Abstract
Human T-lymphotropic viruses types I and II (HTLV-I and HTLV-II) cause chronic infections of T lymphocytes that may lead to leukemia and myelopathy. However, their long-term effects on blood counts and hematopoiesis are poorly understood. We followed 151 HTLV-I–seropositive, 387 HTLV-II–seropositive, and 799 HTLV-seronegative former blood donors from 5 U.S. blood centers for a median of 14.0 years. Complete blood counts were performed every 2 years. Multivariable repeated measures analyses were conducted to evaluate the independent effect of HTLV infection and potential confounders on 9 hematologic measurements. Participants with HTLV-II had significant (P < .05) increases in their adjusted lymphocyte counts (+126 cells/mm3; approximately +7%), hemoglobin (+2 g/L [+0.2 g/dL]) and mean corpuscular volume (MCV; 1.0 fL) compared with seronegative participants. Participants with HTLV-I and HTLV-II had higher adjusted platelet counts (+16 544 and +21 657 cells/mm3; P < .05) than seronegatives. Among all participants, time led to decreases in platelet count and lymphocyte counts, and to increases in MCV and monocytes. Sex, race, smoking, and alcohol consumption all had significant effects on blood counts. The HTLV-II effect on lymphocytes is novel and may be related to viral transactivation or immune response. HTLV-I and HTLV-II associations with higher platelet counts suggest viral effects on hematopoietic growth factors or cytokines.
Introduction
Human T-lymphotropic virus types I and II (HTLV-I and HTLV-II) were first described in 1980 and 1982, respectively,1,2 as the first recognized retroviral human infections.3 HTLV-I is the causative agent of adult T-cell leukemia (ATL),4 HTLV-associated myelopathy (HAM),5,6 and HTLV-associated uveitis.7 HTLV-I infection is associated with several other inflammatory syndromes, including pneumonitis,8 and may also impair the patient's immune response to the helminth Strongyloides stercoralis.9 HAM has been described in participants with HTLV-II, who may also be predisposed to infections such as pneumonia, bronchitis, tuberculosis, urinary tract and kidney infections, and inflammatory syndromes such as neuropathy, asthma, and arthritis.10 Both retroviruses use the following routes of transmission: sexual, blood transfusion, breastfeeding, and intravenously through contaminated needles.11
Several studies have previously examined the complete blood counts (CBCs) of infected participants, generally with cross-sectional designs. Several subclinical findings have been noted, including anemia and lymphocytopenia12 as well as a decreased prevalence of eosinophilia in a tropical setting.13,14 Other cross-sectional epidemiologic studies examining the association between HTLV-I and laboratory abnormalities have also reported conflicting results: lymphocyte counts have been reported as increased,15,16 unchanged,17 or decreased,12 and HTLV-I infection has been associated with both lower erythrocyte counts12,14 and higher erythrocyte counts15 compared with participants without HTLV-I.
This disagreement over the potential of HTLV-I infection to cause laboratory abnormalities may be resolved with larger and longer prospective studies. To this end, we evaluated the effect of HTLV infection on clinical laboratory test results of a cohort of HIV-negative, HTLV-I+, HTLV-II+, and HTLV-negative blood donors whose health status has been prospectively followed for 14 years. In addition to looking for clinically significant alterations in blood counts, we were hoping to generate hypotheses on the potential biologic effects of HTLV on hematopoiesis. This study extends previously published results from the baseline and second cohort visits.16,18
Methods
Participants and study design
Enrollment criteria in the HTLV Outcomes Study (HOST) cohort, which initially began as part of the National Heart, Lung, and Blood Institute's Retrovirus Epidemiology Donor Study (REDS) HTLV cohort,19 have been described in detail elsewhere.16,18 Briefly, blood donors from 5 U.S. blood centers in different geographic regions were recruited. All donors at these 5 blood centers with confirmed positive antibodies to HTLV-I or HTLV-II were recruited. Further characterization of each infection as HTLV-I or HTLV-II was performed via polymerase chain reaction and/or an HTLV type–specific serology test (select HTLV enzyme-linked immunosorbent assay; Beckman Coulter, Miami, FL). HTLV-positive donors were then stratified by 5-year age groups, sex, race/ethnicity, blood center, and blood donation type. Within each defined stratum, seronegative blood donors were randomly selected from blood center records. The final ratio of HTLV-negative to HTLV-positive donors in the cohort was approximately 1.5:1.18 The study protocol was approved by the University of California at San Francisco Committee on Human Research as well as the institutional review board at each participating institution. Informed consent was obtained in accordance with the Declaration of Helsinki from all study participants.
The participants were followed for a total of 7 visits spaced approximately 2 years apart. Visit 4, which occurred at year 6, was excluded from analysis because interviews and blood samples were obtained by telephone and courier, respectively; many participants therefore lacked CBC data for this time point. All participants who completed the baseline visit and at least one follow-up visit were included in this analysis, with the exception of participants with a diagnosis of ATL.
Questionnaires were administered to all study participants to gather information on age, sex, race/ethnicity, education level, past injection drug use (IDU), smoking status, and alcohol consumption. CBCs were performed by licensed clinical laboratories using standard automated analyzers under contract with each participating blood center. The clinical laboratories fulfilled all licensure requirements including quality assurance standards and were blinded to participants' HTLV status.
