Abstract
We previously reported that sphingosine 1-phosphate (S1P) regulates peritoneal B-cell trafficking and subsequent intestinal IgA production, but the underlying mechanisms remain obscure. We demonstrate here that nuclear factor κB–inducing kinase (NIK) is involved in the regulation of S1P-mediated trafficking of peritoneal B cells. Although peritoneal B cells from NIK-mutated alymphoplasia (aly) mice expressed type 1 S1P receptor (S1P1) at comparable levels and demonstrated normal migration toward S1P, aly peritoneal B cells showed decreased sensitivity to FTY720, an S1P1 modulator. NIK-mutated stromal cells showed decreased levels of adhesion molecules (VCAM-1 and ICAM-1) and increased CXCL13 expressions, leading to impaired ability to support S1P-mediated emigration, but not immigration, of peritoneal B cells. Therefore, aly peritoneal B cells exhibited normal S1P-mediated peritoneal B-cell trafficking from peritoneum to intestine for IgA production when they were transferred into severe combined immunodeficient or wild-type mice. However, S1P-mediated emigration of wild-type B cells from the aly peritoneal cavity was impaired without affecting their immigration from the blood. Further, transfer of wild-type stromal cells into the peritoneum restored S1P-mediated trafficking of aly peritoneal B cells. These findings suggest that NIK in stromal cells has a specific role in the regulation of S1P-mediated trafficking of peritoneal B cells.
Introduction
The peritoneal cavity contains numerous B cells, especially B1 cells, which play important roles in protective immunity in the peritoneal cavity and in the production of secretory IgA antibody (S-IgA) in the intestine.1,2 Accumulating evidence has demonstrated that the trafficking of peritoneal B cells is regulated by chemokines (eg, CCL19, CCL21, and CXCL13), cytokines (eg, interleukin [IL]-10), and adhesion molecules (eg, integrins) at various points in the immigration, retention, and emigration of these cells.3-8
Sphingosine 1-phosphate (S1P) is another key molecule in the regulation of lymphocyte trafficking.9,10 Among the 5 types of S1P receptors, type 1 S1P receptor (S1P1) is preferentially expressed on lymphocytes and is required for their emigration from secondary lymphoid organs and the thymus.11,12 FTY720 is an agonist for S1P receptors, except type 2 S1P receptor (S1P2), and blocks S1P-mediated signaling by inducing internalization of S1P receptors.12-16 Therefore, treatment with FTY720 decreases the number of circulating lymphocytes in both blood and lymph by inhibiting their emigration from secondary lymphoid organs and the thymus.12-16
In addition to its role in the systemic immune compartments, we recently reported that S1P is involved in the trafficking of mucosa-associated immunocompetent cells, including peritoneal B cells, intraepithelial T lymphocytes, and intestinal mast cells.17-19 In those studies, we showed that peritoneal B1 and B2 cells expressed comparable levels of S1P1 and that FTY720 treatment impaired trafficking of peritoneal B cells into the intestine by enhancing their emigration from the peritoneal cavity and by inhibiting their immigration from blood into the peritoneal cavity. The FTY720-associated disruption of peritoneal B-cell trafficking into the intestine was associated with impaired intestinal immunoglobulin A (IgA) production by peritoneal B cells.17 These findings provide strong evidence that S1P plays an essential role in the regulation of peritoneal B-cell trafficking into the intestine and subsequent intestinal IgA production.
Alymphoplasia (aly) mice carry a point mutation in nuclear factor κB–inducing kinase (NIK), leading to the inability to bind to IκB kinase α, a molecule essential for nuclear factor κB (NFκB) activation.20-22 aly mice lack lymph nodes and Peyer patches; they have impaired development of the spleen and thymus and showed accumulation of peritoneal B1 cells.20,23 Because the B1 and B2 cells that originate from the peritoneal cavity and Peyer patches are the primary sources of intestinal IgA,1,2 the immunologic defects in aly mice almost completely ablate intestinal IgA production.23 A previous study revealed that the impaired peritoneal B-cell trafficking in aly mice was, at least in part, due to the defect of signaling pathway coupling with G-proteins, such as chemokine receptors, in lymphocytes.23 A subsequent study revealed that the impaired function of stromal cells in aly mice was also attributable to the defective trafficking of bone marrow–derived naive IgM+ IgA− B cells to the intestine.24
Given the defective G-protein signaling pathways in aly mice,23 and the fact that S1P receptors couple to G proteins,25 our findings led us to hypothesize that the defective peritoneal B-cell trafficking and consequent impaired intestinal IgA production in aly mice might be mediated by S1P. We therefore sought here to investigate the interaction between NIK- and S1P-mediated pathways in peritoneal B-cell trafficking and subsequent intestinal IgA production. Our findings provide new evidence that NIK-mediated signaling in stromal cells regulates S1P-mediated trafficking of peritoneal B cells, especially their emigration from the peritoneal cavity.
