Abstract
AL amyloidosis associated with immunoglobulin M (IgM) paraproteinemia is rare. We report 103 consecutive such patients evaluated at the National Amyloidosis Centre (London, United Kingdom) between 1988 and 2006. Renal, cardiac, and lymph node amyloid was present in 53%, 35%, and 21% of patients, respectively, at presentation and 2 or more organs were involved in 54%. Seventy-three percent had an abnormal bone marrow infiltrate (lymphoid in 87%). The median IgM paraprotein was 8 g/L and serum free light chain (FLC) ratio was abnormal in 77 (88%) of 87. The abnormal FLC component was more than 100 mg/L in only 31% cases. Thirty-two percent achieved a partial hematologic response to treatment with no complete responders, and there appeared to be a greater response to combination regimens than single-agent oral alkylators (59% vs 20%, respectively; P = .003). Four achieved amyloidotic organ responses; organ function remained stable in 68%. None with lymph node involvement showed nodal improvement. Median overall survival was 49 months. AL amyloidosis with IgM paraproteinemia represents a distinctive subset of patients with AL amyloidosis who have a wider variety of underlying clonal disorders (often lymphoid) than AL in general, have low-level FLC abnormality, and should be treated with appropriately tailored chemotherapeutic regimens for the underlying clonal disorder.
Introduction
AL amyloidosis is caused by misfolding, aggregation and deposition of certain monoclonal immunoglobulin (Ig) light chains as extracellular insoluble fibrillar deposits that stain with Congo red and produce pathognomonic red-green birefringence when viewed in cross-polarized light. The age-adjusted incidence of AL amyloidosis is 5.1 to 12.8 per million patient years,1 and it is usually associated with very subtle clonal plasma cell dyscrasias2 that are usually best monitored by the very sensitive serum free light chain (FLC) assay.3 When a whole paraprotein can be identified, it is much more commonly either IgG or IgA than IgM.4 Indeed, in contrast to the 20% frequency of IgM paraproteinemia among patients with monoclonal gammopathy of uncertain significance (MGUS),5 remarkably few patients with AL amyloidosis consequent on IgM paraproteinemia have been reported.6 Only 50 such patients were identified among the very large experience of AL amyloidosis at the Mayo clinic over a 22-year period.7,8 Other reports are of single individuals or small case series.9,10 The response to chemotherapy in this rare group of patients is even less well reported.
We report here the manifestations of amyloidosis and characteristics of the underlying clonal disease at diagnosis, and clinical outcome following various types of chemotherapy in 103 consecutive patients with AL amyloidosis complicating IgM paraproteinemia, who were evaluated in our center between 1988 and 2006.
Methods
Patients, diagnosis, and protocol
All patients with AL amyloidosis in whom a single paraprotein of IgM class had been demonstrated in the serum or urine by electrophoresis or immunofixation, who were first seen at the National Amyloidosis Centre (NAC, London, United Kingdom) between 1988 and 2006, were identified retrospectively from the NAC database.
The presence of amyloid was confirmed by characteristic Congo red staining of a tissue biopsy and/or by a diagnostic 123I-labeled serum amyloid P component scintigraphy (SAP) scan in all cases. AL-type amyloidosis was confirmed by immunohistochemical staining that included a panel of antibodies against known amyloid fibrils proteins11 and exclusion of hereditary amyloidosis by demonstration of wild-type sequence for the genes encoding known hereditary amyloidogenic proteins.12 Myeloma and MGUS were defined according to previously published criteria.13 IgM MGUS was defined according to the criteria described by Baldini et al.6
All patients underwent protocolized assessments scheduled at 6 monthly intervals until 6 months after the end of chemotherapy and then at 6 to 12 monthly intervals as clinically indicated. Assessments included a comprehensive clinical examination, blood and 24-hour urine studies, monitoring of whole body amyloid load with serial 123I-SAP scintigraphy,14 electrocardiogram (ECG) and echocardiography, and measurement of monoclonal immunoglobulins in serum and urine. From 2001, additional blood samples were requested at monthly intervals during administration of chemotherapy and 2 monthly thereafter for measurement of serum FLCs using the Freelite assay (The Binding Site, Birmingham, United Kingdom). FLCs were also assayed retrospectively on serum samples that had been obtained at each visit and routinely archived for patients first seen at the NAC before the routine introduction of this assay in 2001.
Approval for retrospective analysis and publication was obtained from the institutional review board of Royal Free Hospital ethics committee (Royal Free Hospital, London, United Kingdom), and written consent for publication of anonymous material was obtained from all patients in accordance with the Declaration of Helsinki.
Outcome measures
Outcome measures comprised overall patient survival (OS), hematologic response to treatment, amyloidotic organ response, and the course of whole body amyloid burden by serial 123I-SAP scintigraphy.14 Hematologic response was assessed by serum and urine electrophoresis and immunofixation and also by FLC assay. FLC response is a strong predictor of survival in AL amyloidosis3 but its significance in IgM-related disorders has been little studied. Conventional responses for patients were defined using the consensus criteria from the second Waldenström workshop.15 FLC values were considered interpretable for assessing response if the pretreatment FLC κ/λ ratio was outside the 95% reference range (0.3-1.2)16 and the concentration of the light chain class (ie, κ or λ) containing the monoclonal component (also called monoclonal class) was 100 mg/L or more, except in patients with renal failure in whom only the κ/λ ratio was used. A FLC partial response (PR) was defined as a 50% or higher fall in the monoclonal class; a FLC complete response (CR) was defined as normalization of the FLC ratio and both light chain classes, unless there was renal failure (defined as creatinine clearance ≤ .5001 mL/s [30 mL/min]) causing polyclonal retention of FLC, in which case the ratio alone was used. Patients who could not be classed as achieving FLC-PR or FLC-CR were categorized as nonresponders. The response was assessed as the best achieved response at least 6 months after completion of chemotherapy, or the best achieved before any further therapy was given.
