Abstract
Controversy exists regarding management of children newly diagnosed with immune thrombocytopenic purpura (ITP). Drug treatment is usually administered to prevent severe hemorrhage, although the definition and frequency of severe bleeding are poorly characterized. Accordingly, the Intercontinental Childhood ITP Study Group (ICIS) conducted a prospective registry defining severe hemorrhage at diagnosis and during the following 28 days in children with ITP. Of 1106 ITP patients enrolled, 863 were eligible and evaluable for bleeding severity assessment at diagnosis and during the subsequent 4 weeks. Twenty-five children (2.9%) had severe bleeding at diagnosis. Among 505 patients with a platelet count less than or equal to 20 000/mm3 and no or mild bleeding at diagnosis, 3 (0.6%), had new severe hemorrhagic events during the ensuing 28 days. Subsequent development of severe hemorrhage was unrelated to initial management (P = .82). These results show that severe bleeding is uncommon at diagnosis in children with ITP and rare during the next 4 weeks irrespective of treatment given. We conclude that it would be difficult to design an adequately powered therapeutic trial aimed at demonstrating prevention of severe bleeding during the first 4 weeks after diagnosis. This finding suggests that future studies of ITP management should emphasize other outcomes.
Introduction
Immune thrombocytopenic purpura (ITP) is one of the most common hematologic disorders affecting children, with an incidence of 4 to 5 cases per 100 000 children per year.1 Although the sudden onset of bleeding is alarming to parents and primary physicians, affected children generally have a good prognosis. Numerous controversies have ensued during the past several decades regarding management of childhood ITP, including the need for a bone marrow aspirate to confirm diagnosis, the need for hospitalization for observation and initiation of treatment, and the requirement for drug therapy aimed at raising the platelet count to prevent hemorrhage. This latter controversy evolves primarily from concerns about severe or life-threatening hemorrhage and whether drug treatment with corticosteroids, intravenous immunoglobulin (IVIG), or anti-D immunoglobulin can prevent severe hemorrhage in affected patients.
The most frequently reported outcome in prior studies of childhood ITP is platelet count.2-4 This is often viewed as a surrogate marker of hemorrhagic risk, for minor bleeding as well as hemorrhage in critical sites, such as the central nervous system. It has recently been recognized, however, that other outcomes in ITP5 are important, including health-related quality of life,6,7 adverse effects of treatment,8 and the cost of therapy.9,10 Recently a 26-item health-related quality-of-life questionnaire was validated in pediatric patients with both acute and chronic ITP to assess disease burden of ITP on children and their families.7 The side effects of medications include weight gain, moodiness, and hyperglycemia related to steroid use, in addition to adverse effects, such as headache and aseptic meningitis associated with IVIG11 and intravascular hemolysis after anti-D immunoglobulin administration.12,13 Lastly, systematic cost analysis methods have been applied to evaluate different treatment modalities.10
The Intercontinental Childhood ITP Study Group (ICIS) was established in 1997 to facilitate development of prospective registries aimed at better understanding the presentation, management, and outcome of ITP.14-17 The ICIS Registry II was specifically designed to determine the frequency, timing, and sites of severe hemorrhage in children with ITP at diagnosis, during the subsequent 4 weeks, and ultimately during the following 2 years. We report here analysis of the ICIS Registry II dataset investigating children with ITP during the first 4 weeks after diagnosis.
Methods
The ICIS Registry II was a prospective cohort study designed to enroll consecutive patients at each participating center who fit the eligibility criteria. To be eligible, children had to be older than 4 months and younger than 20 years of age, be newly diagnosed as having ITP based on standard criteria,18,19 and be under the care of an ICIS investigator who agreed to submit data to the ICIS coordinating office in Basel, Switzerland. Each participating center was required to document approval of the study by its Institutional Review Board and to obtain written informed consent in accordance with the Declaration of Helsinki from the parents of participating patients. Initial medical management of the child was at the discretion of the investigator and not defined by the study protocol.
