Abstract
WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome is an immune deficiency linked in many cases to heterozygous mutations causing truncations in the cytoplasmic tail of CXC chemokine receptor 4 (CXCR4). Leukocytes expressing truncated CXCR4 display enhanced responses to the receptor ligand CXCL12, including chemotaxis, which likely impair their trafficking and contribute to the immunohematologic clinical manifestations of the syndrome. CXCR4 desensitization and endocytosis are dependent on β-arrestin (βarr) recruitment to the cytoplasmic tail, so that the truncated CXCR4 are refractory to these processes and so have enhanced G protein–dependent signaling. Here, we show that the augmented responsiveness of WHIM leukocytes is also accounted for by enhanced βarr2-dependent signaling downstream of the truncated CXCR4 receptor. Indeed, the WHIM-associated receptor CXCR41013 maintains association with βarr2 and triggers augmented and prolonged βarr2-dependent signaling, as revealed by ERK1/2 phosphorylation kinetics. Evidence is also provided that CXCR41013-mediated chemotaxis critically requires βarr2, and disrupting the SHSK motif in the third intracellular loop of CXCR41013 abrogates βarr2-mediated signaling, but not coupling to G proteins, and normalizes chemotaxis. We also demonstrate that CXCR41013 spontaneously forms heterodimers with wild-type CXCR4. Accordingly, we propose a model where enhanced functional interactions between βarr2 and receptor dimers account for the altered responsiveness of WHIM leukocytes to CXCL12.
Introduction
The G-protein–coupled receptor (GPCR) CXC chemokine receptor 4 (CXCR4) and its ligand, the stromal cell-derived factor-1 (CXCL12/SDF-1), regulate leukocyte hematopoiesis and trafficking.1 They initiate signal transduction by activating heterotrimeric Gαβγ-proteins, which then act on effectors to trigger downstream cellular responses.2 CXCL12 also elicits processes causing receptor desensitization, which results in the uncoupling from G-proteins and involves phosphorylation of the CXCR4 cytoplasmic tail (C-tail) by protein kinase C and GPCR kinases (GRKs) and interaction of the phosphorylated receptor with β-arrestins (βarrs).3-5 βarrs then target desensitized CXCR4 to clathrin-coated pits for endocytosis. βarrs also link GPCRs to the stimulation of additional signaling molecules, including members of the mitogen-activated protein kinase (MAPK) family.6 Studies on CXCR4 have demonstrated that β-arrestin2 (βarr2) strengthens activation of the p38 and extracellular signal-regulated kinase (ERK) MAPKs and CXCL12-induced chemotaxis.5,7,8
WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome (WS) is a rare immunodeficiency disease linked to CXCR4 dysfunctions and is characterized by warts, recurrent bacterial infections, hypogammaglobulinemia, lymphopenia, and myelokathexis, a severe neutropenia with abnormal retention of mature neutrophils in the bone marrow (BM).9,10 WS, most often inherited as an autosomal-dominant trait, is linked in many cases to heterozygous mutations in CXCR4, so that receptors with truncations of the C-tail are expressed and likely coexist with wild-type receptors (CXCR4wt) in patients' cells.11-13 WHIM leukocytes have enhanced responses to CXCL12,11,12 and this might affect their trafficking and thus contribute to the clinical manifestations of the WS. For instance, WHIM neutrophils have enhanced chemotactic responsiveness to CXCL12,11,12 which may contribute to their abnormal sequestration in the BM.14 When expressed in model cells, the WHIM-associated C-tail–truncated receptors (CXCR4m) are refractory to desensitization and internalization and exhibit enhanced activity, as do receptors in patients' cells, thus suggesting that the abnormal behavior of WHIM leukocytes is due to the mutant receptor dysfunctions.11,12,15 Nevertheless, the way in which CXCR4m function prevails over that of CXCR4wt in WHIM leukocytes is unknown.
The molecular basis of the functional alterations in CXCR4m also remains incompletely understood. Truncation of the receptor C-tail eliminates phosphorylation sites,4 and it was therefore anticipated that this would diminish βarr binding for desensitization and internalization and hence prolong the activity of receptors and ultimately increase their chemotactic responsiveness to CXCL12. However, this assumption is partly challenged by the fact that CXCR4 requires βarr2 for signaling, including chemotaxis. Indeed, lymphocytes from βarr2-deficient mice fail to be desensitized in response to CXCL12, but have decreased chemotactic responses.8 Here, we show that the inability of CXCR4 to be desensitized and internalized, per se, cannot account for the enhanced chemotaxis in WS. CXCR4m preserves functional interactions with βarr2, and βarr2-mediated signaling contributes to the enhanced CXCL12-mediated migration of WHIM leukocytes. Evidence is also provided that CXCR4m spontaneously forms heterodimers with CXCR4wt. Overall, we propose that enhanced βarr2-mediated signaling and heterodimerization between CXCR4m and CXCR4wt are processes whereby responsiveness to CXCL12 is altered in WS.
Methods
Cell culture, cDNA constructs, and transfections
The HEK 293T (HEK) and A0.01 T cells and the CXCR41013 receptor were described previously.11 The CXCR4wt/Δi3 mutant lacking the SHSK motif is from Dr A. Brelot (Institut Cochin, Paris, France), previously referred to as CXCR4.ΔICL3-A or ΔI3-A.16,17 The CXCR41013/Δi3 cDNA was constructed using the CXCR4wt/Δi3 cDNA as a template. The GFP-CXCR41013, GFP-CXCR4wt/Δi3, and GFP-CXCR41013/Δi3 cDNAs were constructed using the previously described T7-GFP-CXCR4wt cDNA.11 The CXCR4 cDNAs were cloned into the pTRIP vector, and stable expression of receptors was achieved using a lentiviral-based strategy.18 The pβ-arrestin2-eGFP and pN1-eGFP plasmids previously reported19 were from Dr S. Marullo (Institut Cochin). The CXCR4-Rluc, CXCR4-YFP, and CXCR41013-YFP cDNAs were obtained using the CXCR4wt cDNA as a template and cloned in frame at the C-tail with the humanized Renilla luciferase (Rluc) or the yellow fluorescent protein (YFP) variant of the green fluorescent protein (GFP) into the pCDNA3/CMV-RLuc or the pcDNA3/CMV-GFP Topaze vectors previously described20 (from Dr R. Jockers, Institut Cochin). The GABAB2-YFP receptor (GBR2-YFP) expression vector is from Dr M. Bouvier (Université de Montréal, QC). Transient transfections of plasmids were performed using the calcium phosphate–DNA coprecipitation method, the transfection reagent FuGene 6 (Roche, Indianapolis, IN), or the Amaxa Nucleofector technology (Amaxa, Köln, Germany). βarr2-specific SMARTpool and siCONTROL nontargeting no. 1 small interfering RNA (Dharmacon, Lafayette, CO) were transfected in HEK and in A0.01 cells using DharmaFECT 1 (Dharmacon) and the Amaxa Nucleofector technology, respectively. Expression of βarr2, βarr1, and lactate dehydrogenase (LDH) was assessed by Western blot analysis using a rabbit anti-serum to βarr2 (from Dr R. J. Lefkowitz, Durham, NC), a rabbit anti-βarr1 mAb (Epitomics, Burlingame, CA) and a sheep anti-human LDH polyclonal Ab (Biodesign, Kennebunk, ME).
Patient and sample processing
Pedigree and clinical features of the patient with WHIM (P2) were described.11 The clinical course of the disease was fatal, and the patient died in early 2007. All participating institutions' ethics committee approved this study, and the patient gave consent for the investigation in accordance with the Declaration of Helsinki.
Immunoprecipitation assays
In some experiments, 10 × 106 cells were treated with the disuccinimidyl suberate (DSS) cross-linker (Pierce, Rockford, IL) at a final concentration of 1 mM for 60 minutes at room temperature (RT). The reaction was stopped by addition of 20 mM Tris-HCl (pH 7.4) for 15 minutes at RT and washing once in the same buffer. Cells were then washed in PBS and lysed in 0.2 mL ice-cold lysis buffer (LB; 30 mM HEPES, 100 mM KCl, 20 mM NaCl, 2 mM MgCl2, 5% glycerol, 2% heptanetriol [Fluka, Buchs, Switzerland], 0.5% laurylmaltoside, 5 μM GDP, 1 μM microcystin [Sigma-Aldrich, St Louis, MO], 1 mM orthovanadate, and protease inhibitors [Roche]) for 30 minutes at 4°C. Precleared lysates were incubated overnight at 4°C with the anti-CXCR4 mAb 12G5 precoated on γ-bind sepharose beads (GE Healthcare, Little Chalfont, United Kingdom). After 3 washes in LB, immunoprecipitated receptors were eluted in SDS-PAGE sample buffer (25 mM Tris-HCl [pH 6.8], 5% glycerol, 1.5% SDS, and 0.1% bromophenol blue) for 45 minutes at 37°C, detected by Western blot analysis using the anti-CXCR4 SZ1567 rabbit polyclonal antibody (from Dr M. Thelen, Bellinzona, Switzerland), and quantified using a LAS-1000 CCD camera and Image Gauge 3.4 software (both Fuji Film, Tokyo, Japan).
Functional evaluation of receptors
Receptor cell-surface expression was determined by flow cytometric analysis as described11 using the phycoerythrin (PE)–conjugated 12G5 mAb (BD Biosciences, San Jose, CA). Chemotaxis, receptor down-modulation, and [35S]-GTPγS–binding assays were described.11,21 For ERK1/2 phosphorylation, cells were serum-starved for 4 hours and then stimulated by CXCL12 at 37°C in RPMI supplemented with 20 mM HEPES (4 × 106 cells/mL). The reaction was stopped by adding ice-cold RMPI and brief centrifugation at 4°C; cells were then lysed in ice-cold lysis buffer (20 mM Tris-HCl [pH 7.4], 140 mM NaCl, 1 mM EDTA, and 1% NP40, supplemented with phosphatase and protease inhibitors). After centrifugation, ERK2 and phosphorylated ERK1/2 were detected in the supernatants by Western blot analysis using ERK2 (D-2; Santa Cruz Biotechnology, Santa Cruz, CA) and phospho-p44/42 MAPK (E10; Cell Signaling Technology, Danvers, MA) mAbs.
