Abstract
Senicapoc, a novel Gardos channel inhibitor, limits solute and water loss, thereby preserving sickle red blood cell (RBC) hydration. Because hemoglobin S polymerization is profoundly influenced by intracellular hemoglobin concentration, senicapoc could improve sickle RBC survival. In a 12-week, multicenter, phase 2, randomized, double-blind, dose-finding study, we evaluated senicapoc's safety and its effect on hemoglobin level and markers of RBC hemolysis in sickle cell anemia patients. The patients were randomized into 3 treatment arms: placebo; low-dose (6 mg/day) senicapoc; and high-dose (10 mg/day) senicapoc. For the primary efficacy end point (change in hemoglobin level from baseline), the mean response to high-dose senicapoc treatment exceeded placebo (6.8 g/L [0.68 g/dL] vs 0.1 g/L [0.01 g/dL], P < .001). Treatment with high-dose senicapoc also produced significant decreases in such secondary end points as percentage of dense RBCs (−2.41 vs −0.08, P < .001); reticulocytes (−4.12 vs −0.46, P < .001); lactate dehydrogenase (−121 U/L vs −15 U/L, P = .002); and indirect bilirubin (−1.18 mg/dL vs 0.12 mg/dL, P < .001). Finally, senicapoc was safe and well tolerated. The increased hemoglobin concentration and concomitant decrease in the total number of reticulocytes and various markers of RBC destruction following senicapoc administration suggests a possible increase in the survival of sickle RBCs. This study is registered at http://clinicaltrials.gov as NCT00040677.
Introduction
Polymerization of hemoglobin S is the central event in the pathophysiology of sickle cell disease (SCD).1 Because the rate and extent of polymer formation in red blood cells (RBCs) from patients with SCD (ie, sickle erythrocytes) is profoundly influenced by the intracellular concentration of hemoglobin S, the hydration state of these cells is a critical factor in hemoglobin S polymer formation.1-3
Dehydration of sickle erythrocytes, which follows the loss of solute and osmotically obliged water, appears to result from increased efflux of potassium via 2 specific pathways: the potassium-chloride cotransport pathway and the calcium-activated potassium efflux (or Gardos) channel. The Gardos channel, which becomes activated following a rise in cytosolic-free calcium, contributes to the potassium loss from sickle erythrocytes.4,5 For this reason, blockade of the Gardos channel could have a beneficial effect on the pathophysiology of SCD.
Senicapoc (ICA-17043; bis(4-fluorophenyl)phenyl acetamide), a selective, highly potent Gardos channel blocker (IC50 = 11 nM), specifically inhibits the efflux of potassium from the RBCs of both SCD transgenic mice and human sickle cell disease patients.6,7 Senicapoc is well tolerated by both healthy volunteers and patients with SCD and has favorable pharmacokinetics, with a long half-life permitting once-daily dosing.7 The study goals in this 12-week phase 2 study were to assess the effect of senicapoc on total hemoglobin levels and markers of RBC destruction, to obtain additional safety data, and to identify the most appropriate dose for a pivotal phase 3 study.
Methods
Patients
Enrollment took place at 19 medical centers in the United States. Eligible patients were adults (ages 18-60 years) with sickle cell anemia (Hb SS) and at least one prior acute sickle-related painful episode (commonly referred to as painful crisis) that had required hospitalization, but none in the 4 weeks prior to screening. Each female patient was using an adequate method of birth control or was unable to bear children. Patients on hydroxyurea were required to be on a stable dose for a minimum of 3 months at study enrollment.
Patients were excluded if they had (1) a total hemoglobin level less than 40 g/L (4 g/dL) or more than 100 g/L (10 g/dL); (2) received a transfusion within 30 days of enrollment; (3) undergone an exchange transfusion within 60 days of enrollment; (4) a hemoglobin A level of more than 10% at screening; (5) any condition that might affect the absorption (including such malabsorption states as celiac sprue, Whipple disease, short bowel syndrome, etc), distribution, metabolism, or excretion of senicapoc; (6) known hepatitis B or HIV infection; (7) a positive test for illicit drugs at screening; (8) experienced an acute illness within 5 days of study initiation; (9) a diagnosis of cancer (except nonmelanoma skin cancer) within the past 5 years; or (10) taken one or more nonallowed medications within 30 days of enrollment (eg, amiodarone, chlorperazine, disopyramide, dofedilide, haloperidol, procainamide, quinidine, risperidone, sotalol, thioridazine, trifluoperazine, warfarin sodium, and erythropoietin). The Institutional Review Board at each site (see Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) approved the protocol, and all patients provided written informed consent in accordance with the Declaration of Helsinki.