Amyloidotic organ involvement and response were defined according to the international consensus criteria.17 Heart, liver, kidneys, gastrointestinal tract, nerves (autonomic and/or peripheral), lungs, and soft tissue (lymph node, tongue, muscles, factor X deficiency, or any other) were counted as individual organs. Since lymph node involvement was a prominent feature of the current series, these were also reported separately. All organ responses (as defined by the international consensus criteria)17 were assessed 6 months following initial chemotherapy or before the patient received another regimen. All patients underwent SAP scintigraphy, and serial studies were used to quantitatively measure whole body amyloid load, as previously described.14 Labeled SAP studies were interpreted by a single physician (P.N.H.) with experience of more than 5000 SAP scans. Organ involvement and responses by SAP scintigraphy were documented and analyzed separately. Performance status was according to Eastern Cooperative Oncology Group (ECOG) criteria.18
Statistics
Statistical analysis was undertaken using the SPSS 14 software package (SPSS, Chicago, IL) and Minitab version 14 (State College, PA). Survival was assessed by the method of Kaplan and Meier and compared by log-rank test. Categoric variables were compared with chi-square or Fisher tests as appropriate. All P values were 2 sided with a significance level of .05. Multivariate analysis was by Cox or binary logistic regression as appropriate.
Results
One hundred three patients with a solitary IgM paraprotein underlying AL amyloidosis were assessed at the NAC between 1988 and 2006, accounting for 6% of all patients with confirmed AL amyloidosis during this time. Presenting features are summarized in Table 1. Median age at presentation was 65 years (range, 46-88 years). Median number of organs involved by amyloid was 2. Renal, cardiac, and lymph node involvement were present at the diagnostic evaluation in 55 (53%), 36 (35%), and 22 (21%) patients, respectively. Six (5%) patients had isolated lymph node involvement. These 6 patients have been excluded from the response analysis, and only 1 of these patients received chemotherapy and has also not been included in the response analysis. None of these cases progressed to disseminated systemic disease. Liver involvement was present in 15 (14%) patients by consensus criteria, and was identified by SAP scintigraphy in 24 (24%) additional patients. Ten patients (9%) were dialysis dependent at first assessment. Among the 36 (35%) patients with cardiac involvement by consensus criteria, only 4 (3%) had interventricular septal thickness of 15 mm or more on echocardiography, which is widely regarded to represent severe cardiac amyloidosis. Serum bilirubin was more than 20 mM in 11 (10%) patients, serum albumin was less than 30 g/L in 31 (30%) cases, and platelet count was raised in 23 (22%) cases, in many probably reflecting hyposplenism.19 Overall, 2 or more organs were involved by consensus criteria in 56 (54%) patients, and SAP scintigraphy detected at least 1 additional organ involvement in 31 (30%) patients. Twenty-seven (26%) patients had an ECOG performance status of 3 or more at first assessment. The median follow-up was 36 months (range, 6-167 months) with a median 6 assessments at the NAC.
Presenting features
| . | Median (range) . | No. of patients (%) . |
|---|---|---|
| Age, median, y | 65 (46-88) | |
| Sex, male-female ratio | 1.7:1 | |
| IgM paraprotein, g/L | 8 (IF-60) | 103 (100) |
| Monoclonal light chain type | ||
| κ | 48 (46) | |
| λ | 55 (54) | |
| Hemoglobin, g/L | 12.3 (8.2-17.7) | |
| Total white cell count, × 109/L | 7.0 (0.5-23.4) | |
| Platelets, × 109/L | 287 (18-757) | |
| Creatinine, μM | 103 (49-ESRD) | |
| Albumin, g/L | 35 (14-52) | |
| Bilirubin, mM | 9 (4-225) | |
| Alkaline phosphatase, IU/L | 103 (52-3488) | |
| 24-hour proteinuria, g/24 h | 1.0 (< 0.1-21) | |
| Creatinine clearance, mL/min | 56 (ESRD-152) | |
| ECOG performance status | ||
| 1 or less | 26 (25) | |
| 2 | 50 (49) | |
| 3 or more | 27 (26) | |
| Organ involvement | ||
| Liver, consensus criteria | 15 (14) | |
| Liver, SAP scintigraphy | 39 (38) | |
| Renal, consensus criteria | 55 (53) | |
| Renal, SAP scintigraphy | 49 (47) | |
| Cardiac, any involvement | 36 (35) | |
| Cardiac, IVS 15 mm or more | 4 (3) | |
| Lymph nodes | 22 (21) | |
| Peripheral neuropathy | 4 (3) | |
| Autonomic neuropathy | 8 (7) | |
| Soft tissue | 14 (13) | |
| Gastrointestinal tract | 5 (4) | |