Fluorescence imaging
Experiments were performed as described previously.22 Briefly, HEK cells expressing CXCR4wt or its derivative mutants were plated on polylysine-coated glass coverslips at 37°C in CO2. Cells were fixed in 4% paraformaldehyde in PBS at room temperature for 10 minutes. After washing with PBS, cells were mounted in Vectashield medium (Vector Laboratories, Burlingame, CA). Images were acquired with a Zeiss microscope (Zeiss Axioplan 2 imaging; Carl Zeiss, Oberkochen, Germany) using a Plan Apochromat 63×/1.4 oil-immersion objective and were collected with a cooled CCD camera (Axiocam HRc; Carl Zeiss, Jena, Germany) using the Zeiss Axiovision 4.4 imaging acquisition software and the Zeiss ApoTome system (Carl Zeiss, Göttingen, Germany). Image processing was performed with Adobe Photoshop CS 8.0.1 software (Adobe Systems, San Jose, CA).
Microplate BRET assays
HEK cells expressing RLuc- and YFP-tagged receptors were washed once with PBS, and 1 to 2 × 105 cells were distributed into 96-well optiplates (PerkinElmer, Waltham, MA). Coelenterazine H substrate (Interchim, Montluçon, France) was added (5 μM), and readings were performed with a microplate reader Mithras LB940 (Berthold Technologies, Bad Wildbad, Germany), which allows the sequential integration of the signals detected at 480 (± 20) nm and 530 (± 20) nm for luciferase and YFP light emissions, respectively. The bioluminescence resonance energy transfer (BRET) signal represents the ratio of the light intensity emitted at 530 nm over the light intensity emitted at 480 nm, and is corrected by subtracting the background signal detected with the RLuc-tagged receptor expressed alone. BRET signals were measured for increasing YFP/RLuc intensity ratios, expressed as a percentage of the maximal BRET signal (BRETmax), and analyzed with Prism software (GraphPad, San Diego, CA) using nonlinear regression applied to a single binding site model.
Results
CXCR41013 mediates βarr2-dependent chemotaxis
As βarr2 is needed for CXCR4-mediated chemotaxis,7,8 we hypothesized that CXCR4m preserves functional interactions with βarr2. In siblings with WS, we identified the CXCR41013 receptor lacking the last 15 residues of the C-tail as a result of a S338X substitution.11 We stably expressed CXCR41013 or CXCR4wt in HEK cells, and association of receptors with βarr2 was assessed using coimmunoprecipitation studies following transfection of these cells with a plasmid encoding GFP-tagged βarr2 (βarr2-GFP; Figure 1A). βarr2-GFP could be isolated from the CXCR41013 immunoprecipitates. CXCL12 poorly changed the degree of coimmunoprecipitation, but βarr2 recruitment to CXCR41013 was higher under basal conditions compared with CXCR4wt. Altogether, the data suggest that CXCR41013 maintains association with βarr2 and preserves βarr-mediated signaling.
The WHIM-type receptor CXCR41013-mediated chemotaxis is dependent upon βarr2. (A) The CXCR41013 or its counterpart with the entire C-tail CXCR4wt was stably expressed at similar levels in HEK cells, as assessed by flow cytometric analysis using the PE-conjugated anti-CXCR4 mAb 12G5. These cells were then transiently transfected using the calcium phosphate–DNA coprecipitation method either with pN1-eGFP (N1) or pβarr2-eGFP (βarr2-GFP). Efficiency of transfection was in the same range for both cell types, with 40% to 60% of transfected cells, as deduced from flow cytometric counting of cells with green fluorescence. At 48 hours after transfection, cells were treated (+) or not (−) with 100 nM CXCL12 before immunoprecipitating of receptors. Receptors and βarr2-GFP in the immunoprecipitates were detected by Western blot analysis using the anti-CXCR4 SZ1567 and anti-βarr2 polyclonal antibodies (n = 3). (B) At 48 hours after transfection, 3 × 105 transfected cells in 150 μL DMEM supplemented with 20 mM HEPES and 1% BSA were added to the upper chamber of a 8-μm-pore polycarbonate Transwell culture insert, and cell migration toward the indicated CXCL12 concentrations placed in the lower chamber proceeded for 4 hours at 37°C in humidified air with 5% CO2. The fraction of cells that migrated across the polycarbonate membrane was assessed by flow cytometry and was calculated as follows: [(number of cells migrating to the lower chamber in response to CXCL12) / (number of cells added to the upper chamber at the start of the assay)] × 100. The percentage of input cells with green fluorescence that migrated to the lower chamber was compared with that of input CXCR4wt- and N1-expressing cells that migrated toward 0.1 nM CXCL12 (arbitrarily set at 1, and accounting for, on average, 2% of input cells). Spontaneous migrations were marginal in these experiments. The data are means plus or minus SEM (n = 3). (C) Migration of βarr2-GFP– or N1-eGFP–expressing leukocytes from a healthy donor (CTRL) or a patient with WHIM with the CXCR41013 receptor was assessed as in panel B using a 5-μm-pore polycarbonate Transwell culture insert and RPMI supplemented with HEPES and BSA as a migration medium. The percentage of input cells with green fluorescence that migrated to the lower chamber was compared with that of input N1-expressing control cells that migrated toward 0.3 nM CXCL12. Leukocytes were isolated as described11 and transfected using the Amaxa Nucleofector technology. (D) CXCR4wt- or CXCR41013-expressing HEK cells were transfected with siRNA targeting βarr2 or control siRNA (NT). At 48 hours after transfection, cell lysates were prepared and expression of βarr2, βarr1, or LDH was assessed by Western blot analysis as described in “Methods.” (E) Chemotaxis of siRNA-treated cells was carried out as in panel B. The data represent means plus or minus SEM (n = 2).
The WHIM-type receptor CXCR41013-mediated chemotaxis is dependent upon βarr2. (A) The CXCR41013 or its counterpart with the entire C-tail CXCR4wt was stably expressed at similar levels in HEK cells, as assessed by flow cytometric analysis using the PE-conjugated anti-CXCR4 mAb 12G5. These cells were then transiently transfected using the calcium phosphate–DNA coprecipitation method either with pN1-eGFP (N1) or pβarr2-eGFP (βarr2-GFP). Efficiency of transfection was in the same range for both cell types, with 40% to 60% of transfected cells, as deduced from flow cytometric counting of cells with green fluorescence. At 48 hours after transfection, cells were treated (+) or not (−) with 100 nM CXCL12 before immunoprecipitating of receptors. Receptors and βarr2-GFP in the immunoprecipitates were detected by Western blot analysis using the anti-CXCR4 SZ1567 and anti-βarr2 polyclonal antibodies (n = 3). (B) At 48 hours after transfection, 3 × 105 transfected cells in 150 μL DMEM supplemented with 20 mM HEPES and 1% BSA were added to the upper chamber of a 8-μm-pore polycarbonate Transwell culture insert, and cell migration toward the indicated CXCL12 concentrations placed in the lower chamber proceeded for 4 hours at 37°C in humidified air with 5% CO2. The fraction of cells that migrated across the polycarbonate membrane was assessed by flow cytometry and was calculated as follows: [(number of cells migrating to the lower chamber in response to CXCL12) / (number of cells added to the upper chamber at the start of the assay)] × 100. The percentage of input cells with green fluorescence that migrated to the lower chamber was compared with that of input CXCR4wt- and N1-expressing cells that migrated toward 0.1 nM CXCL12 (arbitrarily set at 1, and accounting for, on average, 2% of input cells). Spontaneous migrations were marginal in these experiments. The data are means plus or minus SEM (n = 3). (C) Migration of βarr2-GFP– or N1-eGFP–expressing leukocytes from a healthy donor (CTRL) or a patient with WHIM with the CXCR41013 receptor was assessed as in panel B using a 5-μm-pore polycarbonate Transwell culture insert and RPMI supplemented with HEPES and BSA as a migration medium. The percentage of input cells with green fluorescence that migrated to the lower chamber was compared with that of input N1-expressing control cells that migrated toward 0.3 nM CXCL12. Leukocytes were isolated as described11 and transfected using the Amaxa Nucleofector technology. (D) CXCR4wt- or CXCR41013-expressing HEK cells were transfected with siRNA targeting βarr2 or control siRNA (NT). At 48 hours after transfection, cell lysates were prepared and expression of βarr2, βarr1, or LDH was assessed by Western blot analysis as described in “Methods.” (E) Chemotaxis of siRNA-treated cells was carried out as in panel B. The data represent means plus or minus SEM (n = 2).
We thus evaluated the effect of βarr2 expression on migration of CXCR4wt- or CXCR41013-expressing HEK cells toward CXCL12. Cells were transfected with βarr2-GFP or the pN1-eGFP vector, and CXCL12-induced migration of cells with green fluorescence was counted (Figure 1B). Following βarr2-GFP expression, the sensitivity of CXCR4wt-expressing cells to CXCL12 was strongly increased (ie, the maximal effective concentration of CXCL12 was decreased, as reported).7 As we previously described,11 CXCR41013-expressing cells demonstrated enhanced chemotaxis. In addition, CXCR41013 exhibited higher chemotactic responses with βarr2-GFP. Using this approach, we observed that βarr2 also regulates CXCL12-induced migration of leukocytes from a healthy individual (CTRL) or a patient with WHIM carrying the CXCR41013 receptor (Figure 1C). Notably, WHIM leukocytes displayed stronger chemotactic responses in the presence of βarr2-GFP. Overall, this suggests that the CXCR41013/βarr2 interactions contribute to the enhanced chemotaxis mediated by the receptor.
We directly investigated this possibility using siRNA targeting βarr2, which reduced almost 65% of endogenous βarr2 amounts in CXCR4wt- or CXCR41013-expressing cells (Figure 1D), while expression of βarr1 or LDH was not altered. Silencing βarr2 expression dramatically reduced the chemotaxis of CXCR4wt- or CXCR41013-expressing cells (Figure 1E). Of note, the chemotactic responses of CXCR41013 were no longer augmented in βarr2-depleted cells, thus indicating that βarr2 is critically required for the enhanced chemotactic responsiveness of CXCR41013-expressing cells to CXCL12.
