Abstract
CD47 functions as a marker of self on red blood cells (RBCs) by binding to signal regulatory protein alpha on macrophages, preventing phagocytosis of autologous RBCs by splenic red pulp macrophages, and Fcγ receptor (FcγR)– or complement receptor–mediated phagocytosis by macrophages in general. RBC senescence involves a series of biochemical changes to plasma membrane proteins or lipids, which may regulate phagocytosis by macrophages. Here, we investigated whether CD47 on experimentally senescent murine RBCs affects their phagocytosis by macrophages in vitro. Clustering of CD47 with antibodies was more pronounced in the plasma membrane of untreated RBCs, compared with that in in vitro oxidized RBCs (Ox-RBCs). Phagocytosis of Ox-RBCs was mediated by scavenger receptors (SRs) distinct from SR-A or CD36 and required serum factors. We found that wild-type (WT) and CD47−/− Ox-RBCs were phagocytosed equally well by macrophages in the presence of serum, suggesting that phagocytosis via SRs is not inhibited by CD47. Despite this, FcγR-mediated phagocytosis of IgG-opsonized Ox-RBCs was strongly inhibited by CD47. These data suggest that based on the specific prophagocytic receptors mediating uptake of senescent RBCs, the phagocytosis-inhibitory role of CD47 may be more or less involved.
Introduction
Removal of senescent red blood cells (RBCs) from the circulation is a selective process mediated mainly by macrophages in the liver and spleen.1 Several molecular patterns, which may be involved as ligands in recognition by macrophages, appear on the surface of senescent RBCs. Macrophages can either directly recognize these ligands, or recognition can be dependent on binding of bridging plasma proteins that may interact with the RBC ligands.2-7 As such, these RBC ligands may include alterations in protein carbohydrate moieties,8 loss of plasma membrane phospholipid asymmetry,9-11 and/or clustering of Band 3 with subsequent binding of complement or antibodies.12-14 Phagocytosis by macrophages can for instance be induced following ligation of receptors such as Fcγ receptors (FcγR), complement receptors, or scavenger receptors (SRs).15-17 FcγR recognizes the Fc portion of immunoglobulin G (IgG) bound to a target cell, resulting in rapid phagocytosis of the IgG-opsonized target. SRs make up a large family of receptors with a broad ligand-binding ability, where most known ligands are polyanionic molecules such as proteins, polyribonucleotides, polysaccharides, and lipids.16 “Altered” host molecules (eg, oxidized low-density lipoprotein [oxLDL]) can be recognized by most SRs, whereas other ligands interact more specifically with SRs.16 Oxidative damage is likely to be one important cause of RBC senescence and exposure of senescence-associated ligands.13,18 RBCs oxidized in vitro (Ox-RBCs) are efficiently phagocytosed by macrophages in the absence of further opsonization, a process shown to be inhibited by oxLDL or other SR ligands, such as fucoidan, dextran sulfate, or poly-I, both in vivo and in vitro.4,7,19 Phagocytosis of Ox-RBCs has also been suggested to be serum dependent,6 and involve recognition of phosphatidylserine (PS) on the RBC surface.9,10
Macrophage phagocytosis of target host cells is regulated by the balance between signaling through prophagocytic receptors (eg, FcγR or complement receptors) and inhibitory receptors. Recognition of the cell surface glycoprotein CD47 by the inhibitory receptor signal regulatory protein alpha (SIRPα) inhibits phagocytosis of unopsonized RBCs, as well as IgG or complement-opsonized RBCs, both in vivo and in vitro.20-23 CD47 is highly expressed on RBCs, but ubiquitously expressed by most cells in the body, and can also negatively regulate phagocytosis of platelets and leukocytes.24-26 The amount of CD47 on the cell surface regulates macrophage phagocytosis, since IgG-opsonized RBCs from CD47+/− mice (expressing about 50% of normal levels of CD47) are taken up faster than wild-type (WT) RBCs, but slower than CD47−/− RBCs.27 We have shown that the phagocytosis-inhibitory effect of CD47 does not come into play on apoptotic nucleated cells, where CD47 is clustered into patches on the apoptotic cell surface and segregated away from prophagocytic ligands such as PS or calreticulin.25
Although older circulating RBCs were found to express reduced levels of CD47,28,29 little is known about the function of CD47 during RBC senescence and in regulating macrophage phagocytosis of senescent RBCs. Therefore, the aim of the present study was to investigate the role of CD47 in regulating macrophage phagocytosis of experimentally senescent RBCs.
Methods
Antibodies and reagents
Antimouse CD47 mAb miap43030 (mouse IgG2a), anti-FcγR mAb 2.4G2, and antimouse RBC/Band 3 mAb 34-3C (mouse IgG2a; kindly provided by Dr Shozo Izui, University of Geneva, Geneva, Switzerland) established from autoimmune-prone NZB mice31 were purified from hybridoma supernatants by ammonium sulfate precipitation and protein A/G chromatography (Amersham Pharmacia, Piscataway, NJ). Functional blocking CD36 mAb 63 (mouse IgA) and SR-A mAb 2F8 (mouse IgG2a) were from Chemicon-Millipore (Molsheim, France) and Hycult Biotechnology (Uden, The Netherlands), respectively. Cy3-conjugated goat anti–mouse IgG was from Jackson ImmunoResearch Europe (Newmarket, United Kingdom). Fluorescein isothiocyanate (FITC)–conjugated annexin V was from ImmunoTools (Friesoythe, Germany). The inhibitors SB203580, PD98059, genistein, LY294002, PP2, and PP3 were from Calbiochem (Darmstadt, Germany). QIFIKIT for the quantification of cell surface bound IgG was from Dako Cytomation (Glostrup, Denmark). Dulbecco phosphate-buffered saline (PBS), Dulbecco modified Eagle medium (DMEM), penicillin, streptomycin, and fetal calf serum (FCS) were from Invitrogen (Stockholm, Sweden). Piceatannol, fucoidan, dextran sulfate, chondroitin sulfate, carrageenan, poly-I, poly-C, poly-A, FITC-conjugated anti–mouse IgG, and all other reagents were from Sigma-Aldrich (St Louis, MO).
Mice
Adult (2-6 months old) male or female CD47−/− Balb/c mice,32 back-crossed to Balb/c (The Jackson Laboratory, Bar Harbor, ME) for 16 generations, and their heterozygous and homozygous littermates were from our own breeding colony. The mice were housed in a barrier facility, according to local guidelines. All experiments were performed according to national and local guidelines, and were approved by the local institutional review board at Umeå University, to allow for use of experimental animals, and their cells and tissues in the present study (approval no. A22-06).
