Abstract
T-cell acute lymphoblastic leukemia (T-ALL) is mostly characterized by specific chromosomal abnormalities, some occurring in a mutually exclusive manner that possibly delineate specific T-ALL subgroups. One subgroup, including MLL-rearranged, CALM-AF10 or inv (7)(p15q34) patients, is characterized by elevated expression of HOXA genes. Using a gene expression–based clustering analysis of 67 T-ALL cases with recurrent molecular genetic abnormalities and 25 samples lacking apparent aberrations, we identified 5 new patients with elevated HOXA levels. Using microarray-based comparative genomic hybridization (array-CGH), a cryptic and recurrent deletion, del (9)(q34.11q34.13), was exclusively identified in 3 of these 5 patients. This deletion results in a conserved SET-NUP214 fusion product, which was also identified in the T-ALL cell line LOUCY. SET-NUP214 binds in the promoter regions of specific HOXA genes, where it interacts with CRM1 and DOT1L, which may transcriptionally activate specific members of the HOXA cluster. Targeted inhibition of SET-NUP214 by siRNA abolished expression of HOXA genes, inhibited proliferation, and induced differentiation in LOUCY but not in other T-ALL lines. We conclude that SET-NUP214 may contribute to the pathogenesis of T-ALL by enforcing T-cell differentiation arrest.
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is a thymocyte malignancy, and represents about 15% of pediatric patients with ALL. T-ALL often presents with a high tumor mass, accompanied by a rapid progression of disease. Still, about 30% of patients with T-ALL relapse during therapy or within the first 2 years following treatment and eventually die.1
Over the last few years, great progress has been made in unravelling the genetics of T-ALL, including recurrent chromosomal translocations (TAL1, LYL1, LMO1, LMO2, HOX11/TLX1, HOX11L2/TLX3, MYB, and Cyclin D2), deletions (SIL-TAL1, del(6q), del(9)(p21), and del(11)(p12p13)), amplifications (NUP214-ABL1), duplications (MYB), and mutations (RAS and NOTCH1).2-13 Some of these abnormalities are mutually exclusive and may delineate distinct T-ALL subgroups (ie, TAL1, LMO1, LMO2, HOX11, HOX11L2, CALM-AF10, MLL, and Inv(7)). Others are shared by some of these subgroups and may lead to the deregulation of cell cycle (ie, del(9)(p21) that includes the CDKN2A/p15 and CDKN2B/p16 loci).3,4 Some may be acquired during leukemic growth, like the episomal NUP214-ABL1 amplification.6 NOTCH1 activation mutations are present in more than half of all patients with T-ALL regardless of the presence of other rearrangements.7 It has been hypothesized that activation of NOTCH1 represents one of the most advanced abnormalities in T-ALL that may enable for uncontrolled proliferation and/or inhibition of apoptosis, possibly through up-regulation of the target genes cMYC and DELTEX1.14-16
In contrast to the wide variety of genetic abnormalities in T-ALL, initial microarray studies have revealed only 5 different expression clusters: immature/LYL1, TAL1, HOX11, HOX11L2, and HOXA clusters.8,17 One of the explanations for this phenomenon is that patients with different molecular cytogenetic defects may share a highly similar expression profile and are being recognized as one single expression cluster.8,17 For example, patients with different abnormalities demonstrate high expression of genes of the HOXA cluster (HOXA5, -A9, -A10, and -A11). This cluster includes patients with CALM-AF108,18 or MLL rearrangements,8,19 or patients with an inversion on chromosome 7 due to the rearrangement of the T-cell receptor-beta (TCRβ) locus into the HOXA cluster.8,10 Elevated HOXA gene expression levels have also been reported in the absence of these genetic aberrations,8,20 suggesting that alternative mechanisms of HOXA activation may exist in T-ALL.
Previously, we have studied the incidence and prognostic relevance of recurrent molecular cytogenetic abnormalities for pediatric T-ALL.21 Within our cohort, about half of the patients with T-ALL lack currently known molecular cytogenetic abnormalities. To identify the underlying genetic defects in these patients, we used various high-resolution genomic screening strategies, including microarray-based comparative genomic hybridization (array-CGH). We recently described a new and recurrent deletion (ie, the del(11)(p12p13)), in about 4% of patients with T-ALL.12 This interstitial deletion leads to the loss of a negative regulatory domain upstream of LMO2, resulting in ectopic expression of this oncogene. Array-CGH also led to the identification of a recurrent duplication of MYB in about 10% of patients with T-ALL.2,9
In this study, we combined gene expression profiling and array-CGH analysis to detect a new and recurrent molecular cytogenetic abnormality in patients with T-ALL that coclustered with 5 well-defined HOXA-activated T-ALL samples. We describe the cloning of a recurrent SET-NUP214 fusion product in these samples, and identified a potential mechanism by which SET-NUP214 may activate the HOXA gene cluster as potential leukemogenic event in T-ALL.
Methods
Patient samples
Viably frozen diagnostic bone marrow or peripheral blood samples from 92 pediatric patients with T-ALL and clinical and immunophenotypic data were provided by the German Cooperative Study Group for Childhood Acute Lymphoblastic Leukemia (COALL) and the Dutch Childhood Oncology Group (DCOG). The patients' parents or their legal guardians provided informed consent to use leftover material for research purposes in accordance with the Declaration of Helsinki. The Review Board of the Erasmus Medical Center approved the use of human participants in this study. Leukemic cells were isolated and enriched from these samples as previously described.12 All resulting samples contained 90% or more leukemic cells, as determined morphologically by May-Grünwald-Giemsa–stained cytospins (Merck, Darmstadt, Germany). Viably frozen T-ALL cells were used for DNA and RNA extraction, and a minimum of 5 × 106 leukemic cells were lysed in Trizol reagent (Invitrogen, Life Technologies, Breda, The Netherlands) and stored at −80°C. A total of 25 × 103 leukemic cells was used to prepare cytospin slides for fluorescence in situ hybridization (FISH) and stored at −20°C.
Genomic DNA isolation, RNA extraction, and cDNA synthesis
Genomic DNA and total cellular RNA were isolated using Trizol (Invitrogen) according to the manufacturer's protocol, with minor modifications. An additional phenol-chloroform extraction was performed, and the RNA was precipitated with isopropanol along with 1 μL (20 μg/mL) glycogen (Roche, Almere, The Netherlands). After precipitation, RNA pellets were dissolved in 20 μL RNAse-free TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]). The RNA concentration was quantified spectrophotometrically. Following a denaturation step of 5 minutes at 70°C, 1 μg RNA was reverse transcribed to single-stranded cDNA using a mix of random hexamers (2.5 μM) and oligodT primers (20 nM). The reverse transcriptase (RT) reaction was performed in a total volume of 25 μL containing 0.2 mM of each dNTP (Amersham Pharmacia Biotech, Piscataway, NJ), 200 U Moloney murine leukemia virus (M-MLV) RT (Promega, Madison, WI), and 25 U RNAsin (Promega). Conditions for the RT reaction were 37°C for 30 minutes, 42°C for 15 minutes, and 94°C for 5 minutes. The cDNA was diluted to a final concentration of 8 ng/μL and stored at −80°C.