| Respiratory | 4 (3) | |
| Total no. of organs, international consensus criteria | ||
| 1 organ | 47 (46) | |
| 2 organs | 35 (34) | |
| 3 or more organs | 21 (20) | |
| Total no. of organs, SAP scintigraphy | ||
| 1 organ | 6 (5) | |
| 2 organs | 61 (60) | |
| 3 or more organs | 36 (35) |
| . | Median (range) . | No. of patients (%) . |
|---|---|---|
| Age, median, y | 65 (46-88) | |
| Sex, male-female ratio | 1.7:1 | |
| IgM paraprotein, g/L | 8 (IF-60) | 103 (100) |
| Monoclonal light chain type | ||
| κ | 48 (46) | |
| λ | 55 (54) | |
| Hemoglobin, g/L | 12.3 (8.2-17.7) | |
| Total white cell count, × 109/L | 7.0 (0.5-23.4) | |
| Platelets, × 109/L | 287 (18-757) | |
| Creatinine, μM | 103 (49-ESRD) | |
| Albumin, g/L | 35 (14-52) | |
| Bilirubin, mM | 9 (4-225) | |
| Alkaline phosphatase, IU/L | 103 (52-3488) | |
| 24-hour proteinuria, g/24 h | 1.0 (< 0.1-21) | |
| Creatinine clearance, mL/min | 56 (ESRD-152) | |
| ECOG performance status | ||
| 1 or less | 26 (25) | |
| 2 | 50 (49) | |
| 3 or more | 27 (26) | |
| Organ involvement | ||
| Liver, consensus criteria | 15 (14) | |
| Liver, SAP scintigraphy | 39 (38) | |
| Renal, consensus criteria | 55 (53) | |
| Renal, SAP scintigraphy | 49 (47) | |
| Cardiac, any involvement | 36 (35) | |
| Cardiac, IVS 15 mm or more | 4 (3) | |
| Lymph nodes | 22 (21) | |
| Peripheral neuropathy | 4 (3) | |
| Autonomic neuropathy | 8 (7) | |
| Soft tissue | 14 (13) | |
| Gastrointestinal tract | 5 (4) | |
| Respiratory | 4 (3) | |
| Total no. of organs, international consensus criteria | ||
| 1 organ | 47 (46) | |
| 2 organs | 35 (34) | |
| 3 or more organs | 21 (20) | |
| Total no. of organs, SAP scintigraphy | ||
| 1 organ | 6 (5) | |
| 2 organs | 61 (60) | |
| 3 or more organs | 36 (35) |
IF indicates immunofixation positive only; and ESRD, end-stage renal failure.
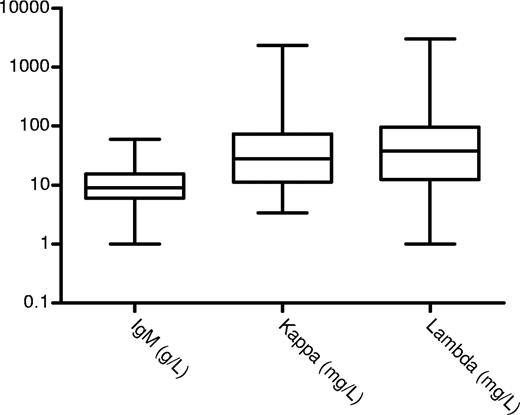
The bone marrow was reported to be abnormal in 77 (74%) cases. The infiltrate was reported as lymphoplasmacytoid in 42 (41%) patients, and was mainly lymphoid in 24 (23%) cases and plasma cells in 10; no infiltrate was seen in 11 and no information, in 16 cases. The bone marrow information is limited due to the retrospective nature of this review spanning 18 years and the lack of availability of the source material for a full lymphoid antibody panel to be performed retrospectively—even though this may not have been sensitive enough to identify low-level clonal infiltration. Immunophenotyping details were available in 16 (38%) of 42 cases with a lymphoplasmacytoid morphology and all were light chain restricted (9 lambda and 7 kappa) and positive for IgM, CD19, and CD20 and negative for CD10, CD5, CD23, or CD138 expression. Immunophenotyping details were available in 6 (25%) of 24 cases with a lymphoid morphology that was positive for CD19 and CD20 and light chain restricted (4 lambda and 2 kappa) but negative for CD10, CD5, or CD23 in all but 3 cases. One case each had typical chronic lymphocytic leukemia, follicular lymphoma, and mantle cell lymphoma (including the appropriate translocations for the latter 2). The rest of the patients did not have any other manifestations of lymphoma or myeloma. Similarly, apart from the 2 cases with follicular and mantle cell lymphoma, all other lymph node biopsies showed replacement with amyloid and no features diagnostic of lymphoma. Median percentage of the lymphoid cells or lymphoplasmacytoid cells in the bone marrow was 12% (range, 1%-70%). Among 10 patients with a bone marrow plasma cell infiltrate (median, 4%; range, 2%-44%), only 1 had clearly documented multiple myeloma. The other 9 had less than 10% plasma cells and no other features of myeloma and may represent a more mature end of the lymphoplasmacytoid spectrum. The median IgM paraprotein concentration at presentation was 8 g/L (range immunofixation positive, 60 g/L) and only 12 (11%) patients had more than 20 g/L serum paraprotein (Figure 1). Serum FLC measurements were available before chemotherapy was administered in 87 patients, yielding an abnormal ratio in 77 (88%) cases. Median kappa and lambda concentrations, for patients with IgM kappa and IgM lambda, respectively, were 28 mg/L (range, 5-2330 mg/L) and 38 mg/L (range, 1-3019 mg/L), respectively (Figure 1). The overall level of the abnormal FLC component appears to be rather low and only 24 (31%) of 77 with creatinine clearance more than .5001 mL/s (30 mL/min) had an aberrant FLC concentration of more than 100 mg/L.