Study design and treatment schedule
This randomized, double-blind, placebo-controlled study consisted of a screening phase and a 12-week treatment phase. Patients who met eligibility criteria were randomized, within 30 days of screening, to 1 of 3 treatment arms: (1) high dose: a single 150-mg senicapoc loading dose followed by 10-mg daily maintenance dose; (2) low dose: a single 100-mg senicapoc loading dose followed by 6-mg daily maintenance dose; or (3) placebo. Treatment assignment and validation of eligibility relied on a centralized randomization protocol using an integrated voice response system. The randomization was stratified to ensure that treatment assignments would be balanced with respect to concomitant use of hydroxyurea by some patients.
The treatment phase began on study day 1 with baseline measurements for efficacy, safety, pharmacokinetics, and pharmacodynamics (Gardos channel inhibition), followed by oral administration of a loading dose of the study drug. Each patient received a daily maintenance dose of the study drug on days 2 to 84. All patients were instructed to ingest the maintenance dose each morning before breakfast. The same efficacy, safety, pharmacokinetic, and pharmacodynamic assessments were obtained at the end of week 1 and then every 2 weeks until completion of the treatment phase. At the end of the treatment phase, each patient was offered access to active drug through enrollment into an open-label extension study. All patients who declined this option returned for a final study visit 8 weeks after completion of the treatment phase for a final assessment of safety.
End points
The primary efficacy end point was change in blood hemoglobin level from baseline to end of study. “Baseline” hemoglobin level was defined as the average of 2 measurements, one performed at screening and one obtained on day 1 just before study medication was dispensed. “End of the study” hemoglobin level was defined to be the average of the 2 measurements obtained at weeks 10 and 12. For those patients who dropped out early, the last available hemoglobin value was used as the imputed value for the end-of-study value.
Secondary efficacy end points included laboratory markers of hemolysis (indirect bilirubin and lactate dehydrogenase), number and proportion of reticulocytes and dense RBCs (defined as the percentage of RBCs with hemoglobin concentration > 410 g/L [41 g/dL]), changes in RBC count and indices, hematocrit level, and frequency of painful crises. To ensure the quality and consistency of the primary and secondary end point data, all clinical and hematologic laboratory samples were sent to a central laboratory (ICON Central Laboratories, Farmingdale, NY) for analysis.
An independent, blinded crisis review committee adjudicated all sickle cell painful crises and related adverse event data (Document S1). A painful crisis was defined as a period of severe pain (with no explanation other than SCD) lasting 4 or more hours in duration, requiring a visit to a health care facility, and requiring parenteral opiate or other narcotic for relief. The Safety Committee, which reviewed both unblinded patient safety data and overall study progress (Document S2), comprised 2 hematologists with expertise in SCD, a cardiologist, a clinical pharmacologist, and a biostatistician.
Pharmacokinetics
During the treatment period, blood samples were collected immediately before and following administration of study drug to measure senicapoc plasma concentrations at the following time points: before dose on day 1, and study days 8, 15, 29, 43, 57, 71, and 85. Senicapoc levels were measured using a high-performance liquid chromatography/mass spectrometric assay developed and validated by MDS Pharma Services (Montreal, QC).
Pharmacodynamics
Blood samples were obtained to measure erythrocyte Gardos channel activity before and after administration of study drug at the following time points: screening visit, before dose on day 1, and on days 8, 15, 29, 43, 57, 71, and 85. Erythrocyte Gardos channel blockade by senicapoc was measured at the laboratories of Icagen. Flux through the Gardos channel was activated by exposing RBCs to calcium and a calcium ionophore, and then measuring the rate of 86Rb+ movement.6,8
Statistical analysis
The choice of the target sample size of 90 patients was guided by considerations of the expected statistical precision, expected power of the test procedure for the primary analysis of efficacy, feasibility, cost, and the aims of the study. This was not a sequential study, and there were no interim analyses. For planning purposes, a 10-g/L (1.0-g/dL) increase in hemoglobin level was considered clinically meaningful. It was anticipated that the stratum of patients receiving concomitant hydroxyurea (up to 8 of the 30 patients in each arm) might not exhibit a benefit from senicapoc, and, therefore, the mean change in hemoglobin level for the high-dose arm in this study was targeted to be 7.5 g/L (0.75 g/dL) higher than placebo. For N = 90 and any true 7.5-g/L (0.75-g/dL) treatment difference in hemoglobin level change from baseline, the power estimate and its 95% confidence interval was 0.90 (0.84-0.93). This and similar expectations of power were based on an estimate of standard deviation (SD) of 8.5 g/L (0.85 g/dL) obtained from the data of 299 participants in the Multicenter Study of Hydroxyurea Trial9 kindly provided by the investigators of that study.