Gene expression array analysis
Integrity of total RNA was checked using the Agilent 2100 Bio-analyzer (Agilent, Santa Clara, CA). Copy DNA and ccRNA syntheses from total RNA, hybridization of Humane Genome U133 plus 2.0 oligonucleotide microarrays (Affymetrix, Santa Clara, CA), and washing steps were performed according to the manufacturers' protocols. Probeset intensities were extracted from CEL files using GeneChip Operating Software (GCOS) version 1.4.0.036 (Affymetrix), and all arrays had a 3′ end to 5′ end GAPDH ratio lower than 3-fold. Probe intensities were normalized using the variance stabilization procedure (Bioconductor package VSN22 ; http://www.bioconductor.org/) in the statistical data analysis environment R, version 2.2.0 (http://www.r-project.org/). Differentially expressed genes between T-ALL subgroups were calculated using a Wilcoxon statistical test, and corrected for multiple testing error according to the false discovery rate procedure as developed by Hochberg and Benjaminin23 using the Bioconductor package Multtest. The fold change was calculated using the formula: e(median value group A − median value group B). Supervised clustering and principal component analyses were performed using GeneMath XT 1.6.1 software (Applied Maths, Austin, TX).
Array-CGH
Array-CGH analysis was performed, as previously described,12,24 on the human genome CGH Microarray 44A (Agilent), which consists of approximately 40 000 60-mer oligonucleotide probes that span both coding and noncoding sequences with an average spatial resolution of approximately 35 kb. Briefly, 10 μg genomic reference or patient DNA was digested overnight at 37°C with AluI (20 U) and RsaI (20 U; Invitrogen). Reference and patient DNA were purified and labeled with Cy5-dUTP and Cy3-dUTP (PerkinElmer, Wellesley, MA). Reference and patient DNA for each hybridization were pooled and mixed with 50 μg human Cot-1 DNA (Invitrogen), 100 μg yeast tRNA (Invitrogen), and 1 × hybridization control targets (SP310; Operon Technologies, Alameda, CA) in a final volume of 500 μL in situ hybridization buffer (Agilent). The hybridization mixtures was denatured at 95°C for 3 minutes, preincubated at 37°C for 30 minutes, and hybridized to the array in a microarray hybridization chamber (Agilent) for 14 to 18 hours at 65°C in a rotating oven (Robbins Scientific, Mountain View, CA) at 20 rpm. The array slides were washed in 0.5 × SSC/0.005% Triton X-102 at room temperature for 5 minutes, followed by 5 minutes at 37°C in 0.1 × SSC/0.005% Triton X-102. Slides were dried and scanned using a 2565AA DNA microarray scanner (Agilent). Microarray images were analyzed using feature extraction software (version 8.1; Agilent), and the data were subsequently imported into array-CGH analytics software v3.1.28 (Agilent). Microarray data are available at http://www.ncbi.nlm.nih.gov/geo/ (accession no. GSE10609).
FISH
FISH analysis was performed on thawed cytospin slides using the LSI BCR-ABL ES translocation probe, according to the manufacturer's protocol (Vysis, Downers Grove, IL). Cells were counterstained with DAPI/Vectashield mounting medium (Vysis). Fluorescence signals were visualized with a Zeiss Axioplan II fluorescence microscope (Zeiss, Sliedrecht, The Netherlands) using Mac Probe Software (version 4.3, Applied Imaging, Newcastle upon tyne, United Kingdom). The combined presence of a clonal del(9)(q34.11q34.13) and an episomal NUP214-ABL1 amplification in patient no. 120 was determined by FISH analysis as previously described12 using bacterial artificial chromosomes (BACs) clones RP11-83J21 (covering ABL1) and RP11-618A20 (covering ASS1, located in the deleted area between SET and ABL1). BACs were obtained from BAC/PAC Resource Center (Children's Hospital, Oakland, CA).
RT-PCR and RQ-PCR
The SET-NUP214 fusions were determined by RT–polymerase chain reaction (PCR) using forward primer 5′-TTCCCGATATGGATGATG-3′ (exon 7 SET) and reverse primer 5′-CTTTGGGCAAGGATTTG-3′ (exon 20 NUP214). PCR reactions were performed using 40 ng cDNA (8 ng/μL), 10 pmol primers, 10 nmol dNTPs, 4 mM MgCl2, 1.25 U ampliTaq gold (Applied Biosystems, Foster City, CA) in 10 × PCR buffer II (Applied Biosystems) in a total volume of 50 μL. After the initial denaturation at 94°C for 10 minutes, PCR was performed for 39 cycles of 95°C for 15 seconds, 60°C for 1 minute, and 68°C for 3 minutes. NUP214-ABL1 fusions were determined as previously described.6
Expression levels of HOXA, SET, and SET-NUP214 transcripts were quantified relative to the expression level of the endogenous housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using quantitative RT-PCR (RQ-PCR) in an ABI 7700 sequence detection system (Applied Biosystems). The HOXA primers were as described previously.8 For SET expression, the forward primer 5′-TTCCCGATATGGATGATG-3′ (exon 7 SET) and the reverse primer 5′-CCCCCCAAATAAATTGAG-3′ (exon 8 SET) were used. For SET-NUP214 expression, the primers used were as described in “RT-PCR and RQ-PCR.”
Cell culture
T-ALL cell lines (DSMZ, Braunschweig, Germany) were cultured in RPMI-1640 medium (Invitrogen) supplemented with 10% fetal calf serum (Integro, Zaandam, The Netherlands), 100 IU/mL penicillin, 100 μg/mL streptomycin, and 0.125 μg/mL fungizone (Invitrogen) and grown as suspension cultures at 37°C in humidified air containing 5% CO2. LOUCY and SKW3 cells (107) were transfected with 50 nM SET siRNA by electroporation in 400 μL RPMI 1640 with L-alanyl-L-glutamine (Invitrogen) in 4 mm electroporation cuvettes (BioRad, Hercules, CA). The SET siRNAs were located in exon 5 (5′-GAAAUCAAAUGGAAAUCUGGAAA-3′), exon 3 (5′-CGAGUCAAACGCAGAAUAA-3′), and exon 8 (5′-AGGAGAAGAUGACUAAAUA-3′). To compensate for the amount of cell death induced as a consequence of the electroporation procedure, control cells were electroporated without siRNA. Electroporation was performed using an EPI 2500 gene pulser (Fischer, Heidelberg, Germany) applying a rectangle pulse of 350 V for 10 ms. After incubating for 15 minutes at room temperature, the cells were diluted 10-fold with RPMI 1640 medium (Invitrogen) supplemented with 10% FCS (Integro), 100 IU/mL penicillin, 100 μg/mL streptomycin, and 0.125 μg/mL fungizone (Invitrogen) and incubated at 37°C and 5% CO2. Cell viability was assessed by annexinV/propidium iodide (PI) staining and determined by flow cytometry using a FACSCalibur (Becton Dickinson, San Jose, CA). Electroporation of a fluorescein isothiocyanate (FITC)–labeled siRNA (Eurogentec, Seraing, Belgium) and subsequent fluorescence-activated cell sorter (FACS) analysis indicated that transfection efficiencies were greater than 90%. Electroporation of this FITC-labeled siRNA also served as negative siRNA control.
Immunophenotyping and cell-cycle analysis was performed by multicolor flow cytometry using an LSR II flow cytometer (BD Biosciences, Franklin Lakes, NJ). A total of 2 different 6-color labeling combinations were performed: labeling 1, anti-TCRαβ (FITC), anti-TCRγδ (peridine chlorophyll protein [PE]), CD3 (phycoerythrin [PerCP]), CD4 (PE-Cy7), CD7 (allophycocyanin [APC]), and CD8 (APC-Cy7); labeling 2, CD3 (FITC), CD10 (PE), CD45 (PerCP), CyCD3 (PE-Cy7), CD5 (APC), and CD2 (APC-Cy7). Data analysis was performed using FACSDiva software version 4.1.2 (BD Biosciences).