We measured lymphocyte surface markers on a subset of participants with HTLV-II selected to have high (n = 10), medium (n = 10), and low (n = 10) total lymphocyte counts averaged across all 7 visits, as well as randomly selected seronegative controls (n = 10). Samples were selected from visits 5, 6, or 7 with total lymphocyte counts closest to the patient's mean across all visits. We thawed 5 × 106 frozen peripheral blood mononuclear cells (PBMCs) from each patient and incubated them with a single tube panel of the following antibody/fluorescent conjugates: CD3 Pacific Blue, CD4 Alexafluor 700, CD8 APC, CD16 PE-Cy5, CD19 PE-Cy7 (all from BD Biosciences-Pharmingen, San Diego, CA), and the LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Invitrogen/Molecular Probes, Eugene, OR). The proportion of PBMCs with each marker was then measured. Data were collected on an LSR II flow cytometer (BD Biosciences-Immunocytometry Systems, San Jose, CA) and analyzed offline with FlowJo Software Version 8.6.3 (Tree Star, Ashland, OR) with gating on viable lymphocytes. A minimum of 3000 viable lymphocytes were analyzed for standard lymphocyte subsets using dual parameter plots (CD3/CD4, CD3/CD8, CD3/CD16, and CD3/CD19). Percentages of lymphocytes with each surface marker were multiplied by the total lymphocyte count to determine absolute numbers of cells with each phenotype. HTLV-II proviral load was determined by quantitative real-time polymerase chain reaction (PCR) with type-specific primers, as previously described.20
Statistical analysis
Means and standard errors were calculated for each laboratory value by visit and HTLV status, and figures illustrate selected results. An initial repeated measures analysis was performed by constructing separate models for each of the 9 laboratory tests under examination as the outcome variable and HTLV status (either HTLV-I or -II vs HTLV-uninfected), visit, center, and a HTLV status-visit interaction term as the independent variables, yielding a total of 18 models. The use of a repeated measures analysis model allowed assessment of the effect of both HTLV status and visit on laboratory tests, while accounting for nonindependent measures repeated on the same participant. Dummy indicators for the 5 centers were included in these analyses to control for any differences between clinical laboratory equipment, with the largest center (Los Angeles) serving as the reference.
Multivariable repeated measures models were then constructed for each laboratory outcome with the following potential confounders entered as predictor variables along with HTLV status, visit and center: age in years; sex; race/ethnicity, coded as white, black, and other; education level dichotomized at high school graduate or less (reference); baseline income dichotomized at more than or equal to $30 000 or less (reference); alcohol consumption coded into 4 categories of drinks per week (zero, 0-1, 2-7, and > 7); smoking status coded in 3 levels of cumulative pack-year usage (zero, > 0-15, and > 15); injection drug use coded never versus ever; baseline HCV antibody status (participants with HTLV-II only); and interaction terms between HTLV status and visit. The center variable was retained in all models. A backward selection process was used for other potential confounders, retaining only variables found to be possibly associated (P < .20) with the outcomes; categoric variables were considered possibly associated and retained if any of the levels had a P value less than .20. Statistical software (SAS 9.1.3; SAS Institute, Cary, NC) was used to perform these analyses using the PROC MIXED command.
Lymphocyte marker data were analyzed by comparing means between the HTLV-II and seronegative control participants using the t test statistic with correction for unequal variance. HTLV-II proviral load was first log10 transformed and then tested for correlation with total lymphocyte count and CD8+ count using linear regression and the Spearman rank statistic. Natural killer (NK; CD3+/CD16+) cell count was also tested for correlation with CD8+ cell count using linear regression and the Spearman rank statistic.
Results
The demographic characteristics of the study population at baseline are shown in Table 1. The Southern California American Red Cross had the largest percentage of study participants due to its large blood donor base. A total of two-thirds of the cohort participants were female. The median age of the participants with HTLV-I at baseline was 44 years. The median age of participants with HTLV-II was 39 years at baseline enrollment, and the seronegative controls had a median age of 42 years. No single race or ethnicity dominated the cohort; whites comprised the largest group of participants, but African-Americans also constituted a significant percentage of the cohort. The cohort had high educational attainment, which is consistent with data on the blood donor population as a whole. The group with HTLV-I and the HTLV-seronegative group were relatively more educated compared with the participants with HTLV-II. The participants with HTLV-II were more likely to report past IDU at baseline, but only 4.4% of HTLV-II participants reported current IDU at baseline. Participants with HTLV had a lower annual income on average than participants without HTLV.