The following data were collected from the medical record at the participating center and submitted to the ICIS coordinating office 28 days or more after diagnosis: (1) demographic information (date of birth and sex), (2) diagnosis information (date of diagnosis, platelet count, hemoglobin concentration, whether or not marrow aspiration was performed, initial hospitalization date, initial platelet count enhancing therapy, sites of bleeding, and severity of bleeding), and, (3) outcome data summarizing the patient's course during the 28 days after diagnosis. The date of diagnosis was defined as the first day that the child was documented as having thrombocytopenia. Initial treatment was defined as therapy (if any) administered within 24 hours of the patient's diagnosis. Follow-up data during the ensuing 28 days included the highest platelet count (value and date), the lowest hemoglobin concentration (value and date), whether or not the patient received red blood cell and/or platelet transfusions, a description of new bleeding manifestations (including bleeding sites and severity) not present at diagnosis but occurring during the next 28 days, and the treatment of such hemorrhage.
Individual investigators categorized bleeding severity using the scale of Bolton-Maggs and Moon.20,21 Using this method, bleeding was categorized as (1) none or mild—no bleeding at all or bruising, petechiae, occasional mild epistaxis with very little or no interference with daily living; (2) moderate—more severe skin manifestations with some mucosal lesions and more troublesome epistaxis or menorrhagia; or (3) severe—bleeding episodes (epistaxis, melena, menorrhagia, and/or intracranial hemorrhage) requiring hospital admission and/or blood transfusions, that is, symptoms interfering seriously with quality of life.
The primary study outcome was the incidence of severe hemorrhage after diagnosis and initial treatment during the subsequent 28 days in those children with no or mild hemorrhage at diagnosis. Secondary outcomes were (1) incidence of moderate bleeding during the subsequent 28 days in patients with no or mild bleeding at diagnosis, (2) incidence of severe hemorrhage during the subsequent 28 days in children with moderate bleeding at diagnosis, (3) comparison of mean platelet counts and child age with bleeding severity at time of diagnosis, and (4) evaluation of the impact of platelet count on physician treatment practices in children who have no or mild bleeding at diagnosis.
Results are descriptive in nature, including 95% exact confidence intervals (CIs), and a Fisher exact test was performed to compare the relationship between initial therapy (none, IVIG, steroids, anti-D, multiple/other treatments) and subsequent development of severe bleeding. An analysis of variance (ANOVA) was computed to compare differences in mean platelet count between initial bleeding severity and between initial therapy choices among those with no or mild bleeding at presentation. ANOVA was also calculated to compare mean ages across initial bleeding severity. An alpha level of 0.050 was used.
Results
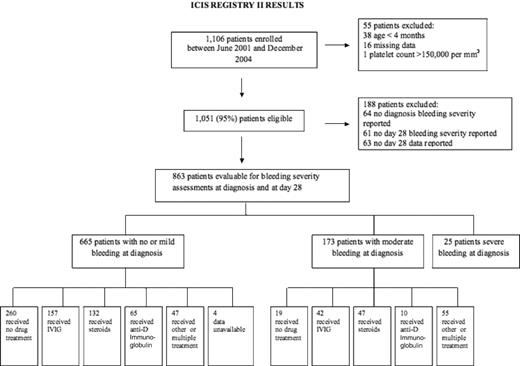
Between June 2001 and December 2004, 1106 patients were enrolled on the ICIS II Registry. Seventy-four pediatric hematology investigators from 54 institutions in 22 different countries participated. A total of 1051 (95%) of these patients met all of the study's eligibility criteria. The subsequent analysis of bleeding severity in newly diagnosed patients was restricted to those 863 patients who were fully evaluable for bleeding severity assessments at diagnosis and again at day 28 (Figure 1). Of the 863 patients evaluated, 685 of them (79%) had a platelet count less than or equal to 20 000/mm3 at diagnosis and 178 (21%) had a platelet count more than 20 000/mm3.
