Abstract
In the HD15 trial of the German Hodgkin Study Group, the negative predictive value (NPV) of positron emission tomography (PET) using [18F]-fluorodeoxyglucose in advanced-stage Hodgkin lymphoma (HL) was evaluated. A total of 817 patients were enrolled and randomly assigned to receive BEACOPP-based chemotherapy. After completion of chemotherapy, residual disease measuring more than or equal to 2.5 cm in diameter was assessed by PET in 311 patients. The NPV of PET was defined as the proportion of PET− patients without progression, relapse, or irradiation within 12 months after PET review panel. The progression-free survival was 96% for PET− patients (95% confidence interval [CI], 94%-99%) and 86% for PET+ patients (95% CI, 78%-95%, P = .011). The NPV for PET in this analysis was 94% (95% CI, 91%-97%). Thus, consolidation radiotherapy can be omitted in PET− patients with residual disease without increasing the risk for progression or early relapse compared with patients in complete remission. The impact of this finding on the overall survival at 5 years must be awaited. Until then, response adapted therapy guided by PET for HL patients seems to be a promising approach that should be further evaluated in clinical trials. This trial is registered at http://isrctn.org study as #ISRCTN32443041.
Introduction
Hodgkin lymphoma (HL) is one of the best curable malignancies in adult oncology, with reported disease-free survival in excess of 80% at 5 years.1,2 Because of the risk of secondary malignancies and other sequelae, the reduction of toxicity has become one of the major goals in the treatment of HL patients, including those in advanced stages.3,4 After establishing 8 cycles of BEACOPP (cyclophosphamide, adriamycin, etoposide, procarbazine, prednisone, vincristine and bleomycin) escalated as new treatment of choice in this group of patients,5 the German Hodgkin Study Group (GHSG) follow-up studies HD12 and HD15 aimed at reducing toxicity while maintaining the improved disease control with an overall survival of 92% and freedom from treatment failure of 88% at 5 years.
One possible approach to reduce toxicity is to omit or substantially reduce the number of patients receiving additional radiotherapy.6-8 More recently, smaller studies suggested that positron emission tomography (PET) using [18F]-fluorodeoxyglucose (FDG) might discriminate between active and inactive tissue in HL, suggesting a high negative predictive value (NPV) for FDG-PET in this malignancy.9-11 Thus, the GHSG HD15 study for advanced-stage HL included a PET question (HD15-PET) evaluating the NPV of PET in patients after BEACOPP. Radiotherapy after chemotherapy was restricted to those patients in partial remission (PR) who had PET-positive (PET+) residual tissue. PET-negative (PET−) patients received no additional radiotherapy.
Here we present the results of HD15-PET demonstrating a NPV of 94% after 6 to 8 cycles of BEACOPP for PET− patients.
Methods
Patients
All patients of the HD15-PET study were recruited into the prospectively randomized HD15 multicenter trial of the GHSG. Inclusion criteria were newly diagnosed, histology-proven HL in clinical stages IIB with extranodal disease and/or large mediastinal mass (≥ one-third of the maximum thoracic diameter), III, and IV. Patients had to be 18 to 60 years and free of other concurrent disease precluding protocol treatment. Patients with HL as part of a composite lymphoma, previous malignancy, chemotherapy or radiotherapy, pregnancy, or lactation were not eligible. Entry criterion for the PET question in this trial was a PR after chemotherapy (6-8 cycles of BEACOPP) and at least one involved nodal site of more than or equal to 2.5 cm in the transversal or longitudinal diameter as measured by computed tomography (CT). Exclusion criteria included diabetes mellitus, elevated fasting blood sugar level more than 130 mg/dL, and skeletal involvement with risk of instability.
Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki. The trial coordinators obtained a vote of approval from the responsible ethical committee of the medical faculty of the University of Köln and have registered the trial with the responsible authorities (state president of Northrhine-Westphalia and BfArM) according to the German drug regulations (AMG V. 1.1.2002). For PET diagnostics, the trial coordinators obtained the approval of the German Authority for Radiation Safety (Bundesamt für Strahlenschutz).
Study design
Patients were contributed by approximately 400 study centers in Germany, Switzerland, Austria, The Netherlands, and the Czech Republic. After clinical staging, patients were randomly assigned to 3 arms: patients in the standard arm A had 8 cycles, patients in arm B had 6 cycles of BEACOPP escalated, and patients in arm C 8 cycles of time-condensed BEACOPP14. After 4 cycles of chemotherapy, an interim staging was performed, including CT of all initially involved regions. PET was performed at a median of 21 days after the last administration of chemotherapy in qualified PET centers using the [18F]-fluorodeoxyglucose technique. Local radiotherapy (30 Gy) was restricted to patients who were PET+ after chemotherapy and had PR with residual disease measuring more than or equal to 2.5 cm, as described in “Central PET review panel.”
Chemotherapy
Central PET review panel
A multidisciplinary panel consisting of a medical oncologist, a radiologist, a radiation oncologist, and a nuclear medicine physician, accompanied by a statistician, reviewed PET and CT scans as well as available conventional X-rays blinded to the treatment arm. Inclusion and exclusion criteria were also assessed. A PET+ was defined according to the standard criteria as focal or diffuse uptake above background incompatible with normal anatomy or physiology, without a specific standardized uptake value cutoff. Exceptions were mild and diffusely increased FDG uptake at the site of moderate-sized or large residual masses with intensity lower than or equal to that of mediastinal blood pool structures.13,14 The further treatment recommendation was based on the central interpretation of PET and CT scans.
Statistics
The planned analysis set for the present analysis (AS1) comprised all patients randomized in HD15 not later than July 1, 2005 (Table 1). The cutoff date for randomization was chosen so that the target population had time to complete treatment, PET, and 12 months' follow-up before the analysis. The planned analysis set (AS2) for the HD15-PET question comprised all AS1 patients deemed qualified for PET in whom PET had been performed and reviewed by the PET panel. For the analysis of progression or relapse, AS2 was restricted to patients with at least 12 months of follow-up or with an event within 12 months (AS3). The NPV in PET− patients was calculated as the proportion of AS3 patients with progression, relapse, or radiotherapy within 12 months of the panel decision. A 95% confidence interval (CI) for the NPV was calculated using the normal approximation to the binomial distribution: ν ± 1.96.SQRT (ν(1 − ν)/n) with ν = NPV and n = total number of cases (SQRT indicates square root). For progression-free survival (PFS), progression, relapse, and death from any cause were defined as failures. For comparison between PET+ and PET− groups, PFS was measured from the date of the PET panel; for comparison of these groups with patients who achieved complete remission (CR), PFS was measured from the date of randomization. Kaplan-Meier analysis and the log-rank test were used to compare PFS of PET− and PET+ patients in PR (with residual mass ≥ 2.5 cm) with those in CR.
Results
Patients
Between January 2003 and May 2007, 1788 patients in HD15 were randomly assigned to treatment arms, 995 of whom before the deadline of July 1, 2005 (Table 1). A total of 33 patients were not qualified for this study and excluded from additional analysis. Reasons for exclusion were review pathology diagnosis not HL (n = 20), wrong stage (n = 9), concurrent disease (n = 1), patient's age more than 60 years (n = 1), and treatment before randomization (n = 2). Documentation was completed for 817 of these 962 patients (85%). Thus, 817 patients were included in the analysis set 1 (AS1). PET was performed and assessed by the PET panel in 355 patients of AS1 (43%). A total of 44 patients were not qualified for the PET question and were excluded from additional analysis. Reasons for exclusion were residual tissue less than 2.5 cm (n = 39), diabetes (n = 1), elevated blood sugar levels (n = 1), or skeletal involvement with risk of instability (n = 3). Consequently, 311 patients (38% of AS1) were included in the analysis set 2 (AS2). Patients in AS2 had been randomly assigned as follows: 95 to 8 cycles of BEACOPP escalated, 117 to 6 cycles of BEACOPP escalated, and 99 to 8 cycles of BEACOPP14. Patient characteristics were well balanced between treatment arms (Table 2).
PET activity in residual mass after 6 to 8 cycles of BEACOPP
Of 311 patients who had residual tissue measuring more than or equal to 2.5 cm by CT after 6 to 8 cycles of BEACCOPP, PET was positive in 66 patients (21% of AS2) and negative in 245 (79% of AS2; Table 3). A total of 244 of 245 PET− cases received no further treatment as defined in the protocol. One patient with a very large initial and residual mediastinal mass had radiation as recommended by the review panel. A total of 66 patients had PET+ residues, with radiotherapy recommended in 63: in 2 cases, the panel assessment came too late and in one patient, the PET+ residue was confined to the liver.
As defined in the protocol, patients were irradiated or not according to the panel decision. The adherence to protocol was good with 242 of 246 AS2 cases in whom “no radiation” was recommended actually received no radiotherapy. Four patients were radiated against the panel decision, 3 of whom had radiotherapy to skeletal lesions that were PET−. All 63 patients for whom irradiation of residual tissues was recommended actually received radiotherapy (Table 3).
Freedom from progression and relapse after PET
Those patients with at least 12 months of follow-up after PET and those with progressive disease or relapse within 12 months after PET were included in this analysis (n = 275; AS3 = 88% of AS2). Table 4 displays events within 12 months of PET. Nine of 216 (4%) patients with PET− residues and 9 of 59 (15%) patients with PET+ residues had an event (ie, progression or relapse). Event rates were significantly different between those patients with PET− residual tissue or those having PET+ residual tissue (P = .005). Of 18 events, 7 were confined to residual tissue sites, whereas 11 occurred outside these sites (Table 4).
PFS of PET− and PET+ patients starting from the date of the PET panel is shown in Figure 1. At 12 months, PFS for PET− patients was 96% (CI, 94%-99%) and 85% (CI, 78%-95%) for those who were PET+ (P = .011). The median observation time for patients included was 18 months. Thus, a positive PET after effective chemotherapy was predictive of subsequent treatment failure, even though the PET+ patients had additional radiotherapy.
Progression-free survival for patients with PET+ and PET− residual tissues starting from the date of the PET panel.
Progression-free survival for patients with PET+ and PET− residual tissues starting from the date of the PET panel.
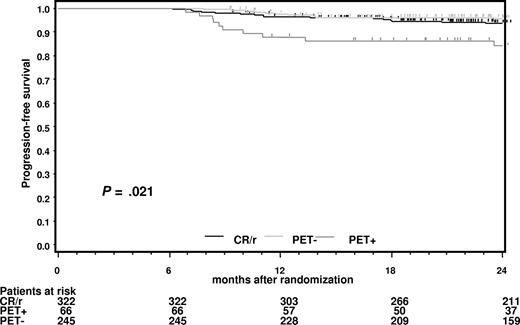
PFS of PET− and PET+ patients starting from the date of randomization was compared with that of patients in CR (with or without CR-compatible residues) at restaging after completing chemotherapy (Figure 2). It is apparent that PET− patients in PR with more than or equal to 2.5 cm residual disease at the end of chemotherapy had a prognosis very similar to those in CR.
Progression-free survival for patients in complete remission (CR) at restaging after completion of chemotherapy starting from the date of randomization.
Progression-free survival for patients in complete remission (CR) at restaging after completion of chemotherapy starting from the date of randomization.
NPV of PET after chemotherapy
All patients in analysis set AS3 with PET− residual mass (n = 216) were included for calculating the NPV. There were 9 patients with progression or relapse within 12 months and 5 patients with radiotherapy after PET, although being PET−, of whom 4 were otherwise failure-free. Thus, the NPV was calculated as follows:
NPV = (216 − 13)/216 = 94% with a 95% CI ranging from 91% to 97%.
Discussion
The HD15-PET trial evaluated the NPV of PET in advanced-stage HL patients with residual disease after 6 to 8 cycles of BEACOPP. Depending on the PET results, those patients who were PET− were followed up without additional radiotherapy, whereas those with PET+ tumors more than or equal to 2.5 cm received radiotherapy (30 Gy) to residual masses. The following findings emerge from this planned analysis, including 311 patients qualified for and receiving PET, of whom 275 had sufficient follow-up: (1) 79% evaluable patients were PET− and 21% were PET+ after 6 to 8 cycles of BEACOPP and received additional radiotherapy; (2) PFS at 18 months after randomization was 96% for PET− patients and 85% for PET+ patients (P = .011) (the corresponding PFS for patients in CR after chemotherapy was 95%); and (3) the NPV for PET in this analysis was 94%.
This is the first randomized trial that has proven a high NPV (94%) of PET in HL. The NPV was defined as the proportion of PET− patients without progression, relapse, or radiotherapy within 12 months of follow-up after central PET review panel recommendation, which is equivalent to 18 months of follow-up after randomization. Because PET− patients who had radiotherapy were counted as failures as predefined in the protocol, the analysis presented here is very conservative.
The follow-up period of 12 months after central PET review panel was chosen for different reasons. First, PET certainly is a sensitive method to detect viable tumor masses in HL as it could be shown in numerous nonrandomized studies. Accordingly, in case of a true-positive PET, indicating a malignant process and residual masses more than or equal to 2.5 cm as defined in our study, relapses are expected to occur within some months. This hypothesis is confirmed in our study, where 8 of 9 relapses were documented within the first 6 months for PET+ patients. In addition, 8 of 9 relapses occurred within the PET+ residual tissue. Thus, PET obviously is able to detect emerging relapses some months before they can be displayed by CT scan, but, for technical reasons, the resolution of PET is presumably not good enough to detect minimal residual disease, which can cause late relapses (> 12 months) in HL.15 Thus, a negative PET should predict PFS for at least 12 months in most cases.
Second, in the preceding HD9 trial for advanced stages HL, 59% of all events during the 5-year follow-up period occurred within the first 12 months after randomization.5 These events are defined as primary progressive disease or early relapse. Therefore, the NPV of PET at the predefined follow-up period of 12 months after PET in our study covered the majority of events expected and was considered a clinically relevant milestone. This hypothesis is supported by the recent data published by Gallamini et al,16 who had a PFS of only 12.8% at 2 years in case of an early positive PET after 2 cycles of chemotherapy. In this study, almost all relapses occurred within the time frame of our observation period reported here.
Third, this study was accompanied by major safety concerns. Patients with large residual tumor masses (eg, 8 cm in one patient) received no radiotherapy in case of a negative PET. To ensure the safety of this concept, an early analysis was regarded to be mandatory.
On the basis of these prerequisites, the 12-month follow-up after PET review panel was chosen for the determination of the NPV. Taking into account the identical PFS of PET− PR patients compared with CR patients in our study, major differences in the final outcome seem to be improbable at least. Nonetheless, the impact on established outcome parameters as PFS or overall survival at 5 years remains to be awaited. The results from our study indicate that PET-guided response adapted therapy is a promising approach in HL patients.17
In contrast to the NPV, the impact of a positive PET after chemotherapy is much weaker.18 Several trials reported positive predictive values (PPVs) ranging between 40% and 50% after doxorubicin, bleomycin, vinblastine, and dacarbazine-based chemotherapy.9-11 To investigate the PPV of a PET+ residual disease after chemotherapy in our study, interventions such as radiotherapy would have been impossible. Because radiotherapy still is the standard of care for patients with residual disease, omission of this intervention was regarded too risky and unethical. Thus, the PPV was not an end point in our study.
PET+ patients after treatment with BEACOPP had a significantly higher risk of progression or relapse (15%) compared with PET− patients (4%) at 12 months. However, this rate was lower than expected. Several factors may have contributed:
One reason might be the PET interpretation. To ensure consistency, a central multidisciplinary review panel was arranged. In accordance with the criteria of the imaging subcommittee of the international harmonization project in lymphoma, PET was also judged positive in cases with residual masses and an uptake slightly higher than the mediastinal blood pool.13,14 This contrasts with recent observations,16 in which such findings were classified as minimal residual uptake and thus PET might result in a higher number of false-positive PET scans in our study.
Many groups have investigated the value of PET showing a poor outcome for PET+ patients compared with PET− patients.16,19-21 One area of uncertainty is the best timing of PET. Although a minimum of 3 weeks between PET and the last chemotherapy had been advocated earlier,13 a minimum of 10 days after chemotherapy seems sufficient in more recent recommendations.22 To allow radiotherapy on time in our study, PET was performed after a median of 3 weeks after the last administration of chemotherapy. Thus, the interval used could result in more false-positive cases because of the obligatory use of prednisone and growth factors in the BEACOPP regimen.23,24
Finally, radiotherapy might be a very effective therapy turning truly positive PET lesions into remission. Because, for the reasons discussed, a treatment arm without radiotherapy was not foreseen, this question cannot be answered by our study.
Notably, 85% of PET+ patients had neither progression nor early relapse, but the rate of progression and relapse was significantly increased despite additional radiotherapy compared with the PET− patients. Taking biopsies from PET+ lesions would be the only way to determine the rate of truly positive PET results. Because relapses occurred not only infrequently, but also both inside PET+ residual masses (8 of 9) and outside (4 of 9), taking biopsies for a histologic proof of residual disease for all PET+ patients is not justified by our results. In addition, the most common site of residual disease in HL is the mediastinum, and a biopsy would require invasive procedures associated with the risk of morbidity in most cases.
So far, our data do not support additional aggressive consolidation regimens such as high-dose chemotherapy with autologous stem cell transplantation for PET+ patients because the majority of patients would be overtreated. These approaches should be evaluated earlier during the treatment course, to further improve the outcome of high-risk patients. In summary, based on the high NPV of PET in this large study, radiotherapy can be omitted in patients with PET− residual tumor without increasing the risk of primary progressive disease or early relapses compared with patients in CR after BEACOPP chemotherapy. The 5-year follow-up period must be completed for a definite conclusion on PFS and overall survival. Until then, response adapted therapy guided by PET for HL patients seems to be a promising approach that should be further evaluated in clinical trials.16,19-24
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the PET Centers in Switzerland (Zurich, Bern, and Basel), Austria (Linz, Innsbruck), Germany (Augsburg, Leipzig, Frankfurt, Heidelberg, Regensburg, Homburg, Munich TU, Munich LMU, Freiburg, Goettingen, Kiel, Ulm, Nuernberg, Stuttgart, Aachen, Koblenz, Juelich, Bad Berka, Dresden, Erlangen, Fulda, Karlsruhe, and Bonn), and Czech Republic (Prague, Brno); as well as A. Gossmann (Radiology); H. Stein and M. Hansmann (Pathology); K. Hansemann and R. Skripnitchenko (Radiotherapy); H. Bredenfeld, A. Josting, L. Nogova, and B. Klimm (GHSG Physicians); B. Pfistner, A. Pluetschow, H. Haverkamp, and C. Brillant (GHSG Statisticians); and B. Koch and H. Nisters-Backes (GHSG Documentation).
Authorship
Contribution: C.K., M.D., P.B., and A.E. wrote the paper; R.-P.M., H.T.E., M.D., M.F., P.B., V.D., and A.E. designed and conceptualized the study; J.F. performed statistical analysis; J.M., A.L., and J.M.Z. enrolled patients and assembled and documented clinical data; and H.A., S.K., W.H.K., A.B., M.W., R.L., M.S., R.B., and H.S. performed and analyzed the PET scans.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andreas Engert, Department I of Internal Medicine, University of Cologne, Kerpener Strasse 62, 50924 Cologne, Germany; e-mail: a.engert@uni-koeln.de.