Baseline characteristics of the study population
| Variable . | HTLV-I, n (%) . | HTLV-II, n (%) . | Controls, n (%) . |
|---|---|---|---|
| Participants | 152 (11) | 387 (29) | 797 (60) |
| Blood center | |||
| ARC Chesapeake | 31 (20) | 51 (13) | 121 (15) |
| ARC SE Michigan | 32 (21) | 38 (10) | 101 (13) |
| ARC Southern California | 43 (28) | 205 (53) | 344 (43) |
| BCP | 30 (20) | 70 (18) | 157 (20) |
| OBI Oklahoma | 16 (11) | 23 (6) | 74 (9) |
| Sex | |||
| Male | 44 (29) | 102 (26) | 257 (32) |
| Female | 108 (71) | 285 (74) | 540 (68) |
| Age, y | |||
| < 30 | 12 (8) | 28 (7) | 77 (10) |
| 30-39 | 30 (20) | 165 (43) | 248 (31) |
| 40-49 | 65 (43) | 129 (33) | 260 (33) |
| 50-59 | 22 (14) | 44 (11) | 128 (16) |
| ≥ 60 | 23 (15) | 21 (5) | 84 (11) |
| Race/ethnicity | |||
| White | 62 (41) | 162 (42) | 370 (46) |
| Black | 60 (39) | 124 (32) | 242 (30) |
| Hispanic | 5 (3) | 76 (20) | 85 (11) |
| Asian | 20 (13) | 6 (2) | 50 (6) |
| Other | 3 (2) | 10 (3) | 39 (5) |
| Unknown | 2 (1) | 9 (2) | 11 (1) |
| Education | |||
| High school or less | 53 (35) | 154 (40) | 146 (18) |
| Some college | 62 (41) | 176 (45) | 357 (45) |
| College | 36 (24) | 53 (14) | 289 (36) |
| Unknown | 1 (1) | 4 (1) | 5 (1) |
| Annual income | |||
| ≤ $30 000 | 57 (38) | 168 (43) | 200 (25) |
| $30 001 to $49 999 | 46 (30) | 121 (31) | 242 (30) |
| ≥ $50 000 | 46 (30) | 91 (24) | 343 (43) |
| Unknown | 3 (2) | 7 (2) | 12 (2) |
| Injection drug use | |||
| Never | 149 (98) | 294 (76) | 786 (99) |
| Ever | 2 (1) | 92 (24) | 10 (1) |
| Missing | 1 (1) | 1 (0) | 1 (0) |
| Variable . | HTLV-I, n (%) . | HTLV-II, n (%) . | Controls, n (%) . |
|---|---|---|---|
| Participants | 152 (11) | 387 (29) | 797 (60) |
| Blood center | |||
| ARC Chesapeake | 31 (20) | 51 (13) | 121 (15) |
| ARC SE Michigan | 32 (21) | 38 (10) | 101 (13) |
| ARC Southern California | 43 (28) | 205 (53) | 344 (43) |
| BCP | 30 (20) | 70 (18) | 157 (20) |
| OBI Oklahoma | 16 (11) | 23 (6) | 74 (9) |
| Sex | |||
| Male | 44 (29) | 102 (26) | 257 (32) |
| Female | 108 (71) | 285 (74) | 540 (68) |
| Age, y | |||
| < 30 | 12 (8) | 28 (7) | 77 (10) |
| 30-39 | 30 (20) | 165 (43) | 248 (31) |
| 40-49 | 65 (43) | 129 (33) | 260 (33) |
| 50-59 | 22 (14) | 44 (11) | 128 (16) |
| ≥ 60 | 23 (15) | 21 (5) | 84 (11) |
| Race/ethnicity | |||
| White | 62 (41) | 162 (42) | 370 (46) |
| Black | 60 (39) | 124 (32) | 242 (30) |
| Hispanic | 5 (3) | 76 (20) | 85 (11) |
| Asian | 20 (13) | 6 (2) | 50 (6) |
| Other | 3 (2) | 10 (3) | 39 (5) |
| Unknown | 2 (1) | 9 (2) | 11 (1) |
| Education | |||
| High school or less | 53 (35) | 154 (40) | 146 (18) |
| Some college | 62 (41) | 176 (45) | 357 (45) |
| College | 36 (24) | 53 (14) | 289 (36) |
| Unknown | 1 (1) | 4 (1) | 5 (1) |
| Annual income | |||
| ≤ $30 000 | 57 (38) | 168 (43) | 200 (25) |
| $30 001 to $49 999 | 46 (30) | 121 (31) | 242 (30) |
| ≥ $50 000 | 46 (30) | 91 (24) | 343 (43) |
| Unknown | 3 (2) | 7 (2) | 12 (2) |
| Injection drug use | |||
| Never | 149 (98) | 294 (76) | 786 (99) |
| Ever | 2 (1) | 92 (24) | 10 (1) |
| Missing | 1 (1) | 1 (0) | 1 (0) |
Percentages may not add to 100 due to rounding. ARC indicates American Red Cross; BCP, Blood Centers of the Pacific; OBI, Okahoma Blood Institute.
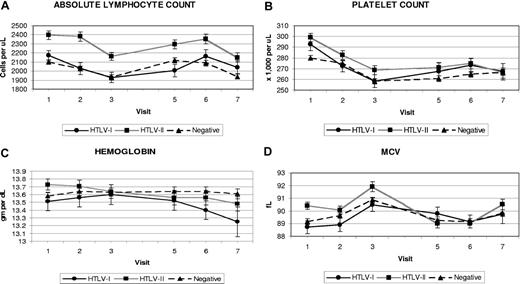
The cohort was followed for a median of 14 years, with no difference in follow-up by HTLV status. Figure 1 shows the mean platelet counts, lymphocyte counts, hemoglobin values, and mean corpuscular volumes (MCVs) by HTLV status and visit. Means and standard errors of each laboratory test result by HTLV status and visit are presented in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). The mean platelet count of participants with HTLV-II was roughly 10 000 platelets/μL higher than that of the seronegative controls at all study time points. The mean platelet count of participants with HTLV-I had more variability but was also higher than the uninfected participants. The lymphocyte counts of participants with HTLV-I and HTLV-II were higher than the seronegative control participants at most visits; however, only the larger HTLV-II difference of 200 to 300 cells/μL was statistically significant (P < .001). The hemoglobin values of uninfected control participants remained relatively stable over the course of the study, whereas the hemoglobin readings of the participants with HTLV-I and HTLV-II declined over time. The mean MCV readings for participants with HTLV-II were up to 1 fL higher at each visit than seronegatives; this was not observed in the participants with HTLV-I.
Means and standard errors of selected CBC measurements by HTLV status and visit. (A) Absolute lymphocyte count. (B) Platelet count. (C) Hemoglobin. (D) MCVs. Visit 4 was not included due to missing CBC data on many participants.