The primary analysis of relative efficacy focused on improvement (increase) in total hemoglobin level from baseline to “end of the study,” and relied on a 2-sided test (α = .05) of the null hypothesis of no difference between high-dose senicapoc and placebo. To control the experiment-wise type I error rate under monotonicity assumptions, statistical significance for this test was a prerequisite for statistical significance of pair-wise comparisons of high-dose versus low-dose and low-dose versus placebo.10 This testing procedure was also applied to the secondary measures of efficacy. Further auxiliary analyses of the primary and secondary efficacy variables relied on analysis of covariance models conditional on baseline hemoglobin level and hydroxyurea therapy as covariates. Hypothesis tests for interaction of treatment with concomitant hydroxyurea therapy were performed.
The analysis of efficacy was applied only to the modified intent-to-treat (ITT) population, which consisted of all patients who received at least one week of study drug and completed their initial efficacy assessments (day 8). The secondary analyses for painful crises used Mantel-Haenszel mean score chi-square tests, with hydroxyurea as a stratification factor on the outcome. The analyses of safety relied on listings and simple descriptive statistical methods.
Results
Patients characteristics
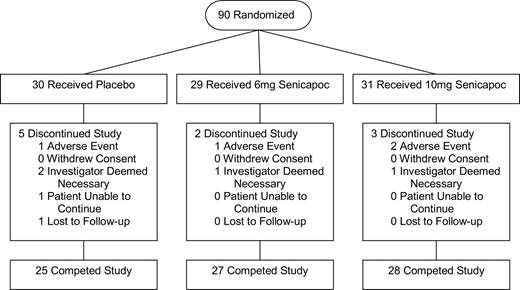
Ninety patients with sickle cell anemia were enrolled in the study between February 2002 and January 2004. Thirty-one were randomly assigned to the high-dose arm (senicapoc, 10 mg/day); 29, to the low-dose arm (senicapoc, 6 mg/day); and 30, to placebo (Figure 1). The 24 patients receiving concomitant hydroxyurea therapy were equally distributed among the 3 treatment arms. Eighty patients completed the full 12-week treatment period and 10 patients withdrew prematurely (Figure 1). Baseline characteristics of study patients are shown in Table 1.
Efficacy
Eighty-eight of 90 patients were suitable for evaluation based on the modified ITT population. Two patients dropped out before the first efficacy assessment was completed. Senicapoc treatment was associated with dose-dependent increases in hemoglobin level, the primary end point (Table 2; Figure 2A). Compared with those in the placebo arm, patients in the high-dose arm exhibited an increase in hemoglobin level of 6.8 g/L (0.68 g/dL) versus 0.1 g/L (0.01 g/dL; P < .001). A corresponding increase in hematocrit level and RBC count was also observed in patients taking senicapoc compared with placebo (Table 2). When the low-dose arm was compared with placebo, the change in hemoglobin level from baseline was smaller, 2.8 g/L (0.28 g/dL), and the difference was not statistically significant.
Time course of senicapoc plasma concentrations and pharmacodynamic response. (A) Mean changes in hemoglobin level during the 12-week treatment period are plotted versus time for the 3 treatment groups. Mean hemoglobin level changes (± SE) were calculated from baseline hemoglobin values (average of screening and day-1 [before dose] samples). (B) Mean plasma concentrations of senicapoc during the 12-week treatment period are plotted versus time in study patients. Senicapoc levels were expressed as mean values (± SE). Measurements were obtained at day 1 (before dose) and then at the indicated time points during the course of the study for those patients assigned to 1 of the 2 active treatment arms. (C) Effect of senicapoc on the rate of 86Rb+ flux across the RBC membrane. RBCs were obtained from each of the treatment groups (placebo, 6 mg, 10 mg). Measurements of 86Rb+ flux due to activation by intracellular calcium were obtained at baseline (average of screening and day 1 [before dose] samples) and then at the indicated time points during the course of the study. Treatment groups are plotted in comparison with whole blood samples spiked with 10 μM senicapoc.
