Abstract
CD20 is expressed in approximately one- half of pediatric acute lymphoblastic leukemia (ALL) cases with B-cell precursor (BCP) origin. We observed that it is occasionally up-regulated during treatment. To understand the impact of this on the potential effectiveness of anti-CD20 immunotherapy, we studied 237 CD10+ pediatric BCP-ALL patients with Berlin-Frankfurt-Munster (BFM)–type therapy. We analyzed CD20 expression changes from diagnosis to end-induction, focusing on sample pairs with more than or equal to 0.1% residual leukemic blasts, and assessed complement-induced cytotoxicity by CD20-targeting with rituximab in vitro. CD20-positivity significantly increased from 45% in initial samples to 81% at end-induction (day 15, 71%). The levels of expression also increased; 52% of cases at end-induction had at least 90% CD20pos leukemic cells, as opposed to 5% at diagnosis (day 15, 20%). CD20 up-regulation was frequent in high-risk patients, patients with high minimal residual disease at end-induction, and patients who suffered later from relapse, but not in TEL/AML1 cases. Notably, up-regulation occurred in viable cells sustaining chemotherapy. In vitro, CD20 up-regulation significantly enhanced rituximab cytotoxicity and could be elicited on prednisolone incubation. In conclusion, CD20 up-regulation is frequently induced in BCP-ALL during induction, and this translates into an acquired state of higher sensitivity to rituximab. This study was registered at http://www.clinicaltrials.gov as #NCT00430118.
Introduction
CD20 is a signature B-cell differentiation antigen strongly expressed on the surface of mature normal as well as malignant B cells. It is also expressed, but at lower levels and with larger variance, on more immature B cells and their malignant counterparts found in B-cell precursor (BCP) acute lymphoblastic leukemia (ALL).1,2 In line with the expression patterns, anti-CD20 directed immunotherapy has been shown to elicit potent antitumor effects specifically in mature B-cell lymphoma and leukemia, where it has been incorporated into standard treatment as a valuable therapy advance.3 To date, the most broadly evaluated compound for CD20 targeting is rituximab, a chimeric antibody that was licensed by the Food and Drug Administration in 1997 as the first anticancer monoclonal antibody. It acts by complement-dependent and antibody-dependent cell-mediated cytotoxicity as well as by inducing apoptosis directly.4 Recently, targeted therapy with rituximab has been implicated also in BCP-ALL for combination with conventional chemotherapy,5 with at least 6 active trials listed at http://www.clinicaltrials.gov (accessed May 2008). In children with BCP-ALL, published usage has been confined mostly to anecdotal reports on relapsed or refractory disease.6-9 Importantly, activity can be anticipated primarily in CD20+ cases, which relevantly limits its applicability in pediatric BCP-ALL supposedly to less than one half of patients as determined at diagnosis.2
During the course of an internationally collaborative study on flow cytometric minimal residual disease (MRD) assessment in childhood ALL, we noted that phenotypic modulation occurred regularly in viable leukemic cells resisting induction treatment with protocol Italian Association of Pediatric Hematology and Oncology/Berlin-Frankfurt-Munster (AIEOP-BFM) 2000.10,11 As one of the most frequently observed phenotypic changes, CD20 expression was found to be up-regulated. Gene expression analysis showed that this increase in surface protein density is paralleled by up-regulation of mRNA expression as early as at day 8 after the start of treatment.12 The phenomenon has largely been attributed to glucocorticoid action.11,12 We hypothesized that this increase in CD20 expression could be exploited for anti-CD20 targeted therapy, setting the stage that more patients with BCP-ALL than assessable on phenotypic analysis at diagnosis could eventually profit from such treatment. To get a more comprehensive view of the frequency and the potential impact of CD20 up-regulation, we analyzed this in a large cohort of pediatric BCP-ALL patients recruited to a nationwide treatment protocol in Austria and also assessed the effects of rituximab in paired in vitro analyses.
Methods
Patients, samples, and treatment
Between December 2000 and June 2006, 306 patients with ALL (age, 1-18 years) were consecutively recruited in Austria to the international treatment trial AIEOP-BFM-ALL 2000 (registered at http://www.clinicaltrials.gov as #NCT00430118).13 Flow cytometric MRD assessment was done in these patients in the Vienna laboratory as part of a 4-center collaborative add-on study. Sampling for immunophenotypic and MRD investigations was approved along with the international trial by the institutional ethical committees and was strictly done according to informed consent guidelines in accordance with the Declaration of Helsinki. In assessing CD20 expression, we focused on the 237 cases with a CD10+ BCP phenotype in which we obtained enough material for analysis, thus excluding from further analysis 38 patients with T-ALL, 12 with a pro-B phenotype, and 19 with inadequate sample. Peripheral blood (PB) and bone marrow (BM) samples were investigated for this study at diagnosis and from several follow-up time points during induction treatment: PB day 8, BM from days 15 and 33. Induction treatment consisted of a 7-day prephase with daily oral prednisolone (60 mg/m2 body surface area/day) and one dose of intrathecal methotrexate (age-adjusted). From day 8 until day 33, patients received a 4-drug regimen containing randomized glucocorticoids (prednisolone 60 mg/m2 or dexamethasone 10 mg/m2), 8 infusions of L-asparaginase, 4 administrations of daunorubicin and vincristine, as well as additional intrathecals.13 Glucocorticoid medication was tapered after day 28. A second induction phase was started not earlier than on day 36.