Means and standard errors of selected CBC measurements by HTLV status and visit. (A) Absolute lymphocyte count. (B) Platelet count. (C) Hemoglobin. (D) MCVs. Visit 4 was not included due to missing CBC data on many participants.
An initial repeated measures analysis was performed for both HTLV-I and HTLV-II versus controls at each visit, adjusted only for visit, blood center, and an HTLV status-visit interaction term (Table 2). Significant associations with HTLV-I status were seen for higher platelet count (+16 326 cells), lower monocytes (−36 cells), and lower eosinophils (−29 cells). HTLV-II status was significantly associated with higher MCV (+1.1 fL), higher platelet count (+18 153 cells), higher white cell count (+430 cells), higher lymphocyte count (+289 cells), and higher monocyte count (+24 cells). Overall, several CBC parameters changed significantly with successive visits independent of HTLV status: hemoglobin, MCV, and monocyte counts increased, while platelet and lymphocyte counts decreased. Interesting trends were evident in the interaction between HTLV status and visit: both HTLV-I and HTLV-II showed negative interactions with later visits for hemoglobin and MCV; similar negative interactions were apparent only for visits 2 and 3 for platelet count. The negative interaction of HTLV status and visit on hemoglobin is also visible upon close examination of Figure 1. More isolated interactions were seen for lymphocyte count and later visits: negative for HTLV-I and positive for HTLV-II.
Simple repeated measures analysis of complete blood counts by HTLV status, adjusted only for visit, center, and HTLV status–visit interaction
| Laboratory test . | HTLV-I versus seronegative . | HTLV-II versus seronegative . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HTLV-I . | Visit . | Interaction of HTLV-I and visit . | HTLV-II . | Visit . | Interaction of HTLV-II and visit . | |||||||||
| 2 . | 3 . | 5 . | 6 . | 7 . | 2 . | 3 . | 5 . | 6 . | 7 . | |||||
| Hemoglobin | +1 | −5 | −8 | +1 | −3 | −5 | −6 | −7 | ||||||
| MCV | +0.5 | −2.4 | −2.3 | +1.1 | +0.5 | −0.8 | −3.1 | −3.5 | −2.3 | |||||
| Platelet count | +16 326 | −5 409 | −15 276 | −23 394 | +18 153 | −5 412 | −13 133 | −23 160 | ||||||
| White cell count | −385 | +430 | ||||||||||||
| Lymphocyte count | −69 | −339 | −299 | +289 | −69 | +211 | +360 | |||||||
| Polymorphs | ||||||||||||||
| Monocytes | −36 | +15 | +24 | +15 | ||||||||||
| Eosinophils | −29 | |||||||||||||
| Basophils | +28 | |||||||||||||
| Laboratory test . | HTLV-I versus seronegative . | HTLV-II versus seronegative . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HTLV-I . | Visit . | Interaction of HTLV-I and visit . | HTLV-II . | Visit . | Interaction of HTLV-II and visit . | |||||||||
| 2 . | 3 . | 5 . | 6 . | 7 . | 2 . | 3 . | 5 . | 6 . | 7 . | |||||
| Hemoglobin | +1 | −5 | −8 | +1 | −3 | −5 | −6 | −7 | ||||||
| MCV | +0.5 | −2.4 | −2.3 | +1.1 | +0.5 | −0.8 | −3.1 | −3.5 | −2.3 | |||||
| Platelet count | +16 326 | −5 409 | −15 276 | −23 394 | +18 153 | −5 412 | −13 133 | −23 160 | ||||||
| White cell count | −385 | +430 | ||||||||||||
| Lymphocyte count | −69 | −339 | −299 | +289 | −69 | +211 | +360 | |||||||
| Polymorphs | ||||||||||||||
| Monocytes | −36 | +15 | +24 | +15 | ||||||||||
| Eosinophils | −29 | |||||||||||||
| Basophils | +28 | |||||||||||||
Numbers represent effect size of each predictor's significant (P < .05 or stronger) association with the outcome, in the following units: hemoglobin, g/L; MCV, fL; cell counts, cells/μL. Unless otherwise indicated, visit is per incremental 2-year follow-up period. Blanks indicate a predictor was not significant or there was no interaction. Coefficients for blood center are not shown.
To account for potential confounding by demographic and behavioral characteristics, we performed multivariable repeated measures analysis for both HTLV-I and HTLV-II versus negative participants. Tables 3 and 4 display adjusted parameter estimates for selected variables; the complete model outputs are available in Document S1. Across all visits, participants with HTLV-II had an approximately 7% higher lymphocyte count (+126 cells; P = .044), higher MCV (+1.0 fL; P = .022), and slightly higher hemoglobin values (+2 g/L [+0.2 g/dL]; P = .008) than seronegative participants. Participants with HTLV-I and HTLV-II had higher platelet counts (16 544 and 21 401 cells/mm3 higher) than seronegative participants. After controlling for potential confounding, HTLV infection status, and HTLV-visit interactions in repeated measures analyses, the effect of each subsequent visit remained significant with decreases in platelet count (−6749 to −6611 cells), lymphocyte count (−67 to −66 cells; both per 2-year interval), and basophils for HTLV-I. Subsequent visits were also associated with increases in hemoglobin, MCV, and monocytes for both viruses and with increases in eosinophils for HTLV-I. Negative interactions between visit and HTLV infection were seen for hemoglobin, MCV, and platelet count, and were similar to those shown in Table 2.