Enhanced CXCR41013-mediated chemotaxis depends on the SHSK motif in ICL3 of the receptor
βarr2 was described to interact with the third intracellular loop (ICL3) of CXCR4.5 Located in the N-terminal portion of ICL3 is an SHSK motif that is needed for mobilization of intracellular calcium16,17 and G protein–independent stimulation of Jak2/STAT3 in response to CXCL12.16 Therefore, we asked whether this motif also plays a role in the enhanced CXCR41013-mediated chemotaxis (Figure 2). We transduced A0.01 cells, which do not express CXCR4,23 to express similar amounts of CXCR4wt or CXCR41013 or their counterparts lacking the SHSK motif, CXCR4wt/Δi3 or CXCR41013/Δi3, at the cell surface (Figure 2A). Cells were then evaluated for their ability to migrate toward different CXCL12 concentrations (Figure 2B). CXCR41013-expressing cells displayed significantly stronger chemotaxis at the lowest CXCL12 concentrations compared with cells expressing CXCR4wt or the SHSK-deleted receptors. The differences in migration were less apparent at higher CXCL12 concentrations, thus indicating that the decrease of the maximal effective chemotactic concentration of CXCL12 is a mechanism whereby CXCR41013 enhances responsiveness of cells to the chemokine. Compared with CXCR4wt-expressing cells, cells expressing the CXCR41013/Δi3 receptor lacking both the C-tail and the SHSK motif did not exhibit enhanced CXCL12-induced migration. This indicates that the SHSK motif is needed for the enhanced chemotaxis mediated by CXCR41013.
CXCR41013-mediated chemotaxis depends on the SHSK motif of the receptor. (A) A0.01 T cells were transduced to express identical amounts of CXCR4wt, CXCR41013, or the receptors lacking the SHSK motif, CXCR4wt/Δi3, or CXCR41013/Δi3. The panel shows typical cell-surface expression levels of CXCR4wt (solid line) and CXCR41013 (dotted line), as assessed by flow cytometric analysis using PE-conjugated 12G5, compared with parental cells (filled peak). The inset depicts the expression levels of CXCR41013 (▩), CXCR4wt/Δi3 (■), and CXCR41013/Δi3 (▧) compared with that of CXCR4wt arbitrarily set at 100% (□). Data are means plus or minus SEM (n = 3). (B) Transduced cells were subjected to CXCL12-induced chemotaxis as in Figure 1C. The transmigrated cells recovered in the lower chamber were counted by flow cytometry. The data (means ± SEM; n = 3) represent chemotactic indexes that were calculated as follows: (number of cells that migrated toward CXCL12) / (number of cells that migrated spontaneously). Spontaneous migrations were in the same range for all cell populations, reaching 4.4% plus or minus 1.8%, 3.8% plus or minus 2.2%, 4.2% plus or minus 2.2%, and 3.6% plus or minus 0.6% of input CXCR4wt-, CXCR41013-, CXCR4wt/Δi3-, and CXCR41013/Δi3-expressing cells, respectively. As also shown in the panel, pretreating CXCR4wt- or CXCR41013-expressing cells with Bordetella pertussis toxin (PTX) at 0.5 μM for 90 minutes abrogated CXCL12-dependent migration. *P < .05 compared with cells with the other receptors in unpaired one-tailed Student t test.
CXCR41013-mediated chemotaxis depends on the SHSK motif of the receptor. (A) A0.01 T cells were transduced to express identical amounts of CXCR4wt, CXCR41013, or the receptors lacking the SHSK motif, CXCR4wt/Δi3, or CXCR41013/Δi3. The panel shows typical cell-surface expression levels of CXCR4wt (solid line) and CXCR41013 (dotted line), as assessed by flow cytometric analysis using PE-conjugated 12G5, compared with parental cells (filled peak). The inset depicts the expression levels of CXCR41013 (▩), CXCR4wt/Δi3 (■), and CXCR41013/Δi3 (▧) compared with that of CXCR4wt arbitrarily set at 100% (□). Data are means plus or minus SEM (n = 3). (B) Transduced cells were subjected to CXCL12-induced chemotaxis as in Figure 1C. The transmigrated cells recovered in the lower chamber were counted by flow cytometry. The data (means ± SEM; n = 3) represent chemotactic indexes that were calculated as follows: (number of cells that migrated toward CXCL12) / (number of cells that migrated spontaneously). Spontaneous migrations were in the same range for all cell populations, reaching 4.4% plus or minus 1.8%, 3.8% plus or minus 2.2%, 4.2% plus or minus 2.2%, and 3.6% plus or minus 0.6% of input CXCR4wt-, CXCR41013-, CXCR4wt/Δi3-, and CXCR41013/Δi3-expressing cells, respectively. As also shown in the panel, pretreating CXCR4wt- or CXCR41013-expressing cells with Bordetella pertussis toxin (PTX) at 0.5 μM for 90 minutes abrogated CXCL12-dependent migration. *P < .05 compared with cells with the other receptors in unpaired one-tailed Student t test.
Deleting the SHSK motif of CXCR41013 does not impair G protein coupling efficiency
Migration of CXCR4wt- or CXCR41013-expressing cells in response to CXCL12 was fully abrogated in the presence of PTX (Figure 2B), thus indicating that receptor-mediated chemotaxis is dependent upon activation of Gi proteins. As deletion of the SHSK motif has been reported to prevent CXCR4 from activating Gi protein–dependent signals,16,17 we explored whether removing the SHSK motif of CXCR41013 impairs coupling to G proteins, thereby providing a molecular basis to explaining our data showing that this motif is required for the enhanced chemotactic responses of this receptor. We thus measured CXCL12-induced 35S-GTPγS binding to activated Gα subunit–containing membranes from HEK cells expressing similar amounts of CXCR4wt, CXCR41013, CXCR4wt/Δi3, or CXCR41013/Δi3 (Figure 3A,B). Exposure of membranes from CXCR4wt-expressing cells to CXCL12 promoted a dose-dependent 35S-GTPγS binding (Figure 3C), with a half-maximal effective concentration (EC50) in the nanomolar range (EC50 = 18 ± 4 nM). Truncation of the C-tail in CXCR41013 improved to some extent CXCL12-induced coupling efficiency and potency of the receptor (EC50 = 12 ± 2 nM), as reported.11 By comparison, CXCL12 demonstrated a dramatically reduced efficiency to activate G proteins from CXCR4wt/Δi3, together with a slightly diminished potency (EC50 = 35.6 ± 2 nM), thus indicating a role for the SHSK motif in regulating G protein coupling. Unexpectedly, deleting the SHSK motif together with the C-tail in CXCR4 (CXCR41013/Δi3) corrected coupling efficiency to a level equivalent to that observed with CXCR4wt or CXCR41013, although the EC50 values remained slightly altered (EC50 = 36.8 ± 3 nM).
The C-tail and the SHSK motif of CXCR4 regulate activation of G proteins and receptor down-modulation. (A,B) CXCR4wt, CXCR41013, CXCR4wt/Δi3, and CXCR41013/Δi3 were transiently expressed in HEK cells. Flow cytometric analysis using PE-conjugated 12G5 confirmed similar expression levels of receptors at the cell surface. Staining of parental cells was used as a negative control (filled peaks). (C) CXCL12-induced [35S]GTPγS binding to membranes from these cells transiently expressing CXCR4wt (■), CXCR41013 (□), CXCR4wt/Δi3 (●), or CXCR41013/Δi3 (○) is shown. Membranes were incubated in assay buffer containing 0.1 nM [35S]GTPγS and the indicated concentrations of CXCL12. The data, which are representative of 4 independent experiments performed in triplicate, are expressed as a percentage of the basal binding to CXCR4wt-expressing membranes. (D) Receptor expression levels following stimulation with 200 nM CXCL12 for 45 minutes at 37°C are shown. Results (means ± SD; n = 3) indicate the amount of receptor that remains at the surface of HEK cells expressing CXCR4wt (□), CXCR41013 ( ), CXCR4wt/Δi3 (■), or CXCR41013/ Δi3 (▧) after incubation with CXCL12 compared with untreated cells (n.s.;
), CXCR4wt/Δi3 (■), or CXCR41013/ Δi3 (▧) after incubation with CXCL12 compared with untreated cells (n.s.;  ).
).
The C-tail and the SHSK motif of CXCR4 regulate activation of G proteins and receptor down-modulation. (A,B) CXCR4wt, CXCR41013, CXCR4wt/Δi3, and CXCR41013/Δi3 were transiently expressed in HEK cells. Flow cytometric analysis using PE-conjugated 12G5 confirmed similar expression levels of receptors at the cell surface. Staining of parental cells was used as a negative control (filled peaks). (C) CXCL12-induced [35S]GTPγS binding to membranes from these cells transiently expressing CXCR4wt (■), CXCR41013 (□), CXCR4wt/Δi3 (●), or CXCR41013/Δi3 (○) is shown. Membranes were incubated in assay buffer containing 0.1 nM [35S]GTPγS and the indicated concentrations of CXCL12. The data, which are representative of 4 independent experiments performed in triplicate, are expressed as a percentage of the basal binding to CXCR4wt-expressing membranes. (D) Receptor expression levels following stimulation with 200 nM CXCL12 for 45 minutes at 37°C are shown. Results (means ± SD; n = 3) indicate the amount of receptor that remains at the surface of HEK cells expressing CXCR4wt (□), CXCR41013 ( ), CXCR4wt/Δi3 (■), or CXCR41013/ Δi3 (▧) after incubation with CXCL12 compared with untreated cells (n.s.;
), CXCR4wt/Δi3 (■), or CXCR41013/ Δi3 (▧) after incubation with CXCL12 compared with untreated cells (n.s.;  ).
).
The SHSK motif prevents CXCR4 from constitutive desensitization and down-modulation
We thus hypothesized that the impaired ability of CXCR4wt/Δi3 to activate G proteins results from an increased desensitization due to a favored recruitment of βarrs to the receptor C-tail. Association of βarrs with the CXCR4 C-tail targets the receptor to clathrin-coated pits for endocytosis.4 Thus we asked whether the SHSK motif could regulate CXCR4 internalization, with the anticipation that the CXCR4wt/Δi3 mutant lacking this motif might display an enhanced endocytosis. We quantified the amount of receptors remaining at the surface of HEK cells stably expressing CXCR4wt, CXCR41013, CXCR4wt/Δi3, or CXCR41013/Δi3 after exposure to CXCL12 (Figure 3D). The chemokine induced down-modulation of 35% to 40% of cell-surface CXCR4wt, but failed to trigger endocytosis of the C-tail–truncated receptors, CXCR41013 or CXCR41013/Δi3. Surprisingly, CXCR4wt/Δi3 was also apparently impaired in its ability to be internalized in response to CXCL12.