Preparation of RBCs
Blood was collected into heparin by heart puncture of CO2-asphyxiated mice, and mixed with an equal volume of PBS containing 5 mM ethylenediaminetetraacetic acid (EDTA). Following centrifugation for 4 minutes at 300g, the platelet-rich plasma and buffy coat were removed. RBCs were washed extensively in PBS, resuspended at 4% to 6% hematocrit (hct) in Alsever solution, and stored at +4°C until used in experiments. RBCs to be used in the present experiments were prepared freshly at least once a week. Ox-RBCs were prepared as previously described.4,5 In brief, RBCs were washed in PBS, resuspended in PBS at 4% hct, and incubated with 0.2 mM CuSO4 and 5 mM ascorbic acid at 37°C for 60 minutes. RBCs were then washed 2 times in PBS with 5 mM EDTA, followed by 2 washes with PBS, and finally resuspended at 2 × 108/mL in PBS. For IgG opsonization, RBCs were resuspended at 5 × 108/mL in PBS and incubated with 1 to 25 μg/mL mAb 34-3C (as indicated in the figure legends) at + 4°C for 30 minutes, and washed 3 times in PBS before use in phagocytosis experiments. Quantification of bound IgG was performed using QIFIKIT beads, according to the manufacturer's instructions.
Preparation of macrophages and phagocytosis experiments
Bone marrow–derived macrophages (BMMs) were prepared as previously described, by culture of nonadherent bone marrow cells in the presence of 15% L-cell supernatant for 6 to 8 days.26 Mature macrophages were seeded at 5 × 104/mL in complete DMEM (DMEM supplemented with 10% [vol/vol] heat-inactivated FCS and 100 units/mL penicillin/streptomycin) without L-cell supernatant onto 11-mm sterile glass coverslips, and incubated at 37°C for 24 hours. To obtain resident peritoneal macrophages (RPMs), the peritoneal cavity of CO2-asphyxiated mice was flushed with 7 mL cold complete DMEM. After sedimentation at 200g for 5 minutes at 4°C, cells were resuspended at 1 × 106/mL in cold complete DMEM. Resident peritoneal cells (300 μL) were seeded onto 11-mm sterile glass coverslips and the macrophages were allowed to adhere for 4 hours at 37°C. Before the addition of RBCs, nonadherent cells were rinsed off with warm DMEM (with or without 10% FCS) and 300 μL DMEM with or without 10% FCS was added per well. In some experiments, IgG-depleted FCS was used, prepared using protein G–sepharose as previously described.33 In experiments where inhibitors were used, macrophages were preincubated with inhibitors at 37°C for 30 minutes prior to addition of RBCs. Following addition of 50 μL RBCs at 2 × 108/mL to each well, the cells were incubated at 37°C for 30 or 60 minutes, as indicated in the figure legends. The wells were then rinsed with cold PBS and noningested RBCs were lysed for 40 seconds, using cold distilled H2O. The cells were then fixed and stained as previously described.27 A phagocytosis index was calculated by counting phagocytosed RBCs in 200 to 300 macrophages per coverslip, and expressed as number of RBCs/100 macrophages.
Immunofluorescence microscopy
Untreated RBCs or Ox-RBCs were incubated in PBS plus 2% FCS with 10 μg/mL mAb miap430 at room temperature for 30 minutes. After 2 washes in PBS, the cells were incubated with Cy3-conjugated goat anti–mouse IgG diluted 1:100 in PBS plus 2% FCS at +4°C for 30 minutes, washed in PBS, fixed for 30 minutes at room temperature in 3% paraformaldehyde and 0.01% glutaraldehyde, and washed 3 times in PBS. Fixed RBCs were adhered to poly-l-lysine–coated coverslips, and mounted in Vectashield medium (Vector Laboratories, Peterborough, United Kingdom). Analysis of CD47 distribution was done using laser scanning confocal microscopy (Leica TSP-2; Leica Geosystems, Sollentuna, Sweden) with a 63×/1.2 NA water objective. To quantify clustering of CD47, visible clusters (larger than 250 nm in diameter) were counted at ×630 magnification (63× optical and 10× digital zoom) in midsections of untreated RBCs or Ox-RBCs, using Photoshop (Adobe Systems, San Jose, CA) and Leica LCM confocal software.
Statistics
Statistical analyses were done using the Student t test for paired or unpaired samples, as described in the figure legends.
Results
Serum-dependent phagocytosis of in vitro Ox-RBCs requires macrophage SRs
Peroxidation of membrane lipids is suggested to be a common feature of aged RBCs in vivo.1 Thus, to study phagocytosis of Ox-RBCs, we used a well-established technique to oxidize RBCs in vitro, using CuSO4 and ascorbic acid.4,7,34 Using WT cells, we found that Ox-RBCs were efficiently phagocytosed by RPM, whereas untreated RBCs were phagocytosed only to a limited extent (Figure 1A). Although phagocytosis of Ox-RBCs was about 10 times less efficient by BMM than by RPM, Ox-RBCs were phagocytosed substantially more than untreated RBCs also by BMM (Figure 1B). Removal of serum abolished phagocytosis of Ox-RBCs by RPM (Figure 1C), and by BMM (data not shown). Preincubation of Ox-RBCs in serum-containing media before addition to macrophages under serum-free conditions did not result in phagocytosis, suggesting that the serum components promoting phagocytosis did not bind to the RBCs (data not shown).
Macrophage phagocytosis of Ox-RBCs is dependent on serum and SRs. Phagocytosis of Cu/ascorbate-treated Ox-RBCs ( ), or PBS-treated RBCs (□), by RPM (A), or bone marrow–derived macrophages (B). (C) Phagocytosis of Ox-RBCs by RPM is strongly inhibited in the absence of FCS. (D) Macrophage phagocytosis of Ox-RBCs is inhibited by the SR ligands dextran sulfate (100 μg/mL), fucoidan (100 μg/mL), carrageenan (100 μg/mL), and poly-I (50 μg/mL), but not by the non-SR ligands poly-A (50 μg/mL), poly-C (50 μg/mL), or chondroitin sulfate (100 μg/mL). (E) Functional blocking antibodies to SR-A or CD36 (25 μg/mL) do not inhibit phagocytosis of Ox-RBCs by RPM. (F) Phagocytosis of Ox-RBCs is not affected in the presence of 10% IgG-depleted FCS. In panels D-F, control refers to incubation in DMEM with 10% FCS. In phagocytosis experiments, RBCs were incubated with adherent macrophages for 60 minutes at 37°C. Following lysis of noningested RBCs and fixation/staining, the number of ingested RBCs was counted in 200 to 300 macrophages per coverslip and expressed as a phagocytosis index (number of ingested RBCs/100 macrophages). Data are mean plus or minus SEM for 3 to 6 separate experiments. *P < .05 and **P < .01, compared with control, using the Student t test for paired comparisons.
), or PBS-treated RBCs (□), by RPM (A), or bone marrow–derived macrophages (B). (C) Phagocytosis of Ox-RBCs by RPM is strongly inhibited in the absence of FCS. (D) Macrophage phagocytosis of Ox-RBCs is inhibited by the SR ligands dextran sulfate (100 μg/mL), fucoidan (100 μg/mL), carrageenan (100 μg/mL), and poly-I (50 μg/mL), but not by the non-SR ligands poly-A (50 μg/mL), poly-C (50 μg/mL), or chondroitin sulfate (100 μg/mL). (E) Functional blocking antibodies to SR-A or CD36 (25 μg/mL) do not inhibit phagocytosis of Ox-RBCs by RPM. (F) Phagocytosis of Ox-RBCs is not affected in the presence of 10% IgG-depleted FCS. In panels D-F, control refers to incubation in DMEM with 10% FCS. In phagocytosis experiments, RBCs were incubated with adherent macrophages for 60 minutes at 37°C. Following lysis of noningested RBCs and fixation/staining, the number of ingested RBCs was counted in 200 to 300 macrophages per coverslip and expressed as a phagocytosis index (number of ingested RBCs/100 macrophages). Data are mean plus or minus SEM for 3 to 6 separate experiments. *P < .05 and **P < .01, compared with control, using the Student t test for paired comparisons.
Macrophage phagocytosis of Ox-RBCs is dependent on serum and SRs. Phagocytosis of Cu/ascorbate-treated Ox-RBCs ( ), or PBS-treated RBCs (□), by RPM (A), or bone marrow–derived macrophages (B). (C) Phagocytosis of Ox-RBCs by RPM is strongly inhibited in the absence of FCS. (D) Macrophage phagocytosis of Ox-RBCs is inhibited by the SR ligands dextran sulfate (100 μg/mL), fucoidan (100 μg/mL), carrageenan (100 μg/mL), and poly-I (50 μg/mL), but not by the non-SR ligands poly-A (50 μg/mL), poly-C (50 μg/mL), or chondroitin sulfate (100 μg/mL). (E) Functional blocking antibodies to SR-A or CD36 (25 μg/mL) do not inhibit phagocytosis of Ox-RBCs by RPM. (F) Phagocytosis of Ox-RBCs is not affected in the presence of 10% IgG-depleted FCS. In panels D-F, control refers to incubation in DMEM with 10% FCS. In phagocytosis experiments, RBCs were incubated with adherent macrophages for 60 minutes at 37°C. Following lysis of noningested RBCs and fixation/staining, the number of ingested RBCs was counted in 200 to 300 macrophages per coverslip and expressed as a phagocytosis index (number of ingested RBCs/100 macrophages). Data are mean plus or minus SEM for 3 to 6 separate experiments. *P < .05 and **P < .01, compared with control, using the Student t test for paired comparisons.
), or PBS-treated RBCs (□), by RPM (A), or bone marrow–derived macrophages (B). (C) Phagocytosis of Ox-RBCs by RPM is strongly inhibited in the absence of FCS. (D) Macrophage phagocytosis of Ox-RBCs is inhibited by the SR ligands dextran sulfate (100 μg/mL), fucoidan (100 μg/mL), carrageenan (100 μg/mL), and poly-I (50 μg/mL), but not by the non-SR ligands poly-A (50 μg/mL), poly-C (50 μg/mL), or chondroitin sulfate (100 μg/mL). (E) Functional blocking antibodies to SR-A or CD36 (25 μg/mL) do not inhibit phagocytosis of Ox-RBCs by RPM. (F) Phagocytosis of Ox-RBCs is not affected in the presence of 10% IgG-depleted FCS. In panels D-F, control refers to incubation in DMEM with 10% FCS. In phagocytosis experiments, RBCs were incubated with adherent macrophages for 60 minutes at 37°C. Following lysis of noningested RBCs and fixation/staining, the number of ingested RBCs was counted in 200 to 300 macrophages per coverslip and expressed as a phagocytosis index (number of ingested RBCs/100 macrophages). Data are mean plus or minus SEM for 3 to 6 separate experiments. *P < .05 and **P < .01, compared with control, using the Student t test for paired comparisons.
Macrophage SRs have been implicated in recognition of oxidatively damaged RBCs.4,7,19 Therefore, we next studied whether serum-dependent phagocytosis of Ox-RBCs by RPM was sensitive to a panel of known SR ligands.16,35 Phagocytosis of Ox-RBCs was inhibited by fucoidan, dextran sulfate, poly-I, and carrageenan, but not by the non-SR ligands poly-A, poly-C, or chondroitin sulfate (Figure 1D). Since the SR-ligands that inhibited uptake of Ox-RBCs may bind to SR-A,16 we studied the role of SR-A using the functional blocking mAb 2F8. Preincubation of RPM with mAb 2F8 did not affect uptake of Ox-RBCs (Figure 1E). A functional blocking antibody (mAb 63) against CD36 was also without effect on phagocytosis (Figure 1E). In addition, phagocytosis was affected neither by Arg-Gly-Asp-Ser (RGDS) nor Arg-Gly-Glu-Ser (RGES), which ruled out that integrins were involved (data not shown). To investigate whether FcγRs were important for serum-dependent phagocytosis of Ox-RBCs, we also used 10% IgG-depleted FCS and found that phagocytosis was not affected, compared with that in 10% untreated FCS (Figure 1F). In addition, treatment of macrophages with the FcγR-blocking mAb 2.4G2 did not inhibit serum-dependent phagocytosis (data not shown).
Reduced mobility of CD47 on the surface of Ox-RBCs
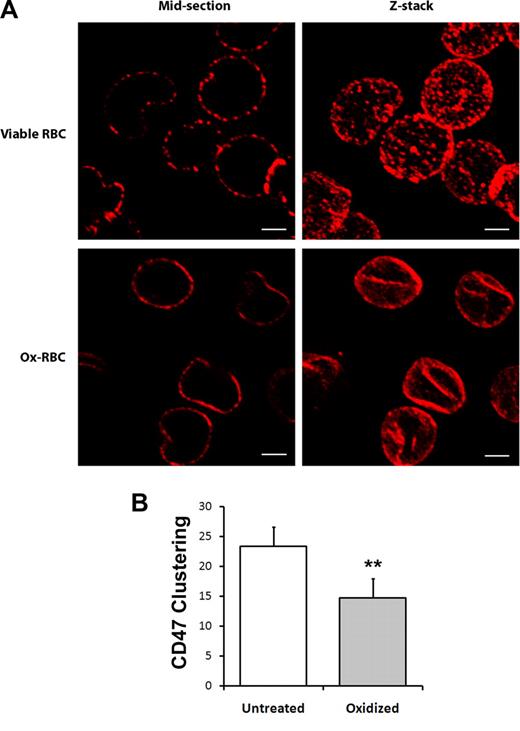
Most CD47 molecules in murine RBCs were shown to be freely mobile within the plasma membrane with minimal attachment to the cytoskeleton. A freely mobile pool of CD47, able to cluster at the contact site with the macrophage, was proposed to be required to generate phagocytosis-inhibitory signals.36,37 In agreement with these previous reports, we found that sequential labeling of CD47 on untreated RBCs with anti-CD47 and secondary antibodies before fixation resulted in clustering of CD47 into distinct patches on the cell surface (Figure 2A). However, similar sequential labeling of CD47 on Ox-RBCs showed reduced patchlike distribution of CD47 (Figure 2A). Quantification of clusters in confocal sections of labeled cells confirmed a significantly lower number of CD47 clusters in Ox-RBCs (P < .01; Figure 2B). Thus, these data indicated that the mobility of CD47 was significantly reduced in the plasma membrane of Ox-RBCs, compared with untreated RBCs.