Methods
Mice, FTY720 treatment, and cell isolation
Female C57BL/6, aly, and severe combined immunodeficient (SCID) mice (7-9 weeks) were purchased from Japan Clea (Tokyo, Japan). All mice were maintained in horizontal laminar flow cabinets and provided with sterile food and water ad libitum. For FTY720 treatment, mice were injected intraperitoneally with FTY720 (1 mg/kg; Novartis Pharma, Basel, Switzerland).17-19 Peritoneal cells were obtained by flushing the peritoneum with 8 mL ice-cold phosphate-buffered saline (PBS).17 All experiments were approved by the Animal Care and Use Committee of the University of Tokyo and conducted in accordance with its guidelines.
Flow cytometry and cell sorting
A standard protocol was used for flow cytometric analysis and cell sorting.17-19 Cells were incubated with anti-CD16/32 antibody (Ab; BD Biosciences, San Diego, CA) and then stained with the appropriate fluorescent-conjugated Abs specific for B220, CD11b, ICAM-1, and VCAM-1 (BD Biosciences). Viaprobe (BD Biosciences) was used to discriminate between dead and live cells. Flow cytometric analysis and cell sorting were performed with FACSCalibur (BD Biosciences) and FACSAria (BD Biosciences), respectively.
In vitro migration assay
In vitro migration assays using purified B1 and B2 cells were performed according to a previously established method.12 Briefly, peritoneal B cells were applied to the upper chambers (pore diameter, 5 μm; Invitrogen, Carlsbad, CA) and 0, 20, 200, or 2000 nM S1P was added to the lower wells. After a 6-hour incubation, the B cells that had migrated into the lower wells were counted with the aid of trypan blue staining.
RT-PCR
To measure mRNA expression for S1P1, quantitative and conventional reverse-transcription–polymerase chain reaction (RT-PCR) using LightCycler (Roche Diagnostics, Mannheim, Germany) were performed.17-19 Briefly, total RNA was isolated using TRIzol reagent (Invitrogen), and cDNA was synthesized using Powerscript reverse transcriptase (BD Biosciences). The oligonucleotide primers and probes specific for S1P1 (forward primer, TACACTCTGACCAACAAGGA; reverse primer, ATAATGGTCTCTGGGTTGTC; FITC-probe, TGCTGGCAATTCAAGAGGCCCATCATC; LCRed 640-probe, CAGGCATGGAATTTAGCCGCAGCAAATC), glyceraldehyde-3-phosphate dehydrogenase (GAPDH; forward primer, TGAACGGGAAGCTCACTGG; reverse primer, TCCACCACCCTGTTGCTGTA; FITC-probe, CTGAGGACCAGGTTGTCTCCTGCGA; LCRed 640-probe, TTCAACAGCAACTCCCACTCTTCCACC), CCL19 (forward primer, GCCAAGAACAAAGGCAACA; reverse primer, CACACTCACATCGACTCTCTA), CCL21 (forward primer, ACAGACACAGCCCTCAA; reverse primer, CATGAGGTGGCTGCTTT), and CXCL13 (forward primer, GAACAGGCATTTAGTGACAAC; reverse primer, TTTTGGAAGCCTGCGTTTT) were designed and synthesized by Nihon Gene Research Laboratory (Sendai, Japan).26
Adoptive cell transfer
For tracing cells in vivo, peritoneal B cells (107 cells) were incubated with 0.25 μM 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) in the dark for 10 minutes at 37°C and then were washed twice with PBS according to a previously described method.17,18 Labeled B cells (5 × 106 cells) were transferred into recipient mice intraperitoneally or intravenously; FTY720 was administered intraperitoneally simultaneously. After 12 hours, peritoneal cells were collected for flow cytometric analysis.
For experiments involving stromal cell transfer, stromal cells were isolated from the small intestines of wild-type (WT) mice as previously described.27 Briefly, cells were isolated from intestinal lamina propria and cultured on 10-cm culture plates in complete RPMI1640 medium. After 1 hour, nonadherent cells were removed by washing with PBS, and remaining adherent cells were cultured overnight in complete RPMI1640 medium. After overnight culture, the plates were washed with PBS, and remaining adherent cells were cultured in complete RPMI1640 medium. After 2 rounds of subculture, confluent cells were used as stromal cells and transferred into the peritoneal cavities of aly mice (107 cells per mouse). Two weeks after transfer, mice were treated with FTY720 for analysis of peritoneal B-cell trafficking. To analyze Ab production from peritoneal B cells, SCID mice were adoptively transferred with peritoneal B cells (5 × 106 cells per mouse) via the intraperitoneal route and treated with FTY720 every 2 days for 2 weeks. Two weeks after adoptive transfer, fecal extracts were collected for the measurement of total IgA levels by enzyme-linked immunosorbent assay (ELISA).