IgM levels for patients with a quantifiable paraprotein (g/L), free kappa, and free lambda levels (mg/L) in cases with abnormal ratios. The boxes represent the 25th to 75th percentiles; the horizontal line is at the median and the vertical line defines the range.
IgM levels for patients with a quantifiable paraprotein (g/L), free kappa, and free lambda levels (mg/L) in cases with abnormal ratios. The boxes represent the 25th to 75th percentiles; the horizontal line is at the median and the vertical line defines the range.
Treatment and response
Hematologic response to first-line chemotherapy was evaluable in 77 (75%) patients, in 75 cases by conventional means and in 22 patients by FLC assay. Eight patients were not treated, and insufficient data were available to determine response in 18 patients. FLCs were not evaluable in 55 of 77 patients due to either a pretreatment aberrant FLC concentration of less than 100 mg/L or a normal ratio.
A variety of chemotherapy regimens were used and are listed in Table 2. Patients received a median of 4 cycles (range, 2-9 cycles) of chemotherapy. Twenty-one (27%) patients without bone marrow plasma cell infiltrate received a regimen typically used for treatment of plasma cell disorders with only 3 patients having a partial response (2 treated with VAD and 1, with CTD). The hematologic responses are shown in Table 3. Using conventional paraprotein measurements, 25 (32%) (18 with lymphoplasmacytoid infiltration and 7 with other) of the 77 evaluable patients achieved a PR, but none achieved a CR. The number of responding patients is too small to allow for meaningful statistical subgroup analysis by the underlying clonal disease type. FLC criteria in the 22 evaluable cases indicated PR in 9 (41%) patients and CR in a further 3 (14%) such patients; 2 patients with a FLC response did not have a response according to conventional criteria. It appeared that combination chemotherapy (VAD, CHOP, CVP, R-CVP) or purine analogues or rituximab (or combinations) (fludarabine, cladribine, FCR, rituximab) showed higher responses compared with oral agents (chlorambucil, oral melphalan, and oral cyclophosphamide) (responses in 13 [59%] of 25 vs 8 [21%] of 37 cases, respectively; P = .003) but these numbers are limited and need interpretation with appropriate caution. Thirty-four (33%) cases received a second-line treatment with a further 14 (41%) patients achieving at least a partial clonal response. A total of 3 cases in the current series underwent an autologous stem cell transplantation (1 as first line and 2 as second line) with complete response in 2 cases and very good partial response in 1.
Hematologic response according to chemotherapy regimen
| Regimen . | No. of patients . | Hematologic response (%) . |
|---|---|---|
| Chlorambucil | 22 | 6 (27) |
| VAD | 9 | 4 (44)* |
| IDM | 8 | 1 (12) |
| Oral melphalan | 6 | 1 (16) |
| Oral cyclophosphamide | 6 | 0 (0) |
| CVP | 3 | 1 (33) |
| R-CVP | 2 | 1 (50) |
| CHOP | 2 | 2 (100)* |
| CTD | 3 | 1 (33) |
| FCR | 3 | 2 (66)* |
| Fludarabine or cladribine | 2 | 2 (100) |
| Others† | 11 | 3 (27)* |
| Regimen . | No. of patients . | Hematologic response (%) . |
|---|---|---|
| Chlorambucil | 22 | 6 (27) |
| VAD | 9 | 4 (44)* |
| IDM | 8 | 1 (12) |
| Oral melphalan | 6 | 1 (16) |
| Oral cyclophosphamide | 6 | 0 (0) |
| CVP | 3 | 1 (33) |
| R-CVP | 2 | 1 (50) |
| CHOP | 2 | 2 (100)* |
| CTD | 3 | 1 (33) |
| FCR | 3 | 2 (66)* |
| Fludarabine or cladribine | 2 | 2 (100) |
| Others† | 11 | 3 (27)* |
VAD indicates vincristine-adriamycin-dexamethasone; IDM, intravenous melphalan (25mg/m2); CVP, cyclophosphamide-vincristine-prednisone; R-CVP, rituximab-cycophosphamide-vincristine-prednisone; CHOP, cyclophosphamide-doxorubicin-vincristine-prednisone; CTD, cyclophosphamide-thalidomide-dexamethasone; and FCR, fludarabine-cyclophopshamide-rituximab.
Organ response; each symbol denoted 1 patient.
Others indicates R-CHOP, single-agent rituximab, intravenous cyclophosphamide, thalidomide-dexamethasone, lomustine-idarubicin-dexamethasone, single-agent dexamethasone, fludarabine-cyclophosphamide, idarubicin-dexamethasone, and stem cell transplantation in 1 patient each.