Reduced mobility of CD47 in the plasma membrane of Ox-RBCs. Untreated (viable RBCs) or Cu/ascorbate-treated (Ox-RBCs) RBCs were incubated sequentially with the anti-CD47 mAb miap430 and Cy3-conjugated goat anti–mouse IgG, followed by fixation in 3% paraformaldehyde and 0.01% glutaraldehyde. (A) RBCs adherent to poly-l-lysine–coated coverslips were analyzed by laser-scanning confocal microscopy. Z-stacks were obtained by scanning focal sections 0.5 μm apart throughout the cells. Scale bar represents 2 μm. Images are representative of 3 independent experiments. (B) The number of visible CD47 clusters was quantified in midsections of untreated RBCs or Ox-RBCs. Data are mean plus or minus SEM for 3 experiments with 50 RBCs analyzed in each experiment. **P < .01, using Student t test for paired comparisons.
Reduced mobility of CD47 in the plasma membrane of Ox-RBCs. Untreated (viable RBCs) or Cu/ascorbate-treated (Ox-RBCs) RBCs were incubated sequentially with the anti-CD47 mAb miap430 and Cy3-conjugated goat anti–mouse IgG, followed by fixation in 3% paraformaldehyde and 0.01% glutaraldehyde. (A) RBCs adherent to poly-l-lysine–coated coverslips were analyzed by laser-scanning confocal microscopy. Z-stacks were obtained by scanning focal sections 0.5 μm apart throughout the cells. Scale bar represents 2 μm. Images are representative of 3 independent experiments. (B) The number of visible CD47 clusters was quantified in midsections of untreated RBCs or Ox-RBCs. Data are mean plus or minus SEM for 3 experiments with 50 RBCs analyzed in each experiment. **P < .01, using Student t test for paired comparisons.
CD47 does not inhibit serum-dependent macrophage phagocytosis of unopsonized Ox-RBCs
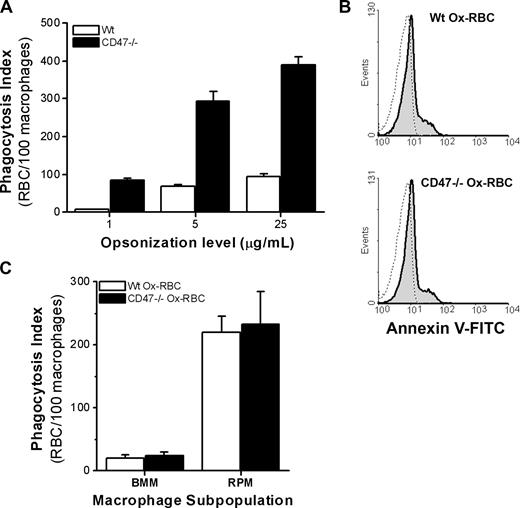
Macrophage phagocytosis of opsonized RBCs is negatively regulated by CD47 on the RBC.22,23 Indeed, we found that IgG-opsonized CD47−/− RBCs were phagocytosed 4 to 5 times more efficiently by RPM over a range of opsonization levels, compared with equally opsonized WT RBCs (Figure 3A). To understand how CD47 regulates macrophage uptake of senescent RBCs, we next investigated phagocytosis of WT or CD47−/− Ox-RBCs. WT and CD47−/− Ox-RBCs bound virtually equal amounts of annexin V (Figure 3B), and displayed the same size after oxidation, as determined by the flow cytometric forward scatter (FSC) profile (data not shown). Interestingly, in contrast to the strongly enhanced phagocytosis of IgG-opsonized CD47−/− RBCs (Figure 3A), we found no difference in the phagocytosis of WT or CD47−/− Ox-RBCs in either BMM or RPM (Figure 3C). Thus, in sharp contrast to that seen with IgG-opsonized RBCs, these experiments suggested that CD47 did not inhibit SR-mediated phagocytosis of Ox-RBCs.
CD47 inhibits FcγR-mediated phagocytosis of untreated RBCs, but not serum-dependent phagocytosis of Ox-RBCs. (A) WT or CD47−/− RBCs were opsonized with 1, 5, or 25 μg/mL mAb 34-3C, washed, and then incubated with RPM for 30 minutes at 37°C. Data are mean plus or minus SEM for 4 coverslips in one representative experiment of 3. (B) Following oxidation with Cu/ascorbate for 60 minutes, WT Ox-RBCs and CD47−/− Ox-RBCs (gray histograms) bound similar levels of FITC-conjugated annexin V, as determined by flow cytometry (dotted line: RBCs incubated in PBS). (C) WT or CD47−/− Ox-RBCs were incubated with adherent BMM or RPM for 60 minutes at 37°C in the presence of serum. Phagocytosis was evaluated as described in the legend to Figure 1. Data represent mean plus or minus SEM for 3 to 6 individual experiments.
CD47 inhibits FcγR-mediated phagocytosis of untreated RBCs, but not serum-dependent phagocytosis of Ox-RBCs. (A) WT or CD47−/− RBCs were opsonized with 1, 5, or 25 μg/mL mAb 34-3C, washed, and then incubated with RPM for 30 minutes at 37°C. Data are mean plus or minus SEM for 4 coverslips in one representative experiment of 3. (B) Following oxidation with Cu/ascorbate for 60 minutes, WT Ox-RBCs and CD47−/− Ox-RBCs (gray histograms) bound similar levels of FITC-conjugated annexin V, as determined by flow cytometry (dotted line: RBCs incubated in PBS). (C) WT or CD47−/− Ox-RBCs were incubated with adherent BMM or RPM for 60 minutes at 37°C in the presence of serum. Phagocytosis was evaluated as described in the legend to Figure 1. Data represent mean plus or minus SEM for 3 to 6 individual experiments.