Measurement of fecal IgA by ELISA
The concentration of fecal IgA was determined by ELISA as previously described.17 Purified murine IgA Ab (BD PharMingen, San Diego, CA) was used as a standard for the quantification. After blocking of coated anti–mouse Ig Ab (Southern Biotechnology Associates, Birmingham, AL) with 5% bovine serum albumin in PBS, diluted fecal extract was added and incubated in the coated wells for 2 hours at room temperature. Bound Ab was quantified using HRP-conjugated anti–mouse IgA (Southern Biotechnology Associates) and 3,3′5,5′-tetramethylbenzidine (Moss, Pasadena, CA), as previously described.17
Statistics
The results were compared using the Student t test or Welch t test. P value of less than .05 was considered statistically significant.
Results
Decreased sensitivity to FTY720 in aly mice
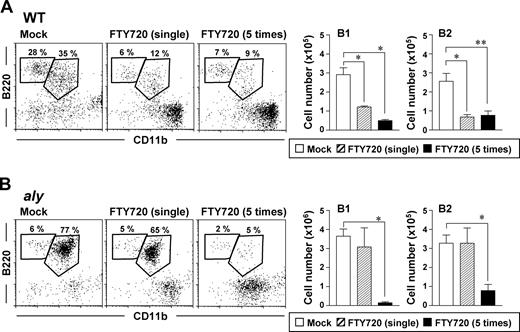
To test whether the defective trafficking of peritoneal B cells in aly mice was attributable to a dysfunctional S1P-mediated pathway, we compared the effect of FTY720 on peritoneal B cells in aly mice with those in WT mice. Consistent with our previous results,17 a single injection of FTY720 induced rapid reductions in the percentages and absolute cell numbers of peritoneal B1 and B2 cells in WT mice (Figure 1A). In contrast, aly mice showed scant reduction in peritoneal B cells after a single injection of FTY720 (Figure 1B). To elucidate whether the FTY720 reactivity of aly mice was complete or partial, mice were injected repeatedly with FTY720 and their peritoneal B cells were examined. In agreement with our previous results,17 treating WT mice with multiple injections of FTY720 did not increase its effect on peritoneal B cells, such that B1- and B2-cell counts and percentages were similar to those of the single-treatment group (Figure 1A). In contrast, repeated, but not single, FTY720 treatment significantly (P < .05) reduced the peritoneal B1- and B2-cell populations of aly mice (Figure 1B). These findings suggest that the peritoneal B cells of aly mice showed reduced sensitivity but are still reactive to FTY720.
Decreased reactivity of peritoneal B cells to FTY720 in aly mice. Cells were isolated from the peritoneal cavities of WT (A) or aly (B) mice 12 hours after single or multiple (that is 5) injections of FTY720 (right) or vehicle only (mock; left), and cell populations were analyzed by flow cytometry. The data are representative of at least 4 independent experiments. The numbers of B220+CD11b+ B1 cells and B220+CD11b− B2 cells were calculated from the total cell number and flow cytometric data. Data are presented as mean plus or minus SEM (n = 4). *P < .01; **P < .05.
Decreased reactivity of peritoneal B cells to FTY720 in aly mice. Cells were isolated from the peritoneal cavities of WT (A) or aly (B) mice 12 hours after single or multiple (that is 5) injections of FTY720 (right) or vehicle only (mock; left), and cell populations were analyzed by flow cytometry. The data are representative of at least 4 independent experiments. The numbers of B220+CD11b+ B1 cells and B220+CD11b− B2 cells were calculated from the total cell number and flow cytometric data. Data are presented as mean plus or minus SEM (n = 4). *P < .01; **P < .05.
Normal S1P1 expression and migration to S1P in aly peritoneal B cells
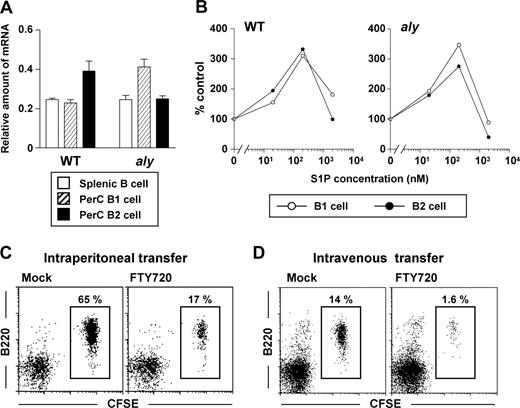
We hypothesized that the decreased reactivity of aly peritoneal B cells to FTY720 was due to their minimal expression of S1P receptors, especially S1P1, whose type was exclusively expressed on peritoneal B cells.17 To test this hypothesis, we performed quantitative RT-PCR analysis and found that the levels of S1P1 in peritoneal B1 and B2 cells and splenic B cells were comparable between aly and WT mice (Figure 2A). Together with a previous report indicating that NIK-mediated signaling is linked to the same G-coupled protein that S1P receptors use,25 our results suggested that NIK mutation abolished S1P1-mediated signaling. To test whether S1P1-mediated signaling in aly peritoneal B cells was functional, we investigated the in vitro migration of peritoneal B cells toward S1P. We found that, like the peritoneal B cells isolated from WT mice, both peritoneal B1 and B2 cells from aly mice migrated to the gradient of S1P (Figure 2B). These data indicated that the reduced reactivity to FTY720 in aly mice was not due to a defect in S1P1 expression or S1P1-mediated signaling.