Multivariable repeated measures analysis of complete blood counts by status, HTLV-I versus seronegative
| Outcome . | HTLV-I . | Visit . | Age . | Female . | Race . | Alcohol . | Smoking . |
|---|---|---|---|---|---|---|---|
| Hemoglobin | NS | +1 | — | −15 | −7* | — | — |
| MCV | NS | +0.4 | +0.1 | −1.2† | −3.0*, −1.2† | +2.2 | — |
| Platelets | +16 544 | 6749 | NS | +37 731 | +17 473† | NS | NS |
| Total WBC | NS | NS | −19 | +575 | −532* | −529‡ | +1248§ |
| Lymphocytes | NS | −67 | −9 | +149 | +163* | −272‡ | +406§ |
| Neutrophils | NS | NS | −9 | +416 | −601* | −220‡ | +697§ |
| Monocytes | NS | +19 | — | — | −30* | −33‡ | +72§ |
| Eosinophils | NS | +3 | — | — | −31* | NS | +26§ |
| Basophils | NS | −2 | NS | +5 | NS | NS | +20§ |
| Outcome . | HTLV-I . | Visit . | Age . | Female . | Race . | Alcohol . | Smoking . |
|---|---|---|---|---|---|---|---|
| Hemoglobin | NS | +1 | — | −15 | −7* | — | — |
| MCV | NS | +0.4 | +0.1 | −1.2† | −3.0*, −1.2† | +2.2 | — |
| Platelets | +16 544 | 6749 | NS | +37 731 | +17 473† | NS | NS |
| Total WBC | NS | NS | −19 | +575 | −532* | −529‡ | +1248§ |
| Lymphocytes | NS | −67 | −9 | +149 | +163* | −272‡ | +406§ |
| Neutrophils | NS | NS | −9 | +416 | −601* | −220‡ | +697§ |
| Monocytes | NS | +19 | — | — | −30* | −33‡ | +72§ |
| Eosinophils | NS | +3 | — | — | −31* | NS | +26§ |
| Basophils | NS | −2 | NS | +5 | NS | NS | +20§ |
Numbers represent effect size of each predictor's significant (P < .05 or stronger) association with the outcome, in the following units: hemoglobin, g/L; MCV, fL; cell counts, cells/μL. Unless otherwise indicated, visit is per incremental 2-year follow-up period. Age is in years; alcohol intake is categorized as 0 to 1 drinks per week, 2 to 7 drinks per week, and more than 7 drinks per week versus none; smoking is categorized as 0 pack-years, more than 0 to 15 pack-years, more than 15 pack-years; education is dichotomized at high school graduate or less; annual baseline income is dichotomized at $30 000; injection drug use is ever versus never. Coefficients for blood center, education, income, injection drug use, donation type, and interaction terms between HTLV infection status and time are not shown.
NS indicates a predictor remained in the model but was not significant; — indicates the predictor was dropped from the final model.
Black race.
Hispanic or other race.
More than 7 drinks per week versus none.
More than 15 pack-years versus none. Complete model outputs are available in Table S1.
Multivariable repeated measures analysis of complete blood counts by status, HTLV-II versus seronegative
| Outcome . | HTLV-II . | Visit . | Age . | Female . | Race . | Alcohol . | Smoking . | HCV . |
|---|---|---|---|---|---|---|---|---|
| Hemoglobin | +2 | +1 | — | −15 | −7* | — | NS | — |
| MCV | +1.0 | +0.4 | +0.1 | −1.0† | −3.0*, −1.0† | +2.2‡ | NS | — |
| Platelets | +21 401 | −6 611 | −430 | +32 263 | +9187 | NS | — | NS |
| Total WBC | NS | NS | −15 | +434 | −474* | −654‡ | +1178§ | +551 |
| Lymphocytes | +126 | −66 | −7 | +151 | +197* | −241‡ | +331§ | +254 |
| Neutrophils | NS | NS | NS | +215 | −620* | −364‡ | +637§ | — |
| Monocytes | NS | +20 | — | NS | −30* | −34‡ | +57§ | +50 |
| Eosinophils | NS | NS | — | — | NS | — | +18§ | — |
| Basophils | NS | NS | — | NS | +8† | −10‡ | +15§ | — |
| Outcome . | HTLV-II . | Visit . | Age . | Female . | Race . | Alcohol . | Smoking . | HCV . |
|---|---|---|---|---|---|---|---|---|
| Hemoglobin | +2 | +1 | — | −15 | −7* | — | NS | — |
| MCV | +1.0 | +0.4 | +0.1 | −1.0† | −3.0*, −1.0† | +2.2‡ | NS | — |
| Platelets | +21 401 | −6 611 | −430 | +32 263 | +9187 | NS | — | NS |
| Total WBC | NS | NS | −15 | +434 | −474* | −654‡ | +1178§ | +551 |
| Lymphocytes | +126 | −66 | −7 | +151 | +197* | −241‡ | +331§ | +254 |
| Neutrophils | NS | NS | NS | +215 | −620* | −364‡ | +637§ | — |
| Monocytes | NS | +20 | — | NS | −30* | −34‡ | +57§ | +50 |
| Eosinophils | NS | NS | — | — | NS | — | +18§ | — |
| Basophils | NS | NS | — | NS | +8† | −10‡ | +15§ | — |
Significant effects were seen for recognized confounding variables (Tables 3 and 4). In both the HTLV-I and HTLV-II models, older age was associated with slightly higher MCV and slightly lower total white blood cell (WBC) and lymphocyte counts. Female sex was associated with lower hemoglobin and higher total WBC, lymphocyte, platelet, and neutrophil counts. Black race/ethnicity was associated with lower hemoglobin, MCV, total WBC, and neutrophil counts, and with higher lymphocyte and platelet counts. Cigarette smoking was associated with higher WBC, lymphocyte, eosinophil, neutrophil, monocyte, and basophil counts. Higher alcohol consumption was associated with lower WBC, lymphocyte, monocyte, and neutrophil counts. MCV values rose as alcohol consumption rose. Positive HCV antibody status at baseline (included in HTLV-II model only) was associated with higher WBC, lymphocyte, and monocyte counts. Higher educational attainment was associated with lower lymphocyte counts in both HTLV-I and HTLV-II models (P = .002 and P = .001, respectively) and with lower WBC counts in HTLV-I only (P = .015). History of IDU was associated with higher hemoglobin and lower MCV in participants with HTLV-I (P = .002 and P = .03, respectively). Finally, higher income at baseline was associated with lower WBC count in participants with HTLV-II (P = .035).