Hemorrhage at diagnosis
Bleeding severity in the 863 evaluable patients and sites of severe hemorrhage are described in Table 1. The majority of patients (77%) had no or mild bleeding at diagnosis. Of those with severe bleeding, only one patient had central nervous system bleeding hemorrhage, and the other 24 patients had mucosal bleeding. These results are similar to those described by Bolton-Maggs and Moon.20
Bleeding severity at presentation: comparative results of current and earlier study
| Bolton-Maggs and Moon bleeding grade classification20 . | ICIS Registry II, % (no.) (n = 863) . | Bolton-Maggs and Moon, % (no.) (n = 427) . |
|---|---|---|
| None/mild | 77 (665) | 76 (324) |
| Moderate | 20 (173) | 21 (90) |
| Severe | 3* (25) | 3 (13) |
| Bolton-Maggs and Moon bleeding grade classification20 . | ICIS Registry II, % (no.) (n = 863) . | Bolton-Maggs and Moon, % (no.) (n = 427) . |
|---|---|---|
| None/mild | 77 (665) | 76 (324) |
| Moderate | 20 (173) | 21 (90) |
| Severe | 3* (25) | 3 (13) |
95% confidence interval, 1.9% to 4.2%.
Sites of bleeding: epistaxis (15 cases), mouth (8 cases), gastrointestinal tract (8 cases), urinary tract (2 cases), multiple sites (12 cases), other (1 case), and menorrhagia and central nervous system (1 case each).
At presentation, 505 of the 685 (74%) evaluable patients with a platelet count less than or equal to 20 000 per mm3 were recorded as having no or mild bleeding and 158 (23%) others had moderate hemorrhage. Twenty-two patients (3%) were reported as having severe bleeding at presentation. There was one nonfatal intracranial hemorrhage (0.15% incidence) in a 9-year-old girl. The mean (SD) of the platelet count at diagnosis for patients with no or mild, moderate, or severe bleeding was 17 000/mm3 (20.1), 10 000/mm3 (12.0), and 9000/mm3 (11.0) respectively. ANOVA detected a significant difference in the mean platelet count across bleeding severity at presentation (P < .001). The mean ages (SD) were 5.7 years (4.4), 6.1 years (4.1), and 7.7 years (5.0) for patients with no or mild, moderate, or severe bleeding at presentation, respectively (P = .06).
Of the 178 patients with a platelet count more than 20 000 per mm3, 160 (90%) had no or mild bleeding at diagnosis and an additional 15 (8%) had moderate bleeding. Three of the 178 patients (2%) had severe bleeding, none with intracranial hemorrhage. The 3 patients with severe bleeding had platelet counts of 23 000, 26 000, and 50 000/mm3.
New hemorrhage after diagnosis and initial treatment in children with no or mild bleeding at diagnosis
Among those 505 patients with a platelet count less than 20 000/mm3 and no or mild bleeding at diagnosis, 3 (0.6%; 95% CI, 0.1%-1.7%) developed a new severe hemorrhagic event during the subsequent 28 days, the primary study outcome. Severe bleeding occurred from the mouth in one child, from the nose in 2 patients, and from the gastrointestinal tract in all 3 patients. Table 2 lists how these 505 patients had been treated at diagnosis before the severe hemorrhage ensued. A total of 146 children (29%) had received IVIG, 113 (22%) corticosteroids, 65 (13%), anti-D immunoglobulin, 41 (8%) combined or other therapy, and 138 (27%) no drug therapy; 2 patients did not have treatment data available. One of the 3 children subsequently demonstrating severe hemorrhage had been treated with IVIG at diagnosis, and 2 others had received no drug therapy at diagnosis. There was no significant relationship between the various initial therapies and subsequent development of severe hemorrhage (P = .82). Among these 505 patients, 9 (1.8%; 95% CI, 0.8%-3.4%) developed new moderate bleeding during the subsequent 28 days. Three each had been treated initially with IVIG, corticosteroids, and no drug therapy.