Clonal response to chemotherapy by serum FLC assay, by conventional immunofixation electrophoresis (IFE), and by combined FLC plus IFE
| Response . | FLC response, n = 22 . | Conventional response, n = 77 . | Combined response, n = 77 . |
|---|---|---|---|
| CR (%) | 3 (14) | 0 (0) | 0 (0) |
| PR (%) | 9 (41) | 25 (32) | 25 (32) |
| Total response (%) | 12 (55) | 25 (32) | 25 (32) |
| Response . | FLC response, n = 22 . | Conventional response, n = 77 . | Combined response, n = 77 . |
|---|---|---|---|
| CR (%) | 3 (14) | 0 (0) | 0 (0) |
| PR (%) | 9 (41) | 25 (32) | 25 (32) |
| Total response (%) | 12 (55) | 25 (32) | 25 (32) |
Numbers in rows do not add up as patients are overlapping or in different response categories for FLC and conventional responses.
Amyloidotic organ responses (according to the consensus criteria) occurred in a total of 4 patients—2 (12%) of 25 conventional hematologic responders, and additionally in both of the patients who achieved an FLC but not a conventional response. Amyloidotic organ function remained stable in 17 (68%) of 25 responders, and worsened in 6 (30%). There were 2 renal responses, comprising 55% and 62% decreases in proteinuria, and 3 hepatic responses each comprising improvement in liver function tests. None of the patients with lymph node involvement showed any decrease in nodal size after treatment, although among the partial responders nor was there any further nodal enlargement. Of these 4 patients with conventional organ response, serial SAP scintigraphy demonstrated regression of hepatic amyloid in 2 patients and of bone amyloid in 1 patient.
Survival
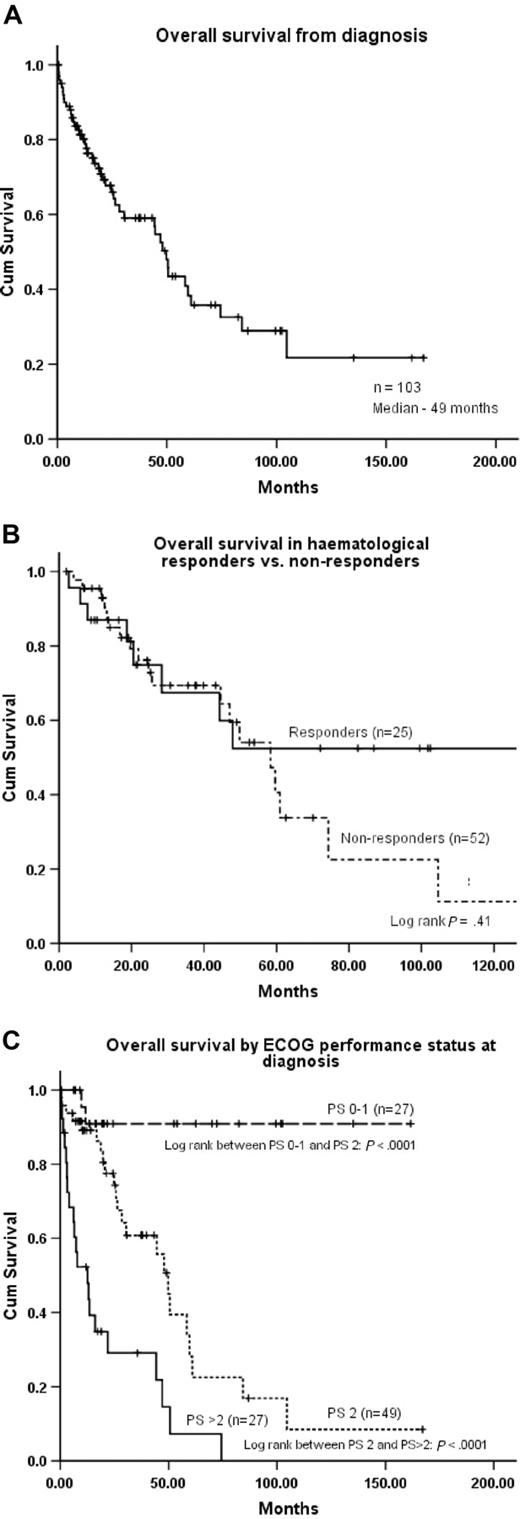
There was no significant difference in the overall survival of patients with a lymphoid, lymphoplasmacytoid, plasma cell, or normal/unknown infiltrate in the bone marrow (median Kaplan-Meier estimate of 47 months, 49 months, 51 months, and 39 months, respectively; log rank: P = .9). Only one of the deaths was directly due to the underlying lymphoma (high-grade transformation). The Kaplan-Meier estimated median OS for the whole cohort from diagnosis was 49 months (Figure 2A). Although the difference did not attain statistical significance, potentially reflecting the relatively small numbers of patients, the estimated 10-year survival for nonresponders was less than 10% compared with more than 50% for responders, with the survival curves diverging at approximately 50 months (Figure 2B). The Kaplan-Meier estimated OS was 44 months for the 26 patients who were not treated or were not evaluable for treatment response. Estimated median survival for patients with single-organ involvement was 84 months compared with 26 and 19 months, respectively, for those with 2 or 3 involved organs (log rank, P = .003). The estimated 10-year survival among patients with ECOG performance status of 1 or lower before treatment was more than 90% (Figure 2C). The various factors associated with survival are detailed in Table 4, of which on univariate analysis, ECOG performance status, number of organs involved by amyloid, and presence of amyloid deposits in the heart or liver were significant. On multivariate analysis, only performance status and liver involvement were significant (P < .001, RR 3.7 and P = .018, RR 2.2, respectively).
Overall survival, effect of treatment, and performance at diagnosis on overall survival. (A) Overall survival from diagnosis (all patients). (B) Overall survival according to clonal response or no response to initial treatment. (C) Overall survival stratified by the ECOG performance status at diagnosis.