We examined the proportion of participants with laboratory values outside of the normal ranges for hemoglobin, lymphocyte, and platelet counts by HTLV status and visit. Due to substantial variation by visit, there were no clear trends in the proportion of participants with values above or below the normal ranges except for lymphocyte counts. As many as 20% to 30% of participants with HTLV-II in any one visit had lymphocyte values above the normal range, whereas only 11% to 18% of seronegative participants and participants with HTLV-I had values above the normal range in any one visit.
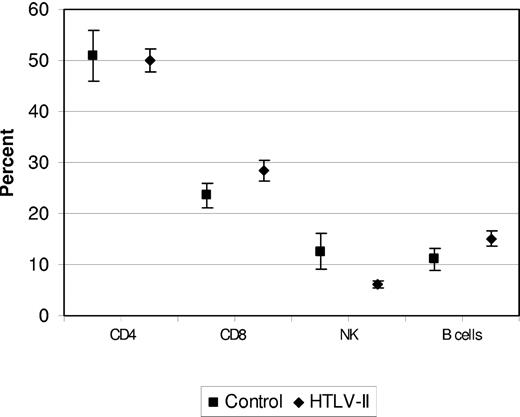
To examine whether a specific lymphocyte subset or subsets were responsible for the observed higher HTLV-II total lymphocyte count, we performed lymphocyte phenotyping by flow cytometry. We detected no significant difference in the proportion of selected surface markers between 30 selected participants with HTLV-II and 10 randomly selected controls. Although the HTLV-II subgroup with the highest mean lymphocyte counts (n = 10) had significantly higher absolute CD3+ (mean, 2740 cells/μL; P = .008) and CD8+ (mean, 968 cells/μL; P = .004) counts compared with controls (means, 1811 and 560 cells/μL, respectively), among all 30 participants with HTLV-II, the relative percentage of PBMCs with each marker did not differ significantly from the controls (P = .85 for CD4+, P = .21 for CD8+, and P = .11 for NK; Figure 2). Mean HTLV-II proviral load was 2.67 log10 copies per 106 PBMCs in the high lymphocyte subset, 3.44 log10 copies per 106 PBMCs in the medium lymphocyte subset, and 1.49 log10 copies per 106 PBMCs in the low lymphocyte subset. There was a weak, inverse correlation between NK cell (CD3+/CD16+) and CD8+ cell count by the Spearman rank test (P = .123) and linear regression (P = .639), but it was not statistically significant. Log10 HTLV-II proviral load was correlated with CD8+ count by both linear regression (2.607; SE = 0.88; P = .005) and the Spearman rank test (0.400; P = .013).
Lymphocyte surface markers on 30 participants with HTLV-II and 10 seronegative participants. Mean and standard error of the percentage of cells positive for each marker are given by HTLV-II versus seronegative status. Visit 4 was not included due to missing CBC data on many participants.
Lymphocyte surface markers on 30 participants with HTLV-II and 10 seronegative participants. Mean and standard error of the percentage of cells positive for each marker are given by HTLV-II versus seronegative status. Visit 4 was not included due to missing CBC data on many participants.
Discussion
A notable finding of this study was the observation that participants with HTLV-II had a significant increase in absolute lymphocyte counts that persisted over 14 years, while participants with HTLV-I had smaller, nonsignificant increases in lymphocytes. Our data also suggest that the platelet counts of participants with HTLV-I and HTLV-II were consistently higher than in HTLV-seronegative controls. Participants with HTLV-II had higher MCV and hemoglobin readings compared with seronegative control participants. Thus, our data suggest that HTLV-I and HTLV-II infection may be associated with persistent, small-to-moderate changes in CBCs even in chronic carriers without ATL.