Data on genotype, clinical risk stratification (low-, medium-, and high-risk), and outcome were retrieved from the Austrian ALL-BFM 2000 study office.
Flow cytometry
The standardized procedure has been recently described in detail, including sample preparation, description of the entire antibody panel, stain-lyse procedure, and flow cytometric analysis.14 A fixed quadruple-stain was used to investigate CD20 expression: CD20/CD10/CD19/CD34 (ordered by channels 1-4: fluorescein isothiocyanate, phycoerythrin, phycoerythrin-cyanin 7, and allophycocyanin). In experiments assessing differences in CD20 expression between viable and apoptotic blast cells, an annexin V-staining reagent (BD Biosciences, San Jose, CA) was substituted for CD34 in channel 4.11 The instrument setup was optimized daily by analyzing Calibrite beads (BD Biosciences) and normal adult PB cells stained with CD4/CD8/CD3/CD45.
Immunophenotyping at diagnosis and quality control evaluations were performed collecting at least 30 000 cellular events, whereas for MRD measurements 300 000 events were acquired from 700 000 stained cells. Cell acquisition was performed with a FACSCalibur cytometer (BD Biosciences) using the CELL Quest software (BD Biosciences). Data analysis was done with the PAINT-A-GATE software (BD Biosciences). Leukemic cells were identified using an immunologic gate (associated with 90-degree scattering), which included all CD19+ cells. MRD was defined as an accumulation of at least 10 clustered events displaying lymphoid-scattering properties and leukemia-associated immunophenotypic characteristics as reported.15-17
CD20 expression of samples was estimated by assessing the proportion of leukemic cells positive for the antigen with a cutoff of more than or equal to 20%. The threshold was set according to the upper limit of the background fluorescence of residual lymphoid cells not expressing B-cell markers within the same acquisition. In addition, CD20 expression levels were quantified on the basis of mean fluorescence intensity (MFI) values using the CELL Quest software as reported.10,15 Dako Fluorospheres (type IIIa beads; Dako Cytomation, Glostrup, Denmark) with assigned values of molecules of equivalent soluble fluorochrome were used for longitudinal monitoring of instrument performance stability.14 Measurements of these beads on an approximately weekly basis (which contain blank as well as fluorescence beads with 5 different emission intensities) were performed. These MFI measurements over a period of 12 months or longer gave 5 different SD values (one per fluorescence level of beads in the fluorescein isothiocyanate channel), which ranged from plus or minus 12.4% to 14.9%. These proportions reflect the low background of technical variance in MFI measurements during our study.
Rituximab experiments
Paired BM samples from 9 patients from diagnosis and follow-up time points during induction were selected for these experiments according to a negative/dim CD20 expression status at diagnosis, and to obvious CD20 up-regulation (various degrees) as well as sufficient MRD proportions at follow-up (≥1%). Mononucleated cells were prepared by gradient separation (Lymphoprep, Fresenius, Oslo, Norway). Cells (∼ 1 × 106 per test) were then incubated for one hour at 37°C with human complement serum (15% v/v; Sigma-Aldrich, Steinheim, Germany) with or without clinical grade rituximab (Mabthera; end-concentration, 0.2 mg/mL), and with or without paired micro-antibodies (MB-55, anti-CD55, and MB-59, anti-CD59, end-concentration, 13 μg/mL for both). Rituximab was kindly provided by P. Krenn and A. Wolfsberger (Vienna, Austria), and the micro-antibodies were a kind gift of P. Macor (Trieste, Italy).18 Notably, in tests investigating the effects of micro-antibodies (“augmented lysis”), cells were preincubated with these for 30 minutes at 37°C to allow for antigen saturation before adding rituximab and complement. After incubations, cells were washed with cold phosphate-buffered saline, transferred into Trucount tubes (BD Biosciences), and stained for 15 minutes at room temperature with CD10/CD19/CD3/CD45 plus 4′6-diamidino-2-phenylindole, dihydrochloride (for dead-cell exclusion). Without a further washing step, samples were acquired on an LSRII cytometer (BD Biosciences; equipped with 3 lasers including one with 405-nm emission). The remaining live leukemic blasts were assessed in absolute counts by recalculating the measured proportions using beads (of Trucount tubes) as internal standards. Rituximab-derived values were denoted as proportional recovery compared with control (ie, incubations with complement alone).