Similar expression of S1P1 and biologic reactivity to S1P among various populations of aly peritoneal B cells. (A) Quantitative RT-PCR analysis for S1P1 expression was performed using RNA isolated from sorted splenic B (□), and peritoneal B1 ( ) and B2 (■) cells. The relative quantity of specific mRNA encoding S1P1 was expressed as a ratio to GAPDH. Data are expressed as the mean plus or minus SD of 4 mice. (B) In vitro migration assay was performed with peritoneal B1 (○) and B2 (•) B cells purified from WT (left) and aly (right) mice. Peritoneal B cells were added to the upper chamber of a transwell plate in the presence of 0, 20, 200, or 2000 nM S1P in the lower chamber. Six hours later, the number of cells that had migrated into the lower chamber were counted. Data are representative of 3 independent experiments. (C,D) CFSE-labeled aly peritoneal B cells were adoptively transferred into SCID mice by the intraperitoneal (C) or intravenous (D) routes: reconstituted SCID mice were treated simultaneously with (right panels) or without (left panels) FTY720. After 12 hours, cells were isolated from the peritoneal cavity for the analysis of CFSE+ B220+ cells. Data are representative of 4 independent experiments.
) and B2 (■) cells. The relative quantity of specific mRNA encoding S1P1 was expressed as a ratio to GAPDH. Data are expressed as the mean plus or minus SD of 4 mice. (B) In vitro migration assay was performed with peritoneal B1 (○) and B2 (•) B cells purified from WT (left) and aly (right) mice. Peritoneal B cells were added to the upper chamber of a transwell plate in the presence of 0, 20, 200, or 2000 nM S1P in the lower chamber. Six hours later, the number of cells that had migrated into the lower chamber were counted. Data are representative of 3 independent experiments. (C,D) CFSE-labeled aly peritoneal B cells were adoptively transferred into SCID mice by the intraperitoneal (C) or intravenous (D) routes: reconstituted SCID mice were treated simultaneously with (right panels) or without (left panels) FTY720. After 12 hours, cells were isolated from the peritoneal cavity for the analysis of CFSE+ B220+ cells. Data are representative of 4 independent experiments.
Similar expression of S1P1 and biologic reactivity to S1P among various populations of aly peritoneal B cells. (A) Quantitative RT-PCR analysis for S1P1 expression was performed using RNA isolated from sorted splenic B (□), and peritoneal B1 ( ) and B2 (■) cells. The relative quantity of specific mRNA encoding S1P1 was expressed as a ratio to GAPDH. Data are expressed as the mean plus or minus SD of 4 mice. (B) In vitro migration assay was performed with peritoneal B1 (○) and B2 (•) B cells purified from WT (left) and aly (right) mice. Peritoneal B cells were added to the upper chamber of a transwell plate in the presence of 0, 20, 200, or 2000 nM S1P in the lower chamber. Six hours later, the number of cells that had migrated into the lower chamber were counted. Data are representative of 3 independent experiments. (C,D) CFSE-labeled aly peritoneal B cells were adoptively transferred into SCID mice by the intraperitoneal (C) or intravenous (D) routes: reconstituted SCID mice were treated simultaneously with (right panels) or without (left panels) FTY720. After 12 hours, cells were isolated from the peritoneal cavity for the analysis of CFSE+ B220+ cells. Data are representative of 4 independent experiments.
) and B2 (■) cells. The relative quantity of specific mRNA encoding S1P1 was expressed as a ratio to GAPDH. Data are expressed as the mean plus or minus SD of 4 mice. (B) In vitro migration assay was performed with peritoneal B1 (○) and B2 (•) B cells purified from WT (left) and aly (right) mice. Peritoneal B cells were added to the upper chamber of a transwell plate in the presence of 0, 20, 200, or 2000 nM S1P in the lower chamber. Six hours later, the number of cells that had migrated into the lower chamber were counted. Data are representative of 3 independent experiments. (C,D) CFSE-labeled aly peritoneal B cells were adoptively transferred into SCID mice by the intraperitoneal (C) or intravenous (D) routes: reconstituted SCID mice were treated simultaneously with (right panels) or without (left panels) FTY720. After 12 hours, cells were isolated from the peritoneal cavity for the analysis of CFSE+ B220+ cells. Data are representative of 4 independent experiments.
To further examine whether aly peritoneal B cells show normal reactivity to S1P in vivo, we isolated aly peritoneal B cells, labeled them with CFSE, and adoptively transferred them into SCID mice that were treated concurrently with FTY720 to disrupt S1P-mediated signaling. Because our recent study demonstrated that FTY720 inhibited B-cell immigration into the peritoneal cavity and enhanced their emigration from it,17 we transferred the labeled B cells through 2 different routes, intraperitoneal and intravenous injection. When aly peritonealB cells were adoptively transferred into the peritoneal cavities of SCID mice, FTY720 treatment resulted in a marked reduction in B-cell numbers, suggesting that, as with WT B cells, FTY720 enhanced the emigration of aly B cells from the peritoneal cavity (Figure 2C). Further, immigration of aly B cells from the blood into the peritoneal cavity was impaired when SCID mice were treated with FTY720 after the intravenous transfer of aly peritoneal B cells (Figure 2D). Taken together with the new in vitro and in vivo data, our findings convincingly show that aly peritoneal B cells can react to S1P and FTY720. However, the sensitivity to FTY720 is lower in aly mice than in WT mice.