Protein extraction and Western blot analysis
Cell pellets stored at −80°C were briefly thawed and resuspended in 50 μL lysis buffer composed of 50 mM Tris buffer, 150 mM NaCl, 100 mM EDTA, 1% Triton X-100, 2 mM PMSF, 3% aprotinine (Sigma, Zwijndrecht, The Netherlands), 4 g/mL pepstatin (Sigma), and 4 μg/mL leupeptin (Sigma). Accordingly, cells were lysed for 15 minutes on ice. The supernatant of the lysed cells was cleared by centrifugation for 15 minutes at 13 000g and 4°C. The protein content of the cleared lysates was determined using the BCA protein assay (Pierce Biotechnology, Rockford, IL) with different concentrations of bovine serum albumin as standards. Cell lysates containing 25 μg protein were separated on 10% polyacrylamide gels topped with 4% stacking gels, and transferred onto nitrocellulose membranes (Schleichler & Schuell, Dassel, Germany). Western blots were probed with mouse anti-SET (provided by K.N.) or with mouse antiactin (Sigma) antiserum. Anti-SET was used in different concentrations for proper detection of both SET (1:1000) and SET-NUP214 (1:250). Accordingly, the blots were labeled with peroxidase-conjugated anti-mouse IgG antibodies (DAKO, Glostrup, Denmark) and visualized using SuperSignal West Femto chemiluminescent substrate (Pierce Biotechnology).
IP and ChIP
For chromatin immunoprecipitation (ChIP) analysis, 20 × 106 cells were crosslinked using formaldehyde to a final concentration of 1% for 15 minutes at room temperature. Cross-linking was stopped by adding glycine to a final concentration of 0.125 M followed by 5 minutes of incubation at room temperature. Fixed cells were washed twice using ice-cold 1 × PBS and harvested in SDS buffer (100 mM NaCl, 50 mM Tris-HCl [pH 8.1], 5 mM EDTA [pH 8.0], 0.2% NaN3, and protease inhibitors). After centrifugation, the pellet was resuspended in immunoprecipitation (IP) buffer (100 mM Tris [pH 8.6], 0.3% SDS, 1.7% Triton X-100, and 5 mM EDTA), and the cells were sonicated yielding genomic DNA fragments with a size of 500 to 1000 bp. After preclearing the lysates with protein A beads (50% slurry protein A–Sepharose; Upstate, Charlottesville, VA), the samples were immunoprecipitated overnight at 4°C with affinity-purified anti-NUP214 antibodies (provided by M.F.25 ), antidimethyl H3K79 (Upstate), antiacetylated H3 (Upstate) or anti-FLAG (Sigma). The immune complexes were recovered by adding 50 μL protein A beads and were incubated for 2 hours at 4°C. Subsequently, beads were washed with low-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl [pH 8.1], and 150 mM NaCl), high-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl [pH 8.1], and 500 mM NaCL), LiCl buffer (250 mM LiCl, 1 mM EDTA, 0.5% NP-40, 10 mM Tris-HCL [pH 8.0], and 0.2% NaN3), and 1 × TE buffer (10 mM Tris-HCL [pH 8.0], and 1 mM EDTA). The immune complexes were eluted from the beads by adding elution buffer (1% SDS and 0.1M NaHCO3) for 15 minutes at room temperature. Cross-links were reversed by overnight incubation at 65°C. The eluted material was phenol/chloroform-extracted and ethanol-precipitated. The immunoprecipitated DNA was quantified by RQ-PCR using HOXA-specific promoter primers as previously described.26
For IP analysis, cells were washed twice using ice-cold 1 × PBS and lysed in a single-detergent lysis buffer (142.5 mM KCl, 5 mM MgCl2, 10 mM HEPES [pH 7.0], 1 mM EDTA, 1% NP-40, and protease inhibitors). After preclearing the lysates with protein A beads (Upstate), samples were immunoprecipitated overnight at 4°C with rabbit anti-NUP214, rabbit anti-PP32 (gift from Dr J. Brody, Johns Hopkins University, Baltimore, MD), mouse anti-SET, rabbit anti-CRM1 (gift from Dr M. Yoshida, University of Tokyo, Japan), and rabbit anti-hDOT1L (gift from Dr Yi Zhang, Lineberger Cancer Center, Chapel Hill, NC). The immune complexes were recovered by adding 50 μL protein A beads and were incubated for 2 hours at 4°C. Subsequently, beads were washed twice by single-detergent lysis buffer and twice by single-detergent lysis buffer without NP-40. The pellets were resuspended in loading buffer and boiled for 5 minutes, and Western blot analysis was performed as described in “Protein extraction and Western blot analysis.”
Results
Gene expression profiling of pediatric T-ALL subgroups
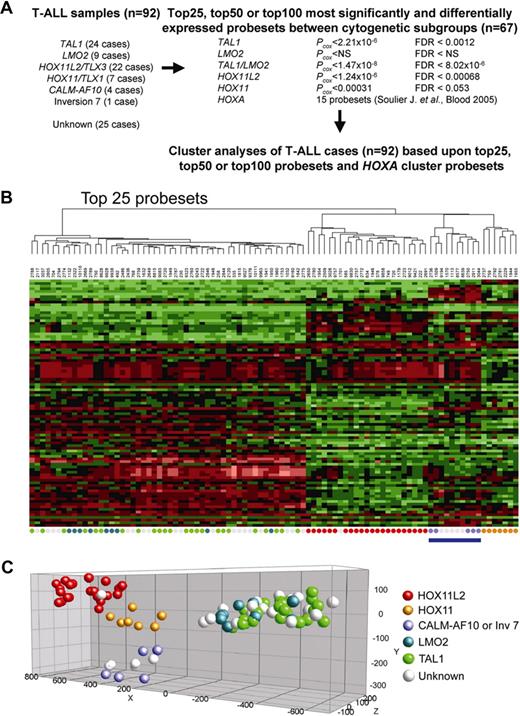
We have used gene expression profiling data to cluster 92 patients with T-ALL: 67 patients with known cytogenetic abnormalities and 25 patients without recurrent aberrations (from this point denoted as unknown patients). For the 67 patients with T-ALL who have one of the major molecular cytogenetic abnormalities (ie, TAL1 [n = 24], LMO2 [n = 9], HOXA [n = 5], HOX11/TLX1 [n = 7], and HOX11L2/TLX3 [n = 22]), differentially expressed probesets were calculated from Affymetrix U133 plus 2.0 data based upon a Wilcoxon analysis and corrected for multiple testing for each probeset. Significant and differentially expressed probesets were obtained for the TAL1, HOX11, and HOX11L2 subgroups (Figure 1A). No significant probesets were obtained for the HOXA subgroup or the LMO2 subgroup (Figure 1A). As TAL1 and LMO2 both participate in the same transcriptional complex,27 activation of these genes may both lead to a highly similar expression profile. Combined analysis of TAL1- and LMO2-rearranged patients revealed significant and differentially expressed probesets that, as expected, almost entirely overlapped with the gene signature obtained for the TAL1 subgroup only.
Gene expression profiles of 92 T-ALL patients. (A) Differentially expressed genes among the major molecular cytogenetic T-ALL subgroups (TAL1, LMO2, HOXA, HOX11/TLX1, and HOX11L2/TLX3; n = 67). The significance level (Wilcoxon P value) and FDR corrected P value for the top 100 genes in each T-ALL subgroup is indicated. TAL1, HOX11, and HOX11L2 subgroups show significant differentially expressedprobesets. (B) Cluster analysis of 92 patients with T-ALL (67 with known, 25 with unknown) based upon the top 25 most significant probesets for the TAL1, TAL1/LMO2, HOX11, and HOX11L2 subgroups combined with 15 HOXA probesets as previously described.8 (C) Principal component analyses shows clustering of the patients with unknown T-ALL along the molecular cytogenetic known patients: 1 HOX11L2-like, 19 TAL1/LMO2-like, and 5 HOXA-like patients.
