptomycin. All cells were cultured at 37°C in 5% CO2 atmosphere and 95% humidity. The P pastoris strain KM71 was obtained from Invitrogen and cultured according to the distributor's instructions at 30°C. All primary and secondary antibodies were purchased from Sigma (Munich, Germany). The anti-RNase rabbit serum was obtained after immunization with the peptide corresponding to the amino acids 87 to 120 of RNase A (DGSRYPNCAYRTSPK; Pineda Ab Service, Berlin, Germany).
Production and purification of CD30-specific antibodies and huTR variants
Production of all CD30-specific huTR and antibody variants except αCD30scFv was done in mammalian HEK293T cells, transfected using HEKfectin (Bio-Rad, Munich, Germany) according to the manufacturer's recommendations. Briefly, 1 day before transfection, HEK293T cells were seeded into petri dishes (10 cm in diameter) using DMEM/high glucose (PAA) supplemented 1% (wt/vol) penicillin/streptomycin and 8% (vol/vol) FCS containing low levels of bovine IgG (Invitrogen). Supernatants were harvested every 48 hours and combined. Purification of all proteins containing an Fc domain was performed by affinity chromatography using a 1 mL protein A HP column on an ÄktaPrime fast performance liquid chromatography (FPLC) system (GE Healthcare, Uppsala, Sweden). After loading the supernatant to the column, it was washed with 20 column volumes of phosphate-buffered saline (PBS; pH 7.4). Elution was done by a pH shift with 100 mM citrate buffer (pH 2.6), followed by immediate neutralization of the solution with 1 M Tris buffer (pH 9.0).
Production of αCD30scFv was done in P pastoris KM71 (Invitrogen) according to the distributor's instructions with inoculation in BMGY medium before switching to BMMY medium for induction of gene expression, followed by an additional incubation for 72 hours at 30°C. The supernatant was harvested and αCD30scFv was purified by protein L affinity chromatography according to the manufacturer's instructions (Pierce, Rockford, IL).
All the samples were analyzed for integrity, purity and degradation by SDS-polyacrylamide gel electrophoresis (PAGE) and silver staining as well as by Western blot and immunostaining using an alkaline phosphate (AP)–conjugated goat anti-human IgG (Fc-specific) antibody except αCD30scFv, which was detected via an anti-pentaHis primary antibody (Qiagen, Hilden, Germany). Protein samples were dialyzed against PBS (pH 7.4) and stored at −20°C. Protein concentration was determined using the Bradford assay (Bio-Rad) and BSA as protein standard.
Gel filtration
Molecular mass of αCD30scFv and αCD30scFv-RNase as well as of bivalent antibodies (αCD30scFv-Fc and IgG) and TR constructs (αCD30scFv-RNase-Fc and αCD30scFv-Fc-RNase) was analyzed on a calibrated Superdex-200 10/300 (GE Healthcare) with PBS (pH 7.4) at a flow rate of 1 mL/minute. Samples were fractionated and stored for further analysis at 4°C.
Binding studies
Affinity and kinetics measurements.
Affinity measurements were performed by surface plasmon resonance (SPR) analysis using a Biacore2000 and a CM5 chip (Biacore, Uppsala, Sweden). Antigen CD30-Fc (R&D Systems, Minneapolis, MN) and control protein lysozyme were bound on flow cells via amine coupling following the manufacturer's instructions. The flow rate was 25 μL/minute at 25°C using 10 mM HEPES buffer (pH 7.4) with 0.15 M NaCl, 3 mM EDTA, and 0.005% (vol/vol) surfactant P20. Chip regeneration was done with 10 mM glycin buffer (pH 2.5). Sample concentrations from 6.6 nM to 33 nM were loaded with an association time of 8 minutes. Curve fitting was done using a bivalent binding model, because both analyte and ligand are homodimeric.
Flow cytometry.
A total of 106 CD30+ Karpas-299 and CD30− HD-MY-Z cells were incubated for 30 minutes on ice with 1 μg/mL αCD30scFv-Fc or αCD30scFv-Fc-RNase. Appropriate controls were included using chimeric 215-IgG1 recognizing the RNA-polymerase of Drosphila melanogaster for negative control and murine αCD30 IgG1 (Chemicon, Temecula, CA) for positive control. After washing with PBS (pH 7.4, 2% [wt/vol] BSA), cells were incubated for 30 minutes on ice with FITC-conjugated anti-human IgG (Fc-specific) or anti-mouse IgG (Fc-specific) antibodies (Sigma) for detection. After washing again 3 times, analysis of the cells was performed using the FC500 flow cytometer (Beckman Coulter, Fullerton, CA).