A previous study demonstrated that S1P lyase, which degrades S1P to phosphoethanolamine, is abundant in secondary lymphoid organs, thus establishing a S1P gradient with lower concentrations in the secondary lymphoid organs than in the blood and lymph.28 These findings suggested to us that the lack of secondary lymphoid organs in aly mice might contribute to their decreased sensitivity to FTY720 owing to the presence of an impaired S1P gradient. But some evidence obtained in our study disproved this hypothesis. We found that Id2−/− mice, which lacked secondary lymphoid organs due to deficiency of a negative regulator of basic helix-loop-helix transcription factors, showed normal sensitivity to FTY720, and that disruption of the S1P gradient by oral feeding of deoxypyridoxine, an inhibitor of S1P lyase, did not affect the peritoneal B-cell trafficking in WT mice (J.K., unpublished data, January 2007). Taken together with a previous report that lymphoid organs were not required for S1P-mediated trafficking of peripheral lymphocytes,29 these findings suggest that the impaired reactivity of aly mice to FTY720 is not attributable to their defective secondary lymphoid organ structure.
Normal S1P-mediated trafficking of aly peritoneal B cells for the intestinal IgA production
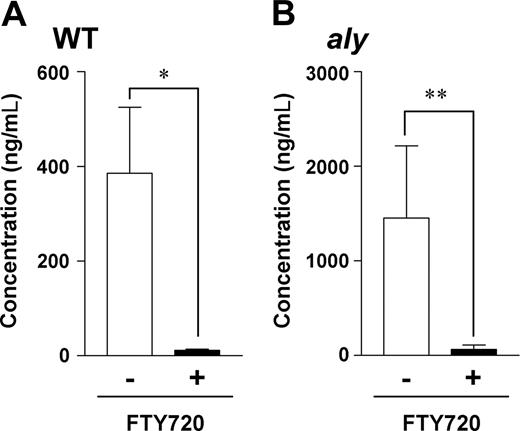
Peritoneal B cells are primarily sources of intestinal IgA production,1,2 and we previously demonstrated that S1P mediates the production of intestinal IgA by peritoneal B cells.17 Therefore, we next examined whether aly peritoneal B cells could produce intestinal IgA in an FTY720-sensitive manner. We addressed this issue by investigating intestinal IgA production in SCID mice adoptively transferred with aly peritoneal B cells. Consistent with our previous findings,17 SCID mice that received WT peritoneal B cells produced considerable amounts of intestinal IgA, and FTY720 treatment inhibited this production (Figure 3A). Similar induction of intestinal IgA production occurred when SCID mice were reconstituted with aly peritoneal B cells (Figure 3B), and, as seen after transfer of WT peritoneal B cells, FTY720 abolished intestinal IgA production in the mice that received aly peritoneal B cells (Figure 3B). These data indicate that aly B cells migrate normally into the intestine and subsequently produce intestinal IgA in an FTY720-sensitive manner in SCID mice. In light of these data, NIK in B cells seems to be redundant in the S1P-mediated trafficking of peritoneal B cells and subsequent intestinal IgA production. Therefore, the impaired sensitivity of aly mice to FTY720 is not due to defective S1P-mediated signaling in B cells.
aly peritoneal B cells show a comparable dependence on S1P for intestinal IgA production. WT (A) or aly (B) peritoneal B cells (5 × 106 cells) were adoptively transferred into SCID mice, which were treated with vehicle only (□) or FTY720 (■) every 2 days. Two weeks after cell transfer, fecal extracts were collected for measurement of IgA levels by ELISA. Data are presented as means plus or minus SEM (n = 4). *P < .01; **P < .05.
aly peritoneal B cells show a comparable dependence on S1P for intestinal IgA production. WT (A) or aly (B) peritoneal B cells (5 × 106 cells) were adoptively transferred into SCID mice, which were treated with vehicle only (□) or FTY720 (■) every 2 days. Two weeks after cell transfer, fecal extracts were collected for measurement of IgA levels by ELISA. Data are presented as means plus or minus SEM (n = 4). *P < .01; **P < .05.