Gene expression profiles of 92 T-ALL patients. (A) Differentially expressed genes among the major molecular cytogenetic T-ALL subgroups (TAL1, LMO2, HOXA, HOX11/TLX1, and HOX11L2/TLX3; n = 67). The significance level (Wilcoxon P value) and FDR corrected P value for the top 100 genes in each T-ALL subgroup is indicated. TAL1, HOX11, and HOX11L2 subgroups show significant differentially expressedprobesets. (B) Cluster analysis of 92 patients with T-ALL (67 with known, 25 with unknown) based upon the top 25 most significant probesets for the TAL1, TAL1/LMO2, HOX11, and HOX11L2 subgroups combined with 15 HOXA probesets as previously described.8 (C) Principal component analyses shows clustering of the patients with unknown T-ALL along the molecular cytogenetic known patients: 1 HOX11L2-like, 19 TAL1/LMO2-like, and 5 HOXA-like patients.
Next, we tried to cluster all 92 patients with T-ALL. Cluster analysis was performed based upon the top 25, 50, or 100 most significant probesets for the TAL1, TAL1/LMO2, HOX11, and HOX11L2 subgroups combined with 15 HOXA probesets identified by Soulier et al8 (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article; Figure 1A,B). Cluster and principal component analyses (PCAs) led to a stable clustering of patients with unknown T-ALL with specific molecular cytogenetic subgroups (Figure 1B,C; Figure S1; Table S2): one patient clustered with HOX11L2-rearranged patients, and this patient uniquely highly expressed the HOX11L2 homologous gene HOX11L1 (data not shown). A total of 19 unknown patients tightly clustered with TAL1- or LMO2-rearranged patients. FISH analysis (not shown) revealed TAL1 and/or LMO2 homologous rearrangements to the TCRβ or TCRαδ loci in 5 of these 19 patients (ie, TAL2 [1 patient]; LMO1 [1 patient]; TAL2/LMO1 [1 patient]; and cMYC [2 patients] in line with karyotypic data. Another 5 unknown T-ALL samples formed a separate cluster with the 5 patients with HOXA-type T-ALL (Figure 1B,C; Table S2), and will be denoted as HOXA-like samples.
New recurrent deletion, del(9)(q34.11q34.13), in HOXA-like T-ALL samples
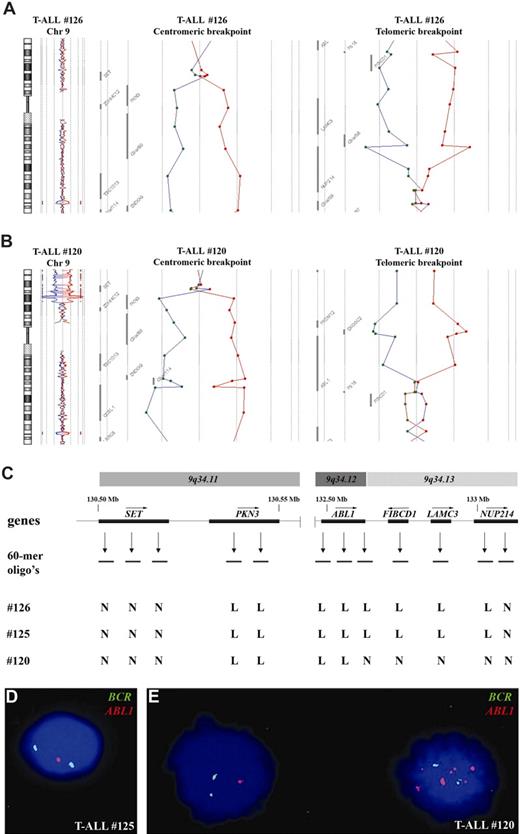
To identify new chromosomal abnormalities in the 5 HOXA-like patients, we screened these patients using oligonucleotide array-CGH. A one-copy loss of an approximately 3-Mb region involving chromosomal band 9q34.11 to 9q34.13 was identified in 3 of 5 HOXA-like patients (patient nos. 126, 125, and 120; Figure 2A-C). Detailed analysis of the centromeric breakpoints in these 3 patients revealed a breakpoint within or in the vicinity of the SET gene. The PKN3 gene just telomeric to SET was consistently lost in all 3 patients (Figure 2A-C). The telomeric breakpoint was located in the NUP214/CAN gene in 2 patients (nos. 125 and 126; Figure 2A-C), whereas the telomeric breakpoint of patient no. 120 seemed situated in the ABL1 oncogene (Figure 2B,C).
Submicroscopic del(9)(q34.11q34.13) in T-ALL. (A) Chromosome 9 ideogram and corresponding oligo array-CGH plots of test DNA–control DNA ratios (blue tracing) versus the dye-swap experiment (red tracing) for patient no. 126. Detailed analyses of the centromeric and telomeric breakpoints show involvement of SET and NUP214. (B) Similar array-CGH plot for patient no. 120. Centromeric and telomeric breakpoints show involvement of SET and ABL1. (C) Overview of oligoarray-CGH results in the potential breakpoint regions for 3 patients with T-ALL with del(9)(q34.11q34.13). The 60-mer oligonucleotide probes present on the array-CGH slide and located in the telomeric and centromeric breakpoint regions, as well as the specific genes located in this region with their transcription direction, are shown. Abbreviations: N; normal, L; loss. Dual-color FISH analysis of patient no. 125 (D) and no. 120 (E) using the LSI BCR-ABL ES translocation probe.
Submicroscopic del(9)(q34.11q34.13) in T-ALL. (A) Chromosome 9 ideogram and corresponding oligo array-CGH plots of test DNA–control DNA ratios (blue tracing) versus the dye-swap experiment (red tracing) for patient no. 126. Detailed analyses of the centromeric and telomeric breakpoints show involvement of SET and NUP214. (B) Similar array-CGH plot for patient no. 120. Centromeric and telomeric breakpoints show involvement of SET and ABL1. (C) Overview of oligoarray-CGH results in the potential breakpoint regions for 3 patients with T-ALL with del(9)(q34.11q34.13). The 60-mer oligonucleotide probes present on the array-CGH slide and located in the telomeric and centromeric breakpoint regions, as well as the specific genes located in this region with their transcription direction, are shown. Abbreviations: N; normal, L; loss. Dual-color FISH analysis of patient no. 125 (D) and no. 120 (E) using the LSI BCR-ABL ES translocation probe.
The presence of the del(9)(q34.11q34.13) in patient nos. 125 and 126 was confirmed by FISH using the LSI BCR-ABL ES translocation probe, resulting in a single-copy loss of the ABL1 gene (Figure 2D). Strikingly, FISH analysis on patient no. 120 also revealed an identical monoallelic loss of ABL1 in all leukemic cells. However, an episomal NUP214-ABL1 amplification6 was detected in a leukemic subclone comprising approximately 5% of the total leukemic cell population (Figure 2E). Subsequent FISH analysis confirmed that the episomal NUP214-ABL1 amplification as well as the del(9)(q34.11q34.13) were both present in this subclone (data not shown). The combined presence of a clonal del(9)(q34.11q34.13) in combination with an episomal NUP214-ABL1 amplification in a leukemic subclone in this patient explains why the telomeric breakpoint of the del(9)(q34.11q34.13) seemed situated in the ABL1 gene according to the array-CGH data. Additional FISH screening of the remaining 87 patients with T-ALL did not reveal other patients with this same deletion.
SET-NUP214 fusion in del(9)(q34.11q34.13)+ patients
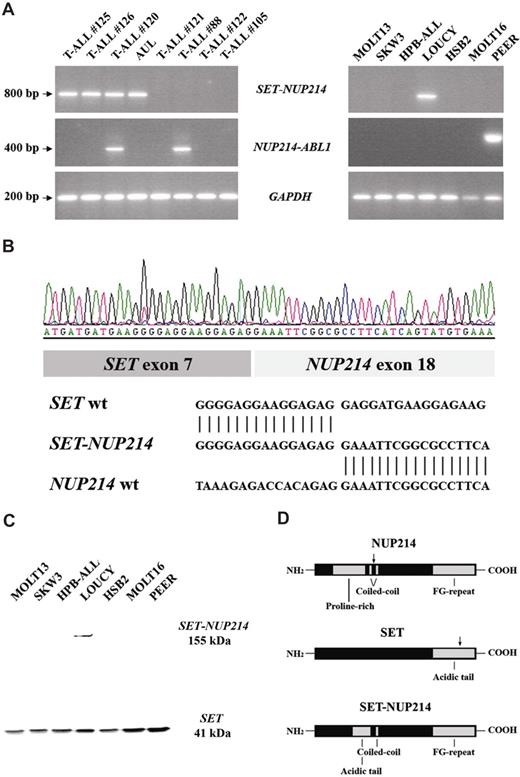
Subsequent RT-PCR analysis to amplify a potential SET-NUP214 fusion product using a SET forward primer (exon 7) in combination with a NUP214 reverse primer (exon 20) revealed an SET-NUP214 fusion product in all 3 del(9)(q34.11q34.13)+ patients with T-ALL that we also identified in the T-ALL cell line LOUCY28 (Figure 3A). A similar SET-NUP214 fusion product due to the reciprocal chromosomal translocation t(9;9)(q34;q34) has been described previously for a patient with an acute undifferentiated leukemia (AUL) by Von Lindern et al.29 Material from this patient with AUL was still available, and RT-PCR analysis revealed a SET-NUP214 fusion product in this patient (Figure 3A). Sequence analyses of the SET-NUP214 PCR products confirmed that these 3 patients with T-ALL and the patient with AUL,29 as well as the cell line LOUCY, all had an identical fusion product fusing SET at exon 7 with the NUP214 gene at exon 18 (Figure 3B). Additional oligonucleotide array-CGH analysis further confirmed that the SET-NUP214 fusion in the LOUCY cell line was indeed due to the presence of a del(9)(q34.11q34.13). This deletion was not present in the patient with AUL, confirming that the SET-NUP214 fusion was the result of a balanced t(9;9)(q34;q34) in this patient.29
SET-NUP214 fusion transcript in T-ALL. (A) RT-PCR analysis using SET- and NUP214-specific primers and GAPDH primers as internal control, reveals a specific SET-NUP214 fusion gene in T-ALL patient nos. 125, 126, and 120; the patient with AUL; and the T-ALL cell line LOUCY. NUP214-ABL1 fusion was detected in patient nos. 120 and no.88 and in the T-ALL cell line PEER (B) Sequence analysis confirmed an identical fusion between exon 7 of SET and exon 18 of NUP214 in all SET-NUP214+ patients with T-ALL, the patient with AUL, and the LOUCY cell line. (C) Western blot analysis of T-ALL cell lines revealed a SET-NUP214 fusion in the cell line LOUCY. (D) At the protein level, the breakpoints are situated in the acidic tail of SET and the coiled-coil domain of NUP214.
SET-NUP214 fusion transcript in T-ALL. (A) RT-PCR analysis using SET- and NUP214-specific primers and GAPDH primers as internal control, reveals a specific SET-NUP214 fusion gene in T-ALL patient nos. 125, 126, and 120; the patient with AUL; and the T-ALL cell line LOUCY. NUP214-ABL1 fusion was detected in patient nos. 120 and no.88 and in the T-ALL cell line PEER (B) Sequence analysis confirmed an identical fusion between exon 7 of SET and exon 18 of NUP214 in all SET-NUP214+ patients with T-ALL, the patient with AUL, and the LOUCY cell line. (C) Western blot analysis of T-ALL cell lines revealed a SET-NUP214 fusion in the cell line LOUCY. (D) At the protein level, the breakpoints are situated in the acidic tail of SET and the coiled-coil domain of NUP214.
RT-PCR analysis also confirmed an episomal NUP214-ABL1 fusion product present in patient no. 120, as well as in control patient material with an episomal NUP214-ABL1 amplification (Figure 3A; patient no. 88). Sequence analysis confirmed an in-frame fusion of NUP214 exon 31 to exon 2 of ABL1 for patient no. 120.
As expected, the presence of a SET-NUP214 fusion protein was detected by Western blotting in LOUCY (Figures 3C), but was absent in other T-ALL cell lines lacking the del(9)(q34.11q34.13). For all patients described, the breakpoints are situated in the acidic tail of SET and the coiled-coil domain of NUP214, generating an in-frame fusion protein with a molecular weight of approximately 155 kDa (Figure 3D).
Clinical and genetic patient characteristics (ie, NOTCH1 mutation status and additional aberrations detected by array-CGH) of the SET-NUP214+ patients with T-ALL and the LOUCY cell line are summarized in Table 1.
Elevated HOXA levels in SET-NUP214+ patients
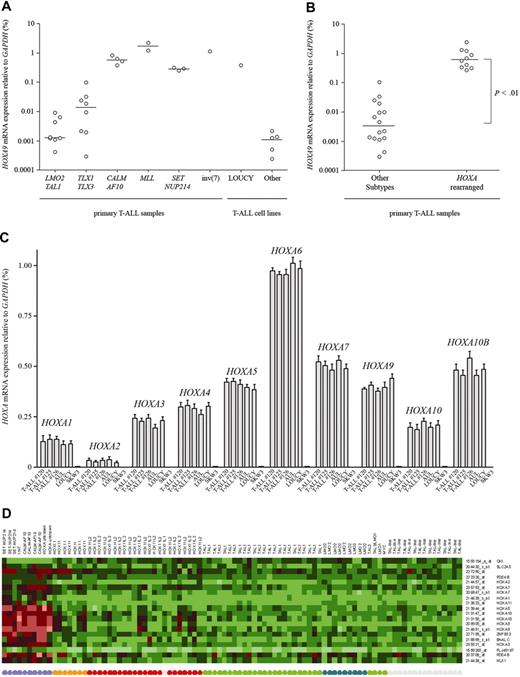
To confirm the clustering of these 3 del(9)(q34.11;q34.13)+ patients within the HOXA cluster based upon the most significant and differentially expressed probesets between the major T-ALL subgroups, we analyzed the expression of the HOXA gene cluster using RQ-PCR. As a control, we also determined the expression levels for these genes in various other patient samples representing other T-ALL subgroups. As described previously,8,10,18,19 MLL-rearranged cases (n = 2), CALM-AF10+ cases (n = 4), and a patient with an inv(7)(p15q34) mutation all highly expressed most genes from the HOXA cluster in contrast to TAL1-, LMO2-, HOX11L2-, or HOX11-rearranged patients (P < .01; Figure 4A,B; only results for HOXA9 are shown). All 3 SET-NUP214+ patients with T-ALL as well as the LOUCY cell line also highly expressed the HOXA cluster of genes. Other T-ALL cell lines, including MOLT13, SKW3, HPB-ALL, HSB2, and PEER, did not express the HOXA gene cluster. Although most HOXA genes were highly expressed in LOUCY, in the patient with AUL and in the SET-NUP214+ patients with T-ALL, expression of HOXA11 and HOXA13 was virtually absent. In addition, the expression of the short HOXA10 isoform, HOXA10B, which previously has been exclusively associated with patients with inv(7)(p15q34) T-ALL,8,20 was also highly expressed in the SET-NUP214+ patients (Figure 4C).