Internalization of TR molecules
For internalization studies, CD30+ Karpas-299 lymphoma cells were incubated in 24-well plates with 5 × 105 cells/well in 250 μL RPMI, CD30− HD-MY-Z cells were grown on coverslips (> 50% confluence). A total of 25 μg/mL scFv-Fc-RNase was added and incubated for 4 hours at 37°C or 4°C. Cells were then fixed with paraformaldehyde and permeabilized with 0.2% (wt/vol) Triton X-100 (Sigma). Internalized antibody fusion proteins were detected using FITC-labeled goat anti-human IgG (Fc-specific) antibody (Sigma). Nucleus staining was done with 1 μg/mL DAPI reagent (Pierce). Internalization was analyzed by confocal laser-scanning microscopy using a CLSM-510META microscope and the LSM imaging software (Zeiss, Göttingen, Germany)
In vitro cytotoxicity of TR
Target cells (CD30+ Karpas-299 cells or CD30− HD-MY-Z control cells) were seeded at 104 cells/well into flat-bottom 96-well plates using culture medium as described. Various concentrations of αCD30-scFv-Fc-RNase or αCD30-scFv-Fc ranging from 0 to 100 nM were added. All samples were performed as triplicates and were incubated for 72 hours at 37°C in 5% CO2 atmosphere. Inhibition of tumor cell growth was measured by counting viable cells after the incubation period.
Serum stability of scFv-Fc and scFv-Fc-Rnase
A total of 5 μg scFv-Fc and scFv-Fc-RNase were diluted in 100 μL PBS containing 10% (vol/vol) heat-inactivated FCS or pooled human AB serum (HS; both sera were obtained from PAA) and incubated at 4°C or 37°C for 1, 2, and 5 days. After incubation, 20 μL of each sample were used for flow cytometry of CD30+ Karpas 299 and CD30− HD-MY-Z cells, respectively. Cells stained by the secondary antibody were used as negative control. For positive controls, scFv-Fc and scFv-Fc-RNase stock solution from the protein preparation stored in PBS at 4°C were used. Samples incubated with FCS were also analyzed by SDS-PAGE and immunoblot using AP-conjugated goat anti-human IgG (Fc-specific) antibody (Dianova, Hamburg, Germany).
Results
Generation and production of CD30-specific antibody and TR variants
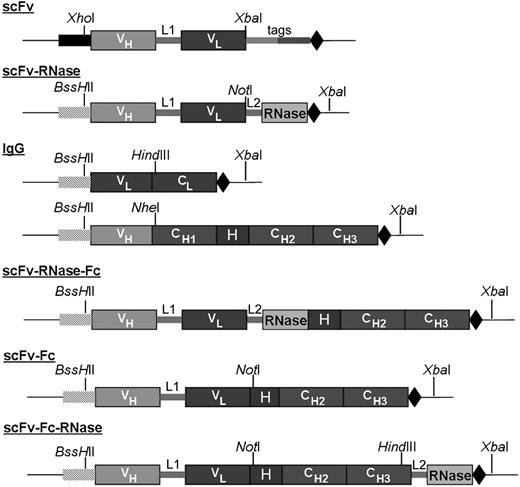
As binding moiety for all constructs, a phage display–derived human CD30-specific scFv fragment was used in which the variable regions of heavy (VH) and light chain (VL) are joined via a 15–amino acid residue linker (Gly4-Ser)3. Based on this scFv fragment, several human antibody formats (scFv, scFv-Fc, and IgG) as well as antibody fusion proteins to human pancreatic RNase (scFv-RNase, scFv-Fc-RNase and scFv-RNase-Fc) were generated (Figure 1). Briefly, the coding region of hinge, CH2, and CH3 of a human IgG1 heavy chain was fused to the C-terminus of the scFv fragment to obtain the scFv-Fc fusion protein. The VH and VL domains of the scFv were fused to the constant regions of human IgG1 heavy and κ light chains, respectively, to generate the IgG1 format. Human pancreatic RNase was fused to the carboxy terminus of the scFv to generate scFv-RNase, and to the scFv-Fc to generate scFv-Fc-RNase, using a 6–amino acid residue peptide linker (AAASSG) as spacer for better flexibility and accessibility of the enzymatic site of the RNase.39 For constructing scFv-RNase-Fc, scFv-RNase was fused to the human IgG1-Fc part. While scFv is a monomeric antibody format with monovalent antigen-binding properties, scFv-Fc and IgG comprise 2 antigen recognition sites that allow bivalent binding.
Schematic setup of different CD30-specific antibody and huTR constructs generated. Signal peptides for yeast (■) and mammalian expression (▒). VH and VL indicate the variable domains of the CD30-specific scFv antibody fragment; CL and CH, constant immunoglobulin domains; H, IgG1 hinge region; L1, (Gly4-Ser)3 linker between VH and VL of the scFv fragment; L2, 6–amino acid linker AAASSG for antibody RNase fusion; tags, tags for purification and detection; and stop codon (◆). Indicated restriction sites were used for cloning into expression vectors and allow modular exchangeability of huTR elements. Constructs are not drawn to scale.