We next transfected HEK cells expressing the CXCR4 variants with βarr2-GFP and then determined cell-surface expression of receptors in GFP+-gated cells in the presence or absence of CXCL12 (Figure 4A,B). βarr2-GFP moderately altered basal cell- surface expression of CXCR4wt, and enhanced the receptor down-modulation by CXCL12 by up to 60%. In contrast, βarr2-GFP expression affected neither basal nor CXCL12-induced down-modulation of CXCR41013 and CXCR41013/Δi3, thus emphasizing that association with the C-tail is critical for βarrs to regulate CXCR4 endocytosis. Finally, βarr2-GFP expression resulted in a remarkable down-modulation of CXCR4wt/Δi3 (approximately 55%) that was poorly increased by CXCL12 (Figure 4B). Receptor cell-surface expression as a function of βarr2-GFP expression was further studied following transitory cotransfection assays in HEK cells. As depicted in Figure 4C, a proportion of the CXCR4wt-expressing cells with a high βarr2-GFP content exhibited down-modulation of receptors. This effect was absent in cells expressing the C-tail–truncated receptors CXCR41013 and CXCR41013/Δi3 (Figure 4D,F), but was dramatically magnified in the cells expressing CXCR4wt/Δi3 (Figure 4E). This basal down-modulation of CXCR4wt/Δi3 strongly suggests that βarr2 constitutively interacts with the receptor C-tail (ie, in an agonist-independent manner), thus providing an explanation for how CXCR4wt/Δi3 is impaired in its ability to activate G proteins.
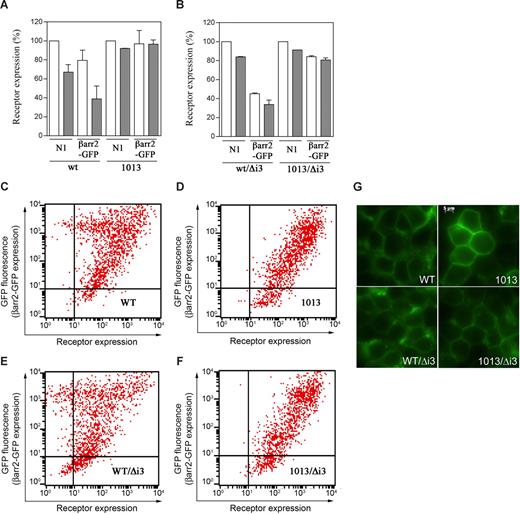
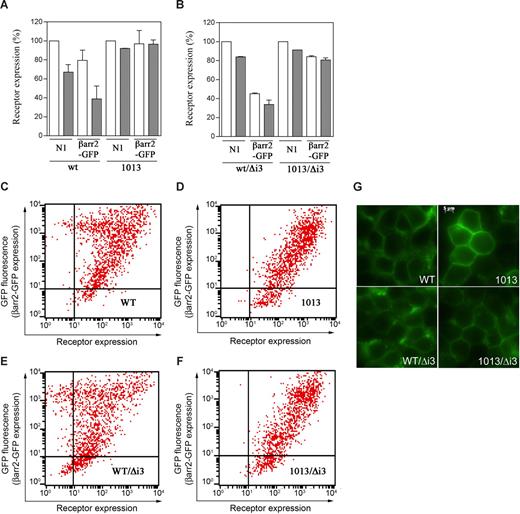
The SHSK motif prevents CXCR4 from constitutive down-modulation. (A,B) Effects of βarr2 on cell-surface expression of CXCR4wt (A), CXCR41013 (A), CXCR4wt/Δi3 (B), and CXCR41013/Δi3 (B). HEK cells stably expressing either of the CXCR4 variants were transiently transfected with pN1-eGFP (N1) or βarr2-GFP. Expression of receptors without (□) or with treatment with 200 nM CXCL12 for 45 minutes at 37°C (■) was then assessed by flow cytometry. Results (means ± SD; n = 3) are receptor expression at the surface of GFP+-gated cells, expressed as percentage of the values in GFP+, pN1-eGFP–transfected cells in the absence of CXCL12 (100%). (C-F) The dot plots from flow cytometric assays represent cell-surface expression of CXCR4wt (C), CXCR41013 (D), CXCR4wt/Δi3 (E), or CXCR41013/Δi3 (F) as a function of βarr2-GFP expression (ie, GFP fluorescence intensity) in transiently cotransfected HEK cells. Experiments (n = 3) were carried out 48 hours after transfection. (G) Fluorescence microscopic imaging of HEK cells stably expressing the GFP-tagged CXCR4 variant receptors.
The SHSK motif prevents CXCR4 from constitutive down-modulation. (A,B) Effects of βarr2 on cell-surface expression of CXCR4wt (A), CXCR41013 (A), CXCR4wt/Δi3 (B), and CXCR41013/Δi3 (B). HEK cells stably expressing either of the CXCR4 variants were transiently transfected with pN1-eGFP (N1) or βarr2-GFP. Expression of receptors without (□) or with treatment with 200 nM CXCL12 for 45 minutes at 37°C (■) was then assessed by flow cytometry. Results (means ± SD; n = 3) are receptor expression at the surface of GFP+-gated cells, expressed as percentage of the values in GFP+, pN1-eGFP–transfected cells in the absence of CXCL12 (100%). (C-F) The dot plots from flow cytometric assays represent cell-surface expression of CXCR4wt (C), CXCR41013 (D), CXCR4wt/Δi3 (E), or CXCR41013/Δi3 (F) as a function of βarr2-GFP expression (ie, GFP fluorescence intensity) in transiently cotransfected HEK cells. Experiments (n = 3) were carried out 48 hours after transfection. (G) Fluorescence microscopic imaging of HEK cells stably expressing the GFP-tagged CXCR4 variant receptors.
Other GPCRs that undergo constitutive βarr-mediated desensitization accumulate intracellularly due to constitutive association with βarr2.24 Similarly, using fluorescence microscopic imaging of HEK cells stably expressing GFP-tagged receptors, we demonstrated that CXCR4wt/Δi3 localized to a higher extent in the cytosol than its wild-type counterpart (Figure 4G). As expected, fluorescence in cells expressing GFP-CXCR41013 or GFP-CXCR41013/Δi3 originated mainly from the plasma membrane. Overall, these findings uncover a critical role for the SHSK motif both in preventing CXCR4 from constitutive desensitization and in stabilizing receptor expression at the cell surface.
Our data also indicate that the C-tail truncation of CXCR4wt/Δi3, which results in the CXCR41013/Δi3 receptor, is sufficient to prevent constitutive desensitization and internalization. However, CXCR41013/Δi3 does not trigger enhanced CXCL12-induced migration (Figure 2), so that it could be that the refractoriness to desensitization and endocytosis does not account per se for the enhanced chemotaxis mediated by CXCR41013.
CXCR41013 triggers enhanced and sustained βarr2-dependent signaling that depends on the SHSK motif
The archetypal role for βarr in signaling is to act as a scaffold for the ERK1/2 MAPK cascade.6 As the enhanced chemotaxis of WHIM cells depends on βarr2, we hypothesized that increased intensity and/or duration of βarr2-dependent signaling occur in WHIM cells. As a first support of this assumption, CXCL12-induced ERK1/2 phosphorylation was dramatically enhanced in leukocytes from a patients with WS harboring CXCR41013 compared with a healthy individual (Figure 5A).
Enhanced and sustained CXCR41013-mediated ERK1/2 activation depends on the SHSK motif. (A) A representative experiment out of 2 showing CXCL12-induced ERK1/2 activation in leukocytes from a healthy subject (CTRL) or a patient with WHIM harboring CXCR41013, with (+) or without (−) 30 nM CXCL12 for 2 minutes at 37°C. Cell lysates were immunoblotted for ERK2 and phosphorylated ERK1/2, and data were quantified as P-ERK/ERK2 ratios. (B,C) Time course of CXCL12-induced ERK1/2 phosphorylation in transduced A0.01 T cells expressing similar amounts of CXCR4wt, CXCR41013, CXCR4wt/Δi3, or CXCR41013/Δi3 (Figure 2A). Cells were stimulated with 30 nM CXCL12 for 2, 5, or 15 minutes, or unstimulated (0), and the ERK2 and phosphorylated ERK1/2 content in the cell lysates was analyzed as in panel A. Immunoblots for ERK2 and phosphorylated ERK1/2 from a representative experiment are depicted in panel B. The values in panel C (means ± SEM; n = 3) represent ERK1/2 activation quantified as P-ERK/ERK2 ratios in cells expressing CXCR4wt, CXCR41013, CXCR4wt/Δi3, or CXCR41013/Δi3, expressed as a percentage of the ERK1/2 activation in CXCR4wt-expressing cells at 2 minutes of stimulation (100%).
Enhanced and sustained CXCR41013-mediated ERK1/2 activation depends on the SHSK motif. (A) A representative experiment out of 2 showing CXCL12-induced ERK1/2 activation in leukocytes from a healthy subject (CTRL) or a patient with WHIM harboring CXCR41013, with (+) or without (−) 30 nM CXCL12 for 2 minutes at 37°C. Cell lysates were immunoblotted for ERK2 and phosphorylated ERK1/2, and data were quantified as P-ERK/ERK2 ratios. (B,C) Time course of CXCL12-induced ERK1/2 phosphorylation in transduced A0.01 T cells expressing similar amounts of CXCR4wt, CXCR41013, CXCR4wt/Δi3, or CXCR41013/Δi3 (Figure 2A). Cells were stimulated with 30 nM CXCL12 for 2, 5, or 15 minutes, or unstimulated (0), and the ERK2 and phosphorylated ERK1/2 content in the cell lysates was analyzed as in panel A. Immunoblots for ERK2 and phosphorylated ERK1/2 from a representative experiment are depicted in panel B. The values in panel C (means ± SEM; n = 3) represent ERK1/2 activation quantified as P-ERK/ERK2 ratios in cells expressing CXCR4wt, CXCR41013, CXCR4wt/Δi3, or CXCR41013/Δi3, expressed as a percentage of the ERK1/2 activation in CXCR4wt-expressing cells at 2 minutes of stimulation (100%).