HOXA activation in SET-NUP214+ T-ALL. (A) Relative HOXA9 expression levels by RQ-PCR for MLL-rearranged, CALM-AF10+, inv(7)(p15q34), TAL1-, LMO2-, HOX11L2-, or HOX11-rearranged patients and T-ALL cell lines including LOUCY, MOLT13, SKW3, HPB-ALL, HSB2, and PEER. (B) Comparison of HOXA9 expression levels between the HOXA T-ALL subgroup (MLL, CALM-AF10, inv(7)(p15q34), and SET-NUP214; n = 10) and other T-ALL subgroups (TAL1, LMO2, HOX11L2, or HOX11). The horizontal lines represent the medians. (C) Relative expression levels of HOXA genes by RQ-PCR for the 3 SET-NUP214+ patients with T-ALL, the LOUCY cell line, the patient with AUL, and SKW3. (D) Heatmap of the 20 significant and differentially expressed probesets with a false discovery rate (FDR) lower than 5% for the HOXA cluster compared with the other patients with T-ALL.
HOXA activation in SET-NUP214+ T-ALL. (A) Relative HOXA9 expression levels by RQ-PCR for MLL-rearranged, CALM-AF10+, inv(7)(p15q34), TAL1-, LMO2-, HOX11L2-, or HOX11-rearranged patients and T-ALL cell lines including LOUCY, MOLT13, SKW3, HPB-ALL, HSB2, and PEER. (B) Comparison of HOXA9 expression levels between the HOXA T-ALL subgroup (MLL, CALM-AF10, inv(7)(p15q34), and SET-NUP214; n = 10) and other T-ALL subgroups (TAL1, LMO2, HOX11L2, or HOX11). The horizontal lines represent the medians. (C) Relative expression levels of HOXA genes by RQ-PCR for the 3 SET-NUP214+ patients with T-ALL, the LOUCY cell line, the patient with AUL, and SKW3. (D) Heatmap of the 20 significant and differentially expressed probesets with a false discovery rate (FDR) lower than 5% for the HOXA cluster compared with the other patients with T-ALL.
From the expression microarray data, the most significant and differentially expressed probesets were calculated for the entire HOXA cluster. A total of 20 significant and differentially expressed probesets with a FDR rate lower than 5% were obtained for this cluster (Figure 4D). Various of these probesets encoding for QKI, HOXA5, HOXA9 (2 probesets), HOXA10 (2 probesets), and HOXA11 were also previously found to be differentially expressed within MLL-rearranged19 or CALM-AF10–rearranged18 patients with T-ALL or in patients with T-ALL belonging to the HOXA subgroup.8
SET-NUP214 activates HOXA expression, increases cellular proliferation, and inhibits cellular differentiation
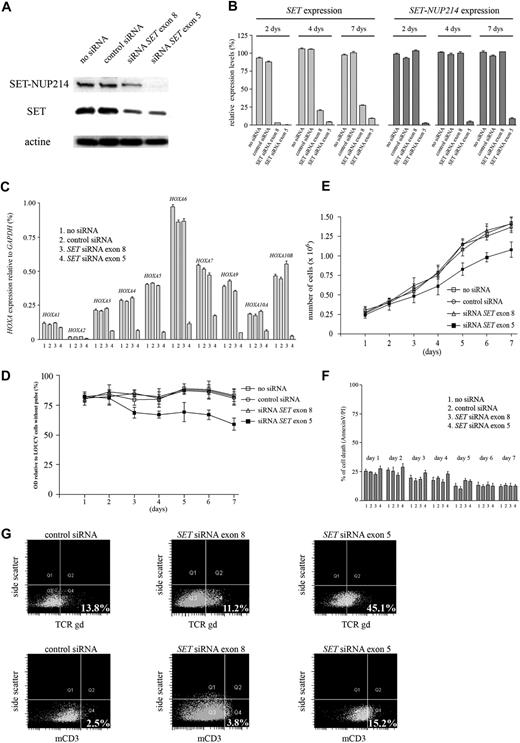
To study the role of the SET-NUP214 fusion transcript in the pathogenesis of T-cell leukemia and its contribution to the activation of the HOXA gene cluster, SET and SET-NUP214 expression were specifically down-regulated in the LOUCY cell line by electroporation of SET-specific siRNAs (Figure 5). Protein expression of SET and SET-NUP214 was specifically reduced using a SET siRNA directed against exon 5, whereas only SET but not SET-NUP214 was down-regulated using a SET siRNA directed against exon 8 (Figure 5A). SET and/or SET-NUP214 mRNA expression levels were specifically targeted, and this effect was sustained for more than 7 days following transfection of SET siRNAs (Figure 5B). Specific down-regulation of both SET and SET-NUP214 resulted in significant reduction in the expression levels of the HOXA gene cluster, while knockdown of SET but not SET-NUP214 had no effect (Figure 5C). This confirms that SET-NUP214, or the combination of SET and SET-NUP214, specifically up-regulates the expression of HOXA genes. Knockdown of SET-NUP214 also reduced cellular proliferation (Figure 5D,E), but the percentage of apoptotic cells did not change over time following inhibition of SET-NUP214 (Figure 5F). In addition, SET-NUP214 down-regulation resulted in the up-regulation of both TCRγδ and membrane CD3 expression in LOUCY (Figure 5G), indicating that repression of SET-NUP214 enforces differentiation. Down-regulation of SET by anti-SET siRNA exon 8 had no effect (Figure 5G). The effects of SET-NUP214 knockdown (HOXA inhibition, mild reduced cell proliferation, and induction of differentiation) were confirmed using a second SET siRNA directed against exon 3 (Figure S3).
siRNA knockdown of SET and SET-NUP214 in the LOUCY T-ALL cell line. (A) SET expression using Western blot analysis 4 days after electroporation with the following conditions: no siRNA, control siRNA, siRNA SET exon 8, or siRNA SET exon 5. (B) Relative SET and SET-NUP214 expression by RQ-PCR after 2, 4, and 7 days for conditions as mentioned in panel A. (C) Relative expression of all members of the HOXA clusters by RQ-PCR (except for HOXA11 and HOXA13) after 4 days for conditions as mentioned in panel A. (D) Optical density (OD) values relative to control cells without pulse after 7 days for conditions as mentioned in panel A. (E) Total cell numbers after 7 days for conditions as mentioned in panel A. (F) Percentage of cell death relative to control cells without pulse after 7 days for conditions as mentioned in panel A. Error bars visualize the standard error of the mean (SEM). (G) FACS analysis 6 days after electroporation with either no siRNA, siRNA SET exon 8, or siRNA SET exon 5 for TCRγδ and membrane CD3. The percentage of cells positive for TCRγδ or mCD3 expression are visualized in quadrant 4.
siRNA knockdown of SET and SET-NUP214 in the LOUCY T-ALL cell line. (A) SET expression using Western blot analysis 4 days after electroporation with the following conditions: no siRNA, control siRNA, siRNA SET exon 8, or siRNA SET exon 5. (B) Relative SET and SET-NUP214 expression by RQ-PCR after 2, 4, and 7 days for conditions as mentioned in panel A. (C) Relative expression of all members of the HOXA clusters by RQ-PCR (except for HOXA11 and HOXA13) after 4 days for conditions as mentioned in panel A. (D) Optical density (OD) values relative to control cells without pulse after 7 days for conditions as mentioned in panel A. (E) Total cell numbers after 7 days for conditions as mentioned in panel A. (F) Percentage of cell death relative to control cells without pulse after 7 days for conditions as mentioned in panel A. Error bars visualize the standard error of the mean (SEM). (G) FACS analysis 6 days after electroporation with either no siRNA, siRNA SET exon 8, or siRNA SET exon 5 for TCRγδ and membrane CD3. The percentage of cells positive for TCRγδ or mCD3 expression are visualized in quadrant 4.