Time course of senicapoc plasma concentrations and pharmacodynamic response. (A) Mean changes in hemoglobin level during the 12-week treatment period are plotted versus time for the 3 treatment groups. Mean hemoglobin level changes (± SE) were calculated from baseline hemoglobin values (average of screening and day-1 [before dose] samples). (B) Mean plasma concentrations of senicapoc during the 12-week treatment period are plotted versus time in study patients. Senicapoc levels were expressed as mean values (± SE). Measurements were obtained at day 1 (before dose) and then at the indicated time points during the course of the study for those patients assigned to 1 of the 2 active treatment arms. (C) Effect of senicapoc on the rate of 86Rb+ flux across the RBC membrane. RBCs were obtained from each of the treatment groups (placebo, 6 mg, 10 mg). Measurements of 86Rb+ flux due to activation by intracellular calcium were obtained at baseline (average of screening and day 1 [before dose] samples) and then at the indicated time points during the course of the study. Treatment groups are plotted in comparison with whole blood samples spiked with 10 μM senicapoc.
At the high-dose senicapoc arm, the stratum of patients who were not receiving hydroxyurea exhibited a statistically significant increase in hemoglobin level compared with placebo that was of comparable magnitude with that in the overall high-dose arm (5.8 g/L [0.58 g/dL] vs −.5 g/L [−0.05 g/dL], P = .004). For those patients taking concomitant hydroxyurea, the increase in hemoglobin level compared with placebo was also of similar magnitude (9.6 g/L [0.96 g/dL] vs 1.9 g/L [0.19 g/dL]). The test for hydroxyurea therapy-by-treatment interactions was not statistically significant at level α = .10.
The percentage and absolute number of reticulocytes and dense RBCs were lower on average when patients in both the high- and low-dose arms were compared with those on placebo (Table 2). Compared with those in the placebo arm, patients receiving high-dose senicapoc exhibited a decrease in percentage of reticulocytes of −4.12% versus −0.46% (P < .001) and a decrease in percentage of dense RBCs of −2.41% versus −0.08% (P < .001). Reductions in both lactate dehydrogenase and indirect bilirubin were also observed when both the high- and low-dose arms were compared with placebo (Table 2). Compared with those in the placebo arm, patients receiving high-dose senicapoc exhibited a decrease in lactate dehydrogenase of −121 U/L versus −15 U/L (P = .002) and a decrease in indirect bilirubin of − 20.18 μmol/L (−1.18 mg/dL) versus 2.05 μmol/L (0.12 mg/dL; P < .001).
The RBC indices, MCV and MCH, were decreased when patients in the high-dose arm were compared with placebo, while only the MCH showed a statistically significant decrease when the low-dose arm was compared with placebo (Table 2). No differences among the treatment regimens were detected in overall frequency of painful crises. However, approximately half of the patients experienced either no pain crises or only one crisis in the previous year.
Senicapoc plasma concentrations and Gardos channel inhibition
Plasma concentrations of senicapoc achieved following administration of the loading doses approached steady-state levels that were then maintained throughout the course of the study (Figure 2B). The mean plasma concentration of senicapoc over the 12-week treatment period appeared to be dose proportional: 56.0 ng/mL in the low-dose (6 mg) group and 100.8 ng/mL in the high-dose (10 mg) group. Finally, Gardos channel inhibition, as measured by the calcium-induced 86Rb+ flux across the RBC membrane, was seen for both the low- and high-dose groups and appeared to be dose dependent with a maximal reduction in this flux of approximately 70% at the highest dose (Figure 2C).
Safety
All 90 patients were included in the safety analysis. Ten patients were prematurely discontinued from the study, 5 of whom were in the placebo group. Three of the 10 patients dropped out due to adverse events: 1 in the low-dose treatment group for weakness and shortness of breath, and 2 in the high-dose treatment group for pain crises and acute chest syndrome (Figure 1). Excluding painful crises, the most common treatment-emergent adverse events are shown in Table 3. Diarrhea and nausea occurred more frequently in both active treatment groups than in the placebo group, and the incidence of these adverse events appeared to be dose related. However, all of the cases of diarrhea and nausea were rated as either mild or moderate in severity, and no patients dropped out of the study due to gastrointestinal events.