NIK-mediated pathway in non-B cells is essential for the sensitivity of S1P-mediated peritoneal B-cell emigration but not immigration
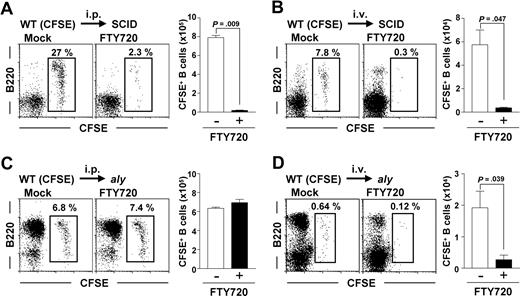
In light of our current findings that aly peritoneal B cells react to FTY720 and S1P, we hypothesized that their decreased sensitivity to FTY720 was due to the NIK mutation in the non-B cells. We therefore examined the FTY720 reactivity of WT peritoneal B cells adoptively transferred into SCID and aly mice. Regardless of the injection route, WT peritoneal B cells showed normal reactivity to FTY720 and thus were decreased after treatment with FTY720 when they were adoptively transferred into SCID mice (Figure 4A,B). In contrast, WT peritoneal B cells transferred intraperitoneally into aly mice lacked reactivity to FTY720. Indeed, the numbers of peritoneal B cells were similar with or without FTY720 treatment (Figure 4C).
NIK-mediated signaling in non-B cells controls S1P-mediated peritoneal B-cell emigration of peritoneal B cells, but not their immigration. (A,B) Peritoneal B cells were isolated from WT mice, labeled with CFSE, and adoptively transferred via the intraperitoneal (i.p.) (A) or intravenous (i.v.) (B) routes into SCID mice. (C,D) Similarly, CFSE-labeled peritoneal WT B cells were transferred into aly mice from which peritoneal cells were removed 8 hours before transfer. The reconstituted mice were treated simultaneously with (right panels) or without (left panels) FTY720. After 12 hours, cells were isolated from the peritoneal cavity for the analysis of CFSE+ B220+ cells. Flow cytometric data are representative of 3 independent experiments and are presented as means plus or minus SEM (n = 3).
NIK-mediated signaling in non-B cells controls S1P-mediated peritoneal B-cell emigration of peritoneal B cells, but not their immigration. (A,B) Peritoneal B cells were isolated from WT mice, labeled with CFSE, and adoptively transferred via the intraperitoneal (i.p.) (A) or intravenous (i.v.) (B) routes into SCID mice. (C,D) Similarly, CFSE-labeled peritoneal WT B cells were transferred into aly mice from which peritoneal cells were removed 8 hours before transfer. The reconstituted mice were treated simultaneously with (right panels) or without (left panels) FTY720. After 12 hours, cells were isolated from the peritoneal cavity for the analysis of CFSE+ B220+ cells. Flow cytometric data are representative of 3 independent experiments and are presented as means plus or minus SEM (n = 3).
We next addressed whether NIK mutation affected B-cell immigration from the blood into the peritoneal cavity. In this experiment, we removed the peritoneal cells from recipient aly mice 8 hours before adoptive transfer, because the aly peritoneal B cells were too numerous to allow detection of intravenously transferred B cells. Twenty-four hours after depletion of the peritoneal B cells, the peritoneal cavities of aly mice contained more B2 cells than untreated aly peritoneal B cells (data not shown), demonstrating that peritoneal B cells were removed and that many cells were derived from the blood. Removing peritoneal cells from recipient mice before adoptive transfer enabled us to detect intravenously injected WT peritoneal B cells in aly mice (Figure 4D). Unlike the case with intraperitoneally transferred WT B cells (Figure 4C), FTY720 prevented the immigration of intravenously transferred WT peritoneal B cells from the blood into the peritoneal cavity (Figure 4D). The number of adoptively transferred B cells was decreased consistently and significantly (P < .05) in the peritoneal cavities of FTY720-treated aly mice (Figure 4D). These findings suggest that NIK-mediated signaling in non-B cells participates in the regulation of S1P-mediated emigration of B cells from the peritoneal cavity but not in their immigration from the blood.
Requirement of NIK-mediated pathway in stromal cells for S1P-mediated emigration of peritoneal B cells
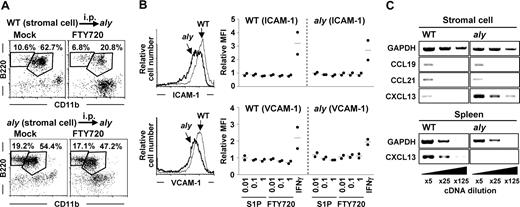
Because both aly and WT B cells showed normal emigration from the peritoneal cavities of SCID mice (Figures 2C and 4A, respectively), T cells likely do not play a role in this pathway. We therefore speculated that NIK-mediated signaling in stromal cells was involved in the emigration of peritoneal B cells. To test this hypothesis, we transferred WT stromal cells into the peritoneal cavities of aly mice treated with FTY720 and noted a subsequent reduction in the number of peritoneal B cells (Figure 5A top panels). In contrast, FTY720 had no discernible effect when aly stromal cells were transferred into aly mice (Figure 5A bottom panels). These data suggest that NIK-mediated signaling in stromal cells participates in the regulation of S1P-mediated peritoneal B-cell emigration.