New severe hemorrhagic events during the first 28 days in patients with no or mild bleeding and a platelet count less than 20 000/mm3 at initial diagnosis
| Initial therapy . | No. (%) of patients with no or mild bleeding at diagnosis . | No. (%) of such patients with new severe bleeding episode before day 28 . |
|---|---|---|
| IVIG | 146 (29) | 1 |
| Corticosteroids | 113 (22) | 0 |
| Anti-D immunoglobulin | 65 (13) | 0 |
| No drug therapy | 138 (27) | 2 |
| Multiple drug treatments/other | 41 (8) | 0 |
| Data unavailable | 2 (< 1) | 0 |
| Total | 505 | 3 (0.6%) |
| Initial therapy . | No. (%) of patients with no or mild bleeding at diagnosis . | No. (%) of such patients with new severe bleeding episode before day 28 . |
|---|---|---|
| IVIG | 146 (29) | 1 |
| Corticosteroids | 113 (22) | 0 |
| Anti-D immunoglobulin | 65 (13) | 0 |
| No drug therapy | 138 (27) | 2 |
| Multiple drug treatments/other | 41 (8) | 0 |
| Data unavailable | 2 (< 1) | 0 |
| Total | 505 | 3 (0.6%) |
Among the subset of 160 children with a platelet count more than 20 000/mm3 and no or mild bleeding at diagnosis, none developed severe hemorrhage and only one developed moderate bleeding during the subsequent 28 days. There were no deaths reported in the 863 study patients.
New severe hemorrhage after diagnosis and initial treatment in children with moderate or bleeding at diagnosis
Among those 173 patients with moderate bleeding at diagnosis, 3 (1.7%; 95% CI, 0.4%-5.0%) developed a new severe hemorrhagic event during the subsequent 28 days. Table 3 lists how these 173 patients had been treated at diagnosis before the severe hemorrhage ensued. Forty-two (24%) had received IVIG, 42 (27%) corticosteroids, 10 (6%) anti-D immunoglobulin, 55 (32%) combined or other therapy, and 19 (11%) no drug therapy. Of the 3 children who subsequently demonstrated severe hemorrhage, one had been treated with corticosteroids, one with anti-D immunoglobulin, and one with corticosteroids and IVIG at diagnosis. There was no significant relationship between the various initial therapies and subsequent development of severe hemorrhage (P = .27).
New severe hemorrhagic events during the first 28 days in patients with moderate bleeding at initial diagnosis
| Initial therapy . | No. (%) of patients with moderate bleeding at diagnosis . | No. (%) of such patients with new severe bleeding episode before day 28 . |
|---|---|---|
| IVIG | 42 (24) | 0 |
| Corticosteroids | 47 (27) | 1 |
| Anti-D immunoglobulin | 10 (6) | 1 |
| No drug therapy | 19 (11) | 0 |
| Multiple drug treatments/other | 55 (32) | 1 |
| Total | 173 | 3 (1.7%) |
| Initial therapy . | No. (%) of patients with moderate bleeding at diagnosis . | No. (%) of such patients with new severe bleeding episode before day 28 . |
|---|---|---|
| IVIG | 42 (24) | 0 |
| Corticosteroids | 47 (27) | 1 |
| Anti-D immunoglobulin | 10 (6) | 1 |
| No drug therapy | 19 (11) | 0 |
| Multiple drug treatments/other | 55 (32) | 1 |
| Total | 173 | 3 (1.7%) |
Platelet count and treatment of patients with none/mild bleeding at diagnosis
Treatment was evaluated for 653 of the 665 patients with no or mild bleeding at diagnosis (Figure 1). Patients who received other treatment (n = 8) or for whom no data were available (n = 4) were excluded. The mean platelet count for patients receiving no drug therapy was 28 000/mm3 (SD, 26.2). Mean (SD) platelet counts for anti-D immunoglobulin, IVIG, steroids, and combination therapy were 7000/mm3 (4.8), 9000/mm3 (7.5), 12 000/mm3 (10.2), and 8000/mm3 (7.8), respectively. The ANOVA detected a significant difference in platelet count across therapy (P < .001). Post-hoc analysis showed this difference to be significant only between no drug therapy and each of the other treatment modalities (IVIG, steroids, anti-D immunoglobulin, and combination therapy). There was no statistically significant difference among platelet count and treatment with IVIG, steroids, anti-D immunoglobulin, and combination therapy. Of the 160 patients with a platelet count more than 20 000/mm3 and no or minor bleeding at diagnosis, 36 (23%) received drug treatment.