SET-NUP214 directly activated HOXA expression, possibly by recruitment of DOT1L
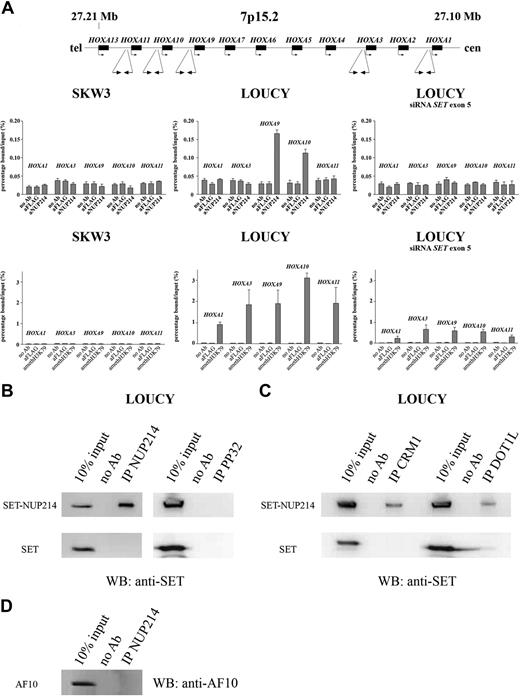
Our siRNA-mediated knockdown experiments indicated that SET-NUP214 regulates the transcription of the HOXA gene cluster. To investigate whether this activation was caused by direct interaction of SET-NUP214 with HOXA promoter sequences, ChIP analyses with the LOUCY cell line and the negative control cell line SKW3 were performed. No enrichment of HOXA (HOXA1, HOXA3, HOXA9, HOXA10, and HOXA11) promoter sequences was detected in NUP214 immunoprecipitates obtained for SKW3 control cells (Figure 6A). For LOUCY cells, HOXA9 and HOXA10 promoter sequences were enriched in NUP214 immunoprecipitates, but not HOXA1, HOXA3, and HOXA11 promoter sequences, indicating that SET-NUP214 may only bind to specific members of the HOXA cluster (Figure 6A). Enrichment of HOXA9 and HOXA10 promoter sequences in the ChIP analysis could be completely reversed using SET siRNA molecules directed against exon 5.
ChIP and coIP analysis of T-ALL cell lines LOUCY and SKW3. (A) ChIP analysis of SKW3, LOUCY, and LOUCY 4 days after electroporation with siRNA SET exon 5, for promoter sequences of HOXA1, HOXA3, HOXA9, HOXA10, and HOXA11. The amount of bound DNA was calculated relative to the 5% input DNA in anti-NUP214 and anti-acetyl histone H3 immunoprecipitates, whereas no antibody and anti-FLAG immunoprecipitates were used as negative control. Error bars represent SEM. (B) Western blot analysis of NUP214 and PP32 immunoprecipitates of the cell line LOUCY using anti-SET. (C) Similar Western blot analysis as in panel B for CRM1 and DOT1L immunoprecipitates in LOUCY. (D) Western blot analysis of NUP214 immunoprecipitates of the cell line LOUCY using anti-AF10.
ChIP and coIP analysis of T-ALL cell lines LOUCY and SKW3. (A) ChIP analysis of SKW3, LOUCY, and LOUCY 4 days after electroporation with siRNA SET exon 5, for promoter sequences of HOXA1, HOXA3, HOXA9, HOXA10, and HOXA11. The amount of bound DNA was calculated relative to the 5% input DNA in anti-NUP214 and anti-acetyl histone H3 immunoprecipitates, whereas no antibody and anti-FLAG immunoprecipitates were used as negative control. Error bars represent SEM. (B) Western blot analysis of NUP214 and PP32 immunoprecipitates of the cell line LOUCY using anti-SET. (C) Similar Western blot analysis as in panel B for CRM1 and DOT1L immunoprecipitates in LOUCY. (D) Western blot analysis of NUP214 immunoprecipitates of the cell line LOUCY using anti-AF10.
Additional ChIP analysis using antiacetyl histone H3 (Figure S2)– and antidimethyl histone H3K79 (Figure 6A)–specific antibodies revealed histone H3 acetylation and histone H3K79 methylation of HOXA1, HOXA3, HOXA9, HOXA10 and HOXA11 promoters in the LOUCY cell line, which was absent in SKW3. This further strengthens the idea that binding of SET-NUP214 as a specific transcriptional cofactor for some HOXA gene members may result in an open chromatine structure of the entire HOXA cluster. Upon SET-NUP214 inactivation, the histone H3K79 methylation was partially lost (Figure 6A), indicating that sustained histone H3K79 methylation of the HOXA locus depended on the presence of SET-NUP214. The level off histone H3 acetylation of the HOXA locus remained unaltered within the time frame of the experiment (Figure S2). As SET normally associates with the HOXA gene cluster30 and has an inhibitory role on gene transcription as part of the INHAT complex,31 we investigated whether SET-NUP214 may substitute for SET in this complex, rendering this complex inactive. However, IP experiments failed to demonstrate a direct interaction between SET-NUP214 and components of the INHAT complex (ie, SET and PP32; Figure 6B).
CALM-AF10, MLL-AF10, MLL-ENL, and MLL-AF4 fusion proteins have been shown to recruit the methyltransferase DOT1L, leading to aberrant methylation of histone H3 at lysine 79, thereby facilitating transcriptional activation of the HOXA gene cluster.32-35 For CALM-AF10 and MLL-AF10, this interaction with DOT1L depends on the octapeptide and leucine zipper domain (OM-LZ region) in the AF10 part of these fusion proteins.32,33 With respect to the histone H3–K79 methylation of the HOXA locus in our SET-NUP214+ patients, we could demonstrate an interaction between SET-NUP214 and DOT1L in vivo (Figure 6C). However, this interaction seemed independent of the presence of AF10, since an interaction between SET-NUP24 and AF10 could not be demonstrated in LOUCY (Figure 6D). Reciprocal DOT1L IP experiments to study AF10 involvement could not be performed, as suitable DOT1L antibodies for IP experiments are not available. Whether the interaction between DOT1L and SET-NUP214 is a direct interaction or depends on the participation of additional proteins remains to be established. As shown by others,36,37 we could confirm an in vivo interaction between SET-NUP214 and CRM1 (Figure 6C), which normally binds to the FG-repeat of wild-type NUP214 as part of the nuclear pore complex.36
Discussion
Gene expression profiling studies in T-ALL have shown that patients with a CALM-AF10 translocation, an MLL rearrangement, or an inv(7)(p15q34) mutation share a gene expression signature characterized by elevated expression levels of HOXA genes. Cluster analysis of 25 patients with T-ALL lacking known cytogenetic abnormalities with 67 cytogenetically well-characterized patients led to the identification of 5 patients with unknown disease that clustered with HOXA-activated samples having CALM-AF10 translocations or an inv(7)(p15q34) mutation.