In vitro incubations with glucocorticoids
Mononucleated BM cells from 10 ALL patients at diagnosis were prepared by gradient separation (as in rituximab experiments), and incubated with or without clinical grade prednisolone (0.00, 0.05, 0.5, 5 μg/mL) at 2 × 105 cells per well (96-well plates) on human mesenchymal stroma cells using GIBCO AIM V medium without serum supplements (Invitrogen, Lofer, Austria). As stroma cells, we used a human BM mesenchymal cell line immortalized by enforced expression of telomerase, which was kindly donated by D. Campana, St Jude Children's Cancer Research Hospital.19,20 After 3 days of culture, we assessed the CD20 expression (MFI values) on viable cells (recovery in controls: mean ± SD, 66% ± 33%) in the different incubations using a CD20/CD10/CD19/annexin V stain.
Statistical analyses
Concordance and correlations between sample cohorts or time points were estimated using either the Shrout-Fleiss reliability assay or the Pearson correlation coefficient (PCC). The Wilcoxon signed-rank test (2-tailed) was used to assess the significance of observed differences between cohorts in paired data. Comparisons between the following datasets of paired BM or PB samples were performed: BM day 0 versus day 15 (cBM0/15), PB day 0 versus day 8 (cPB0/8), BM day 0 versus day 33 (cBM0/33), and BM day 15 versus day 33 (cBM15/33). The χ2 test was used to screen for differences in proportional distributions between cohorts. The Spearman rank correlation coefficient was used to assess the correlation between levels of CD20 expression (MFI values) and efficacy of rituximab-lysis.
Results
CD20 expression at diagnosis
We investigated 237 BM and 202 PB samples of CD10+ BCP-ALL patients at diagnosis. We found expression of CD20 (≥ 20% positive blasts) in 46% (109 BMs) and 51% (104 PBs) of samples, respectively. The mean proportion of CD20+ blasts per sample was low: 27% in BM (± 29% SD; n = 237) and 33% in PB (± 32% SD; n = 202). In paired analysis, there was a good correlation between the proportions of CD20+ blasts in BM and PB (P < .001; Pearson correlation coefficient = 0.83), but with some variance of values (difference: mean ± SD, 13% ± 17%).
CD20 expression in follow-up
Paired samples from diagnosis and early follow-up, which had sufficient MRD (≥ 0.1%) to test reliably for CD20 expression, were available in 159 (BM from day 15; 67% of all) and 138 (PB from day 8; 68% of all) cases, respectively. Paired samples from diagnosis and late induction (BM day 33) had sufficient MRD in the latter in 27 cases (11%). Hence, all follow-up samples with very low or even negative MRD and their counterparts from diagnosis were excluded from further analyses. Compared with the total available sample cohort at diagnosis, those with paired follow-up samples had very similar proportions of CD20+ cases at diagnosis (BM, 45%; PB, 52%).
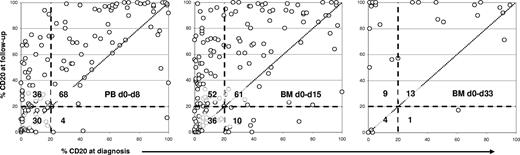
Follow-up specimens showed significantly increased CD20 expression compared with their paired initial samples (Table 1). This up-modulation was seen as an overall increase in CD20+ samples as early as at day 8 in PB (75% positive; cPB0/8: P < .001), and also in follow-up BM samples (day 15, 71% positive, cBM0/15: P < .001; day 33: 81%, cBM0/33 P < .01). There was even a trend toward a further increase from day 15 to day 33 (cBM15/33 P = .05; n = 25 pairs). The mean proportion of CD20+ blasts in BM was 25% at day 0 (± 28% SD), rose to 47% at day 15 (± 34%), and to 72% at day 33 (± 36%). Regarding fluorescence levels, CD20 expression of blasts increased from a mean MFI value of 14 in initial samples (± 27 SD) to 45 (± 100) at day 15, and to 109 (± 187) at day 33. Only 5.7% of patients had a CD20-MFI more than 50 at diagnosis, but the proportion increased to 22.2% at day 15 and to 48% at day 33.