Next, we compared the time course of CXCL12-stimulated ERK1/2 activation in A0.01 cells expressing similar amounts of CXCR4wt, CXCR41013, CXCR4wt/Δi3, or CXCR41013/Δi3 (Figure 5B,C). In CXCR4wt-expressing cells, CXCL12-induced ERK1/2 phosphorylation was rapid and transient, reaching a maximum at 2 minutes and then declining over time. In CXCR41013-expressing cells, ERK1/2 activation was increased at 2 minutes and persisted at longer time points. Interestingly, CXCR41013/Δi3, which similarly to CXCR41013 lacks CXCL12-mediated desensitization and endocytosis, triggered an early peak of ERK1/2 phosphorylation that was also enhanced, but the amount of activated ERK1/2 returned rapidly to levels close to those observed in CXCR4wt-expressing cells. This indicates that refractoriness of receptors to desensitization does not contribute to the overall sustained duration of the ERK1/2 phosphorylation observed in CXCR41013-expressing cells. In contrast, the SHSK motif of CXCR41013 is involved in this process. ERK1/2 activation at 2 minutes was strongly reduced in cells expressing the constitutively desensitized receptorCXCR4wt/Δi3, and remained at a low level over 15 minutes. This result, together with the fact that CXCR41013 and CXCR41013/Δi3 elicited an enhanced signal at 2 minutes, strongly suggests that the early peak of CXCL12-mediated ERK1/2 activation is primarily dependent upon coupling to G proteins and as such is sensitive to desensitization.
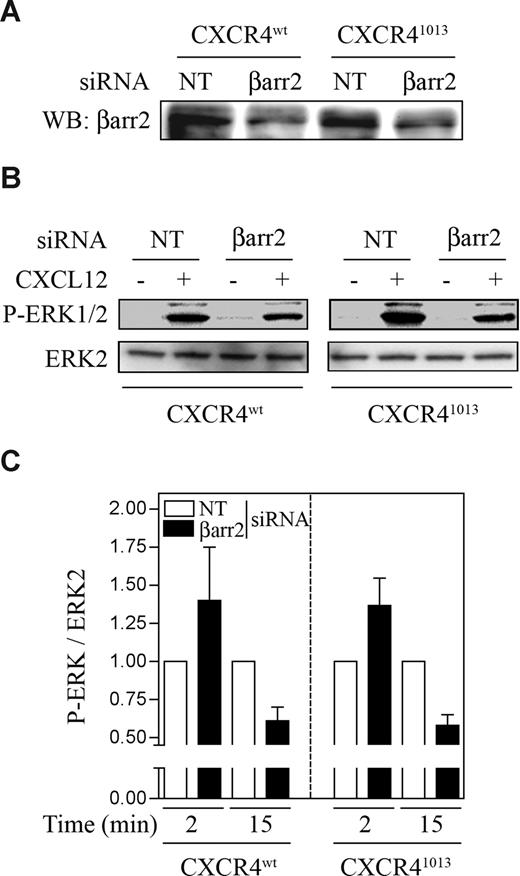
The time course of ERK1/2 activation mediated by CXCR41013 resembles those reported for other GPCRs,25,26 which stimulate ERK1/2 by 2 distinct pathways: one depending on G protein activation, which is transient and occurs shortly after ligand stimulation, and the other, which is a G-protein–independent, βarr-mediated pathway, leading to a slower but much more prolonged ERK1/2 activation. Thus, we evaluated whether βarr2 could contribute to the sustained duration of the CXCR41013-mediated ERK1/2 activation. Reduction of endogenous βarr2 amounts (approximately 40%) in CXCR4wt- or CXCR41013-expressing A0.01 cells (Figure 6A) effectively diminished the prolonged CXCL12-induced ERK1/2 activation in CXCR41013-expressing cells to levels comparable with those measured in CXCR4wt-expressing cells (Figure 6B). This suggests that βarr2 contributes to the enhancement of the ERK1/2 phosphorylation observed in CXCR41013-expressing cells at the late time points. βarr2 depletion also attenuated to some extent CXCR4wt-mediated ERK1/2 activation at 15 minutes of stimulation. Nonetheless, when experiments were repeated at 2 minutes after CXCL12 stimulation, βarr2 depletion did not reduce ERK1/2 activation (Figure 6C), thus indicating that βarr2 does not contribute to the early ERK1/2 phosphorylation mediated by CXCR4wt or CXCR41013. ERK1/2 activation at 2 minutes was even higher in βarr2-silenced cells, thus suggesting that βarr2 can exert a dual effect on ERK1/2 activation downstream of these receptors (ie, preventing the early activation of ERK1/2 and triggering it at later time points).
Effects of βarr2 siRNA on CXCR41013-mediated ERK1/2 activation. A0.01 T cells expressing CXCR4wt or CXCR41013 were nucleoporated with siRNA targeting βarr2 or control siRNA (NT). At 48 hours after transfection, cells were stimulated or not with 10 nM CXCL12 for 15 (B,C) or 2 minutes (C). Cell lysates were prepared, and 20 μg of proteins was used to visualize expression of βarr2 (A) and ERK1/2 phosphorylation (B,C). Representative results (n = 3) are shown in panels A and B. (C) ERK1/2 phosphorylation is expressed as a ratio of activated ERK1/2 over total ERK2, and ratios with βarr2 siRNA are compared with those from cells with control siRNA (arbitrarily set at 1). Results are means plus or minus SEM.
Effects of βarr2 siRNA on CXCR41013-mediated ERK1/2 activation. A0.01 T cells expressing CXCR4wt or CXCR41013 were nucleoporated with siRNA targeting βarr2 or control siRNA (NT). At 48 hours after transfection, cells were stimulated or not with 10 nM CXCL12 for 15 (B,C) or 2 minutes (C). Cell lysates were prepared, and 20 μg of proteins was used to visualize expression of βarr2 (A) and ERK1/2 phosphorylation (B,C). Representative results (n = 3) are shown in panels A and B. (C) ERK1/2 phosphorylation is expressed as a ratio of activated ERK1/2 over total ERK2, and ratios with βarr2 siRNA are compared with those from cells with control siRNA (arbitrarily set at 1). Results are means plus or minus SEM.
Spontaneous heterodimerization between CXCR41013 and CXCR4wt
Heterozygous mutations of CXCR4 in WS lead to the expression of CXCR4m, which likely coexist with CXCR4wt in patients' cells.11 These cells have enhanced CXCL12-induced chemotaxis and ERK1/2 phosphorylation, as do CXCR4m-expressing cell lines, thus suggesting that CXCR4m alters the CXCR4wt functioning in WHIM cells. In line with this, CXCR41013 was found to prevent CXCL12-induced endocytosis of CXCR4wt,11 thus implying that the receptors heterodimerize when they coexist in the same cell.
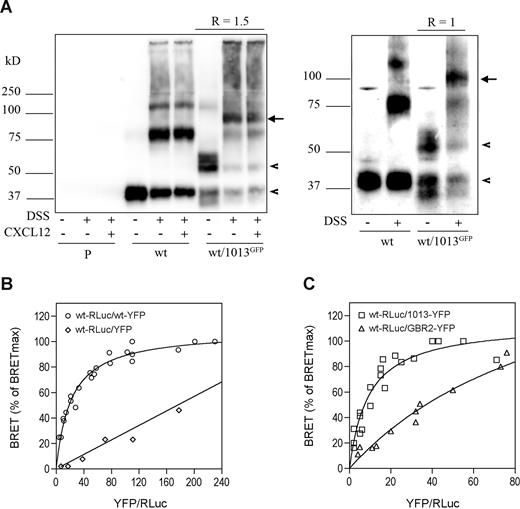
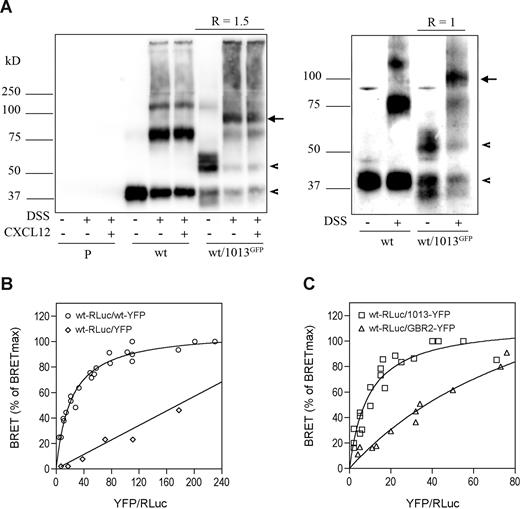
To investigate this possibility, we used HEK cells expressing CXCR4wt with or without the GFP-CXCR41013 receptor, and association of receptors was assessed using coimmunoprecipitation studies with the DSS cross-linker (Figure 7). In the absence of DSS, immunodetection of precipitated receptors revealed bands with molecular weights close to those expected for monomeric CXCR4wt (41 kDa) and GFP-CXCR41013 (approximately 59 kDa). Following cross-linking of CXCR4wt receptors, we detected additional bands of higher molecular masses (88 and 123 kDa) corresponding to CXCR4wt dimers and trimers. Similar results were obtained with CXCR41013 (data not shown). CXCL12 did not alter the degree of receptor association, thus confirming previous works showing that CXCR4 exists as constitutive oligomers.27,28 In the presence of GFP-CXCR41013 we detected a new molecular species at approximately 100 kDa that was not altered by CXCL12, thus indicating that CXCR4wt and CXCR41013 form constitutive heterodimers. The relative abundance of heterodimers was higher than that of CXCR4wt or GFP-CXCR41013 homodimers (barely detectable at approximately 128 kDa), thus suggesting that heterodimers are preferentially formed. Quantification of immunoreactive bands when both receptors are expressed at a 1:1 ratio (Figure 7A right panel) reveals that the extent of heterodimer formation was 2-fold higher compared with CXCR4wt homodimers, which is an expected result if CXCR4wt has the same propensity to self-associate or to interact with CXCR41013.