Discussion
Intracranial hemorrhage is the most severe complication of childhood ITP and the primary cause of death.22 Its incidence is very low, estimated at 1 in 800 cases based on national survey data from the United Kingdom23 and substantiated by reports from ICIS and other investigators.14,20,24,25 Given the rarity of this complication, there is widespread agreement that a randomized clinical study aiming to prevent intracranial hemorrhage is not feasible. However, the central nervous system is not the only site of major hemorrhage in ITP. Profuse bleeding from the nose, mouth, gastrointestinal, or genitourinary tract is not only frightening but may also result in profound anemia requiring red blood cell and platelet transfusions and emergency drug treatment to raise the platelet count.26 To our knowledge, previous clinical trials have not incorporated such severe bleeding events as a primary outcome or have even described their frequency.
In this investigation, we were able to meet our primary study outcome and demonstrated that children with acute ITP, regardless of platelet count or initial treatment, whose hemorrhage was graded as mild or less at diagnosis, infrequently develop severe hemorrhage during the subsequent 4 weeks (3 of 665 cases, 0.5%). Children with no or mild hemorrhage at diagnosis received diverse therapies from their physician. However, it was not possible to define any benefits from corticosteroid, IVIG, or anti-D immunoglobulin treatment in preventing severe bleeding during the next 4 weeks. Given the rarity of severe bleeding, the use of drug therapy as “prophylaxis” against severe hemorrhage in newly diagnosed ITP patients can be questioned based on these data.
Treatment of ITP with corticosteroids, IVIG, or anti-D immunoglobulin is provided either to treat hemorrhage or to prevent its occurrence in children thought to be at risk. No one would question the administration of platelet count enhancing therapy to children with ITP who are having active hemorrhage. However, the value of using drugs for prevention of major bleeding in thrombocytopenic children exhibiting little or no clinical hemorrhage has not been established. Our large and prospective ITP cohort provides new and valuable information on the risk of developing significant hemorrhage in children with no or mild bleeding at diagnosis, which raises questions about the merit of such prophylactic treatment.
Children with ITP and platelet counts less than 10 000 per mm3 may exhibit virtually no hemorrhage except for scattered petechiae, whereas others have profuse mucous membrane or very rarely intracranial hemorrhage. In our study, although the difference in platelet count was statistically significant between bleeding severity groups (none/mild, moderate, severe), it was not clinically significant because all groups had a platelet count less than 20 000/mm3, the cutoff value often used for prophylactic drug treatment and randomized controlled trials of various intervention strategies.2,4,25 Not only do clinical bleeding manifestations differ among children, for reasons that are unclear, but their response to treatment does as well. A subset of patients, 10% to 30%, fails to exhibit a prompt platelet count increase after IVIG or anti-D immunoglobulin.2,25 Moreover, children with severe hemorrhage probably don't have an increase in their platelet count after drug treatment compared with those with mild cutaneous bleeding alone.27
Although there are many reasons why physicians prescribe drug treatment to newly diagnosed patients with ITP,28 the main rationale is probably prevention of intracranial or other serious hemorrhage.3 If one uses a composite outcome of severe hemorrhage in all clinical sites, not just in the central nervous system, development of a therapeutic trial to identify the optimal preventive strategy might be considered feasible. However, the baseline incidence of severe bleeding in multiple sites must be established to appropriately power a randomized trial. The ideal clinical setting for conduct of such a study would be immediately after diagnosis of ITP and during the subsequent few weeks after the initial management decisions.29,30 It is this period when ITP management is most controversial and during which most randomized ITP therapeutic trials have been conducted.2,3,18,19,25