In individual patients, the proportions of CD20+ blasts increased with time irrespective of whether a given leukemic case at diagnosis had been CD20+ or not (P < .001 for all correlations; Figure 1). The proportions of CD20+ blasts in paired PB and BM samples from days 8 and 15 correlated well (PCC = 0.80; P < .001) but with some variance (difference: mean ± SD, 15% ± 18%). Notably, the CD20 expression neither correlated with amounts of MRD in day 8 PB (PCC = 0.22) nor day 15 BM (PCC = 0.18).
Proportions of CD20+ blasts in paired samples from diagnosis and follow-up. Comparisons of PB samples from day 0 versus day 8 (left plot), as well as of BM samples from diagnosis versus day 15 (center) and day 33 (right), are shown. Each data point represents one BCP-ALL case. Thresholds for determining CD20 expression on leukemic cells were set by using the background fluorescence of residual non-B lymphocytes within the same acquisition. Follow-up samples only with more than or equal to 0.1% residual leukemic cells were analyzed. Dashed lines mark the cutoff of 20% used to describe a sample as positive or negative. The numbers of samples located within each of the 4 quadrants, which are built by the cutoff lines, are shown. Identical results in paired samples would fit closely to the dotted diagonal line. In all comparative plots, it can be seen that follow-up samples have higher proportions of CD20+ leukemic cells than the paired samples at diagnosis.
Proportions of CD20+ blasts in paired samples from diagnosis and follow-up. Comparisons of PB samples from day 0 versus day 8 (left plot), as well as of BM samples from diagnosis versus day 15 (center) and day 33 (right), are shown. Each data point represents one BCP-ALL case. Thresholds for determining CD20 expression on leukemic cells were set by using the background fluorescence of residual non-B lymphocytes within the same acquisition. Follow-up samples only with more than or equal to 0.1% residual leukemic cells were analyzed. Dashed lines mark the cutoff of 20% used to describe a sample as positive or negative. The numbers of samples located within each of the 4 quadrants, which are built by the cutoff lines, are shown. Identical results in paired samples would fit closely to the dotted diagonal line. In all comparative plots, it can be seen that follow-up samples have higher proportions of CD20+ leukemic cells than the paired samples at diagnosis.
CD20 up-modulation occurs in viable cells
We recruited paired BM and PB samples from 10 consecutive patients with CD10+ BCP-ALL to investigate and compare the levels of CD20 expression (MFI values) on viable (annexin−) and apoptotic (annexin+) leukemic blasts within the same specimens.
Apoptotic blasts showed slightly but significantly higher MFI values in the CD20 channel than viable cells, as reflected by a mean ratio of 1.28 (± 0.3 SD; P < .01) in BM samples at diagnosis and 1.32 (± 0.34 SD; P = .01) at day 15. Similarly, in PB samples, we found mean increase factors of 1.32 (± 0.76 SD; P < .01) at diagnosis and of 1.17 (± 0.21 SD; P = .01) at day 8. However, compared with these differences between live and apoptotic cells, the increases in CD20 expression on blast cells between diagnosis and follow-up were much higher, and these latter differences were found comparing viable as well as apoptotic cells separately. The mean (SD) increase factors in the BM samples (cBM0/15) were 5.84 (± 7.0; P < .01) in viable blast cells and 5.32 (± 6.0; P < .01) in apoptotic blast cells. Similarly, mean (SD) increased factors in PB samples (cPB0/8) were 4.46 (± 5.2; P < .01) in annexin− blasts and 4.40 (± 4.7; P < .01) in annexin+ leukemia cells. All ratios built on comparisons between cells at diagnosis and at follow-up were significantly higher than those from viable vs dead cell comparisons (P < .05 and lower in Wilcoxon ranking test for paired data).
CD20 expression in subgroups of patients
The 2 patient cohorts receiving different glucocorticoids (days 8 until 33) on initial randomization were very similar in CD20 expression at baseline and also reacted similar as estimated at day 15 and day 33 in BM (P = not significant).
There was no significant correlation between CD20 expression and age (as tested in groups of 1-5, 6-10, and >10 years) or ALL-phenotype (C vs pre-B subtype). In addition, we found no significant difference in CD20 expression in BM samples between the 3 BFM risk groups (Table 1) either at diagnosis (P = .06) or at day 15 (P = .32). CD20 up-regulation occurred in follow-up in all 3 BFM risk groups (P < .001 for each risk group), with high-risk patients showing very high proportions of positive cases at all time points (range, 83%-88%).