CXCR4wt and CXCR41013 form constitutive heterodimers. (A) Coimmunoprecipitation of CXCR41013 and CXCR4wt. HEK cells stably expressing CXCR4wt (wt) were transfected or not with a CXCR41013 receptor variant fused to GFP at its N-tail (1013GFP). At 48 hours after transfection, cells were incubated without (left and right panels) or with (left panel) 100 nM CXCL12 for 15 minutes in PBS at 37°C and treated or not with the DSS covalent cross-linker; receptors were immunoprecipitated using the anti-CXCR4 mAb 12G5. Immunoprecipitated receptors were analyzed by Western blotting as described in Figure 1. Left and right panels are representative experiments performed with 2 different GFP-CXCR41013 to CXCR4wt ratios (R), which were calculated using a LAS-1000 CCD camera with the Image Gauge 3.4 software. Experiments with parental cells (P) are also shown. The arrowheads indicate monomeric CXCR4wt and GFP-CXCR41013 at 41 and 59 kDa, respectively, and the arrows indicate GFP-CXCR41013/CXCR4wt heterodimers at 100 kDa. (B,C) Detection of receptor dimerization using BRET titration experiments. HEK cells were transfected using the transfection reagent FuGene 6 with constant amounts of cDNA coding for CXCR4wt-RLuc (50 ng/well in 6-well dishes) and increasing amounts (from 25 ng up to 1000 ng) of cDNA encoding for CXCR4wt-YFP (panel B; ○), YFP alone (panel B; ◇), CXCR41013-YFP (panel C; □) or GBR2-YFP (panel C; ▵). Experiments were carried out 48 hours after transfection. Total fluorescence (determined using an excitation filter at 485 nm) and luminescence were used as relative measures of total expression of the RLuc- and YFP-tagged proteins. The CXCR4wt-RLuc receptor amounts were roughly similar in the experimental conditions tested. BRET values, expressed as percent of the maximal BRET reached (BRETmax), are plotted as a function of the ratio of YFP/RLuc fusion proteins. Plotted results are from 3 independent experiments.
CXCR4wt and CXCR41013 form constitutive heterodimers. (A) Coimmunoprecipitation of CXCR41013 and CXCR4wt. HEK cells stably expressing CXCR4wt (wt) were transfected or not with a CXCR41013 receptor variant fused to GFP at its N-tail (1013GFP). At 48 hours after transfection, cells were incubated without (left and right panels) or with (left panel) 100 nM CXCL12 for 15 minutes in PBS at 37°C and treated or not with the DSS covalent cross-linker; receptors were immunoprecipitated using the anti-CXCR4 mAb 12G5. Immunoprecipitated receptors were analyzed by Western blotting as described in Figure 1. Left and right panels are representative experiments performed with 2 different GFP-CXCR41013 to CXCR4wt ratios (R), which were calculated using a LAS-1000 CCD camera with the Image Gauge 3.4 software. Experiments with parental cells (P) are also shown. The arrowheads indicate monomeric CXCR4wt and GFP-CXCR41013 at 41 and 59 kDa, respectively, and the arrows indicate GFP-CXCR41013/CXCR4wt heterodimers at 100 kDa. (B,C) Detection of receptor dimerization using BRET titration experiments. HEK cells were transfected using the transfection reagent FuGene 6 with constant amounts of cDNA coding for CXCR4wt-RLuc (50 ng/well in 6-well dishes) and increasing amounts (from 25 ng up to 1000 ng) of cDNA encoding for CXCR4wt-YFP (panel B; ○), YFP alone (panel B; ◇), CXCR41013-YFP (panel C; □) or GBR2-YFP (panel C; ▵). Experiments were carried out 48 hours after transfection. Total fluorescence (determined using an excitation filter at 485 nm) and luminescence were used as relative measures of total expression of the RLuc- and YFP-tagged proteins. The CXCR4wt-RLuc receptor amounts were roughly similar in the experimental conditions tested. BRET values, expressed as percent of the maximal BRET reached (BRETmax), are plotted as a function of the ratio of YFP/RLuc fusion proteins. Plotted results are from 3 independent experiments.
We explored this issue in intact cells using BRET-based titration experiments, as described for CXCR428,29 and other receptors.30-32 CXCR4wt was fused at the C-tail with Renilla luciferase used as an energy donor (CXCR4wt-RLuc) and then expressed at a constant amount in HEK cells together with increasing concentrations of C-tail–tagged CXCR4wt (Figure 7B) or CXCR41013 (Figure 7C) with the BRET acceptor YFP. Then, the extent of energy transfer between RLuc and YFP was plotted as a function of increasing acceptor-donor ratios. As previously reported,28,29 CXCR4wt homodimerization is indicated by BRET signals between CXCR4wt-RLuc and CXCR4wt-YFP that varied as a hyperbolic function, reaching an asymptote when all CXCR4wt-RLuc molecules are associated with CXCR4wt-YFP. In contrast, coexpressing CXCR4wt-RLuc with YFP alone resulted in nonspecific signals that progressed linearly (Figure 7B). Energy transfer between CXCR4wt-RLuc and CXCR41013-YFP also increased hyperbolically with the YFP/RLuc ratios, thus confirming that the receptors spontaneously form heterodimers. The BRET50 values, which correspond to the acceptor-donor ratios resulting in half-maximal saturation of RLuc and represent the propensity of the protomers to interact with one another,33 were found to be in the same range for CXCR4wt homodimer and CXCR4wt/CXCR41013 heterodimer formation (BRET50 = 21.7 ± 2.1 and 9.4 ± 1.6, respectively). In contrast, CXCR4wt-RLuc barely associated with the unrelated GPCR, GBR2-YFP, as previously reported,29 and as revealed by substantially right-shifted titration curves (BRET50 > 100; Figure 7C). Overall, these results extend to intact cells that CXCR4wt/CXCR41013 heterodimers form as efficiently as CXCR4wt homodimers.
Discussion
CXCL12/CXCR4 regulate migration, homing, and retention of leukocytes within primary lymphoid organs and leukocyte homeostasis in the periphery.1 For instance, the BM constitutively expresses CXCL12, and disrupting interaction of the chemokine with CXCR4 cause leukocytosis, with the release of hematopoietic progenitor cells, neutrophils, and lymphocytes into the blood.34,35 In contrast, increased expression of CXCR4 at the surface of leukocytes augments responsiveness to CXCL12 and improves their accumulation in the BM.14,36,37 Individuals with WS suffer from lymphopenia and neutropenia that presumably favor the development of infections and warts. Heterozygous mutations of CXCR4 causing truncations of the receptor C-tail in WS are linked to enhanced responsiveness of leukocytes to CXCL12,11,12 thus suggesting that the clinical manifestations may be due to alterations in CXCR4 functioning. In line with this, expression of WHIM-type mutated CXCR4 in healthy human CD34+ stem cells enhances chemotactic responses to CXCL12 and BM engraftment of these cells in a nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse xenograph model, and myeloid cells with the mutant receptor exhibit reduced release in the blood.14 These data support the notion that increased CXCL12-induced chemotaxis, possibly together with greater adherence properties,12 contribute to the abnormal sequestration of WHIM neutrophils in the BM. The CXCR4m receptors, including CXCR41013, are refractory to desensitization and internalization (this work and others11,15 ), and it has been anticipated that these results are due to the removal of Ser/Thr residues in the CXCR4 C-tail that are needed for GRK- and βarr-mediated down-modulation of the receptor.4 Due to the impaired desensitization, the receptors activate G proteins more efficiently (Figure 3; Balabanian et al11 ) and have improved G protein–dependent signals in response to CXCL12, such as calcium mobilization13,15 or the early ERK1/2 phosphorylation (Figure 5). However, our findings now reveal that physical and functional interactions of CXCR4m receptors with βarr2 are actually preserved, and together with heterodimerization with CXCR4wt, are mechanisms by which CXCR4 activity is altered in WHIM cells and the chemotactic responsiveness to CXCL12 is enhanced.
We found that the enhanced CXCR41013-mediated chemotaxis is not exclusively related to the impaired receptor desensitization and internalization, but critically requires βarr2-dependent signaling that depends on the SHSK motif in ICL3 of the receptor. Indeed, using ERK1/2 phosphorylation as a readout, we demonstrated that βarr2-dependent signaling is enhanced and prolonged downstream of CXCR41013. Removing the SHSK motif from CXCR41013 abolished βarr2-dependent signaling (ie, the late ERK1/2 phosphorylation; Figure 5) and the enhanced chemotactic responsiveness (Figure 2), although the resulting receptor (CXCR41013/Δi3) retains G protein–dependent signaling together with defective desensitization and internalization (Figures 3,Figure 4–5). A clue to explaining the requirement for the SHSK motif in the enhanced CXCR41013-mediated chemotaxis may lie in its ability to help βarr2 to act as a platform for the recruitment and the functional regulation of molecules involved in this signaling pathway.38 The requirement for βarrs in CXCR4-mediated chemotaxis was first documented in cells from βarr2-deficient mice or after inhibition of βarr2 ex-pression by siRNA,7,8 and βarr2 expression has also been found to strengthen the CXCL12-induced chemotaxis of CXCR4-expressing HEK cells.7 Here, we demonstrate that the last 15 residues of the CXCR4 C-tail are not required for βarr2 to regulate CXCL12-mediated chemotaxis. Indeed, CXCR41013-mediated chemotaxis is substantially attenuated in βarr2-deficient cells and conversely is increased after βarr2 overexpression. Moreover, βarr2 expression magnifies the enhanced migration of WHIM leukocytes (Figure 1). βarr is involved in chemotaxis induced by other GPCRs, including chemokine receptors,7,21 angiotensin-II type 1a receptor (AT1AR),39 and protease-activated receptor-2 (PAR-2);40,41 in some cases this process can occur independently of G protein coupling. Regarding CXCR4, βarr2 is more likely to be involved in a G protein–dependent chemotaxis, as this process requires the release of Gβγ subunits from activated Gi proteins2 and is abrogated by PTX, which inactivates Gi proteins (Figure 2B). In fact, βarrs can influence chemotaxis downstream of activated GPCRs by virtue of their ability to temporally coordinate and spatially control signaling of molecules involved in cytoskeleton reorganization, including members of the MAPK family, RhoA, PI3K, and actin-binding proteins.38 To take PAR-2–mediated chemotaxis as an example, βarrs bound to activated receptors sequester phosphorylated ERK1/2 along with the actin-filament–severing protein cofilin to locally control actin assembly/disassembly at the leading edge of migrating cells.40,42 Both ERK and p38 MAPKs were found to be regulated by βarr2 downstream of activated CXCR4.5,7 However, regulation of CXCR4-mediated migration by either of these 2 proteins differs between cell types (our unpublished observations, April 2004 and September 2007),7,43-45 thus suggesting that the components of the βarr2-dependent signaling pathway involved in cell migration may be cell-type specific. Recently, interactions between CXCR4 and the actin-binding protein filamin-A were suggested to play a role in regulating CXCL12-induced activation of the RhoA/ROCK pathway, myosin light chain phosphorylation, cofilin activity, and finally chemotaxis.46 Interestingly, filamin-A was first identified as a βarr-binding protein that provides a link between GPCR-induced βarr-mediated signaling and cytoskeletal regulation.47,48 This raises the possibility that the filamin-A–dependent signaling cascade may be set in motion downstream of activated CXCR4m.