Requirement of NIK-mediated signaling in stromal cells for the emigration of peritoneal B cells. (A) aly mice were intraperitoneally (i.p.) transferred with WT (top panels) or aly (bottom panels) stromal cells. Two weeks after cell transfer, mice were treated with FTY720 for the analysis of peritoneal B-cell populations. Flow cytometric data are representative of 3 independent experiments and are presented as means plus or minus SEM (n = 3). (B) Expression of ICAM-1 (top panels) and VCAM-1 (bottom panels) on WT (thin lines) and aly (thick lines) stromal cells was determined by flow cytometry (left). Twenty-four hours after treatment of stromal cells with various concentrations of S1P, FTY720, or IFNγ (50 units/mL), expression of ICAM-1 and VCAM-1 was determined by flow cytometry. Relative mean fluorescence intensity (MFI) was expressed as a ratio to MFI of untreated cells. Data are representative of 2 independent experiments, and bars indicate mean values. (C) Expression of chemokines (CCL19, CCL21, and CXCL13) in stromal and spleen cells was examined by RT-PCR. Data are representative of 3 independent experiments.
Requirement of NIK-mediated signaling in stromal cells for the emigration of peritoneal B cells. (A) aly mice were intraperitoneally (i.p.) transferred with WT (top panels) or aly (bottom panels) stromal cells. Two weeks after cell transfer, mice were treated with FTY720 for the analysis of peritoneal B-cell populations. Flow cytometric data are representative of 3 independent experiments and are presented as means plus or minus SEM (n = 3). (B) Expression of ICAM-1 (top panels) and VCAM-1 (bottom panels) on WT (thin lines) and aly (thick lines) stromal cells was determined by flow cytometry (left). Twenty-four hours after treatment of stromal cells with various concentrations of S1P, FTY720, or IFNγ (50 units/mL), expression of ICAM-1 and VCAM-1 was determined by flow cytometry. Relative mean fluorescence intensity (MFI) was expressed as a ratio to MFI of untreated cells. Data are representative of 2 independent experiments, and bars indicate mean values. (C) Expression of chemokines (CCL19, CCL21, and CXCL13) in stromal and spleen cells was examined by RT-PCR. Data are representative of 3 independent experiments.
To investigate the mechanisms of peritoneal B-cell trafficking mediated by NIK in stromal cells, we compared the expression of vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1, adhesion molecules regulating peritoneal B-cell trafficking, between WT and aly stromal cells. The expression of these adhesion molecules was lower on aly stromal cells than on WT stromal cells (Figure 5B). A previous report that S1P regulated the expression of VCAM-1 on endothelial cells30 led us to hypothesize that S1P affects the expression of VCAM-1 and ICAM-1 on stromal cells. However, unlike endothelial cells, WT and aly stromal cells showed negligible expression of all types of S1P receptors (data not shown). Therefore, treatment of stromal cells with S1P or FTY720 influenced neither VCAM-1 nor ICAM-1 expression, although IFNγ increased the expression of both of these molecules in both WT and aly stromal cells (Figure 5B).
We then examined the expression of chemokines that were reported to be involved in peritoneal B-cell trafficking (CCL19, CCL21, and CXCL13).3,4 Our results showed that expression of CCL19 and CCL21 was comparable between WT and aly stromal cells (Figure 5C). In contrast, CXCL13 expression was increased in aly stromal cells compared with WT stromal cells (Figure 5C), although CXCL13 expression in the aly spleen was lower than in the WT spleen (Figure 5C), as previously reported.23 These findings collectively indicate that, in S1P-mediated peritoneal B-cell trafficking, S1P directly affects peritoneal B cells, not stromal cells, but stromal cells are involved in the S1P-mediated pathway through the expression of adhesion molecules and chemokines. Decreased expression of VCAM-1 and ICAM-1 on stromal cells, or unbalanced CXCL13 expression between the peritoneal cavity and other sites (eg, spleen) in aly mice (or both mechanisms), may explain the impaired S1P-mediated trafficking of peritoneal B cells in aly mice.
Discussion
Lymphocyte trafficking into and from lymph nodes and nonlymphoid organs is regulated through several bioactive molecules (eg, chemokines and adhesion molecules).9 We previously demonstrated that a lipid mediator, S1P, regulated mucosa-associated lymphocyte trafficking of peritoneal B cells, intraepithelial T lymphocytes, and mast cells into the intestine.17-19 To this end, our recent study17 showed that S1P plays important roles in both the immigration and emigration of B cells into and from the peritoneal cavity. Our current study extends this observation by showing that NIK-mutated aly mice were less sensitive (∼5 times) to FTY720 than WT mice (Figure 1). We found that NIK-mediated signaling in stromal cells was involved in the emigration, but not immigration, step of S1P-mediated trafficking of peritoneal B cells (Figure 4). Although our results showed that the specific involvement of NIK-mediated signaling in stromal cells in the emigration of peritoneal B cells is a critical and major factor determining less sensitivity (∼5 times) of aly mice to FTY720, it is simply possible that elevated numbers of peritoneal B cells in aly mice (∼10 times) may at least partly provide additional explanation for the lower sensitivity of aly mice to FTY720.