The most common serious adverse event was sickle cell crisis followed by pneumonia and acute chest syndrome, with a similar incidence across the active and placebo arms for each of these events (Table 4). None of these serious adverse events were thought attributable to senicapoc. Five patients on placebo experienced at least one serious adverse event during dosing, while 7 patients in the low-dose arm and 6 patients in the high-dose arm experienced at least one serious adverse event.
There were no notable changes in vital signs, physical examinations, ophthalmologic examinations, or electrocardiograms during the 12-week treatment period. Of particular importance, there were no differences in corrected QT interval (QTc) (based on either Bazett or Fredericia corrections of QT intervals for heart rate) when either of the senicapoc treatment groups was compared with the placebo group. The median γ-glutamyl transferase (GGT) value was increased in both the high-dose arm (10 U/L) and low-dose arm (2 U/L) compared with the placebo arm (−0.2 U/L). There were no notable changes in any other laboratory tests monitored during the study. Finally, no meaningful differences were noted between treatment groups for any adverse events for those patients who did not enter the open-label extension but returned for a final study visit 8 weeks after the conclusion of dosing with study medication.
Discussion
On the basis of our current understanding of the molecular pathogenesis of SCD, several independent treatment approaches have been proposed.1 To date, however, the only available cure is allogeneic bone marrow transplantation,11 a form of therapy that is limited by the availability of potential donors and by toxicity. Hydroxyurea, the one drug that has been approved by the Food and Drug Administration specifically for the treatment of severe SCD, decreases the frequency of painful events, episodes of acute chest syndrome, and the requirements for blood transfusions.9 In addition, while it does appear to prolong survival, many hydroxyurea-treated SCD patients continue to experience vaso-occlusive complications, develop end-organ damage, require blood transfusion, and exhibit life expectancies that remain substantially decreased compared with healthy individuals.12
In this study, we observed that, compared with placebo, treatment with senicapoc at the high dose produced increases in hemoglobin level, with concomitant decreases in the number of reticulocytes, and the chemical markers of hemolysis, lactate dehydrogenase, and indirect bilirubin. Similar but smaller changes in all of these parameters were seen at the lower dose of senicapoc, suggesting a dose-related response. The dose-related decrease in dense RBCs provides additional support for the hypothesis that blocking the Gardos channel leads to a lessening in the dehydration of the sickle erythrocytes. As dense sickle RBCs are reported to have a very short survival in vivo,13 all these findings are consistent with an improvement in the life span of the sickle erythrocyte and an amelioration of the anemic state. In addition to the decreased markers of hemolysis and increased hemoglobin concentrations that likely resulted from an improvement in the hydration state of the sickle erythrocyte, it is quite possible that the observed decrease in dense RBCs could provide further beneficial effects due to a reduction in the adhesivity of sickle RBCs to both the vascular endothelium and to the proteins of the subendothelial matrix.14-16 Furthermore, because reticulocytes are particularly adhesive and express the integrin complex α4β1 that binds to both fibronectin17 and vascular-cell adhesion molecule-1,18,19 it is conceivable that a decrease in circulating reticulocytes could also reduce the adhesion of sickle erythrocytes to the vascular endothelium.
While the decrease in overall MCV is somewhat surprising, we suspect that this finding is, in part, a result of the concomitant decrease in reticulocyte count observed in the high- and low-dose arms compared with placebo, as reticulocytes have a higher MCV than do mature erythrocytes. The reason for the decreased MCH observed in the high-dose arm compared with placebo is not clear based upon the results obtained from this study.
In addition to its hematologic benefit, senicapoc exhibited a good overall safety profile. The most frequent adverse events, diarrhea and nausea, occurred sporadically, appeared to be dose-dependent, and were described as “mild” to “moderate” in intensity. Finally, although GGT levels did increase in the senicapoc-treated groups, these increases were quite modest and the clinical significance is not known.
We did not detect any differences in the overall frequency of painful crises when the high-dose arm was compared with placebo. Furthermore, a phase 3 study of senicapoc in SCD patients was recently terminated due to the low probability of achieving a reduction in crisis rate, the primary end point of that study. As a result, the observed hematologic changes following senicapoc administration may not translate into benefit as measured by reduction in painful crises.