Here, we report that CXCL12-induced ERK1/2 phosphorylation is enhanced in leukocytes from a patient with WS with CXCR41013 (Figure 5A), and we confirm in A0.01 cells the causative role of CXCR41013. Phosphorylated ERK1/2 plays roles in numerous cellular activities, including migration, cell proliferation, and cell survival; these depend on its duration, intensity, and subcellular compartmentalization.49 Cytoplasmic and nuclear ERK1/2 have different functions and βarr retains ERK1/2 in the cytoplasm,50,51 alters the time course of ERK1/2 phosphorylation, and can modify ultimately the transcriptional response to receptor activation.52 These observations together with our results raise the possibility that ERK1/2-mediated responses are different in WHIM and control cells. In line with this, a recent study has revealed that CXCL12 acts as an antiapoptotic factor for circulating neutrophils from patients with WHIM, but not for neutrophils from a healthy individual, and the MEK1 inhibitor PD98059 suppressed this effect, thus indicating that survival of WHIM cells may be linked to MEK activation and the subsequent ERK1/2 phosphorylation.53
Previous studies have shown that βarr2 interacts with the C-tail and ICL3 of CXCR4,5 and the results shown here allow for the possibility that the SHSK motif is required for βarr2 binding to ICL3. The deletion of either the C-tail or the SHSK motif allowed us to propose that each domain is dispensable for βarr2 binding, and that the interactions of βarr2 with either of these regions of CXCR4 can mediate distinct functions. Regulation of receptor desensitization and internalization is mediated by the receptor C-tail, and βarr2 does not trigger endocytosis of the C-tail–truncated receptors CXCR41013 and CXCR41013/Δi3. On the contrary, the C-tail is dispensable for βarr2-mediated signaling, but this process requires the integrity of ICL3. Our observations that CXCR41013 exhibits enhanced βarr-mediated signaling are consistent with a model where the interactions between βarr2 and ICL3 of CXCR41013 are favored. It has been proposed that the C-tail covers ICL3 in the CXCR4wt inactive state and then moves away to expose the loop when the receptor is activated.54 Accordingly, truncation of the CXCR4 C-tail might continuously expose ICL3 for βarr binding, thus providing a molecular basis for the enhanced constitutive interactions that CXCR41013 exhibits with βarr2 (Figure 1). Alternatively, but not exclusively, in accordance with a sequential multisite binding model,55 βarr2 may associate with both or either of the 2 regions of CXCR4 when βarr2 encounters the receptor, so that truncation of the C-tail in CXCR41013 results in the privileged association with ICL3. This model is in line with our data showing that in the converse situation, in which the SHSK motif of CXCR4wt is deleted (giving rise to CXCR4wt/Δi3), the receptor is constitutively desensitized and internalized (Figures 3 and 4), possibly due to constitutive interactions of βarr with the C-tail. Indeed, deleting the C-tail in CXCR4wt/Δi3 (CXCR41013/Δi3) abolishes constitutive desensitization and internalization. Thus, these results explain previous works showing that deleting the SHSK motif prevents CXCR4 from activating Gi protein–dependent signals,16,17 and uncover a key role for the SHSK motif in preventing CXCR4 from desensitization. They also suggest that βarrs bound to ICL3 and G proteins engage together on the receptor to concomitantly activate distinct βarr- and G protein–dependent signals.
Evidence is accumulating that GPCRs exist as dimers or as larger oligomers,56 and our results extend previous work showing that CXCR4 also forms constitutive oligomers.27-29 Dimerization plays roles in numerous receptor functions, including ligand binding,57 G protein coupling,58,59 and endocytosis,60 and GPCRs can form heterodimers with new pharmacologic properties compared with homodimers.56 Here, we show that CXCR4wt/CXCR41013 heterodimers form as efficiently as CXCR4wt homodimers and are at least 2-fold more abundant than homodimers when both receptors coexist in the same cell at a 1:1 ratio, thus suggesting that CXCR4wt/CXCR41013 heterodimers prevail in the cells of patients with WHIM. These data also explain how CXCR41013 has a dominant-negative effect on CXCR4wt endocytosis.11 Similarly to CXCR41013, other endocytosis-resistant GPCRs have been reported to prevent internalization by the formation of heterodimers,61,62 and in some cases this process could result from impaired βarr recruitment by the heterodimers. These data are consistent with the notion that binding of βarrs to GPCRs would require homodimer formation. As an example, activation of both subunits in an M3 muscarinic receptor dimer is required for βarr recruitment, and association of this receptor with a mutated M3 receptor lacking βarr binding abrogates this recruitment.63 Similarly, one can speculate that βarr2 must engage 2 CXCR4 molecules with an intact C-tail for an efficient receptor endocytosis, so that this process is impaired when CXCR4wt associates with CXCR41013. On the other hand, expression of CXCR41013 in model cells reproduces the CXCR4 dysfunctions detected in WHIM leukocytes, thus suggesting that CXCR4wt/CXCR41013 heterodimers behave similarly to CXCR41013 homodimers, and as such maintain the interactions with βarr2 for signaling. In other words, it might be that a single CXCR41013 receptor in the CXCR41013 homodimer or the CXCR4wt/CXCR41013 heterodimer preserves its ability to bind βarr2, thus providing a mechanism by which CXCR4m alters the functioning of CXCR4wt in WHIM leukocytes.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are indebted to the patient and his family for their cooperation and to Prof J.-F. Nicolas (Service de Pneumologie, CHU Lyon, France) for the provision of blood samples. We thank Dr M. Bouvier, Dr A. Brelot, Dr R. Jockers, Dr R. J. Lefkowitz, Dr S. Marullo, and Dr M. Thelen for providing cDNAs and reagents as detailed in the text. We are grateful to Dr L. Burleigh (Unité de Pathogénie Virale Moléculaire, Institut Pasteur, Paris, France) for critical reading of the manuscript.
This work was supported by Inserm, GIS-Network for rare diseases, la Ligue Contre le Cancer, Ensemble Contre le SIDA (SIDACTION), and the Agence Nationale de Recherches sur le SIDA (ANRS). K.Y.C.C. was supported by a scholarship from the Croucher foundation (Hong Kong), K.B. by a Young Investi-gator Fellowship from Inserm, A.L. by a fellowship from SIDACTION, and J.H. by a grant from ANRS.
Authorship
Contribution: B.L. designed and performed research, collected, analyzed, and interpreted data, and wrote the manuscript; F. Bachelerie designed research, analyzed data, performed the coordination and funding of the study, and wrote the manuscript; K.Y.C.C. and K.B. performed experiments, analyzed data, and edited the manuscript; A.L., J.H., and T.P. contributed to experiments; T.P. and F.A.S. edited the manuscript; F.A.S. and N.G.S. coordinated the patient cohort; and F. Baleux performed chemokine synthesis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernard Lagane, Institut Pasteur, 25 rue du docteur roux, Paris 75724, France; e-mail: lagane@pasteur.fr.