Schematic setup of different CD30-specific antibody and huTR constructs generated. Signal peptides for yeast (■) and mammalian expression (▒). VH and VL indicate the variable domains of the CD30-specific scFv antibody fragment; CL and CH, constant immunoglobulin domains; H, IgG1 hinge region; L1, (Gly4-Ser)3 linker between VH and VL of the scFv fragment; L2, 6–amino acid linker AAASSG for antibody RNase fusion; tags, tags for purification and detection; and stop codon (◆). Indicated restriction sites were used for cloning into expression vectors and allow modular exchangeability of huTR elements. Constructs are not drawn to scale.
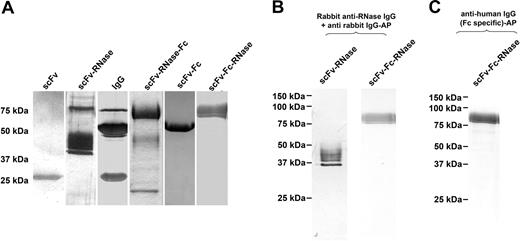
With the exception of αCD30scFv, all CD30-specific antibodies and huTR constructs were transiently expressed in HEK293T cells and purified to homogeneity by protein A affinity chromatography. The αCD30-scFv was expressed in P pastoris and purified by protein L affinity chromatography. SDS-PAGE analysis using purified CD30-specific antibody and derived huTR variants under reducing conditions confirmed purity and protein bands at the expected molecular weight (Figure 2A). In both silver staining and Western blot analysis, no major degradation products or impurities were detected. Minor impurities were found in IgG and scFv-RNase preparation samples, most likely due to BSA from the culture medium. A possible degradation product was detected in the scFv-RNase-Fc sample at 50 kDa and below 25 kDa. Western blot analysis using an RNase or an Fc-specific antibody for detection verified that the 75-kDa band found for scFv-Fc-RNase represents the complete huTR construct (Figure 2B,C). All RNase-containing constructs show one sharp band with some material of slightly higher apparent molecular mass, presumably representing differentially glycosylated forms of the protein.
SDS-PAGE analysis of αCD30 antibody and huTR variants after purification. SDS-PAGE analysis of αCD30 antibody variants and huTR after protein L (scFv, scFv-RNase) or protein A purification. SDS-PAGE was performed under reducing conditions followed by silver staining (A) or Western blot analysis using RNase specific polyclonal rabbit serum and anti-rabbit IgG AP conjugate (B) or using human Fc-specific IgG AP conjugate (C) for detection.
SDS-PAGE analysis of αCD30 antibody and huTR variants after purification. SDS-PAGE analysis of αCD30 antibody variants and huTR after protein L (scFv, scFv-RNase) or protein A purification. SDS-PAGE was performed under reducing conditions followed by silver staining (A) or Western blot analysis using RNase specific polyclonal rabbit serum and anti-rabbit IgG AP conjugate (B) or using human Fc-specific IgG AP conjugate (C) for detection.
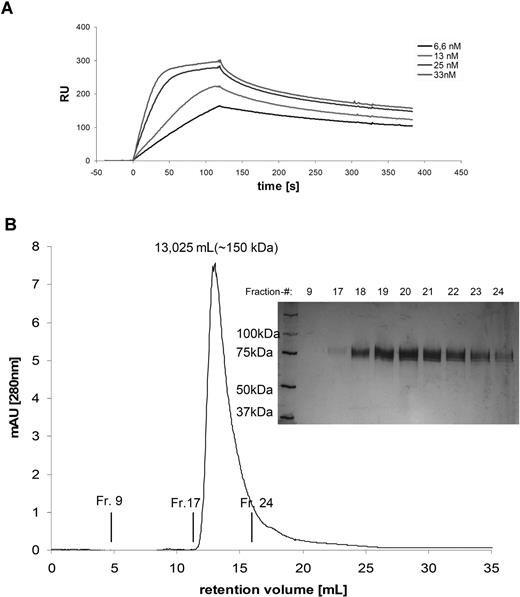
As some scFv fragments were shown to dimerise or aggregate, the molecular mass of the soluble native proteins was investigated by calibrated size exclusion chromatography (data not shown). The scFv-Fc-RNase molecules were present as homodimers with a molecular mass of approximately 150 kDa (Figure 3B). No multimeric variants or aggregates were detected for any of the constructs. Aggregation of scFv-Fc-RNase proteins did not occur, even after storage of purified TR samples for 4 weeks at 4°C (data not shown). The total yield after purification obtained from transient expression of the αCD30–scFv-Fc-RNase protein in HEK293T cells was 4 mg/L. Expression levels of the other variants were in a similar range, yielding from 2 to 4 mg/L. Further properties of the antibody and huTR constructs are summarized in Table 1.
Biochemical properties of scFv-Fc-Rnase. (A) Plasmon resonance. Overlay plot of various concentrations (scFv-Fc-RNase, 6.6–33 nM) were applied on CM5 chip with immobilized recombinant CD30-Fc. Samples volumes of 50 μL were injected at a flow rate of 25 μL/minute. Control flow cell was immobilized with lysozyme to observe unspecific protein protein interaction. (B) Size exclusion chromatography on a calibrated Superdex-200. The single peak at 13.03 mL corresponds to a molecular mass of 150 kDa. Fractionated samples were analyzed by SDS-PAGE (10%; reducing conditions, silver staining).