Subsequent array-CGH analysis revealed an identical interstitial deletion on chromosome 9 [ie, the del(9)(q34.11q34.13)], in 3 of 5 patients as a novel chromosomal aberration in pediatric T-ALL that we also identified in the T-ALL cell line LOUCY. This deletion gives rise to a similar SET-NUP214 fusion gene in all cases, and was identical to a SET-NUP214 fusion as described 15 years ago for a single acute undifferentiated leukemia patient with a reciprocal translocation t(9;9)(q34;q34),29,38 and most recently for a single patient with acute myeloid leukemia.39
We studied the role of SET-NUP214 in the pathogenesis of T-ALL by siRNA-mediated knockdown of SET-NUP214 in the LOUCY cell line. Down-regulation of SET-NUP214 reduced HOXA expression levels, indicating that SET-NUP214 could function as a transcriptional regulator of the HOXA gene cluster. Our ChIP data in fact provide evidence that SET-NUP214 directly interacts with the promotor regions of specific HOXA members itself, especially with HOXA9 and HOXA10, and therefore may function as a transcriptional coactivator. SET-NUP214 does not bind to promotor sequences of HOXA1, HOXA3, HOXA11, and possibly others despite their SET-NUP214 dependency for transcriptional activation in LOUCY. Binding of SET-NUP214 to HOXA9 and HOXA10 promoter regions presumably may lead to an open chromatin structure and transcriptional activation of the entire HOXA cluster.
Based upon the ChIP analyses using antiacetylated histone H3 and antidimethyl histone H3–K79 before and after siRNA-mediated SET-NUP214 knockdown, we hypothesize that binding of SET-NUP214 at the HOXA promoters may facilitate the recruitment of the H3–K79 methyltransferase DOT1L, resulting in local H3–K79 methylation as a first direct chromatine modification effect. This was supported by our data, in which histone H3–K79 methylation of the HOXA locus was partially lost upon inhibition of SET-NUP214 within the (limited) time frame of the experiment. This modification may further trigger the acquisition of other “open state” chromatin modifications, such as H3-acetylation possibly enabling the recruitment of other transcriptional cofactors and transcriptional activation of various HOXA gene members.
For MLL-AF10 and CALM-AF10 fusion proteins, it has already been demonstrated that the oncogenicity of these proteins depends on binding of DOT1L to the OM-LZ region of AF10.32,33 MLL-AF10 and CALM-AF10 both bind to the promoter regions of the HOXA gene cluster, and it was shown that recruitment of DOT1L results in aberrant methylation of Lys79 in histone H3 and transcriptional activation of especially HOXA9 for MLL-AF1033 and HOXA5 for CALM-AF10.32 In addition, recent reports also show interaction of DOT1L with the fusion proteins MLL-ENL and MLL-AF4.34,35 Our data suggest that HOXA9 may also represent a bona fide target of the CALM-AF10 fusion protein, as HOXA9 is highly expressed in CALM-AF10+ T-ALL cells (Dik et al18 and this study). We propose a similar mechanism for SET-NUP214 in the activation of HOXA genes, and HOXA9/HOXA10 in particular, as we could demonstrate binding of DOT1L to the SET-NUP214 fusion protein. An OM-LZ region as present in AF1033 was not identified in SET-NUP214, and therefore another OM-LZ–like region in SET-NUP214 or an indirect interaction between SET-NUP214 and DOT1L may be facilitated by other SET-NUP214–interacting proteins. In this respect, we confirmed that CRM1 also binds to SET-NUP214,37 possibly to the FG repeats as retained in this fusion protein.
SET-NUP214 is highly similar to the DEK-NUP214 fusion as previously identified in t(6;9)(p23;q34)+ patients with AML.40 As DEK-NUP214 AML samples also have an activated HOXA gene signature (P. J. Valk, oral communication, January 2008), DEK-NUP214 may function in a similar fashion compared with SET-NUP214 by binding to the promotor regions of specific HOXA gene members in t(6;9)(p23;q34)+ patients with AML.
SiRNA knockdown experiments in LOUCY led to complete absence of SET-NUP214 and down-regulation of HOXA expression levels that sustained for more than 7 days. Ablation of SET-NUP214 had a mild effect on cellular proliferation without inducing apparent apoptosis in this timeframe. In fact, inhibition of SET-NUP214 may have resulted in cellular differentiation and promoted mCD3 and TCRγδ expression. Our results are in agreement with previous data by others in which overexpression of SET-NUP214 inhibits differentiation in vitro41 as well as in vivo.42
During normal T-cell development, HOXA expression (HOXA7, HOXA9, HOXA10) is restricted to the earliest stages of differentiation.43,44 We therefore propose that SET-NUP214 will sustain HOXA gene expression and therefore impair T-cell differentiation. This differentiation arrest may encourage the acquisition of additional genetic hits, eventually leading toward the development of T-cell leukemia. In mouse studies, overexpression of Hoxa10 inhibits both myeloid and lymphoid cell differentiation,45 whereas overexpression of Hoxa9 results in defective T-cell development.46
T-cell leukemia depends on multistep pathogenic events.3-5 Cooperative genetic abnormalities affecting cell cycle and proliferation, differentiation, and survival initiate leukemic transformation of thymocytes. Since SET-NUP214 fails to cause T-ALL in transgenic mice,42,47 additional mutations will be required for the induction of T-cell leukemia. We identified a number of cooperative aberrations in the SET-NUP214+ T-ALL samples. NOTCH1 mutations, generally present in about 50% of T-ALL,7 were found in all 3 SET-NUP214+ T-ALL samples. Besides the SET-NUP214 fusion (differentiation arrest) and NOTCH1 mutations, patient no. 120 also showed a homozygous CDKN2A/CDKN2B deletion (cell-cycle defect) and an episomal NUP214-ABL1 amplification (proliferation and survival), showing the multiple molecular pathways that are involved in the pathogenesis of T-ALL.4 It is remarkable that in this patient, 2 different genetic rearrangements (SET-NUP214 and NUP214-ABL1) target the same gene (NUP214) in a single leukemic cell. The SET-NUP214 fusion was present as a clonal genetic rearrangement present in all leukemic cells, whereas NUP214-ABL was only present in a leukemic subclone. So SET-NUP214 probably acts as a primary oncogenic event, whereas NUP214-ABL1 rather functions as a further dedifferentiating event in T-ALL. In patient no. 125 and the LOUCY cell line, terminal deletions of chromosome 16 were identified (Table 1). Because these 16p deletions were not previously identified as a recurrent abnormality in T-ALL,48 it is likely that they cooperate in SET-NUP214–mediated leukemogenesis. Nevertheless, the target genes of this aberration remain to be identified.
In conclusion, we identified SET-NUP214 as a novel recurrent fusion gene in T-cell leukemia. Our experiments show that SET-NUP214 may contribute to T-ALL pathogenesis by inhibition of T-cell maturation through the transcriptional activation of the HOXA genes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by the Ter Meulen Fund, Royal Netherlands Academy of Arts and Sciences, and the Foundation “De Drie Lichten.” P.V.V. is financed by the Sophia Foundation for Medical Research (SSWO-440), M.v.G. was financially supported by the Quality of Life and KOCR foundations, C.L. was supported in part by a National Cancer Institute grant (CA11560) and a Leukemia and Lymphoma Society Translational grant (6161-05), and J.T. was supported by a German Research Foundation Fellowship Award (Tc-57/1-1).
National Institutes of Health
Authorship
Contribution: P.V.V., J.T., and M.v.G. designed and performed research and wrote the paper; C.L. collaborated on the array-CGH study and wrote the paper; E.v.W. and M.H. collected and made available patient samples; J.G. performed and designed FISH analysis; P.J.S. and A.S. analyzed gene expression data; K.N. and M.F. provided antisera; J.C. and J.S. designed research and wrote the paper; and J.P.P.M, H.B.B., and R.P. wrote the grant, designed research, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jules P. P. Meijerink, Erasmus MC/Sophia Children's Hospital, Department of Pediatric Oncology/Hematology, Rm Sp 2456, Dr Molewaterplein 60, PO Box 2060, 3000 CB Rotterdam, the Netherlands; e-mail: j.meijerink@erasmusmc.nl.