Our current study also revealed that aly peritoneal B cells are functionally normal and therefore show normal S1P1 expression and reactivity to S1P (Figure 2). In addition, aly peritoneal B cells show normal expression of CXCR5, a receptor for CXCL13.23 In contrast, functional defects of aly stromal cells led to impaired S1P-mediated peritoneal B-cell trafficking in aly mice, although stromal cells did not express any types of S1P receptors (Figure 5 and data not shown). Regarding this issue, we found 2 possible major defects in stromal cell expression of adhesion molecules (ICAM-1 and VCAM-1) and chemokine, CXCL13. First, in agreement with previous findings that the expression of VCAM-1 and ICAM-1 was positively regulated by NFκB/NIK pathway,31,32 aly stromal cells showed reduced expression of VCAM-1 and ICAM-1, thereby weakening the attachment of peritoneal B cells to stromal cells in their trafficking pathway (Figure 5B). Second, although CXCL13 expression was decreased in the spleens of aly mice compared with WT control (Figure 5C), which is in agreement with a previous work,23 aly stromal cells showed increased CXCL13 expression compared with WT stromal cells (Figure 5C). Underlying mechanisms of the opposite effect of NIK mutation on CXCL13 expression between stromal and spleen cells remain enigmatic and represent challenges for future studies. But our current findings indicate that NIK-mediated signalings are involved in both positive and negative regulation of CXCL13 expression and which is used depends on cell types. This idea is supported by a previous report that the regulation of inflammatory cytokine–mediated CXCL13 expression was different among cells types (eg, bone marrow stromal cells and osteoblasts).33 Taken together with these facts that S1P and CXCL13 mutually regulate marginal B-cell trafficking and the S1P function is dominant to CXCL13,34 it seems that, under normal conditions, the disruption of S1P1-mediated signaling by FTY720 treatment may allow peritoneal B cells to react to the CXCL13 gradient between the peritoneal cavity and other sites (eg, spleen), leading to B-cell emigration from the peritoneal cavity through the interaction with stromal adhesion molecules (eg, ICAM-1 and VCAM-1). Our results indicated that the functional defects of stromal cells in this pathway caused decreased sensitivity of aly mice to FTY720 and thus replacement of aly stromal cells with WT rescued normal S1P-mediated emigration of peritoneal B cells.
The molecular mechanisms of peritoneal B-cell trafficking for intestinal IgA production remain enigmatic.1,2 In this regard, the enhanced expression of β7 integrin induced by peritoneal environment plays a role in establishing the commitment of peritoneal B cells to home back to the peritoneal cavity as well as migrate to the intestine.8 In addition, gut-associated dendritic cells (eg, Peyer patches and mesenteric lymph nodes) can allow B cells to migrate into the intestine by inducing the expression of α4β7 integrin and CCR9 through retinoic acid.35 However, the molecules involved in the peritoneum-dependent gut tropism of peritoneal B cells remain unknown. In a previous study,24 NIK-dependent stromal cell activation was required for the direct migration of bone marrow–derived B cells to the intestinal lamina propria but not for the migration of B cells primed in the Peyer patches. Our current results similarly suggest that the peritoneum-mediated trafficking of B cells into the intestine involves NIK-dependent stromal cells. The present study therefore shows that both NIK-dependent signaling in stromal cells and S1P were required for B-cell trafficking from the peritoneal cavity to the intestine for intestinal IgA production, especially in the step of B-cell emigration from the peritoneal cavity (Figure 4). Therefore, molecular interaction among S1P, NIK in stromal cells, and unknown gut-imprinting molecules likely uniquely coordinates the trafficking of B cells from the peritoneum into the intestine for subsequent intestinal IgA production.
Considering all previous and current findings together, we suggest that the destiny of peritoneal B cells is controlled by a pleonastic regulatory network comprising S1P, chemokines, and integrins. In this pathway, NIK-mediated signaling in stromal cells regulates the S1P-mediated emigration of B cells from the peritoneum to the intestine for subsequent production of intestinal IgA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Yoshifumi Yokota (University of Fukui, Japan) for providing Id2−/− mice. We also thank Novartis Pharma for providing FTY720.
This work was supported by grants from Core Research for Evolutional Science and Technology of the Japan Science and Technology Corporation; the Ministry of Education, Science, Sports, and Culture of Japan; the Ministry of Health and Welfare of Japan; the Waksman Foundation; Mochida Memorial Foundation for Medical and Pharmaceutical Research; and the Yakult Bio-Science Foundation.
Authorship
Contribution: J.K. and M.G. designed and performed research, analyzed data, and wrote the paper; Y.K., M.H., and I.I. performed research and analyzed data; H.K. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hiroshi Kiyono, Division of Mucosal Immunology, Department of Microbiology and Immunology, The Institute of Medical Science, University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan; e-mail: kiyono@ims.u-tokyo.ac.jp.
References
Author notes
*J.K. and M.G. contributed equally to this work.