CD47 on Ox-RBCs can still inhibit FcγR-mediated phagocytosis
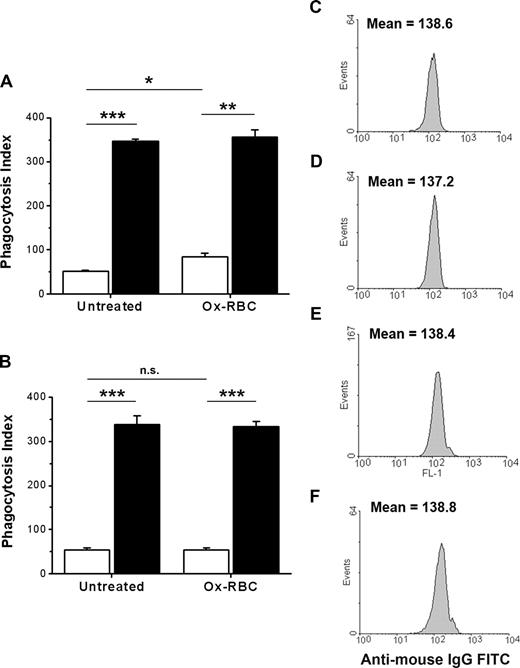
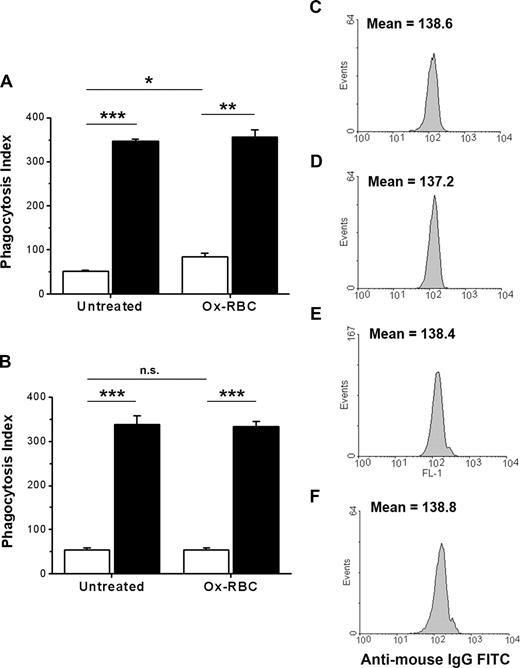
The findings that CD47 could inhibit FcγR-mediated phagocytosis, but not SR-mediated phagocytosis, suggest that the function of CD47 on Ox-RBCs could be compromised or that uptake by SRs could be resistant to CD47-mediated inhibition. To test this, we compared phagocytosis of IgG-opsonized untreated WT or CD47−/− RBCs with that of IgG-opsonized WT or CD47−/− Ox-RBCs. There is strong evidence that naturally occurring antibodies recognizing senescent RBCs in vivo recognize Band 3.38 Thus, we used the antimouse Band 3 mAb 34-3C for opsonization, a mAb that induces RBC phagocytosis both in vivo and in vitro and shows enhanced binding to aged murine RBCs in vivo.23,28,31 In the presence of serum, phagocytosis of IgG-opsonized WT Ox-RBCs was increased, compared with equally opsonized untreated WT RBCs (84.3 ± 8.0 vs 52.3 ± 0.5 RBCs/100 macrophages, respectively; P < .05) (Figure 4A). Phagocytosis of IgG-opsonized CD47−/− RBCs was strongly increased for both untreated RBCs and Ox-RBCs, compared with equally opsonized corresponding WT RBCs (P < .001 and P < .01, respectively; Figure 4A). The experiments presented in Figure 4A were performed in the presence of serum, meaning that the phagocytosis of Ox-RBCs was likely dependent on both SRs and FcγRs. Therefore, to specifically study how CD47 on Ox-RBCs affected FcγR-mediated phagocytosis, we performed similar experiments in the absence of serum. As expected, phagocytosis of IgG-opsonized untreated RBCs was not significantly affected by serum-free conditions (Figure 4A,B). However, in the absence of serum, phagocytosis of IgG-opsonized WT Ox-RBCs was reduced to the levels seen with untreated WT RBCs (Figure 4B). Equal opsonization of untreated WT or CD47−/− RBCs (Figure 4C,D), or WT or CD47−/− Ox-RBCs (Figure 4E,F), was confirmed by flow cytometry. Using calibration beads and flow cytometry, we also determined that this level of opsonization resulted in a deposition of 31 310 plus or minus 1696 IgG molecules/RBC. Thus, although CD47 on Ox-RBCs did not affect SR-dependent phagocytosis, it could still inhibit FcγR-mediated phagocytosis.
CD47 on Ox-RBCs effectively inhibits FcγR-mediated phagocytosis. Untreated RBCs or Ox-RBCs from WT mice (□) or CD47−/− mice (■) were opsonized with mAb 34-3C, washed, and incubated with RPM (A) in the presence of 10% FCS, or (B) in the absence of FCS for 30 minutes at 37°C. Following lysis of uningested RBCs, phagocytosis was determined as described in the legend to Figure 1. Data are mean plus or minus SEM for 4 separate coverslips per group in one representative experiment of 3. Untreated WT RBCs (C), untreated CD47−/− RBCs (D), WT Ox-RBCs (E), and CD47−/− Ox-RBCs (F) were analyzed by flow cytometry to confirm equal opsonization, using FITC anti–mouse IgG. *P < .05; **P < .01; and ***P < .001, using the Student t test for paired comparisons. ns indicates not significant.
CD47 on Ox-RBCs effectively inhibits FcγR-mediated phagocytosis. Untreated RBCs or Ox-RBCs from WT mice (□) or CD47−/− mice (■) were opsonized with mAb 34-3C, washed, and incubated with RPM (A) in the presence of 10% FCS, or (B) in the absence of FCS for 30 minutes at 37°C. Following lysis of uningested RBCs, phagocytosis was determined as described in the legend to Figure 1. Data are mean plus or minus SEM for 4 separate coverslips per group in one representative experiment of 3. Untreated WT RBCs (C), untreated CD47−/− RBCs (D), WT Ox-RBCs (E), and CD47−/− Ox-RBCs (F) were analyzed by flow cytometry to confirm equal opsonization, using FITC anti–mouse IgG. *P < .05; **P < .01; and ***P < .001, using the Student t test for paired comparisons. ns indicates not significant.
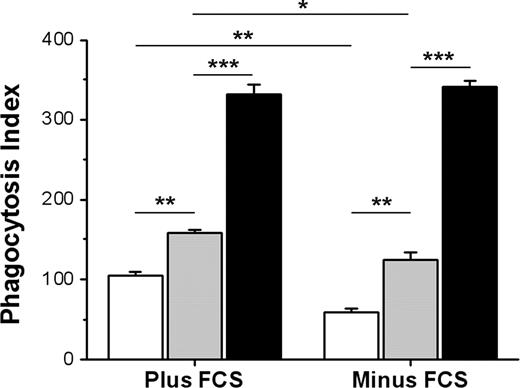
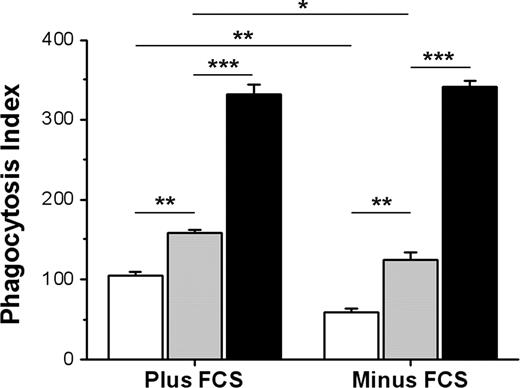
RBCs from CD47+/− mice express about 50% less CD47 compared with WT RBCs,27,32 resulting in a gene-dose effect of CD47 in terms of FcγR inhibition.27 Aged RBCs in circulation were also shown to express reduced levels of CD47.28,29 We therefore compared phagocytosis of IgG-opsonized WT, CD47+/−, or CD47−/− Ox-RBCs. Both in the presence and absence of serum, phagocytosis of CD47+/− Ox-RBCs was significantly elevated compared with that of WT Ox-RBCs, but significantly reduced compared with CD47−/− Ox-RBCs (Figure 5). Similar to the data presented in Figure 4, uptake of WT and CD47+/− Ox-RBCs was significantly reduced in the absence of serum. Thus, CD47 on Ox-RBCs maintained the capacity to dose-dependently inhibit FcγR-mediated phagocytosis.
Gene-dose effect of CD47 on inhibition of FcγR-mediated phagocytosis of Ox-RBCs. Ox-RBCs from WT (□), CD47+/− ( ), or CD47−/− (■) mice were opsonized with mAb 34-3C, washed, and incubated with RPM in the presence or absence of 10% FCS for 30 minutes at 37°C. Following lysis of uningested RBCs, phagocytosis was determined as described in the legend to Figure 1. Equal opsonization was confirmed following labeling of opsonized RBCs with anti–mouse IgG FITC, using flow cytometry, showing the following mean fluorescence intensity (MFI) values: WT Ox-RBCs = 135.1, CD47+/− Ox-RBCs = 134.0, and CD47−/− Ox-RBCs = 134.4. Data are mean plus or minus SEM for 4 separate coverslips per group in one representative experiment. *P < .05; **P < .01; and ***P < .001, using the Student t test for paired comparisons. ns indicates not significant.
), or CD47−/− (■) mice were opsonized with mAb 34-3C, washed, and incubated with RPM in the presence or absence of 10% FCS for 30 minutes at 37°C. Following lysis of uningested RBCs, phagocytosis was determined as described in the legend to Figure 1. Equal opsonization was confirmed following labeling of opsonized RBCs with anti–mouse IgG FITC, using flow cytometry, showing the following mean fluorescence intensity (MFI) values: WT Ox-RBCs = 135.1, CD47+/− Ox-RBCs = 134.0, and CD47−/− Ox-RBCs = 134.4. Data are mean plus or minus SEM for 4 separate coverslips per group in one representative experiment. *P < .05; **P < .01; and ***P < .001, using the Student t test for paired comparisons. ns indicates not significant.
Gene-dose effect of CD47 on inhibition of FcγR-mediated phagocytosis of Ox-RBCs. Ox-RBCs from WT (□), CD47+/− ( ), or CD47−/− (■) mice were opsonized with mAb 34-3C, washed, and incubated with RPM in the presence or absence of 10% FCS for 30 minutes at 37°C. Following lysis of uningested RBCs, phagocytosis was determined as described in the legend to Figure 1. Equal opsonization was confirmed following labeling of opsonized RBCs with anti–mouse IgG FITC, using flow cytometry, showing the following mean fluorescence intensity (MFI) values: WT Ox-RBCs = 135.1, CD47+/− Ox-RBCs = 134.0, and CD47−/− Ox-RBCs = 134.4. Data are mean plus or minus SEM for 4 separate coverslips per group in one representative experiment. *P < .05; **P < .01; and ***P < .001, using the Student t test for paired comparisons. ns indicates not significant.
), or CD47−/− (■) mice were opsonized with mAb 34-3C, washed, and incubated with RPM in the presence or absence of 10% FCS for 30 minutes at 37°C. Following lysis of uningested RBCs, phagocytosis was determined as described in the legend to Figure 1. Equal opsonization was confirmed following labeling of opsonized RBCs with anti–mouse IgG FITC, using flow cytometry, showing the following mean fluorescence intensity (MFI) values: WT Ox-RBCs = 135.1, CD47+/− Ox-RBCs = 134.0, and CD47−/− Ox-RBCs = 134.4. Data are mean plus or minus SEM for 4 separate coverslips per group in one representative experiment. *P < .05; **P < .01; and ***P < .001, using the Student t test for paired comparisons. ns indicates not significant.
Signaling pathways involved in mediating uptake of Ox-RBCs or IgG-opsonized RBCs
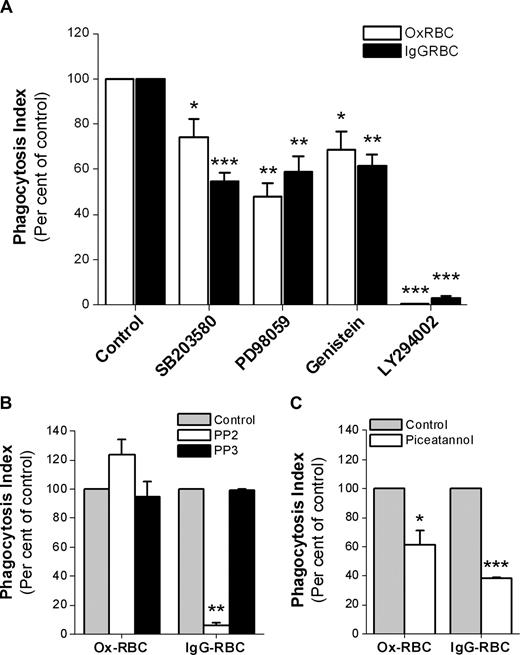
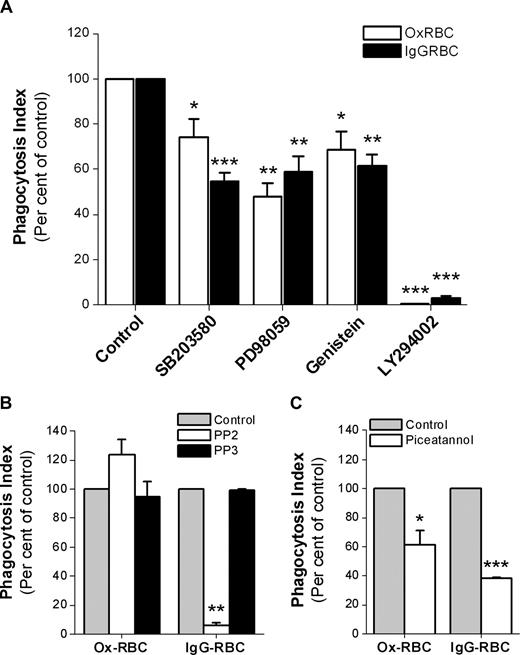
Since CD47 did not seem to inhibit SR-mediated phagocytosis, but strongly prevented FcγR-mediated phagocytosis, we next investigated some of the signaling pathways involved for each mode of uptake. Using WT Ox-RBCs or IgG-opsonized WT RBCs, we found that uptake of both types of targets was sensitive to the phosphatidylinositol 3 (PI3)–kinase inhibitor LY294002, the tyrosine kinase inhibitor genestein, the extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitor PD98059, and the p38 MAPK inhibitor SB203580 (Figure 6A). Interestingly, the Src-family kinase inhibitor PP2 at 10 μM had a strong inhibitory effect on FcγR-mediated phagocytosis, but did not inhibit SR-mediated phagocytosis of Ox-RBCs (Figure 6B). Even at a PP2 concentration of 30 μM, we found no inhibitory effects on SR-mediated uptake of Ox-RBCs (data not shown). Experiments using the Syk-inhibitor piceatannol showed that phagocytosis of both types of targets required Syk activation (Figure 6C).