Absolute lymphocyte counts in participants with HTLV-II were approximately 12.5% (unadjusted) to 7% (adjusted) higher than those of HTLV-seronegative controls. The increase was not attributable to a particular lymphocyte subset. Participants with HTLV-I had only small increases in absolute lymphocyte count (approximately 4%) compared with controls, and the difference was not statistically significant. The strength of the HTLV-II finding relative to that of HTLV-I was unexpected, and it stimulates speculation regarding its cause and possible pathogenic effects. Spontaneous lymphocyte proliferation has been reported previously in HTLV-II infection and could account for the elevated lymphocyte counts seen in participants with HTLV-II.21,22 It has also been speculated that the elevated lymphocyte counts noted in HTLV-II infection are due to an inflammatory response to the viral infection, but little research has been done in this area;19 the association of CD8+ counts with higher HTLV-II proviral load may be indicative of this response. A later report speculated that HTLV-II infection may inhibit immunologic responses to respiratory infections and that both HTLV-I and HTLV-II may induce inflammatory or autoimmune reactions.23,24
Kondo and associates demonstrated that HTLV-II Tax protein could induce interleukin-2 (IL-2)–independent growth in a T-cell line.25 In addition, Niinuma et al reported that HTLV-II Tax induces an IL-2 autocrine loop in vitro in infected cell lines that augments the effects of IL-2 under low concentrations,26 which could produce the elevated lymphocyte counts seen in vivo in HTLV infection.27 The mechanism may also be related to downstream effects of the Tax protein,28 which has been found to affect major signal transduction pathways through activation of antiapoptotic factors such as NFκB, CREB, and SRF and up-regulation of proliferative factors such as IL-2 and IL-15, among others.29 Prospective examination of the in vivo effects of IL-2, sIL-2R, IL-5, and IL-10 in participants with HTLV-I and HTLV-II and their potential correlation with anti-Tax antibody levels, HTLV proviral load, and HTLV-related disease outcomes is warranted. More in-depth studies of lymphocyte surface marker phenotypes might also reveal subtle increases in lymphocyte subpopulations not apparent in our initial analysis.
Our equivocal data on HTLV-I lymphocyte count echo past studies of the HOST cohort,16,18 data reported from the Japanese Miyazaki cohort,15 and a Hawaiian study of Okinawan immigrants.12 The Jamaican cohort of participants with HTLV-I found slightly lower lymphocyte counts, but these results were not statistically significant.14 Other cohorts did not study lymphocyte counts in participants with HTLV-II, thereby making comparisons difficult.
The finding that participants with HTLV-I and HTLV-II had significantly higher platelet counts over 14 years agrees with earlier findings from the HOST cohort,16,18 but we were unable to find other reports in the literature of platelet levels in participants with HTLV. The reason for the observed differences in platelet values is not known but may be related to an up-regulation of either IL-6, thrombopoietin (TPO), or its receptor monophosphoryl lipid A through HTLV-I and HTLV-II Tax transactivation.30 A report by Majka and colleagues indicated that both IL-6 and TPO were capable of protecting megakaryocytes from apoptosis and stimulating various cell types responsible for megakaryopoiesis.31 HTLV-I studies in Japan have shown that IL-6 may be constitutively up-regulated by Tax transactivation32 via activation of an NFκB promoter site.33 We found no reports on the ability of HTLV-II Tax to do likewise, but HIV-1, another retrovirus with a similarly structured genome, also induces well-established changes in cytokine levels.34 Higher platelet levels induced by HTLV may induce activation of platelet-derived growth factor, which is known to be involved in malignant pathways such as ras/ERK.35,36 We therefore recommend further laboratory investigation of possible Tax-mediated effects on cytokine pathways that could be influencing blood platelet levels in ongoing HTLV-I and HTLV-II infection.
Throughout the study, HTLV-II infection was associated with slightly higher hemoglobin (2 g/L [0.2 g/dL]) than seronegative participants. This effect was strengthened by interaction between HTLV-II infection and time. There is disagreement in the literature regarding the effects of HTLV infection on red blood cells. The Miyazaki cohort study found higher hematocrit readings in HTLV-I carriers with phenotypically abnormal lymphocytes.15 A cohort study in Jamaica reported a higher prevalence of anemia in participants with HTLV-I than seronegative controls.14 The Jamaican results may have been complicated by coexisting malnutrition or hemoglobinopathies. Similarly, Ho and colleagues reported lower hemoglobin values in their study of Hawaiian HTLV-I carriers of Japanese ancestry,12 but their analysis was cross-sectional in nature and did not control for alcohol and tobacco use. HTLV-II has been previously linked with recurrent pulmonary disease,37 which could possibly be related to an increase in hemoglobin. On the other hand, we were unable to demonstrate any measurable changes in pulmonary function testing in participants with HTLV-II compared with seronegatives.38 Further inquiry into possible cytokine mechanisms that could be affecting hemoglobin levels in participants with HTLV via IL-6 seems warranted.
HTLV-II association with increased MCV may be due to either a direct effect of HTLV infection on red blood cell production or a secondary effect of HTLV-II on nutritional intake or metabolic pathways related to vitamin B12 or folate. Emerging research indicates that viral effects on red blood cells may be cytokine-mediated; inflammatory cytokines, including IL-6, may affect the relative levels or action of erythropoietin and hepcidin.39,40 Abnormal MCV levels in the elderly have been linked to malabsorption of iron, vitamin B12, and folate; HTLV-II could exacerbate such effects.39 Finally, although we controlled for the effects of alcohol intake and IDU on MCV using multivariable analysis, residual confounding by these behaviors could remain. Although individuals with vitamin and mineral deficiencies have been diagnosed as part of workups for neurologic findings, we have no evidence that they are widespread in the cohort.
The significant association between time and decreases in platelet count and lymphocyte count as well as increases in monocytes and MCV is consistent with research on the effects of aging on hematologic parameters.41 The observed declines in lymphocyte count with age may be consistent with previous research on immunosenescence, namely a series of changes in both the innate and adaptive immune systems. Aging leads to thymic involution, decreasing the body's ability to replenish the dynamic population of naive peripheral T cells; these cells are replaced by memory and effector memory T cells.41,42 Hematopoietic stem cells are also affected by the aging process via the down-regulation of genes governing lymphoid lineage and the up-regulation of myeloid lineage genes.43 Significant interactions between HTLV-I and HTLV-II infection and time for most hematologic parameters indicate these viruses may accelerate age-related changes.