Biochemical properties of scFv-Fc-Rnase. (A) Plasmon resonance. Overlay plot of various concentrations (scFv-Fc-RNase, 6.6–33 nM) were applied on CM5 chip with immobilized recombinant CD30-Fc. Samples volumes of 50 μL were injected at a flow rate of 25 μL/minute. Control flow cell was immobilized with lysozyme to observe unspecific protein protein interaction. (B) Size exclusion chromatography on a calibrated Superdex-200. The single peak at 13.03 mL corresponds to a molecular mass of 150 kDa. Fractionated samples were analyzed by SDS-PAGE (10%; reducing conditions, silver staining).
Determination of binding kinetics (kon, koff, KD) by SPR
The antigen-binding properties of TR are crucial for their in vitro and in vivo activity. In order to select the most suitable antibody format for the huTR constructs, binding of each of the purified antibody and TR formats to immobilized soluble CD30-Fc was analyzed by measuring the binding kinetics via plasmon resonance (Table 1). No unspecific binding to immobilized control antigen (hen egg lysozyme) was detectable, and mass transfer limitations were not present at the flow rate of 25 μL/minute. Due to high dissociation rates, affinity of the monovalent scFv and surprisingly also the bivalent IgG1 format had to be determined at equilibrium state. KD values of IgG and scFv variants were between 3- and 85-fold worse compared with the scFv-Fc, which had an affinity of 6.7 × 10−9 M. Consequently, IgG1 was ruled out as antibody moiety for the TR setup due to superior binding properties of the scFv-Fc format. The affinity of the scFv-Fc–based TR proteins to CD30 was found to be in the low nanomolar to high picomolar range (scFv-RNase-Fc, 7.43 × 10−10 M; scFv-Fc-RNase, 9.81 × 10−10 M; Figure 3A).
Binding to native CD30 on lymphoma cell lines
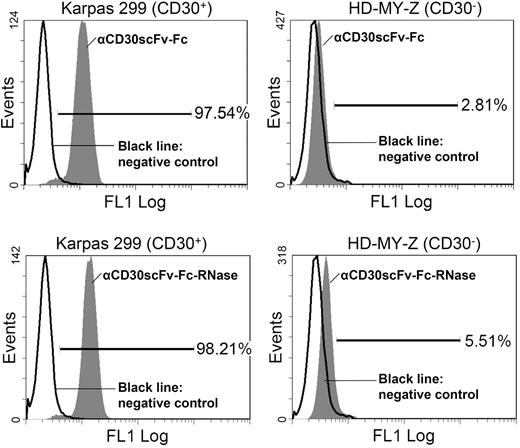
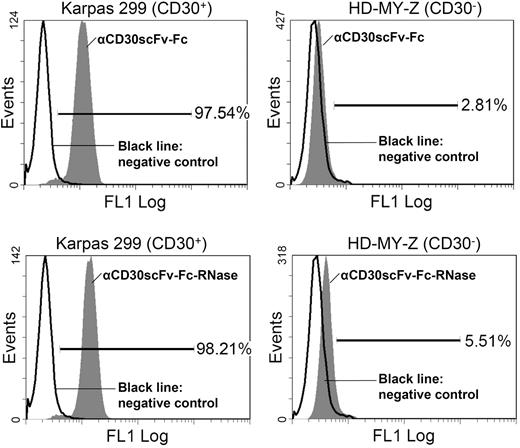
In order to mediate cytotoxicity, huTR derivatives must bind to the native antigen on tumor cells; in addition, they must be internalized and released into the cytoplasm of the target cells. We used flow cytometry to analyze binding of the remaining huTR formats αCD30scFv-RNase and αCD30scFv-Fc-RNase as well as the respective unfused antibody moieties αCD30scFv and αCD30scFv-Fc to CD30 on the surface of CD30+ Karpas-299 lymphoma cells. CD30− HD-MY-Z lymphoma cells were used as negative control. Neither αCD30scFv nor αCD30scFv-RNase revealed significant binding to CD30+ Karpas-299 cells (data not shown). Consequently, the monovalent format was not further considered for the huTR setup. In contrast, αCD30scFv-Fc as well as its huTR derivative αCD30scFv-Fc-RNase showed more than 97% binding to CD30+ Karpas-299 cells, but only a negligible binding to CD30- HD-MY-Z cells (huTR, 5.5%; αCD30scFv-Fc, 2.8%; Figure 4).
Flow cytometric analysis of binding to CD30 on lymphoma cells. Top histograms show αCD30scFv-Fc ( ) staining of CD30+ Karpas cells (left) and of CD30− HD-MY-Z cells (right). Bottom histograms show TR (
) staining of CD30+ Karpas cells (left) and of CD30− HD-MY-Z cells (right). Bottom histograms show TR ( ) staining of CD30+ Karpas cells (left) and of CD30− HD-MY-Z cells (right). Bound αCD30scFv-Fc and huTR were detected with anti-human IgG (Fc-specific) antibody FITC conjugate. The chimeric 215-IgG served as negative control (black solid line).