One problem with using severe hemorrhage as an outcome is defining “severe.” Several grading or scoring instruments for hemorrhage in thrombocytopenic subjects have been described.31-35 A method already reported when the ICIS Registry II was being designed was that of Bolton-Maggs and Moon.20 Since then, more quantitative measurement instruments that assess bleeding in different anatomic sites have been developed.31,34 When Bolton-Maggs and Moon20 applied their scoring severity method to a cohort of newly diagnosed ITP patients in the United Kingdom, they defined 3% of the children as having severe hemorrhage. As validation of this scoring method, the frequency of severe hemorrhage in our ICIS Registry II ITP patients was virtually identical, that is, 25 of 863, or 2.9%. This current study also confirmed the rarity of intracranial hemorrhage, with only one event among the 685 patients (0.15%) with a platelet count less than or equal to 20 000/mm3 at diagnosis and follow-up through day 28. Bolton-Maggs and Moon observed no cases of intracranial hemorrhage in their cohort of 427 newly diagnosed patients.20
This analysis clearly has some limitations. Although the bleeding scoring instrument used here was validated in this study, the measure has not undergone more formal validation and reliability testing. Since the initiation of this trial, one of the more quantitative grading instruments that have been developed31 has been subsequently modified for use in several other studies of childhood ITP.7,36 In addition, we did not examine in this study other potential merits of “prophylactic” drug therapy in newly diagnosed patients, such as prevention of mild or moderate bleeding as a result of promptly increasing the platelet count. Despite the intention of the Registry to enroll all consecutive patients at participating institutions, this cannot be verified. Moreover, no day 28 data were reported in 63 patients, and an additional 61 did not have a bleeding severity score reported at day 28, even though the remaining day 28 data were submitted by the institution. These 61 patients were evaluated by a study investigator making it less probable that they had a severe bleeding event that went unrecognized or unreported by the investigator. The same is not true, however, for the 63 patients with no day 28 data available. It is unknown whether these patients improved and therefore did not obtain follow-up or whether they had a severe or even fatal bleeding event and were seen outside the study institution.
The extremely low incidence of new severe bleeding during the first 28 days after diagnosis and initial treatment will probably make it difficult, if not impossible, to design a therapeutic trial aimed at showing a reduction in the frequency of such an outcome. Neither a superiority trial nor an equivalence design (which would require an extremely large number of patients) would probably be feasible. For example, if it is assumed, based on the data reported here, that at day 28 severe and moderate bleeding combined occur in approximately 3% of patients and the goal of a drug trial is to decrease the frequency to 1% (superiority trial), using an alpha of 0.05 and power of 80%, 865 patients per arm would be required. Patient numbers would be even larger if the study assessed severe bleeding alone or used an equivalence design. Therefore, future research regarding clinical management of ITP should more carefully examine other outcomes, such as adverse effects of drug treatment, cost of alternative treatment approaches, health-related quality of life, or a composite endpoint. Additional studies are also necessary to better define clinical and/or laboratory risk factors for severe bleeding in ITP patients.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Research Service Award (Institutional Training Grant T32-CA009640) and North and Central Texas Clinical and Translational Science Initiative (Clinical and Translational Science Award Grant 1 UL1 RR024982-01).
National Institutes of Health
Authorship
Contribution: C.E.N. analyzed data and wrote the manuscript; P.I., T.K., and G.R.B. designed and performed the research; G.R.B., P.H.B.B.-M., E.J.N., C.M.B., and V.S.B. performed the research; S.K.V. performed statistical analysis; L.A. analyzed and organized data; and all authors were involved in manuscript preparation.
Conflict-of-interest disclosure: P.H.B.B.-M. receives financial support from Glaxo Smith Kline, and the United Kingdom Immune Thrombocytopenic Purpura support association. V.S.B. is a member of the Baxter Bioscience Corporation Global Steering Committee for Protein-Free Recombinant Factor VIII, a member of the Bayer International Hemophilia Advisory Board, and a member of the AMG 531 advisory board. T.K. receives unrestricted grant support from F. Hoffmann-La Roche Ltd and from Amgen. C.E.N., G.R.B., P.I., E.J.N., C.M.B., S.K.V., and L.A. declare no competing financial interests.
Correspondence: Cindy E. Neunert, University of Texas Southwestern Medical Center at Dallas, 5323 Harry Hines Blvd, Dallas, TX 75390-9063; e-mail: cindy.neunert@utsouthwestern.edu.
References
Author notes
Presented in a preliminary fashion at a platform session at the Pediatric Academic Societies and the American Society of Pediatric Hematology-Oncology, Washington, DC, April 2005.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal