Abstract
Humanized mice with a functional human immune system would be very useful for in vivo studies of human immunobiology. We have previously shown that cotransplantation of human fetal thymus/liver tissues and CD34+ fetal liver cells into immunodeficient nonobese diabetic severe combined immunodeficiency (NOD/SCID) mice leads to the development of multiple lineages of human lymphohematopoietic cells and formation of secondary lymphoid organs with normal architecture. Here, we evaluated the ability of these humanized mice to develop antigen-specific, T cell–dependent antibody responses after in vivo immunization with T-dependent antigen, 2,4-dinitrophenyl hapten-keyhole limpet hemocyanin (DNP23-KLH). Human T cells from DNP23-KLH–immunized mice showed strong proliferation in response to KLH in vitro. Furthermore, T cell–dependent production of DNP-specific human antibodies (mainly IgG1 and IgG2) was detected in all immunized mice. These results confirm that a functional human immune system can be established in immunodeficient mice through cotransplantation of human fetal thymus/liver tissues and CD34+ hematopoietic stem/progenitor cells.
Introduction
Small animal models allowing for systematic in vivo studies to address important questions relevant to human immunology have been long awaited.1 In the past 20 years, much effort has been put into developing better protocols to establish a functional human immune system in immunodeficient mice, and the main methodologic approaches include transplantation of human fetal tissues (thymus and liver) or hematopoietic stem/progenitor cells.2-6 Although human thymopoiesis and human T-cell development can be achieved by transplantation of human CD34+ cord blood cells into BALB/c RAG2nullγcnull or nonobese diabetic severe combined immunodeficiency (NOD/SCID)/γcnull newborn mice,5,6 transplantation of hematopoietic stem/progenitor cells has been inefficient in achieving human T-cell development in adult mice. In a recent study, human T-cell development was detected in adult NOD/SCID/IL2Rγnull mice receiving human CD34+ cord blood cells.7 However, the levels of human T cells in these mice were low, with approximately 3% and 5% of human CD3+ T cells in the blood and spleen, respectively, 4 months after transplantation. Furthermore, although a small portion of the grafted mice showed production of antigen-specific antibodies after HIV infection, the failure to produce anti-HIV human IgG in these mice suggests a lack of efficient class switching in this humanized mouse (hu-mouse) model
Considering the essential role of the thymus in determining the major histocompatibility complex (MHC) restriction of human T cells and in the development of regulatory T cells,8 we have established a hu-mouse model in which human T cells are generated in an autologous human thymus. Although transplantation of fetal human thymus (Thy) and liver (Liv) tissues alone can lead to human thymopoiesis and T-cell development in NOD/SCID mice, our previous studies showed that combined transplantation of human fetal Thy/Liv and intravenous administration of CD34+ cells is required to establish a functional human immune system capable of mediating in vivo immune responses.9 NOD/SCID mice receiving combined human Thy/Liv and CD34+ fetal liver cell (FLC) transplants, but not those receiving human Thy/Liv alone, showed long-term repopulation with multilineage human lymphohematopoietic cells, including T cells, B cells, and antigen-presenting cells (APCs); the formation of secondary lymphoid organs with normal structural features; and the production of high levels of human IgM and IgG. Consistent with our findings, Melkus et al subsequently reported that NOD/SCID mice reconstituted with human fetal Thy/Liv plus CD34+ cells mediate strong cellular immune responses against Epstein-Barr virus (EBV) and toxic shock syndrome toxin-1 (TSST-1).10 However, the ability of human fetal Thy/Liv/CD34+ cell–grafted hu-mice to mediate T cell–dependent antibody responses has not been well established.
Methods
Hu-mouse preparation and human chimerism analysis was performed as previously described.9 Hu-mice were immunized 14 weeks after humanization with 2,4-dinitrophenyl hapten-keyhole limpet hemocyanin (DNP23-KLH) or phosphate-buffered saline (PBS; controls), followed by a booster shot 3 weeks later, and killed for analyses at 2 or 4 weeks after the booster injection. To quantify human T-cell responses, splenocytes from immunized and control hu-mice were stained with CFSE and cultured with medium alone, 12.5 μg/mL KLH, or 2 μg/mL Con A. On the fifth day, cells were stained with APC-conjugated anti-human CD3 mAb, and proliferation of human CD3+ cells was analyzed by flow cytometry using the FlowJo Proliferation Platform software (TreeStar, Ashland, OR).11 The levels of DNP-specific human IgG and its subclass antibodies were measured by enzyme-linked immunosorbent assay (ELISA). Histological slides were viewed under a Nikon Eclipse TE2000-U fluorescent microscope using a 20×/0.5 NA objective, and were photographed by a DS-U1 digital camera (Nikon, Melville, NY) using Adobe Photoshop Elements software version 3.0 (Adobe Systems, San Jose, CA). The Student t test was used to determine statistically significant differences between groups. A detailed description of materials and methods is provided in Document S1, available on the Blood website (see the Supplemental Materials link at the top of the online article). Protocols involving the use of human tissues and animals were approved by the Massachusetts General Hospital Human Research Committee and Subcommittee on Research Animal Care, respectively, and all experiments were performed in accordance with the protocols.