Overall survival, effect of treatment, and performance at diagnosis on overall survival. (A) Overall survival from diagnosis (all patients). (B) Overall survival according to clonal response or no response to initial treatment. (C) Overall survival stratified by the ECOG performance status at diagnosis.
Factors affecting overall survival
| Factor . | Univariate significance (odds ratio; 95% CI) . | Multivariate significance (odds ratio; 95% CI) . |
|---|---|---|
| No. of organs | .0001 (1.8; 1.3-2.4) | |
| Performance status* | .0001 (4.0; 2.5-6.5) | .0001 (3.7; 2.2-6.2) |
| Cardiac involvement | .026 (2.3; 1.0-3.7) | 0.072 (3.8; 0.8-2.5)† |
| Liver involvement (consensus criteria) | .0001 (3.2; 1.6-6.4) | .018 (2.2; 1.1-4.5) |
| Renal involvement (consensus criteria) | 0.28 (1.3; 0.76-2.5) | |
| Amyloid load on SAP scan | 0.28 (1.03; 0.97-1.1) | |
| Hematologic response | 0.42 (0.8; 0.3-1.6) | |
| Age | 0.15 (1.02; 0.9-1.05) | |
| IgM level | 0.54 (0.9; 0.9-1.2) | |
| Abnormal FLCs | 0.41 (1.6; 0.4-5.3) |
| Factor . | Univariate significance (odds ratio; 95% CI) . | Multivariate significance (odds ratio; 95% CI) . |
|---|---|---|
| No. of organs | .0001 (1.8; 1.3-2.4) | |
| Performance status* | .0001 (4.0; 2.5-6.5) | .0001 (3.7; 2.2-6.2) |
| Cardiac involvement | .026 (2.3; 1.0-3.7) | 0.072 (3.8; 0.8-2.5)† |
| Liver involvement (consensus criteria) | .0001 (3.2; 1.6-6.4) | .018 (2.2; 1.1-4.5) |
| Renal involvement (consensus criteria) | 0.28 (1.3; 0.76-2.5) | |
| Amyloid load on SAP scan | 0.28 (1.03; 0.97-1.1) | |
| Hematologic response | 0.42 (0.8; 0.3-1.6) | |
| Age | 0.15 (1.02; 0.9-1.05) | |
| IgM level | 0.54 (0.9; 0.9-1.2) | |
| Abnormal FLCs | 0.41 (1.6; 0.4-5.3) |
Bold face numbers in columns 2 and 3 indicate significant P values.
ECOG performance status 2 or less or more than 2.
Not significant.
Discussion
We report the presenting features, response to treatment, and clinical outcome in the largest series to date of patients with AL amyloidosis associated with IgM paraproteinemia, and for the first time analysis of serum FLCs in this clinical setting.
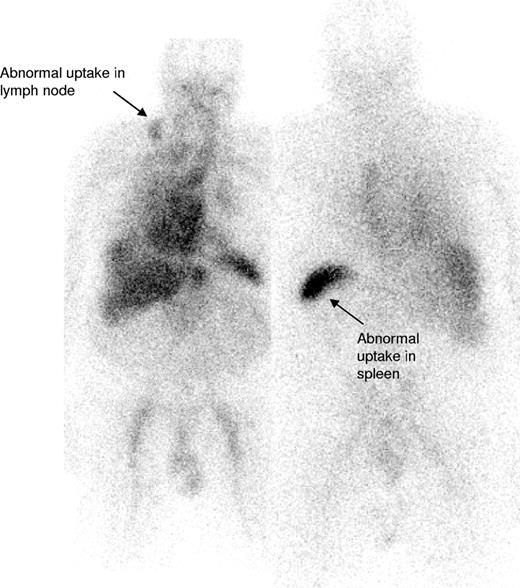
IgM paraproteinemias account for 17% to 20% of patients with MGUS,5,20 and although Waldenström macroglobulinemia (WM) and other lymphomas have been described in association with systemic and localized AL amyloidosis,7,10,21-30 the largest reported series of IgM-associated amyloidosis is 50 patients from the Mayo Clinic in whom treatment outcomes have not been clearly reported.7 The current series of 103 patients accounted for 6% of patients with confirmed AL amyloidosis seen at the NAC between 1988 to 2006, similar to the 5% frequency at the Mayo Clinic.4 In our series, 54% of patients had 2 or more organs involved by amyloid, most frequently the kidneys (53%) followed by the heart (35%), similar to the large previous Mayo series of patients with AL associated with non-IgM monoclonal proteins.4 Lymph node involvement is uncommon in AL amyloidosis associated with non-IgM paraproteins4 but was evident in 21% of patients in our series (Figure 3). Conversely, peripheral neuropathy was less common at presentation, in just 3%, than in previously reported non-IgM series.4,31 None of our patients had lymphoma related “B” symptoms or hyperviscosity syndrome, likely reflecting the observed low clonal burden of their B-cell disorder.
123I-labeled SAP scan from a patient with IgM-associated AL amyloidosis showing uptake of the radiotracer within the involved right cervical lymph node (arrow) in addition to some splenic uptake. There is a normal blood pool tracer signal from the heart and liver.
123I-labeled SAP scan from a patient with IgM-associated AL amyloidosis showing uptake of the radiotracer within the involved right cervical lymph node (arrow) in addition to some splenic uptake. There is a normal blood pool tracer signal from the heart and liver.