TEL/AML1-rearranged cases (n = 46) showed significantly lower CD20 expression in BM samples both at diagnosis and at day 15 compared with the complementary cohort (n = 113; P < .01 for all correlations), as reflected by the number of CD20+ cases (Table 1), proportions of CD20+ blasts, and MFI values (data not shown). Hyperdiploid ALLs (n = 39) had higher CD20 expression than TEL/AML1-rearranged cases at day 15 (P < .01 for all correlations), but at diagnosis only with respect to MFI values (P < .01). Non-TEL/AML1/nonhyperdiploid samples (n = 74) had higher CD20 values than TEL/AML1 cases in all items both at diagnosis and day 15 (P < .01 in all correlations) but were not significantly different in similar comparisons from hyperdiploid cases. CD20 was up-regulated during induction both in TEL/AML1 and non-TEL/AML1 patients (P < .01 for both; Table 1), but the former reached lower positivity (in 46%-56% of cases) than the latter (81%-85%).
Fourteen patients of the cohort of 159 had a relapse within a median observation period of 4.5 years. There was no significant correlation between the relapse status and CD20 expression of leukemic cells from BM either at diagnosis (P = .17) or at day 15 (P = .32). Of the 14 relapse cases only, 4 were CD20+ initially compared with 11 at day 15. The mean (SD) proportions of CD20+ blasts increased from 13% (± 15%) at diagnosis to 44% (± 28%) at day 15. The 3 relapsed patients, who were included in the group of 27 with high MRD also at day 33, had 75% (± 17%) CD20+ blasts at this time point.
CD20 up-regulation enhances rituximab-inducible complement-lysis
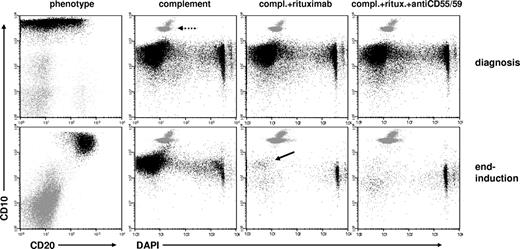
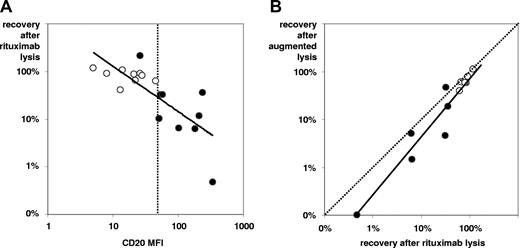
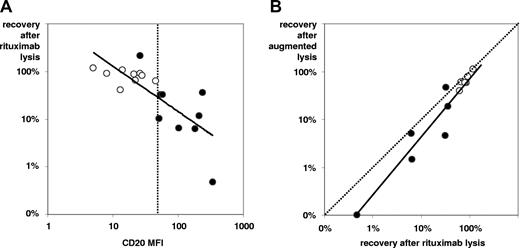
In 9 sample pairs of BM from diagnosis and follow-up, we tested whether CD20 up-regulation is strong and specific enough to translate into relevant complement-dependent cytolysis induced by rituximab in in vitro incubation. There were sufficient leukemic cells in these follow-up samples to do the test reliably (MRD proportion: mean ± SD, 17.9% ± 21.6%). The selected sample pairs showed low CD20 expression at diagnosis (MFI: mean ± SD, 20 ± 12) and significantly higher levels at follow-up (MFI: mean ± SD, 140 ± 107; P < .01 for difference by Wilcoxon's signed rank test). As exemplified in Figure 2, rituximab induced significantly more efficient cytolysis in follow-up samples (recovery: mean ± SD, 39% ± 67%) than in initial specimens (recovery: mean ± SD, 82% ± 23%; P < .05 for difference). Stronger cytolysis was seen in all samples with CD20 MFI more than 50 compared with those with lower MFI values (Figure 3A). Furthermore, we tested whether rituximab action could be augmented by neutralizing the complement-regulatory proteins CD55 and CD59. Using blocking antibodies to these antigens in 6 sample pairs, the “augmented” lysis was slightly more efficient than rituximab-lysis alone in 11 of the 12 samples investigated (recovery: mean ± SD, 40% ± 36% vs 51% ± 38%; P = .01; Figure 3B).
CD20 up-regulation in follow-up translates into efficient rituximab-induced complement-lysis. Dot plots are derived from analyses of paired BM samples of a high-risk BCP-ALL patient at diagnosis and at end-induction. Phenotypic comparisons and cell recovery analyses after in vitro incubations with complement alone, with complement plus rituximab (0.2 mg/mL), and with complement plus rituximab plus micro-antibodies neutralizing CD55 and CD59, are shown. The dashed arrow points at the cluster of Trucount beads that were used as internal standards for absolute cell recovery assessment. Up-regulation of CD20 expression can be seen on the CD10+ leukemic cells (black), which remained after therapy. An almost complete reduction of intact (4′6-diamidino-2-phenylindole, dihydrochloride-negative) CD10+ leukemic cells can be seen on rituximab incubations, with a small remnant fraction of viable leukemic cells marked with an arrow. Of note, cells lysed by complement mostly disappear from dot plots on severe cellular disruption.
CD20 up-regulation in follow-up translates into efficient rituximab-induced complement-lysis. Dot plots are derived from analyses of paired BM samples of a high-risk BCP-ALL patient at diagnosis and at end-induction. Phenotypic comparisons and cell recovery analyses after in vitro incubations with complement alone, with complement plus rituximab (0.2 mg/mL), and with complement plus rituximab plus micro-antibodies neutralizing CD55 and CD59, are shown. The dashed arrow points at the cluster of Trucount beads that were used as internal standards for absolute cell recovery assessment. Up-regulation of CD20 expression can be seen on the CD10+ leukemic cells (black), which remained after therapy. An almost complete reduction of intact (4′6-diamidino-2-phenylindole, dihydrochloride-negative) CD10+ leukemic cells can be seen on rituximab incubations, with a small remnant fraction of viable leukemic cells marked with an arrow. Of note, cells lysed by complement mostly disappear from dot plots on severe cellular disruption.
Dependency of rituximab-induced complement-lysis. Efficacy increases with higher intensity of CD20 expression (A) and with inhibition of complement-regulatory antigens (B). In panel A, 18 samples (9 pairs) of BCP-ALL at diagnosis (○) and from follow-up (•) were analyzed for CD20 expression levels (MFI values) and blast cell recovery on in vitro rituximab/complement incubations. The (continuous) regression line and the MFI channel = 50 (dotted line) are shown. Note that all but one follow-up sample show higher CD20 expression than initial samples along with more efficacious rituximab-lysis (best separator apparently at MFI = 50). In panel B, comparisons of complement-lysis efficacy with rituximab alone vs rituximab plus additional mini-antibodies against CD55 and CD59, denoted “augmented” lysis, are shown. Twelve samples (from 6 of the 9 pairs, as earlier in this figure legend) were tested. Differences between incubations were minor but statistically significant.
Dependency of rituximab-induced complement-lysis. Efficacy increases with higher intensity of CD20 expression (A) and with inhibition of complement-regulatory antigens (B). In panel A, 18 samples (9 pairs) of BCP-ALL at diagnosis (○) and from follow-up (•) were analyzed for CD20 expression levels (MFI values) and blast cell recovery on in vitro rituximab/complement incubations. The (continuous) regression line and the MFI channel = 50 (dotted line) are shown. Note that all but one follow-up sample show higher CD20 expression than initial samples along with more efficacious rituximab-lysis (best separator apparently at MFI = 50). In panel B, comparisons of complement-lysis efficacy with rituximab alone vs rituximab plus additional mini-antibodies against CD55 and CD59, denoted “augmented” lysis, are shown. Twelve samples (from 6 of the 9 pairs, as earlier in this figure legend) were tested. Differences between incubations were minor but statistically significant.
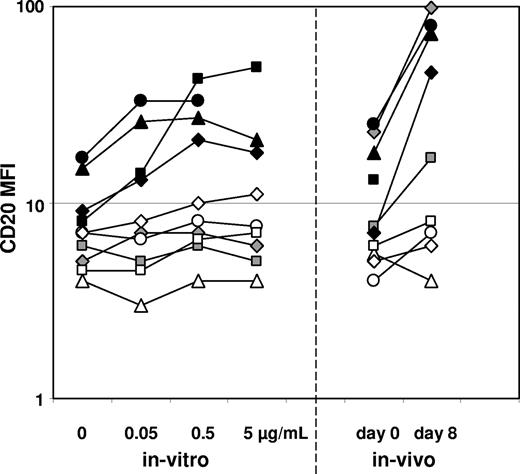
CD20 expression is modulated by prednisolone in vitro
We incubated 10 initial ALL samples with various concentrations of prednisolone for 3 days and assessed thereafter the CD20 expression levels on viable (annexin−) leukemic cells. Expression changes were compared with those found in the same patients in samples from diagnosis and day 8 of induction after only the prednisolone prephase (Figure 4). Four samples showed significant up-regulation in vitro, and the 3 of these that had sufficient leukemic cells on day 8 for analysis also showed up-regulation in vivo. Furthermore, 6 samples showed only minor or no modulation in vitro, 4 of which (3 TEL/AML-1–rearranged cases) also showed no up-regulation in vivo. Two TEL/AML-1–negative samples showed no up-regulation after 3 days in vitro incubation but clear up-modulation in vivo at day 8.
Prednisolone increases CD20 expression in vitro. CD20 expression levels (MFI values) of viable leukemic cells of 10 ALL samples after incubation for 3 days with various concentrations of prednisolone (none; 0.05-5 μg/mL) are shown (individual patients are characterized by specific symbols). In vivo expression changes in samples taken at diagnosis and day 8 (after the prednisolone prephase) among the same patients are shown for comparison. Note patient-specific modulation patterns.
Prednisolone increases CD20 expression in vitro. CD20 expression levels (MFI values) of viable leukemic cells of 10 ALL samples after incubation for 3 days with various concentrations of prednisolone (none; 0.05-5 μg/mL) are shown (individual patients are characterized by specific symbols). In vivo expression changes in samples taken at diagnosis and day 8 (after the prednisolone prephase) among the same patients are shown for comparison. Note patient-specific modulation patterns.
Discussion
Immunophenotypic characterization of ALL is important for directing therapy and predicting outcome. Except for very recent developments, however, the antigenic peculiarities of ALL cells, as determinable by immunophenotyping, have not been exploited for direct therapeutic interventions; this situation is about to change.
CD20, an important differentiation-related surface antigen of B cells, is commonly used as a marker in leukemia phenotyping as well as for assessment of MRD in BCP-ALL.14,16,21,22 Its expression has been associated with a better,2 or an inferior outcome in ALL,23 probably depending on the treatment used. With substantial similarity between observations, CD20 expression (based on either a cutoff of 20% positivity or relevant fluorescence intensity) was found in approximately 48% of patients with BCP-ALL,2,23 which favorably compares to the proportion noted in our present study (46% of initial BM samples). There was no significant relation of CD20 expression to outcome in terms of relapses in our BFM-protocol–based patient cohort with BCP-ALL. In addition to these results, we observed in many patients and among all risk groups that CD20 expression is significantly up-regulated on leukemic cells during the phase of induction chemotherapy. Because CD20 is the target of well-established immunotherapeutic agents, such as rituximab, our observation appears relevant toward using antigenic patterns of leukemia for treatment. Two aspects seem important herein.
The first is that leukemic cells can be influenced to alter their cellular processes and to acquire qualities exploitable for their eradication. There is now sufficient evidence to consider that leukemic phenotypes (of individual clonal cases) are not stable but may vary within certain limits on outer influences.10,11,24 In this respect, these changes in leukemia phenotypes may well be considered just as surrogate appearance of the cellular processes in general.12 This property could be deployed for future treatment specifically in cases in which certain subcellular pathways, as for example, those leading to apoptosis, are blocked or insufficient, so that leukemia could be tackled via alternative routes. Importantly, we found that in particular high-risk BCP-ALLs (which include those with a poor glucocorticoid response or even with a poor response to a 4-drug induction) were CD20+ in follow-up in 83% of cases. More generally, 81% of all patients with significant MRD after induction (≥ 0.1%), an amount known to correlate with dismal outcome,16 were CD20+ even at quite high levels of intensity (day 33 MFI: mean ± SD, 109 ± 187). In the in vitro experiments, we found efficient cytolysis only when CD20 expression intensities surpassed a MFI value of 50. This corresponds in our system roughly to more than or equal to 80% to 90% positive blasts: ie, a fluorescence distribution of leukemic cells almost nonoverlapping with control cells. Hence, mere CD20 positivity at diagnosis based on the cutoff value of more than or equal to 20% of blasts positive with the marker was found insufficient to predict cytolysis in vitro. Nevertheless, the up-regulation seen in follow-up was specific and efficient enough to translate into relevant rituximab activity. Hence, using a cutoff value of more than or equal to 90% or more than or equal to 80% positive cells, 52% to 67% of the MRD-positive (≥ 0.1%) patients with BCP-ALL in our series met the criteria at end-induction, as opposed to only approximately 5% to 8% of cases at diagnosis and 20% to 25% at day 15, respectively. Notably, a dependency of rituximab-inducible complement-lysis on levels of CD20 expression was reported also in B-cell chronic lymphocytic leukemia.25 Compared with the latter study, we used a higher rituximab concentration similar to that reached in serum after a single standard in vivo application of 375 mg/m2.26 However, complement-lysis is not the only mechanism by which rituximab induces cytotoxicity.27 It may therefore not be possible to deduce an algorithm from our in vitro results, which would allow determining the potential in vivo success of rituximab in ALL.