Signaling pathways involved in macrophage FcγR- or SR-mediated phagocytosis. Phagocytosis of WT Ox-RBCs or IgG-opsonized WT RBCs by RPM was studied in the presence or absence of (A) the p38 MAPK-inhibitor SB203580 (30 μM; n = 8), the ERK1/2-inhibitor PD98059 (30 μM; n = 4), the tyrosine kinase–inhibitor genistein (100 μM; n = 4), or the PI3 kinase–inhibitor LY294002 (50 μM; n = 4); (B) the Src kinase family–inhibitor PP2 (10 μM; n = 3), or the inactive control substance PP3 (10 μM; n = 3); or (C) the Syk tyrosine kinase–inhibitor piceatannol (25 μM; n = 3). In the controls, equal amounts of dimethylsulfoxide (DMSO) were added. Macrophages were preincubated with the indicated inhibitors at 37°C for 30 minutes prior to addition of RBCs in the presence of 10% FCS for another 30 minutes. Following lysis of uningested RBCs, phagocytosis was determined as described in the legend to Figure 1. Data are mean plus or minus SEM for the indicated number of experiments. *P < .05; **P < .01; and ***P < .001, using the Student t test for paired comparisons.
Signaling pathways involved in macrophage FcγR- or SR-mediated phagocytosis. Phagocytosis of WT Ox-RBCs or IgG-opsonized WT RBCs by RPM was studied in the presence or absence of (A) the p38 MAPK-inhibitor SB203580 (30 μM; n = 8), the ERK1/2-inhibitor PD98059 (30 μM; n = 4), the tyrosine kinase–inhibitor genistein (100 μM; n = 4), or the PI3 kinase–inhibitor LY294002 (50 μM; n = 4); (B) the Src kinase family–inhibitor PP2 (10 μM; n = 3), or the inactive control substance PP3 (10 μM; n = 3); or (C) the Syk tyrosine kinase–inhibitor piceatannol (25 μM; n = 3). In the controls, equal amounts of dimethylsulfoxide (DMSO) were added. Macrophages were preincubated with the indicated inhibitors at 37°C for 30 minutes prior to addition of RBCs in the presence of 10% FCS for another 30 minutes. Following lysis of uningested RBCs, phagocytosis was determined as described in the legend to Figure 1. Data are mean plus or minus SEM for the indicated number of experiments. *P < .05; **P < .01; and ***P < .001, using the Student t test for paired comparisons.
Discussion
Macrophage phagocytosis of normal RBCs is negatively regulated by CD47. The results of the present study show that phagocytosis of experimentally senescent RBCs may also be negatively regulated by CD47 on the RBCs. However, whether this is the case seems to be determined by the way the RBC is recognized by the macrophage. We found that phagocytosis of Ox-RBCs was strongly dependent on serum and involved recognition by SRs, which was not affected by CD47 on the RBCs. In contrast, despite a reduced mobility of CD47 in the plasma membrane of Ox-RBCs, CD47 could still inhibit FcγR-mediated phagocytosis to virtually the same extent as seen with untreated RBCs.
RBC senescence is associated with several physical and chemical alterations, such as an increased density, loss of lipid asymmetry, accumulation of lipid peroxidation products in the plasma membrane, formation of senescent cell antigens, and an increased amount of immunoglobulins and complement factor C3b on the cell surface.1,14 Although physical changes to the senescent RBCs can reduce their filterability through narrow passages, and result in subsequent hemolysis, most senescent RBCs are recognized and phagocytosed by macrophages in the spleen and liver.1 The mechanisms behind the recognition and uptake of senescent RBCs by macrophages are not yet fully understood, but may involve mechanisms described for macrophage interaction with apoptotic cells in general.39,40 Lipid peroxidation increases with time during RBC aging.1,41,42 As such, oxidation creates RBC surface ligands, which can be recognized by macrophages by mechanisms that are similar to recognition of oxLDL.4 In vitro oxidation of RBCs results in aldehyde formation and membrane protein cross-linking, lipid peroxidation, and possibly also conjugation of lipid fragments to proteins, and protein degradation.4,5 OxLDL and other ligands of SRs (eg, poly-I, fucoidan, and dextran sulfate) can inhibit macrophage recognition of Ox-RBCs,4,7,19 which we confirmed in the present study. Sialosaccharide chains of glycophorin, which were found to cluster or aggregate in the membrane of senescent RBCs, represent one candidate ligand for recognition of oxidized or aged RBCs by macrophage SRs.43 However, the exact nature of the SRs and RBC ligands involved in recognition and uptake of Ox-RBCs is still not completely understood. Based on the SR inhibitors capable of interfering with phagocytosis of Ox-RBCs, SR-A would be one candidate.16 However, SR-A deficiency does not affect uptake of Ox-RBCs in vivo or in vitro,7,44 which is in line with our findings that a blocking SR-A antibody does not inhibit phagocytosis of Ox-RBCs in vitro. Another SR, CD36, can function as a receptor for PS,16 but our experiments with a blocking CD36 antibody showed no effects on uptake of Ox-RBCs. Thus, the specific macrophage SRs mediating uptake of Ox-RBCs still need to be identified.
We found that phagocytosis of Ox-RBCs was critically dependent on serum. Indeed, serum proteins such as complement or IgG were shown to be involved in uptake of senescent RBCs.6,12 However, since we used heat-inactivated serum, complement proteins were most likely not involved in our experiments. Although we have found that the phagocytosis-promoting effect of serum is associated with serum proteins larger than 100 kDa (M.O. and P.-A.O., unpublished data, February 2008), it is unlikely that serum IgG could be important, since we found that neither IgG-depleted serum nor anti-FcγR antibodies abolished phagocytosis of Ox-RBCs. The latter observation is in agreement with a previous report using Ox-RBCs and RPM.4 Our data also indicate that the phagocytosis-promoting factor(s) in serum does not bind to the surface of Ox-RBCs, since preincubation of RBCs with serum did not result in phagocytosis under serum-free conditions. Thus, the exact nature of the phagocytosis-promoting serum proteins needs to be further investigated.
Interestingly, despite the known function of CD47 to inhibit complement receptor or FcγR-mediated phagocytosis in macrophages, we found that WT and CD47−/− Ox-RBCs were taken up equally well by the macrophages. The vast majority of the CD47 molecules seem to be freely mobile within the plasma membrane of murine RBCs, and it has been proposed that a freely mobile pool of CD47 might be required to generate phagocytosis-inhibitory signals at the contact site with the macrophage.36,37 Our present data support previous findings, indicating a high capacity of CD47 to form clusters on the RBC surface following antibody cross-linking. However, clustering of CD47 was much less evident on Ox-RBCs, possibly due to oxidation-mediated aldehyde formation and membrane protein cross-linking. Thus, if the mobility of CD47 in the plasma membrane would be important for inhibition of phagocytosis, we would have expected an increased uptake of IgG-opsonized WT Ox-RBCs, compared with opsonized untreated RBCs. The fact that CD47 could inhibit FcγR-mediated phagocytosis of untreated RBCs or Ox-RBCs to virtually the same extent strongly suggests a potent inhibitory function despite reduced mobility of CD47 in the plasma membrane. In addition, these data also rule out that the phagocytosis-inhibitory function of CD47 is affected by oxidation of RBC membrane proteins or lipids.