Results and discussion
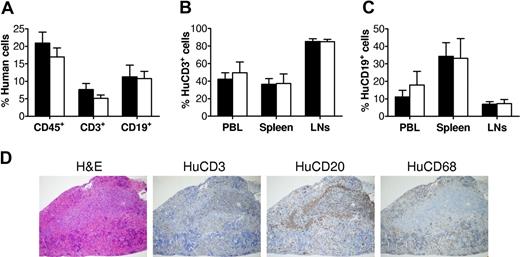
We first confirmed successful human lymphohematopoietic cell reconstitution in NOD/SCID mice after fetal human Thy/Liv/CD34+ FLC transplantation. Consistent with our previous studies,9 flow cytometric analysis of peripheral blood mononuclear cells (PBMCs) revealed the development of multilineage human lymphohematopoietic cells in the hu-mice prior to immunization (Figure 1A). Then, 2 groups of hu-mice with comparable levels of human cell repopulation were immunized with T cell–dependent antigen DNP23-KLH12,13 or PBS, as detailed in “Methods.” We also analyzed human chimerism in various tissues, including peripheral blood, spleen, and lymph nodes (LNs), when these hu-mice were killed for measurement of T-cell responses. As shown in Figure 1B and C, the immunized and control hu-mice had similar levels of human T cells (Figure 1B) and B cells (Figure 1C) in all tissues examined.
Human lymphohematopoietic cell reconstitution in human Thy/Liv/CD34+ FLC-grafted NOD/SCID mice. (A) Levels of total human lymphohematopoietic (CD45+) cells, CD3+ T cells, and CD19+ B cells in PBMCs were analyzed by flow cytometry at week 9 after human tissue/cell transplantation. ■ and □ represent hu-mice that were subsequently used for immunization with DNP23-KLH (n = 7) or adjuvant alone (ie, PBS controls; n = 5), respectively. (B,C) Levels of human CD3+ cells (B) and human CD19+ cells (C) in PBMCs, spleen, and LNs of DNP23-KLH–immunized (n = 7) and PBS-injected control (n = 5) hu-mice were analyzed by flow cytometry at time of death (ie, week 2 or week 4 after booster immunization). Error bars represent SEM. (D) White pulp formation in hu-mouse spleen. Shown are sections prepared from a representative hu-mouse spleen stained with hematoxyin and eosin, anti-human CD3, CD20, and CD68.
Human lymphohematopoietic cell reconstitution in human Thy/Liv/CD34+ FLC-grafted NOD/SCID mice. (A) Levels of total human lymphohematopoietic (CD45+) cells, CD3+ T cells, and CD19+ B cells in PBMCs were analyzed by flow cytometry at week 9 after human tissue/cell transplantation. ■ and □ represent hu-mice that were subsequently used for immunization with DNP23-KLH (n = 7) or adjuvant alone (ie, PBS controls; n = 5), respectively. (B,C) Levels of human CD3+ cells (B) and human CD19+ cells (C) in PBMCs, spleen, and LNs of DNP23-KLH–immunized (n = 7) and PBS-injected control (n = 5) hu-mice were analyzed by flow cytometry at time of death (ie, week 2 or week 4 after booster immunization). Error bars represent SEM. (D) White pulp formation in hu-mouse spleen. Shown are sections prepared from a representative hu-mouse spleen stained with hematoxyin and eosin, anti-human CD3, CD20, and CD68.
Histologic analysis demonstrated the formation of secondary lymphoid tissues with normal structural feature in the hu-mice. Spleens from these hu-mice were separated into red pulp containing human CD68+ macrophages and white pulp, in which human T-cell areas and B-cell follicles were clearly detected (Figure 1D). Previous studies have shown that follicular dendritic cells (FDCs) of nonhematopoietic origin play an essential role in the organization of follicular structure.14,15 Furthermore, mouse FDCs have been reported to provide costimulation to human lymphoid cells.16,17 Thus, it is likely that effective cross-species interactions between mouse FDCs and human B and T cells occurred in these hu-mice.
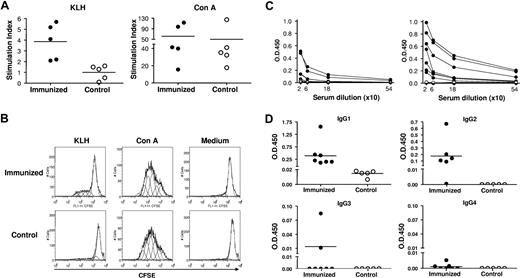
KLH-specific T-cell proliferation was analyzed by flow cytometric analysis of CFSE dilution at 2 and 4 weeks after booster immunization. As shown in Figure 2A and B, human CD3+ T cells from spleens of DNP23-KLH–immunized mice, but not from control hu-mice, proliferated in response to KLH stimulation (P < .01). However, human CD3+ T cells from DNP23-KLH–immunized and control hu-mice showed similar proliferation to Con A stimulation. These results demonstrate that KLH-specific human T cells were primed in vivo in the DNP23-KLH–immunized hu-mice.