In a series of 382 patients reported from the M. D. Anderson Cancer Center (Houston, TX), IgM paraproteinemia was associated with lymphoplasmacytic lymphoma (LPL)/WM in 58%, chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) in 20%, and other lymphoproliferative disorders in 20% of cases.32 The same variability is likely to exist in IgM-associated amyloidosis patients—there was a suggestion that lymphoplasmacytoid morphology was the most common followed by lymphoid, but this will need further prospective study for confirmation. However, it is important to recognize this marked variability of the underlying clonal disorder in these cases—which contrasts from those with AL amyloidosis in general where the underlying clonal cell population is usually a plasma cell dyscrasia—a fact that is not widely recognized outside of specialized centers. IgM amyloidosis patients often do not undergo full immunophenotyping to characterize the lymphoid clone—investigations instead are focused on characterizing the plasma cell disorder; this is also seen in the current series and hence, in some cases, leads to less than appropriate treatment selection. Immunophenotyping by multiparameter flow cytometry is critical for all cases with IgM-associated AL (ideally in every AL case) to accurately diagnose the underlying clonal disorder. In other respects, the situation in the current series was analogous to the typical situation of AL amyloidosis. Most patients in the current series had low-level bone marrow infiltration that was associated with a median circulating IgM paraprotein concentration of 8 g/L. Among 364 non-IgM AL amyloidosis patients seen at NAC over a similar period, in whom a paraprotein was detectable, the median paraprotein concentration was 7 g/L, and in 10% of IgM and non-IgM AL patients assessed in our center who have had monoclonal protein the median was more than 20 g/L (NAC, unpublished observations).
Measurement of serum FLCs (Freelite) is the most sensitive method for detecting, quantifying, and monitoring the underlying clonal disease in patients with AL amyloidosis.3 This assay has, however, been little studied in IgM-associated lymphoid disorders or IgM-associated amyloidosis, the first reported series comprising 6 patients with AL complicating non-Hodgkin lymphoma (NHL) in whom abnormal FLC values were recorded in each case.10 Although the frequency of abnormal FLC results was high in our series, at 88%, the concentration of the clonal FLC class was generally low and exceeded 100 mg/L, the currently defined lower limit for assessing treatment response in the international consensus criteria,17 in only 27% of patients. This is much lower than the AL in general where the FLC levels are assessable for response (> 100 mg/L) in approximately 65% of patients with non–IgM-associated AL amyloidosis (NAC, unpublished data, 2008); although the current series has a selection bias since it was a cohort defined by the presence of a detectable whole paraprotein, which is often absent in AL patients generally. The kappa bias in the current series (as opposed to the usual lambda bias in AL amyloidosis) remains unexplained and may either be due to the nature of the underlying clone or simply reflect patient referral bias. An international registry of patient with IgM amyloidosis would be a step toward gathering accurate information about this condition. The presence and concentration of abnormal FLCs in this cohort is, however, comparable to that reported in a series of individuals with uncomplicated IgM MGUS in whom FLCs were abnormal in 74% of cases, with the clonal FLC concentration usually less than 50 mg/L.17 This and the present findings suggest that low-grade monoclonal IgM-secreting disorders may be oligosecretory for FLCs. This low-level abnormality in FLC often makes it more difficult for FLCs to be used as a marker to track the clonal responses in IgM amyloidosis patients.
Literature is scanty on outcomes of treatment in IgM-associated AL amyloidosis, and response was not examined in the 50-patient Mayo series.8 Two of 5 patients at Memorial Sloan-Kettering Hospital (New York, NY) responded to rituximab-based regimens.10 The Mayo group recently reported favorable outcomes of autologous stem cell transplantation in 12 patients, among whom there was 1 treatment-related death, 7 partial hematologic responses, and 1 complete response.9 The latter, however, compares poorly with a widely reported CR rate of 40% following stem cell transplantation (SCT) in non-IgM amyloidosis,33 but is in keeping with the relatively low complete response rates following SCT in WM compared with myeloma.34,35
The wide variety of chemotherapy regimens received by our patients reflects the lack of standardization of treatment in this condition, and we emphasize the critical need to recognize the distinct underlying clonal disorder in this subgroup of patients from that of AL amyloidosis in general for selection of regimens appropriate for a lymphoid clonal disorder rather than a plasma cell disorder. The overall hematologic response rate to initial treatment was poor (32%)—which may reflect, in part, the fact that 27% of patients received regimens that are not very effective in lymphoproliferative disorders. The small number of responding cases in each group limits the interpretation of response data in relation to the underlying disorder. It was encouraging that patients who received a combination regimen or purine analogues with/without rituximab (defined here as combination chemotherapy such as CHOP or similar combinations, purine analogues, or rituximab or its combination with other agents) had a 3-fold higher response rate than those single-agent oral alkylators or thalidomide and its combinations. These numbers are small and need interpretation with caution. The response rate of 20% following oral alkylator therapy is similar to rather poor responses also seen in non–IgM-associated AL amyloidosis treated with oral melphalan and prednisone.36,37 The regimens with purine analogous or chemoimmunotherapeutic combinations appear to have similar hematologic response rates to some for the more recently described regimens in non-IgM AL amyloidosis38-40 and merit further prospective study. The lack of hematologic complete responses in the present IgM series was striking, given that a fifth of patients can expect a CR following the more recent combination chemotherapy for non-IgM amyloidosis. These findings are not surprising since CR is known to be rare in WM or CLL treated with alkylators,41 single-agent purine analogues,42,43 or single-agent rituximab.44 These findings and also the fact the hematologic responses in the current series did not translate into survival improvement identify an urgent need to study more effective chemoimmunotherapeutic or other novel treatment approaches.