) staining of CD30+ Karpas cells (left) and of CD30− HD-MY-Z cells (right). Bound αCD30scFv-Fc and huTR were detected with anti-human IgG (Fc-specific) antibody FITC conjugate. The chimeric 215-IgG served as negative control (black solid line).
Flow cytometric analysis of binding to CD30 on lymphoma cells. Top histograms show αCD30scFv-Fc ( ) staining of CD30+ Karpas cells (left) and of CD30− HD-MY-Z cells (right). Bottom histograms show TR (
) staining of CD30+ Karpas cells (left) and of CD30− HD-MY-Z cells (right). Bottom histograms show TR ( ) staining of CD30+ Karpas cells (left) and of CD30− HD-MY-Z cells (right). Bound αCD30scFv-Fc and huTR were detected with anti-human IgG (Fc-specific) antibody FITC conjugate. The chimeric 215-IgG served as negative control (black solid line).
) staining of CD30+ Karpas cells (left) and of CD30− HD-MY-Z cells (right). Bound αCD30scFv-Fc and huTR were detected with anti-human IgG (Fc-specific) antibody FITC conjugate. The chimeric 215-IgG served as negative control (black solid line).
Internalization of αCD30scFv-Fc-RNase by CD30+ Karpas-299 cells
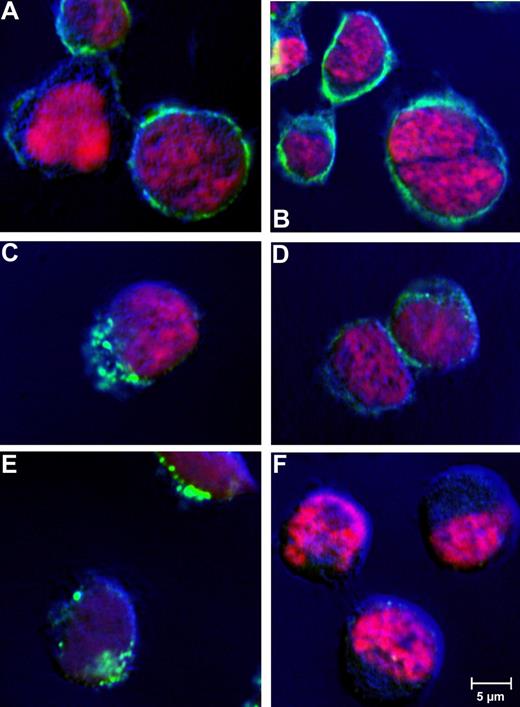
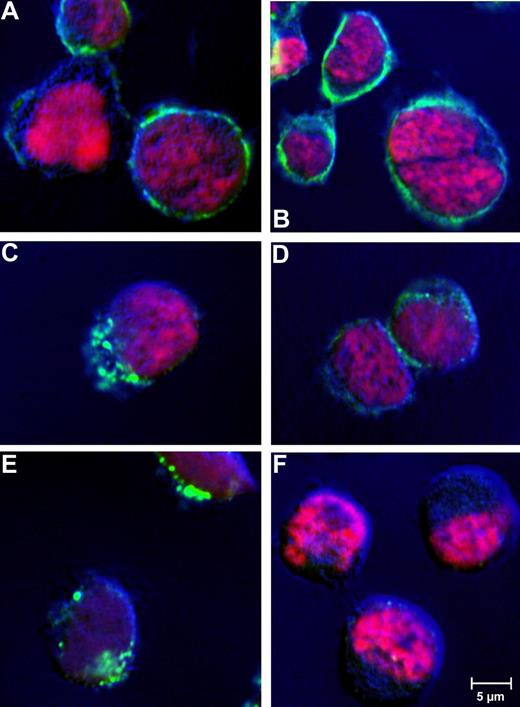
An earlier report on CD30 malignancies already indicated receptor-mediated endocytosis of antibody bound to CD30.40 To confirm cellular uptake of the huTR, CD30+ tumor cell lines were incubated with huTR (25 μg/mL) for 1 hour at 37°C or 4°C. Incubation at 4°C was done to evaluate dependency of internalization on metabolic activity, which is characteristic for receptor mediated endocytosis. The huTR was detected via fluorescently labeled antibody in confocal laser-scanning microscopy (Figure 5). Depending on the incubation temperature, different distribution patterns were found. After incubation at 4°C, the huTR staining was uniformly localized on the plasma membrane, which is particularly visible around the cell shape. On the other hand, cell samples incubated at 37°C showed intracellular staining with a pattern typical for a vesicular pathway after receptor mediated internalization. Controls with CD30− HD-MY-Z cells lacked any huTR uptake.
Receptor-mediated internalization of huTR by CD30+lymphoma cells. Receptor mediated internalization was analyzed by confocal laser-scanning microscopy. CD30+ Karpas 299 (A-E) or CD30− HD-MY-Z cells (F) were incubated with 25 μg/mL huTR (A,C,E,F) or anti-CD30 IgG (B,D) for 4 hours at 4°C (A,B) or 37°C (C-F). Goat anti-human IgG (Fc-specific) antibody FITC conjugate was used for detection (green). Cell nuclei were counter stained with DAPI (channel is shown in pink as false color). Laser-scanning microscopy was performed using a CLSM-510META with a Plan Neofluar 40×/1.3 oil DIC objective (Zeiss). The Argon laser 488-nm line was used for excitation of FITC and the UV laser with 364 nm was used for excitation of DAPI (beam splitter for UV/488 nm). Emitted light of FITC and DAPI was detected using the band pass filters 505 to 550 nm and 385 to 470 nm, respectively.
Receptor-mediated internalization of huTR by CD30+lymphoma cells. Receptor mediated internalization was analyzed by confocal laser-scanning microscopy. CD30+ Karpas 299 (A-E) or CD30− HD-MY-Z cells (F) were incubated with 25 μg/mL huTR (A,C,E,F) or anti-CD30 IgG (B,D) for 4 hours at 4°C (A,B) or 37°C (C-F). Goat anti-human IgG (Fc-specific) antibody FITC conjugate was used for detection (green). Cell nuclei were counter stained with DAPI (channel is shown in pink as false color). Laser-scanning microscopy was performed using a CLSM-510META with a Plan Neofluar 40×/1.3 oil DIC objective (Zeiss). The Argon laser 488-nm line was used for excitation of FITC and the UV laser with 364 nm was used for excitation of DAPI (beam splitter for UV/488 nm). Emitted light of FITC and DAPI was detected using the band pass filters 505 to 550 nm and 385 to 470 nm, respectively.
In vitro cytotoxic effects of huTR
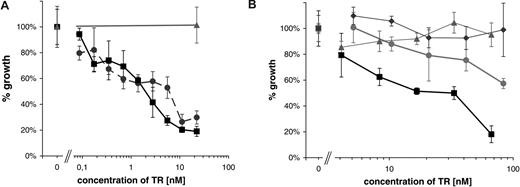
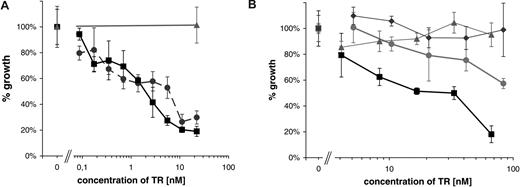
To assess the in vitro cytotoxicity of the αCD30scFv-Fc-RNase huTR construct, the CD30+ lymphoma cell line Karpas-299 and the CD30− cell line HD-MY-Z were incubated with increasing concentrations of purified huTR. After 72 hours, the number of living cells was determined using trypan blue staining to exclude dead cells. The huTR showed a dose-dependent impact on the viability of CD30+ lymphoma cells. At a huTR concentration of 60 nM, the number of viable cells decreased to 15% compared with the controls. No effect of huTR on the proliferation of CD30− HD-MY-Z cells was observed. To further control that the effect is dependent on the CD30 binding, a second, completely different human scFv fragment (2A1) to CD30 was used to construct an alternative variant of the huTR. Having similar biochemical properties and affinity after purification (data not shown), this variant inhibited the cell growth in a similar way (Figure 6A).
Growth inhibition by huTR. A total of 104 CD30+ Karpas 299 cells or CD30− HD-MY-Z cells per well were incubated with increasing amounts of the huTR or scFv-Fc. The number of viable cells was counted after 72 hours. Each data point represents triplicate cell counting of samples; error bars indicate standard deviation. (A) Two different variants of huTRs using either scFv 2A1 (•) or scFv 4E3 (■) were used. Negative control: huTR with 2A1 on CD30− cells (▴). (B) huTR with scFv 4E3 as targeting domain (■, ▴) or its scFv-Fc part without RNase moiety (•, ◆) were used on either CD30+ (■, •) or CD30− lymphoma cells (▴, ◆).
Growth inhibition by huTR. A total of 104 CD30+ Karpas 299 cells or CD30− HD-MY-Z cells per well were incubated with increasing amounts of the huTR or scFv-Fc. The number of viable cells was counted after 72 hours. Each data point represents triplicate cell counting of samples; error bars indicate standard deviation. (A) Two different variants of huTRs using either scFv 2A1 (•) or scFv 4E3 (■) were used. Negative control: huTR with 2A1 on CD30− cells (▴). (B) huTR with scFv 4E3 as targeting domain (■, ▴) or its scFv-Fc part without RNase moiety (•, ◆) were used on either CD30+ (■, •) or CD30− lymphoma cells (▴, ◆).
To demonstrate that the effect is due to the RNase moiety of the huTR and to assess a possible influence of CD30 binding alone on the cell proliferation, the scFv-Fc part of the huTR was produced and used as an equimolar control. Both reagents did not affect the growth of control CD30− HD-MY-Z cells. Incubation with αCD30scFv-Fc without RNase moiety showed only a slight effect on cell proliferation. However, the fusion protein with the RNase moiety showed a strong inhibitory effect on the growth of the CD30+ lymphoma cell line (Figure 6B), indicating a RNase-dependent cytotoxicity.
Stability of huTR
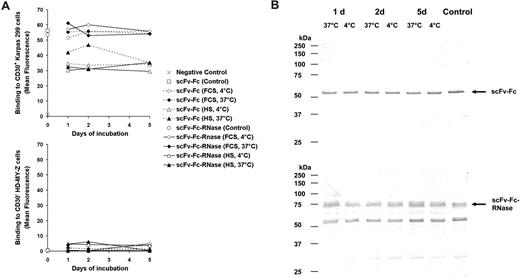
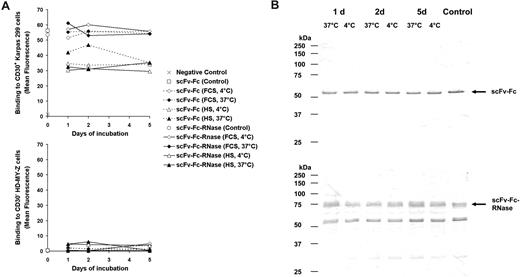
To assess the stability, the scFv-Fc-RNase and its antibody moiety scFv-Fc were incubated in 10% FCS or HS at 37°C or 4°C, respectively. After 5 days of incubation with serum, scFv-Fc and scFv-Fc-RNase still showed specific binding to CD30+ Hodgkin cells analyzed by flow cytometry indistinguishable from the controls (Figure 7A). Furthermore, scFv-Fc and scFv-Fc-RNase samples treated at 37°C for 5 days did not show increased degradation compared with the controls (Figure 7B).
Stability of scFv-Fc and scFv-Fc-RNase. (A) Samples of the CD30 specific scFv-Fc (dotted lines) and scFv-Fc-RNase proteins (solid lines) were incubated with 10% (vol/vol) FCS or HS at 37°C and 4°C for 1 to 5 days and used for flow cytometry analysis of CD30+ Karpas-299 cells and CD30− HD-MY-Z. Cells only stained with secondary antibody were used as negative control. A total of 1 μg scFv-Fc and scFv-Fc-RNase of the protein preparation stored in PBS at 4°C were used as positive controls. Mean fluorescence values are shown. (B) Samples of scFv-Fc (top blot) and scFv-Fc-RNase (bottom blot) incubated for 1 to 5 days with 10% (vol/vol) FCS were also analyzed by SDS-PAGE, Western blot, and immunostaining using an AP-conjugated goat anti-human IgG (Fc-specific) antibody.
Stability of scFv-Fc and scFv-Fc-RNase. (A) Samples of the CD30 specific scFv-Fc (dotted lines) and scFv-Fc-RNase proteins (solid lines) were incubated with 10% (vol/vol) FCS or HS at 37°C and 4°C for 1 to 5 days and used for flow cytometry analysis of CD30+ Karpas-299 cells and CD30− HD-MY-Z. Cells only stained with secondary antibody were used as negative control. A total of 1 μg scFv-Fc and scFv-Fc-RNase of the protein preparation stored in PBS at 4°C were used as positive controls. Mean fluorescence values are shown. (B) Samples of scFv-Fc (top blot) and scFv-Fc-RNase (bottom blot) incubated for 1 to 5 days with 10% (vol/vol) FCS were also analyzed by SDS-PAGE, Western blot, and immunostaining using an AP-conjugated goat anti-human IgG (Fc-specific) antibody.
Discussion
Immunoenzymes combining the specificity of antibodies with enzymes catalyzing cytotoxic reactions are promising for the immunotherapy of tumor diseases.41,42 However, therapeutic approaches with recombinant immunotoxins using plant or bacterial toxins suffer from severe side effects caused by high immunogenicity and nonspecific cytotoxicity.43,44 RNases do not mediate these side effects, but nevertheless are highly cytotoxic if they can find their way into the cytoplasm of a target cell. Several RNases can directly mediate antitumor effects, but most of them require dimerization or at least modifications that prevent binding of the cytosolic RNase inhibitor. The fusion of RNases with tumor-specific antibodies or other tumor-binding ligands resulted in enhanced tumor targeting of RNases.6,9
In this study we generated a novel, completely human TR construct with specificity toward CD30+ lymphoma cells. As an effector RNase, we used human pancreatic RNase, which has been proven to kill tumor cells after being internalized via CD71. In that study, the RNase-scFv fusion protein was very difficult to produce, it required tedious refolding from E coli inclusion bodies, and yields of functional molecules were very poor. Second, the CD71 target, being responsible for the transferrin internalization and thus iron supply, is not a good prototype for a tumor target, as it is present in high copy numbers on the surface and has a very high internalization rate. To cure these problems, we generated various variants binding to the lymphatic tumor antigen CD30 and compared them in respect to (1) specificity and sufficient affinity for CD30 antigen on CD30+ lymphoma cells, (2) receptor-mediated internalization, and (3) specific cytotoxicity to CD30+ tumor cell lines.
Interestingly, the kinetics measurement by SPR indicated that by conversion into the IgG format no major decrease of the dissociation rate due to the fact that expected bivalent binding was obtained compared with the monovalent scFv format. In contrast, the bivalent scFv-Fc format showed a significantly slower dissociation rate, resulting in an approximately 85-fold increase of affinity/avidity (to a KD of 6 nM) compared with the monovalent scFv format. According to these results, the conversion of the scFv fragment into the IgG format probably led to an improper conformational arrangement of the V regions, resulting in a loss of affinity that could not be compensated by avidity. Therefore, the IgG format was excluded from further testing as a TR construct. Of the other various constructs we tried, only the scFv-RNase and scFv-Fc-RNase constructs with C-terminally fused RNase showed RNase activity in vitro, while embedding the human pancreatic RNase between scFv and Fc in the scFv-RNase-Fc construct completely abolished its enzymatic activity (data not shown). Furthermore, the scFv-Fc-RNase is stable in serum at 37°C.
An affinity of 0.6 nM has been described for the human CD30-specific mAb 5F11 (MDX-060), which is derived from the human mAb (HuMAb) mouse.45 Both bivalent huTR constructs using phage display–derived antibody fragments from our study showed a similar apparent affinity of 0.8 to 1 nM. Interestingly, the addition of the RNase domain improved the affinity by a factor of 7 to 8. This effect cannot result from additional multimerization as it should have been observed in size exclusion chromatography. However, dimeric RNase reported to have improved tumor binding by itself.46,47
In contrast to the insufficient binding of the monovalent scFv-RNase, both the scFv-Fc and scFv-Fc-RNase proteins showed strong specific binding to CD30+ Karpas-299 cells. Interestingly, the binding was slightly increased for RNase fusion protein, also on a CD30− lymphoma cell line, which could be due to the intrinsic binding capacity of the RNase domain to tumor cell lines.46,47
Internalization experiments demonstrated the uptake of the scFv-Fc-RNase by CD30+ Karpas-299 cells at 37°C, whereas at 4°C, the huTR molecules remained at the cell surface. The distribution pattern was typical for receptor-mediated endocytosis.48,49 After endocytosis, the translocation into the cytosol necessary for cytotoxic activity most likely occurs from the endosome.11
The αCD30–scFv-Fc-RNase construct had a profound inhibitory effect on the growth of CD30+ Karpas-299 cells, with an IC50 of 3.3 nM. Targeting of a CD30 ligand angiogenin fusion protein on the CD30+ Hodgkin-derived cell line L540 comparably affected proliferation.50 Inhibitory effects on proliferation upon antibody binding to CD30 on Karpas-299, which was reported for other CD30-specific mAbs, can be excluded for the human antibody used in this study.25,51 No effects on viability of the CD30+ ALCL cells and no impact on cell proliferation in vitro was found upon incubation with scFv-Fc. Furthermore, no influence on the proliferation of CD30− HD-MY-Z cells was detectable in the presence of either scFv-Fc or its derived huTR. Thus, cytotoxic activity is proven to be target specific and to be caused by the RNase moiety of the huTR molecules.
De Lorenzo and colleagues described a fully human anti–ErbB-2 scFv-RNase showing specific binding and cytotoxicity to ErbB-2+ epithelial tumor cell lines in a low nanomolar range.52 However, sensitivity of this construct to the cytosolic ribonuclease inhibitor was apparent, but could be neutralized by entering the tumor cells in access.11
In this study, we found that the scFv-Fc-RNase format was most favorable for the construction of an entirely human CD30-specific TR, allowing production of significant amounts in mammalian cell culture. The format can be used as a prototype to exploit the TR concept to treat other cancer types by exchanging the antigen-binding domain for antibodies targeting different internalizing tumor markers.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Dr Hänsch for his advice on confocal microscopy and to Mrs Menzel as well as Dr Hust for reading the manuscript.
This work was supported by the German ministry of research (BMBF) with the SMP Antibody Factory grant of the National Genome Research Network (NGFN).
Authorship
Contribution: C.M., T.S., Z.K., and T.J. performed research and analyzed data; T.S., T.J., S.D., and C.M. designed the research and wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan Dübel, Technical University of Braunschweig, Institute of Biochemistry and Biotechnology, Spielmannstrasse 7, D-38106 Braunschweig, Germany; e-mail: s.duebel@tu-bs.de.
References
Author notes
C.M. and T.S. are dual first authors.
T.J. and S.D. contributed equally to this paper.





 ) staining of CD30+ Karpas cells (left) and of CD30− HD-MY-Z cells (right). Bottom histograms show TR (
) staining of CD30+ Karpas cells (left) and of CD30− HD-MY-Z cells (right). Bottom histograms show TR (