That alcohol and tobacco use had significant effects on several hematologic parameters is consistent with previous research and lends validity to our analysis. A large study by Whitehead and colleagues found that smoking was associated with increased white cell counts, and that both alcohol and tobacco use were linked to increased MCV levels.44 Nordin and associates found increased leukocyte counts associated with smoking and increased MCV levels associated with alcohol consumption.45 Nakanishi et al found a negative dose-response relationship of alcohol consumption on white cell count.46 Finally, our previous research on proviral load found associations with alcohol and tobacco use, which is consistent with our downstream findings.20
These findings generally agree with previous reports on this same cohort,16,18 but may be contrasted with those on other cohorts. The Miyazaki cohort reported by Welles et al included Japanese participants who are more likely to acquire HTLV infection at a younger age via vertical transmission, as opposed to sexual transmission in many of our participants with HTLV.15,47,48 Subtle differences in research methodology could also have caused discrepancies. Our cohort collected CBC samples from a group of former blood donors expressly for this study, whereas the Miyazaki studies used the results from blood samples collected during routine community-based health screenings. Our findings largely disagree with those reported by Ho and colleagues from a nested case-control study on Okinawan immigrants who were enrolled in the prospective Honolulu Heart Program study12 and those reported from the Jamaican cohort study of participants with HTLV-I.14 Like the Miyazaki cohort, this may be due to differences stemming from the route of transmission or genetic background of the infected participants.49,50
This study has several strengths, including its lengthy follow-up and excellent participant retention. Detailed clinical information was collected via self-reported questionnaires, which allowed us to control for potential confounding. CBCs were performed on fresh specimens in local licensed clinical laboratories to minimize shipping artifacts on test results. Finally, the repeated measures analytic technique used in this study maximized our ability to detect HTLV effects on longitudinally measured CBCs while accounting for intraindividual correlation. However, the study also had several potential limitations. As noted previously,15 only 50% of eligible HTLV-seropositive blood donors identified at the participating blood centers joined the cohort. Because the demographic characteristics of participants and nonparticipants were similar across all 3 cohort groups, we do not believe that enrollment bias was significant. Second, there were differences in socioeconomic status and lifetime injection drug use by HTLV status at baseline. Although we controlled for these factors with multivariable modeling, residual confounding may remain. Third, our participants were enrolled from blood donors, who may be healthier than the general population because predonation screening eliminates potential donors with health problems or certain clinical laboratory abnormalities such as low hemoglobin. Finally, because multiple comparisons were performed in this analysis, some findings could have been due to chance and should be interpreted in the context of their biologic plausibility.
In conclusion, these data suggest that participants with HTLV-II have persistently elevated lymphocyte counts compared with uninfected participants, suggesting interesting avenues for investigation into the molecular mechanisms and immunology of this retrovirus heretofore thought to be more benign than HTLV-I. Our data also confirm previous reports that participants with HTLV-I and HTLV-II have increased platelet counts. Overall, these results call for virologic and immunologic studies to elucidate the mechanisms of HTLV hematopathogenesis, particularly the relationship of HTLV Tax expression to cytokine production. Further research in these areas may help illuminate whether these hematologic abnormalities have prognostic value for the development of serious HTLV-related diseases.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Susan Yuen for administrative help, Daniel Hindes for data entry, and Dr Jack Levin for his critical reading of the manuscript. Finally, we are particularly indebted to our research participants for their faithful participation in this long-term study.
HOST is currently the responsibility of the following persons: Study headquarters: University of California, San Francisco and Blood Systems Research Institute (San Francisco, CA): E. L. Murphy (Principal Investigator), J. Engstrom, D. DeVita. Centers: American Red Cross Blood Services Greater Chesapeake and Potomac Region (Baltimore, MD): J. Gibble, D. Behan; American Red Cross Blood Services Southeastern Michigan Region (Detroit, MI): B. Newman, C. Marosi; American Red Cross Blood Services Southern California Region (Los Angeles, CA): G. Garratty, S. Hutching, A. Ziman, D. Littner, N. Cox, A. Rodney; Blood Centers of the Pacific (San Francisco, CA); and Oklahoma Blood Institute (Oklahoma City, OK): J. W. Smith, E. Moore and M. Kaiser. Central laboratory: Blood Systems Research Institute (San Francisco, CA): M. P. Busch, P. Norris, L. H. Tobler, T. H. Lee, L. Pitina, and D. Hirschkorn. Diagnostic review panel: E. L. Murphy, R. Sacher (Hoxworth Blood Center, Cincinnati, OH), and J. Fridey (City of Hope National Medical Center, Duarte, CA).
This work was supported by grants R01-HL-062235-06 and K24-HL-75036 from the National Heart, Lung, and Blood Institute and by Blood Systems Research Institute.
National Institutes of Health
Authorship
Contribution: M.B. analyzed and interpreted data and wrote the manuscript; Z.K. performed statistical analysis; D.H. performed lymphocyte phenotyping, analyzed and interpreted data, and wrote a portion of the manuscript; R.S. and J.F. performed research and analyzed and interpreted data; G.G., J.G., J.S., and B.N. performed research; A.Y. performed statistical analysis and wrote the initial manuscript draft; and E.M. designed and led the study, performed research, and analyzed and interpreted data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edward Murphy, Blood Systems Research Institute, 270 Masonic Avenue, San Francisco, CA 94118; e-mail: murphy@ucsf.edu.
References
Author notes
Presented in part at the 13th International Conference of Human Retrovirology: HTLV and Related Viruses, Hakone, Japan, May 22-25, 2007 (abstract O-6-4 and poster P-B-53).