Median overall survival for IgM amyloidosis was about 2 years in the 1993 Mayo series,7 similar to that reported in non–IgM-associated AL amyloidosis at the time.4 There was no significant difference in the overall survival according to the underlying disorder, which is in keeping with the fact that most cases had a low-grade disorder with progressive lymphoma the actual cause of death in only one case. Survival in AL amyloidosis in general appears to be improving, and median survival of 600 AL patients assessed at the NAC over the last decade has been 3.3 years (NAC, unpublished data, 2008), a value comparable with the outcome of the present IgM series. ECOG performance status at presentation was the most significant single factor associated with survival in the current cohort, and patients with single-organ involvement by amyloid had a notably good prognosis. Only 4 patients had advanced cardiac amyloidosis in the current cohort, which may account for the fact that cardiac involvement was not a significant independent prognostic factor as opposed to liver involvement—the latter accounting for a group of patients who had advanced multiorgan involvement, often with poor performance status. Although it is again tempting to speculate that clonal biology may have some role to play, this outcome likely reflects a referral bias. It is clear that a hematologic response independently predicts for better survival in most AL series,3,45 and the complete responders appear to have even better outcomes especially for cases with cardiac involvement.46 In the current series, although survival of hematologic responders was not statistically better than nonresponders, there was a distinct impression that in a small number of responders benefit might well become evident with more prolonged follow-up beyond 50 months. The former is likely to be accounted by the poor overall response rate of only 32% with no complete responses. In a small number of later cases, the Kaplan-Meier survival curves began to diverge later—consistent with the well-recognized phenomenon that regression of AL amyloid and clinical benefit can occur very slowly after suppression of the underlying clonal disorder. The poor hematologic response also led to only a limited number of organ responses and was not enough to prevent early deaths for those with advanced organ dysfunction—a clear case in need of rapidly effective therapeutic approaches. However, a majority of the surviving hematologic responders had stable function, suggesting that the response was adequate to prevent progression but not enough to achieve regression of amyloid deposits. This is in keeping with earlier observations for AL in general.3,47 The adequacy of the hematologic response for achieving an organ response may be a complete clonal response in some cases, although others may well achieve the same functional improvement with a good partial response avoiding excessive exposure to treatment-related toxicity. However, there are no clearly defined determinants of this adequacy of hematologic response, but early data indicate that functional markers such as NT-ProBNP may well act as surrogates and plainly there is also a need to prospectively study larger populations.
In summary, IgM-associated AL amyloidosis should be recognized as a rare but distinct subtype of AL amyloidosis. Although the presenting features of this subgroup are not vastly different from those of non–IgM-associated AL amyloidosis, lymph node involvement is more frequent. A critical distinction from non-IgM amyloidosis patients is the possibility of a wide variety of underlying lymphoproliferative disorders instead of the usual culprit—a plasma cell clone. Investigations for the clonal disorder in IgM amyloidosis should focus on accurate characterization of the underlying clone to select an appropriate treatment regimen. While recognizing that the current series has limitation in terms of being retrospective and having limited numbers of patients in each treatment group, hematologic response rates with traditional alkylator-based treatment regimens appear to be poor and clearly regimens used for treatment of myeloma, such as standard thalidomide combinations or oral melphalan, are likely to be inappropriate since there is usually an underlying lymphoid clone. There have been significant improvements in lymphoma outcomes with chemoimmunotherapeutic combinations and these approaches should be considered for treatment of these patients, especially if they are not eligible for autologous stem cell transplantation. The rarity of IgM paraprotein-associated AL amyloidosis not only makes prospective trials difficult but also poses a substantial challenge to studying the phenotypic and genetic profiles of the underlying clonal disorder. Establishment of an international registry, including a repository for tissue and marrow samples, would be a valuable step toward achieving these goals.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge Ms Dorothea Gopaul for undertaking SAP scintigraphy, Ms Dorota Rowczenio for performing all relevant genotyping, Ms Janet Gilbertson for histology, and Ms Jean Berkley for expert preparation of the paper. We acknowledge all of the hematologists who took care of these patients.
This study was supported by Medical Research Council (London, United Kingdom) Program Grant G97900510 (P.N.H.), University College London (United Kingdom) Amyloidosis Research Fund, and National Health Service Research and Development Funds.
Authorship
Contribution: A.D.W. performed research, analyzed data, and wrote the paper; H.J.B.G. performed research and wrote the paper; H.J.L. performed research; A.B. contributed to vital reagents for free light chain assay reported in this paper; and P.N.H. and J.G.D. performed research and wrote the paper.
Conflict-of-interest disclosure: A.B. has ownership interest in Freelite, the serum free light chain assay produced by The Binding Site, that has been reported in this paper. The other authors declare no competing financial interests.
Correspondence: Ashutosh Wechalekar, Department of Medicine, University College London Medical School (Royal Free Campus), Rowland Hill Street, London NW3 2PF, United Kingdom; e-mail: a.wechalekar@medsch.ucl.ac.uk.