In conclusion, we found that 12 weeks of treatment with senicapoc at the dose of 10 mg/day produced an increase in hemoglobin level and concomitant decreases in markers of hemolysis. Furthermore, we found this drug to be safe and well tolerated in this study. The hematologic and pharmacodynamic data (ie, hemoglobin level, reticulocyte count, percentage of dense RBCs) provide strong evidence that administration of senicapoc produces inhibition of the Gardos channel that leads to beneficial effects on hematologic parameters in patients with SCD. We hypothesize that preservation of RBC hydration with decreased hemolysis and the concurrent improvement in hematologic parameters may lead to the reduction of certain complications observed in SCD. By decreasing hemolysis, senicapoc may prove to be of particular benefit in pulmonary hypertension, a severe complication thought due at least in part to hemolysis and the resultant scavenging of nitric oxide in SCD patients.20-22 The data presented here suggest that Gardos channel inhibition constitutes a new potential therapeutic approach for SCD, one that might be additive or perhaps even synergistic when combined with other therapeutic agents.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Paul W. Stewart for his helpful comments on the paper. The authors also acknowledge the efforts of the General Clinical Research Center at University of North Carolina-Chapel Hill, which is funded in part by National Institutes of Health grant RR00046.
This work was supported by Icagen (Research Triangle Park, NC). The statistical computations and analyses were performed by Theresa Jimenez, PhD, and Stacy Woodard, PhD, both of whom were employed by Quintiles Transnational.
Authorship
Contribution: K.I.A., E.P.O., G.C.R., and J.W.S. participated in the study design and writing of the paper; K.I.A., W.R.S., L.M.D., P.S., Y.S., O.C., E.V., A.K., and E.P.O. were significant clinical contributors to the trial and have reviewed the paper; G.C.R. and J.W.S. were involved in all aspects of trial conduct including analysis of the data.
A complete list of the ICA-17043-05 Investigators and Study Coordinators can be found in Document S1; available on the Blood website; see the Supplemental Materials link at the top of the online article.
Conflict-of-interest disclosure: G.C.R. and J.W.S. are employed by Icagen, Inc. P.S., O.C., and E.P.O. have been consultants for Icagen, Inc. All remaining authors declare no competing financial interests.
Correspondence: Kenneth I. Ataga, MBBS, Division of Hematology/Oncology, University of North Carolina at Chapel Hill, CB no. 7305, 3009 Old Clinic Bldg, Chapel Hill, NC 27599-7305; e-mail: kataga@med.unc.edu.

![Figure 2. Time course of senicapoc plasma concentrations and pharmacodynamic response. (A) Mean changes in hemoglobin level during the 12-week treatment period are plotted versus time for the 3 treatment groups. Mean hemoglobin level changes (± SE) were calculated from baseline hemoglobin values (average of screening and day-1 [before dose] samples). (B) Mean plasma concentrations of senicapoc during the 12-week treatment period are plotted versus time in study patients. Senicapoc levels were expressed as mean values (± SE). Measurements were obtained at day 1 (before dose) and then at the indicated time points during the course of the study for those patients assigned to 1 of the 2 active treatment arms. (C) Effect of senicapoc on the rate of 86Rb+ flux across the RBC membrane. RBCs were obtained from each of the treatment groups (placebo, 6 mg, 10 mg). Measurements of 86Rb+ flux due to activation by intracellular calcium were obtained at baseline (average of screening and day 1 [before dose] samples) and then at the indicated time points during the course of the study. Treatment groups are plotted in comparison with whole blood samples spiked with 10 μM senicapoc.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/8/10.1182_blood-2007-08-110098/6/m_zh80060816810002.jpeg?Expires=1769305683&Signature=hnjNstOOA98ugg5K3WzhavNvsNdlLMIVoWXADjku98xG~Dd2nSoMrWIjGZ~F7A4MDn8bCLt1xeOvAnO3zbhT5ExDT4UAQGe3ySPmzZI~xKIMXTwnoOVf8UK0bqsynitYvCa2wNARrYfL752OHet-nhK6-hsCuu1CzdoyhT9WltEffLCuSh-yq9PESMjVs4y5E80w0zYLf6NINsPQXhVKjO7C~CbMO5A9BpzNGFPzDz6ReJsc-KK6T6y~asGq9hvF4GYCM4rERc6-WFn4ukqRCLnzz1IHcA7i7ID2v1fX4k7wNZM8EWk8Js7eJ24QADU~KGiBu9Gb8~22Q7-dDw6rxA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