![Figure 1. The WHIM-type receptor CXCR41013-mediated chemotaxis is dependent upon βarr2. (A) The CXCR41013 or its counterpart with the entire C-tail CXCR4wt was stably expressed at similar levels in HEK cells, as assessed by flow cytometric analysis using the PE-conjugated anti-CXCR4 mAb 12G5. These cells were then transiently transfected using the calcium phosphate–DNA coprecipitation method either with pN1-eGFP (N1) or pβarr2-eGFP (βarr2-GFP). Efficiency of transfection was in the same range for both cell types, with 40% to 60% of transfected cells, as deduced from flow cytometric counting of cells with green fluorescence. At 48 hours after transfection, cells were treated (+) or not (−) with 100 nM CXCL12 before immunoprecipitating of receptors. Receptors and βarr2-GFP in the immunoprecipitates were detected by Western blot analysis using the anti-CXCR4 SZ1567 and anti-βarr2 polyclonal antibodies (n = 3). (B) At 48 hours after transfection, 3 × 105 transfected cells in 150 μL DMEM supplemented with 20 mM HEPES and 1% BSA were added to the upper chamber of a 8-μm-pore polycarbonate Transwell culture insert, and cell migration toward the indicated CXCL12 concentrations placed in the lower chamber proceeded for 4 hours at 37°C in humidified air with 5% CO2. The fraction of cells that migrated across the polycarbonate membrane was assessed by flow cytometry and was calculated as follows: [(number of cells migrating to the lower chamber in response to CXCL12) / (number of cells added to the upper chamber at the start of the assay)] × 100. The percentage of input cells with green fluorescence that migrated to the lower chamber was compared with that of input CXCR4wt- and N1-expressing cells that migrated toward 0.1 nM CXCL12 (arbitrarily set at 1, and accounting for, on average, 2% of input cells). Spontaneous migrations were marginal in these experiments. The data are means plus or minus SEM (n = 3). (C) Migration of βarr2-GFP– or N1-eGFP–expressing leukocytes from a healthy donor (CTRL) or a patient with WHIM with the CXCR41013 receptor was assessed as in panel B using a 5-μm-pore polycarbonate Transwell culture insert and RPMI supplemented with HEPES and BSA as a migration medium. The percentage of input cells with green fluorescence that migrated to the lower chamber was compared with that of input N1-expressing control cells that migrated toward 0.3 nM CXCL12. Leukocytes were isolated as described11 and transfected using the Amaxa Nucleofector technology. (D) CXCR4wt- or CXCR41013-expressing HEK cells were transfected with siRNA targeting βarr2 or control siRNA (NT). At 48 hours after transfection, cell lysates were prepared and expression of βarr2, βarr1, or LDH was assessed by Western blot analysis as described in “Methods.” (E) Chemotaxis of siRNA-treated cells was carried out as in panel B. The data represent means plus or minus SEM (n = 2).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/1/10.1182_blood-2007-07-102103/4/m_zh80140820850001.jpeg?Expires=1767720971&Signature=EDrk5dN9bYi6Araw~K6Xik8OhNc9ZcTz1dA9SswY1Cqvz1pjng3fsC0nNQm8Gm0IpcKwijbxKpxoAnphcEgItAN4wEfbzEgW7VX-vQJdxXsC2kNEy9YYIglrLMsz~yPsE3VsU8~nzIb2FX6mAr796ETiCllHKQ-hOQLJvInEl75QfulGf2-7RyVP26y0pCFgdp7oibrMiVbFthbrDyzIVzwbLKgC6drP2PrD0oeodoD1xmYfE6zW2VtYOXUW0CEhXXISNwoC8JCgAz04udjN5KCoqtG6ySe5QiHRGKIFlNrcfBgKIOcOH651kzhxb3aIdNeampjflyQU3CAwys46WA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. The C-tail and the SHSK motif of CXCR4 regulate activation of G proteins and receptor down-modulation. (A,B) CXCR4wt, CXCR41013, CXCR4wt/Δi3, and CXCR41013/Δi3 were transiently expressed in HEK cells. Flow cytometric analysis using PE-conjugated 12G5 confirmed similar expression levels of receptors at the cell surface. Staining of parental cells was used as a negative control (filled peaks). (C) CXCL12-induced [35S]GTPγS binding to membranes from these cells transiently expressing CXCR4wt (■), CXCR41013 (□), CXCR4wt/Δi3 (●), or CXCR41013/Δi3 (○) is shown. Membranes were incubated in assay buffer containing 0.1 nM [35S]GTPγS and the indicated concentrations of CXCL12. The data, which are representative of 4 independent experiments performed in triplicate, are expressed as a percentage of the basal binding to CXCR4wt-expressing membranes. (D) Receptor expression levels following stimulation with 200 nM CXCL12 for 45 minutes at 37°C are shown. Results (means ± SD; n = 3) indicate the amount of receptor that remains at the surface of HEK cells expressing CXCR4wt (□), CXCR41013 (), CXCR4wt/Δi3 (■), or CXCR41013/ Δi3 (▧) after incubation with CXCL12 compared with untreated cells (n.s.; ).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/1/10.1182_blood-2007-07-102103/4/m_zh80140820850003.jpeg?Expires=1767720971&Signature=tRpL3aWGiJgkmkXxybpx-bquAUuYvONO~mGlwLpIAXBOZ7HUUfAs8dkq38Hqmv5Iz6WiqKENtn7UpHHBFZHXMLqs8YG6zNdwtEGqlFf5A3YdYq8UYDP-fyCFcfBospGnDxn0XPr01NCATnqEoHVxEhigksodVlahWNC2bWDF-2jf4pZKC6hZDQwWOsuke4LJuv44LarA1CrxqToYwT4zLeInF9j609biLYuanb9r5V5TiKLE2WTZ3AcAC-h4PLVhxGr00g2~8k3Ew5PpoN41UDWMRbtSn0msmu4jYW2WmaC2cCf-ppcY4Gm3QYE3U9MggvpHUTc~sE0egFyWnZ7eOw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 1. The WHIM-type receptor CXCR41013-mediated chemotaxis is dependent upon βarr2. (A) The CXCR41013 or its counterpart with the entire C-tail CXCR4wt was stably expressed at similar levels in HEK cells, as assessed by flow cytometric analysis using the PE-conjugated anti-CXCR4 mAb 12G5. These cells were then transiently transfected using the calcium phosphate–DNA coprecipitation method either with pN1-eGFP (N1) or pβarr2-eGFP (βarr2-GFP). Efficiency of transfection was in the same range for both cell types, with 40% to 60% of transfected cells, as deduced from flow cytometric counting of cells with green fluorescence. At 48 hours after transfection, cells were treated (+) or not (−) with 100 nM CXCL12 before immunoprecipitating of receptors. Receptors and βarr2-GFP in the immunoprecipitates were detected by Western blot analysis using the anti-CXCR4 SZ1567 and anti-βarr2 polyclonal antibodies (n = 3). (B) At 48 hours after transfection, 3 × 105 transfected cells in 150 μL DMEM supplemented with 20 mM HEPES and 1% BSA were added to the upper chamber of a 8-μm-pore polycarbonate Transwell culture insert, and cell migration toward the indicated CXCL12 concentrations placed in the lower chamber proceeded for 4 hours at 37°C in humidified air with 5% CO2. The fraction of cells that migrated across the polycarbonate membrane was assessed by flow cytometry and was calculated as follows: [(number of cells migrating to the lower chamber in response to CXCL12) / (number of cells added to the upper chamber at the start of the assay)] × 100. The percentage of input cells with green fluorescence that migrated to the lower chamber was compared with that of input CXCR4wt- and N1-expressing cells that migrated toward 0.1 nM CXCL12 (arbitrarily set at 1, and accounting for, on average, 2% of input cells). Spontaneous migrations were marginal in these experiments. The data are means plus or minus SEM (n = 3). (C) Migration of βarr2-GFP– or N1-eGFP–expressing leukocytes from a healthy donor (CTRL) or a patient with WHIM with the CXCR41013 receptor was assessed as in panel B using a 5-μm-pore polycarbonate Transwell culture insert and RPMI supplemented with HEPES and BSA as a migration medium. The percentage of input cells with green fluorescence that migrated to the lower chamber was compared with that of input N1-expressing control cells that migrated toward 0.3 nM CXCL12. Leukocytes were isolated as described11 and transfected using the Amaxa Nucleofector technology. (D) CXCR4wt- or CXCR41013-expressing HEK cells were transfected with siRNA targeting βarr2 or control siRNA (NT). At 48 hours after transfection, cell lysates were prepared and expression of βarr2, βarr1, or LDH was assessed by Western blot analysis as described in “Methods.” (E) Chemotaxis of siRNA-treated cells was carried out as in panel B. The data represent means plus or minus SEM (n = 2).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/1/10.1182_blood-2007-07-102103/4/m_zh80140820850001.jpeg?Expires=1767770242&Signature=zGleV92dTPzWepnuDlDV0Gv49r8JQ~QXtwQAqzV3uTveEvnHCI1uiFhA2bdymVHWWmu8NpwjAZc8TFb4msBfJkfs4lXNnUEqEPUxVwgZ7RSVGG9YZvGwD6kfFI~C3C26YvCoTjrZMmfjiVpVJ3Vt-pP9C9o-xKaW55ub2ec9lZOlbUXRtD0C7CMb~tDajFyAzmaFUy8i~kyIgfSIgjkpLEeamCoO2RVeGAMuEQhWniEHCx0cVRHvHwXIIaPTZQ~x0AQSF9WxsRopyZMHihxWyCcIKY0y1trvMvTSqz0ZwgLxr3~LxHrbe1RG6-BIeW225Dd5WueYhxfVvO8RMwlGsg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. The C-tail and the SHSK motif of CXCR4 regulate activation of G proteins and receptor down-modulation. (A,B) CXCR4wt, CXCR41013, CXCR4wt/Δi3, and CXCR41013/Δi3 were transiently expressed in HEK cells. Flow cytometric analysis using PE-conjugated 12G5 confirmed similar expression levels of receptors at the cell surface. Staining of parental cells was used as a negative control (filled peaks). (C) CXCL12-induced [35S]GTPγS binding to membranes from these cells transiently expressing CXCR4wt (■), CXCR41013 (□), CXCR4wt/Δi3 (●), or CXCR41013/Δi3 (○) is shown. Membranes were incubated in assay buffer containing 0.1 nM [35S]GTPγS and the indicated concentrations of CXCL12. The data, which are representative of 4 independent experiments performed in triplicate, are expressed as a percentage of the basal binding to CXCR4wt-expressing membranes. (D) Receptor expression levels following stimulation with 200 nM CXCL12 for 45 minutes at 37°C are shown. Results (means ± SD; n = 3) indicate the amount of receptor that remains at the surface of HEK cells expressing CXCR4wt (□), CXCR41013 (), CXCR4wt/Δi3 (■), or CXCR41013/ Δi3 (▧) after incubation with CXCL12 compared with untreated cells (n.s.; ).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/1/10.1182_blood-2007-07-102103/4/m_zh80140820850003.jpeg?Expires=1767770242&Signature=EDyYZF3qyUursazUcDeCBrzrAHVXsIUon5x2YqJi17AWU688CLlNm~IJ-84X7qPqgOJJouiK-NDeIWMNCY2v3hBA9dSHKGOW8Z0DoqOYTkCh8MZ3vWgeYSO1tCY522whU9mqL~N8YmRj6Znti2fMqfGcfonWFSi1fd0FCP5QVUXufZGTyVEJCNqFFM96MD7lePyzUluS-r91U1wqLWsaAWcgriEc1QCX4W0zXg3p2TOwS6ZLnwtXB-4lPZHlJ3AMe1G7Dd0-os9nWFUzFt10fxGND3uoqkfX7D30nGNzW3hrVSX66z1-I1YZMf~-tk9xlOqKRxMKACDO7LmmRr7--g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
 ), CXCR4wt/Δi3 (■), or CXCR41013/ Δi3 (▧) after incubation with CXCL12 compared with untreated cells (n.s.;
), CXCR4wt/Δi3 (■), or CXCR41013/ Δi3 (▧) after incubation with CXCL12 compared with untreated cells (n.s.;  ).
).