Recent reports have shown that CD47 is lost from RBCs during storage,45,46 and studies in mice have also shown that older circulating RBCs express less CD47 on their surface.28,29 It was speculated that loss of CD47 on older RBCs might be one factor facilitating their removal from the circulation. However, our present findings indicate that the role of CD47 in this process might be dependent on how the senescent RBC is interacting with the macrophage. At least for serum-dependent prophagocytic mechanisms that require SR recognition, but are operating independent of complement or IgG, the presence or absence of CD47 does not seem to matter. On the other hand, when senescent RBCs are opsonized with IgG, the protective role of CD47 becomes apparent. Indeed, we find that phagocytosis of IgG-opsonized Ox-RBCs is gene dose–dependently inhibited by CD47, which is well in line with our previously published data on FcγR-mediated phagocytosis of untreated RBCs.27 In humans, Band 3–deficient cells and Rhnull cells have very little or no CD47, and circulating Band 3–deficient cells are coated with large amounts of IgG with increasing cell age.47,48 It is tempting to speculate that the combination of increased IgG opsonization and reduced CD47 expression makes these cells more prone to elimination by macrophages. Circulating antibodies, mostly anti–Band 3 antibodies, bind to senescent RBCs in vivo, possibly as a result of Band 3 aggregation in the plasma membrane of senescent RBCs.12,13 However, there is a large variation in the literature regarding the amount of IgG reported to bind senescent RBCs in vivo, which may be the result of methodological differences. Thus, using density fractionation to separate presumably senescent RBCs, only a few hundred IgG molecules were reported to bind each RBC.49 On the other hand, using in vivo biotinylation, it was found that up to 10 000 IgG molecules can bind to each senescent RBC.50 These numbers should be compared with the around 30 000 IgG molecules bound to the IgG-opsonized RBCs used in our in vitro phagocytosis assays to get reliable results on FcγR-mediated phagocytosis. It has been questioned whether the low level of opsonization occurring in vivo can induce efficient FcγR-mediated phagocytosis per se. It is more likely that anti–Band 3 bound to senescent RBCs will require deposition of C3b/C3bi for phagocytosis to occur.51 It is therefore interesting to note that CD47 can also inhibit phagocytosis of complement-opsonized RBCs.22
Phagocytosis of senescent RBCs is likely dependent on several separate mechanisms operating in parallel, all dependent on cell surface changes associated with the RBC senescence process. Phagocytosis through FcγR involves Src-mediated tyrosine phosphorylation of the FcγR immunoreceptor tyrosine-based activation motifs (ITAMs), recruitment of the tyrosine kinase Syk, and downstream activation of PI3 kinase and the MAPK pathways.15,52 We found that both FcγR and SR-mediated uptake was sensitive to inhibitors of Syk, PI3 kinase, MAPK, and tyrosine kinases. However, only FcγR-mediated, but not SR-mediated, uptake was also sensitive to the Src-family kinase inhibitor PP2, suggesting that Syk is activated by alternative mechanisms during uptake of Ox-RBCs. Although results from the present and previous studies suggest that SR-A is not involved in uptake of Ox-RBCs,7,44 it is interesting to note that SR-A–mediated AcLDL endocytosis is insensitive to Src inhibition by PP2.53 Although the prophagocytic receptors involved may differ, the signal transduction events found to be important for uptake of Ox-RBCs by RPM in the present study are in agreement with that described for uptake of apoptotic T cells by the same macrophage population.54
In conclusion, our findings extend the previous knowledge on CD47 and its function to inhibit phagocytosis of RBCs, to also include experimentally senescent RBCs. Although CD47 can potently inhibit FcγR-mediated phagocytosis of Ox-RBCs, SR-mediated Ox-RBC phagocytosis is not inhibited by CD47. Thus, depending on the exact prophagocytic receptors engaged on the macrophage, CD47 on a senescent RBC may have more or less effect in mediating inhibition of phagocytosis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Barbro Borgström for excellent technical assistance.
This work was supported by grants from the Swedish Research Council (31X-14286), the Swedish Society of Medicine, the J.C. Kempe Foundation, and the Faculty of Medicine, Umeå University.
Authorship
Contribution: M.O. designed and performed experiments, analyzed data, and edited the paper; and P.-A.O. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Per-Arne Oldenborg, Department of Integrative Medical Biology Section for Histology and Cell Biology, Umeå University, SE-901 87 Umeå, Sweden; e-mail: per-arne.oldenborg@histocel.umu.se.




 ), or PBS-treated RBCs (□), by RPM (A), or bone marrow–derived macrophages (B). (C) Phagocytosis of Ox-RBCs by RPM is strongly inhibited in the absence of FCS. (D) Macrophage phagocytosis of Ox-RBCs is inhibited by the SR ligands dextran sulfate (100 μg/mL), fucoidan (100 μg/mL), carrageenan (100 μg/mL), and poly-I (50 μg/mL), but not by the non-SR ligands poly-A (50 μg/mL), poly-C (50 μg/mL), or chondroitin sulfate (100 μg/mL). (E) Functional blocking antibodies to SR-A or CD36 (25 μg/mL) do not inhibit phagocytosis of Ox-RBCs by RPM. (F) Phagocytosis of Ox-RBCs is not affected in the presence of 10% IgG-depleted FCS. In panels D-F, control refers to incubation in DMEM with 10% FCS. In phagocytosis experiments, RBCs were incubated with adherent macrophages for 60 minutes at 37°C. Following lysis of noningested RBCs and fixation/staining, the number of ingested RBCs was counted in 200 to 300 macrophages per coverslip and expressed as a phagocytosis index (number of ingested RBCs/100 macrophages). Data are mean plus or minus SEM for 3 to 6 separate experiments. *P < .05 and **P < .01, compared with control, using the Student t test for paired comparisons.
), or PBS-treated RBCs (□), by RPM (A), or bone marrow–derived macrophages (B). (C) Phagocytosis of Ox-RBCs by RPM is strongly inhibited in the absence of FCS. (D) Macrophage phagocytosis of Ox-RBCs is inhibited by the SR ligands dextran sulfate (100 μg/mL), fucoidan (100 μg/mL), carrageenan (100 μg/mL), and poly-I (50 μg/mL), but not by the non-SR ligands poly-A (50 μg/mL), poly-C (50 μg/mL), or chondroitin sulfate (100 μg/mL). (E) Functional blocking antibodies to SR-A or CD36 (25 μg/mL) do not inhibit phagocytosis of Ox-RBCs by RPM. (F) Phagocytosis of Ox-RBCs is not affected in the presence of 10% IgG-depleted FCS. In panels D-F, control refers to incubation in DMEM with 10% FCS. In phagocytosis experiments, RBCs were incubated with adherent macrophages for 60 minutes at 37°C. Following lysis of noningested RBCs and fixation/staining, the number of ingested RBCs was counted in 200 to 300 macrophages per coverslip and expressed as a phagocytosis index (number of ingested RBCs/100 macrophages). Data are mean plus or minus SEM for 3 to 6 separate experiments. *P < .05 and **P < .01, compared with control, using the Student t test for paired comparisons.