Antigen-specific T-cell and antibody responses in immunized hu-mice. (A) Proliferation of human CD3+ T cells in response to KLH (left) and Con A (right). Stimulation index of each individual hu-mouse in DNP23-KLH–immunized (●) and control (○) groups are shown. (B) Representative histograms showing CFSE levels in gated human CD3+ T cells from DNP23-KLH–immunized (top) and control (bottom) groups. Cells stimulated with KLH, Con A, and medium are shown in left, middle, and right panels, respectively. (C) Serum levels of DNP-specific IgG in DNP23-KLH immunized (●) and PBS control (○) mice at week 1 after booster immunization (left) and at time of death (ie, 2 or 4 weeks after booster immunization; right). Each symbol represents an individual hu-mouse. (D) Serum levels of DNP-specific human IgG1, IgG2, IgG3, and IgG4 in DNP23-KLH–immunized (●) and PBS control (○) hu-mice at time of death (ie, 2 or 4 weeks after booster immunization). Each symbol represents an individual mouse. Horizontal lines in Figure 2A,D represent mean values.
Antigen-specific T-cell and antibody responses in immunized hu-mice. (A) Proliferation of human CD3+ T cells in response to KLH (left) and Con A (right). Stimulation index of each individual hu-mouse in DNP23-KLH–immunized (●) and control (○) groups are shown. (B) Representative histograms showing CFSE levels in gated human CD3+ T cells from DNP23-KLH–immunized (top) and control (bottom) groups. Cells stimulated with KLH, Con A, and medium are shown in left, middle, and right panels, respectively. (C) Serum levels of DNP-specific IgG in DNP23-KLH immunized (●) and PBS control (○) mice at week 1 after booster immunization (left) and at time of death (ie, 2 or 4 weeks after booster immunization; right). Each symbol represents an individual hu-mouse. (D) Serum levels of DNP-specific human IgG1, IgG2, IgG3, and IgG4 in DNP23-KLH–immunized (●) and PBS control (○) hu-mice at time of death (ie, 2 or 4 weeks after booster immunization). Each symbol represents an individual mouse. Horizontal lines in Figure 2A,D represent mean values.
The levels of DNP-specific human IgG and IgG subclasses in the sera of immunized and control hu-mice were determined by ELISA. As shown in Figure 2C, 4 of 7 immunized hu-mice showed detectable levels of DNP-specific IgG at week 1 after DNP23-KLH immunization. The levels of DNP-specific IgG in these hu-mice increased over time, and all mice became positive by the time they were killed at week 2 or 4 after immunization. In contrast, DNP-specific IgG was not detected in any of the PBS control hu-mice. DNP-specific IgG in immunized hu-mice was mainly of the IgG1 and IgG2 subclasses (Figure 2D). Also, 2 hu-mice showed detectable levels of DNP-specific IgG3, but none was positive for DNP-specific IgG4. In fact, the development and distribution of DNP-specific human IgG subclasses in these immunized hu-mice were similar to that of antibody responses in humans after KLH immunization, in which IgG3 antibody production is less frequent and IgG4 antibodies develop very slowly.18 These data show clearly that class switch of antigen-specific antibody response takes place in these hu-mice. In humans, IgG1 responses are frequently associated with T-dependent antigens.18 While T-independent antigens tend to induce IgG2 antibodies in humans, IgG2 can also be detected in T cell–dependent humoral immune responses.18,19 Previous studies have shown that immunization with DNP-KLH stimulates the production of antigen-specific human IgM, but not IgG in hu-mice with poor human T-cell reconstitution.20 Taken together, these results indicate that functional human T cell–B cell interactions resulting in B-cell activation, antibody production, and class switching occurred in our hu-mice. Further studies are needed to evaluate the efficiency of antibody affinity maturation and generation of memory B cells upon immunization in these hu-mice.
In summary, our data demonstrate that hu-mice created by cotransplantation of human fetal Thy/Liv and CD34+ FLCs can mount strong antigen-specific T-cell responses and T cell–dependent IgG production after in vivo antigen immunization. This is a powerful model for the in vivo study of human immune system and function. In addition, these hu-mice may also be used to develop therapeutic human antibodies for clinical use.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Emmanuel Zorn and Fabienne Haspot for critical review of this manuscript, Mr Orlando Moreno for outstanding animal husbandry, and Ms Kelly Walsh for expert assistance with the manuscript.
This work was supported by grants from Juvenile Diabetes Research Foundation International (1-2005-72) and the National Institutes of Health (P01 AI 045897 and P30 AI 060354); K.H. is partially supported by a fellowship from JDRF (3-2007-667).
National Institutes of Health
Authorship
Contribution: N.T. designed and performed research; K.H. designed and performed research and wrote the paper; A.S. performed histologic analysis; M.S. designed the research; and Y.Y. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yong-Guang Yang, Transplantation Biology Research Center, Massachusetts General Hospital, Harvard Medical School, MGH-East, Bldg 149-5102, 13th St, Boston, MA 02129; e-mail: yongguang.yang@tbrc.mgh.harvard.edu.
References
Author notes
*N.T. and K.H. contributed equally to this work.