The second important aspect is that this intervention, which influences the leukemic cellular processes in a way that blast cells acquire qualities exploitable for their eradication, comes along without a need for any extra medication. The process seems to be induced by the induction chemotherapy for ALL, and because obvious as early as at day 8 of therapy (after 1 week of prednisolone and a single intrathecal methotrexate instillation) most probably is triggered in this phase by glucocorticoids.12 This is corroborated by our finding that CD20 is up-modulated by prednisolone incubation in vitro, an effect we found to be nonuniform and patient-specific. We are aware that, after the prednisolone-prephase, other medications may also add to the observed effect. Other substances than steroids have already been shown to influence CD20 expression. For example, bryostatin-1, a protein kinase C modulator, enhances both CD20 mRNA and protein levels, and a combined therapy with rituximab was suggested recently.28 In cell line experiments, this effect was found to be even insensitive to steroid action. Such effects add to strategies aiming at enhancing anti-CD20–directed cytotoxicity. The Memorial Sloan-Kettering Cancer Center currently screens the possibility to use orally available β-glucans, which prime complement binding to the cell surface and thus enhance rituximab activity.29 Increasing complement-lysis by neutralizing the inhibitory surface molecules CD55 and CD5918,25 or by driving CD20 to segregate into lipid rafts via cross-linking by a new polymer formulation of rituximab are further evaluated strategies.30,31 Notably, other anti-CD20 antibodies, such as tositumomab, may have higher affinity to CD20 as well as different mechanisms of action compared with rituximab.32 Immunotherapy directed to other antigens, such as CD52, also complements the novel armamentarium against lymphoid malignancies.33,34 Target validation on a case-by-case basis has been suggested in this context.35 Of note, the TEL/AML1-rearranged cases in our study had much lower CD20 expression at baseline and also poorly up-regulated the antigen in follow-up. Of 46 TEL/AML1 cases, none at diagnosis, only one at day 15 (2%) and also not the single case with relevant MRD at day 33 showed CD20 expression on more than or equal to 90% of blasts, as opposed 7%, 27%, and 54% of the non-TEL/AML1 cases at these time points. These results together with the observations of Golay et al, who showed that TEL/AML1 cases are very sensitive to anti-CD52 treatment as opposed to non-TEL/AML1 leukemias,36 create the possibility to choose rituximab or alemtuzumab based on genotype.
In conclusion, we have shown that CD20 is up-regulated during induction therapy with BFM-type protocols in a very significant proportion of patients with non-TEL/AML1 BCP-ALL. Most relevantly, the process is continued and sustained until end-induction (and even thereafter; M.N.D., manuscript submitted) so that, in particular, patients with high MRD at this time point could profit from anti-CD20–directed immunotherapy. Because up-regulation of CD20 is induced during chemotherapy (most obviously because of glucocorticoid action) without a need for extra medication, this is a simple and straightforward way to influence leukemic cells so that they acquire qualities exploitable for their eradication. Based on this rationale, clinical trials in pediatric ALL could now be designed to assess whether rituximab would result in a clinical benefit.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank P. Macor and F. Tedesco (Trieste, Italy) for providing the mini-antibodies MB-55 and MB-59; P. Krenn and A. Wolfsberger (Vienna) for donating rituximab; D. Campana (Memphis, TN) for the immortalized human stroma cell line HBMS; all doctors, nurses, and technicians of the participants of the Austrian BFM for their close collaboration; D. Janousek from the Austrian BFM study office for clinical data supply; R. Panzer-Grümayer (Vienna), M. Veltroni (Padova), and L. Karawajew (Berlin) for very useful discussions; and G. Fröschl (Vienna) for excellent technical assistance.
This work was supported by the Jubiläumsfonds (Project No. 10962) of the Austrian National Bank (M.N.D.); Fondazione Citta' della Speranza, Consiglio Nazionale delle Ricerche, Ministero dell'Universita a Ricerca Scientifica a Tecnologica, Associazione Italiana per la Ricerca sul Cancro (G.B.); Fondazione Tettamanti, Fondazione Cariplo, Associazione Italiana per la Ricerca sul Cancro, Consiglio Nazionale delle Ricerche (G.G.); and Wilhelm Sander Stiftung 2004.072.1 (R.R.). This study was promoted by the International BFM Study Group.
A complete list of study participants can be found on the Blood website.
Authorship
Contribution: M.N.D. designed research, collected data, analyzed and interpreted data, and drafted the manuscript; A.S. did most sample analyses and experimentation; D.P. provided experimental guidance; U.P. performed statistical analyses; Z.H. and A.A. contributed in analyses and experimentation; G.B., G.G., and R.R. designed research and participated in manuscript drafting; G.M. collected samples and provided clinical data; and H.G. designed research and secured funding.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael N. Dworzak, Department of Pediatric Hematology and Oncology, Children's Cancer Research Institute and St Anna Kinderspital, Kinderspitalgasse 6, A-1090 Vienna, Austria; e-mail: dworzak@stanna.at.